Abstract
The mechanisms of radiation-induced bystander effects (RIBE) have been investigated intensively over the past two decades. Although quite a few reports demonstrated that cytokines such as TGF-β1 are induced within the directly irradiated cells and play critical roles in mediating the bystander effects, little is known about the signaling pathways that occur in bystander cells. The crucial question as to why RIBE signals cannot be infinitely transmitted, therefore, remains unclear. In the present study, we showed that miR-663, a radiosensitive microRNA, participates in the regulation of biological effects in both directly irradiated and bystander cells via its targeting of TGF-β1. MiR-663 was downregulated, while TGFB1 was upregulated in directly irradiated cells. The regulation profile of miR-663 and TGFB1, on the other hand, was reversed in bystander cells, in which an elevated miR-663 expression was exhibited and led to downregulation of TGF-β1. Further studies revealed that miR-663 interacts with TGFB1 directly and that through its binding to the core regulation sequence, miR-663 suppresses the expression of TGFB1. Based on the results, we propose that miR-663 inhibits the propagation of RIBE in a feedback mode, in which the induction of TGF-β1 by reduced miR-663 in directly irradiated cells leads to increased level of miR-663 in bystander cells. The upregulation of miR-663 in turn suppresses the expression of TGF-β1 and limits further transmission of the bystander signals.
Keywords: microRNA, TGF-β1, miR-663, bystander effects, ionizing radiation
Introduction
Radiation-induced bystander effects (RIBE), which are biological responses in cells that are not themselves in the path of ionizing radiation but receive signals transmitted from directly hit cells, will amplify or exaggerate the action of low dose radiation and thus can significantly increase radiation risk and tissue damage.1 In view of RIBE, existing radiotherapy models should be reconsidered and the carcinogenesis risk of low-dose radiation should be re-assessed. Therefore, a better understanding of the mechanisms underlying RIBE is essential.
It has been demonstrated that radiation-induced bystander effects can only be propagated within limited distance. Cells located as far as 1 mm in 3D cultured tissue2 and 7.5 mm in 2D cultured cells3 from the directly irradiated cells were shown to display cytotoxic and genotoxic effects that were believed to be the consequences of RIBE. It was proposed that both gap junction intercellular communication (GJIC)4 and soluble factors such as reactive oxygen species (ROS),5 nitric oxide (NO),6 calcium fluxes,7 and cytokines such as interleukin-8 (IL-8),8 tumor necrosis factor-α (TNF-α),9 and transforming growth factor-β1 (TGF-β1)10-12 generated by directly irradiated cells participate in the bystander effects. However, most of the studies were focused on signals exiting irradiated cells and likely detected by bystander cells. Questions as to whether bystander cells also send out signals and why RIBE can only be transmitted within a certain distance remain largely unanswered.
In recent years, accumulating evidences have shown that microRNAs (miRNAs) can act as modulators of biological processes including cellular responses to radiation.13 It was also demonstrated that miRNAs may participate in RIBE. For instance, miR-194 was demonstrated to be upregulated in bystander spleen.14 Irradiation of rat cranium triggered gender-specific deregulation of the microRNAome in the non-irradiated bystander spleen.15 Moreover, Kovalchuk et al. showed that the microRNAome profile is altered in bystander 3D human tissue models.16 Several major bystander endpoints were suggested to be mediated by changed expression of miRNAs. Since miRNAs are secreted to the outside of the cells and circulated in blood and body fluid,17,18 they may serve as long-distance signal transmitters of RIBE. However, the exact function of miRNAs in RIBE is yet to be determined.
TGF-β1 is a well-established sensor and transducer of radiation stress.19 Since the observation that TGF-β1 was increased in a dose-dependent manner in irradiated rat liver,20 multiple studies have demonstrated the involvement of TGF-β1 in direct radiation responses21-28 and as an important mediator in RIBE.10-12 It was suggested that TGF-β1 is induced by radiation at both transcriptional23 and post-transcriptional levels.29 However, the role of miRNAs in TGF-β1-mediated responses toward radiation is still largely unknown.
In a previous study, we observed altered miRNA expression profile in human cervical carcinoma cells (HeLa) irradiated with X-rays, suggesting a regulatory role of miRNAs upon radiation treatment.30 Here, we focused our studies on miR-663 which was previously shown to be downregulated in renal carcinoma tissues exposed to X-ray irradiation31 and showed that miR-663 is significantly downregulated upon irradiation but increased in bystander cells. Further, the miRNA targets TGFB1 in a feedback mode.
Results
MiR-663 was downregulated in directly irradiated cells
A previous microRNA chip analysis showed that miR-663 was significantly downregulated in renal carcinoma tissues exposed to X-ray irradiation, regardless of the dosage used.31 These changes were validated in HeLa cells using qRT-PCR. Consistent with chip results, a significant decrease of miR-663 expression was observed following irradiation of cells with 4 Gy of X-rays. Interestingly, miR-663 level in cells irradiated with 8 Gy X-rays was similar with that of the control, non-irradiated cells (Fig. 1A). In addition, alteration of miR-663 expression was time-dependent. Its level was decreased by 40% of the sham control two hours after irradiation, remained relatively unchanged till 24 h post-irradiation and started to increase afterwards. Expression of miR-663 was returned back to control level by 48 h after irradiation (Fig. 1B).
Figure 1.
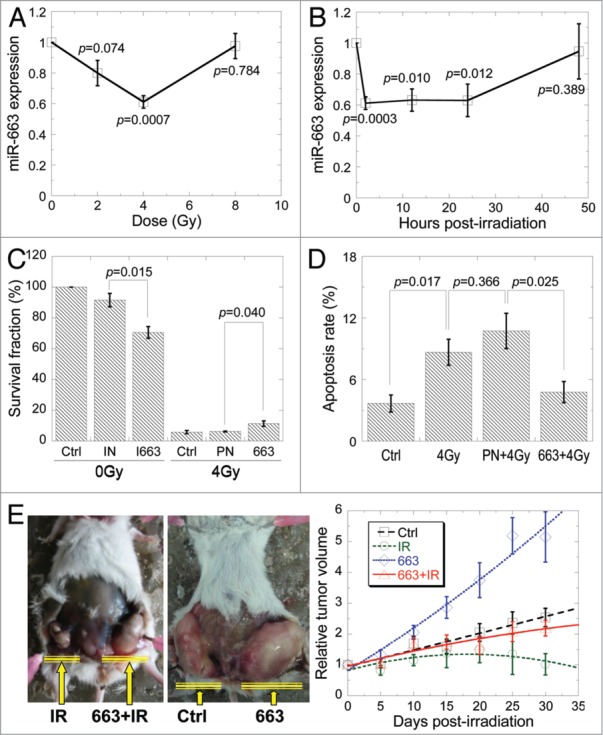
MiR-663 was downregulated in directly irradiated cells. (A) qRT-PCR was performed to detect miR-663 expression in HeLa cells 2 h after exposure to different doses of X-rays. (B) MiR-663 levels in HeLa cells exposed to 4 Gy X-ray irradiation at different time-points were detected using qRT-PCR. (C) Colony forming assay was performed on HeLa cells subjected to different treatments, after which cells were allowed to grow for 13 d. 663, miR-663 mimics; I663, miR-663 inhibitors; PN, negative control for miR-663 mimics; IN, negative control for miR-663 inhibitors. (D) Apoptosis rates of HeLa cells subjected to 4 Gy X-ray irradiation plus transfection with either miR-663 mimics or negative control (PN) were assayed with Hoechst33342/PI double staining. (E) Tumors generated by a stable HeLa cell line with inducible miR-663 expression in NOD/SCID mice 1 mo after 5 Gy X-ray irradiation and/or the induction of miR-663 expression. Tumor volumes were measured every 5 d and normalized to those obtained immediately before irradiation. The tumor volumes were measured using the formula ab2 × π/6, whereby a represents the length and b is the width. n = 10 flanks. Data were obtained from at least three independent experiments, and presented as means ± SE P values between the indicated samples and sham control (Ctrl) are additionally presented.
To investigate the biological significance of miR-663 downregulation, we measured cell survival and apoptosis of cultured cells transfected with miR-663 inhibitors. As shown in Figure 1C, cell survival rate was significantly decreased in miR-663 inhibitor transfected cells. When cells were treated with 4 Gy X-rays, the survival rate was decreased to 5.88 ± 1.05% (P < 0.01) and the level of apoptosis increased from 3.68 ± 0.83% to 8.66 ± 1.27% (P < 0.05). However, when cells were simultaneously transfected with miR-663, the survival rate was increased (to 11.38 ± 1.76%) while the level of apoptosis reduced (from 8.66 ± 1.27% to 4.79 ± 1.03%) (Figs. 1C and D). To further verify these effects on molecular levels, Bcl-2, Ki67 and caspase-3 were detected. It is shown that miR-663 restored Bcl-2 and Ki67 expression while inhibited caspase-3 activity in directly hit cells (Figs. S1–3). Moreover, to confirm these findings in vivo, we established an animal model with inducible HeLa-TetR-663 cell lines based on the tetracycline operator system (Fig. S4). Our results showed that tumors overexpressing miR-663 grow significantly faster than control tumors (Fig. 1E). X-ray irradiation led to significant suppression of tumor growth up to 30 d in our study. However, overexpression of miR-663 abolished the anti-tumor effects of radiation (Fig. 1E). These results implied that miR-663 is likely an oncogene and that ionizing radiation may suppress its expression, which suggests a possible involvement of this microRNA in cellular radiosensitivity.
MiR-663 upregulation was observed in bystander cells
Since miR-663 was downregulated in directly irradiated HeLa cells, we next wanted to see if this is also the case in bystander cells. To our surprise, miR-663 level was increased instead in cells treated with the medium harvested from the cultures for directly irradiated cells (conditioned medium, CM) (Fig. 2A). 53BP1 foci and MN formation analysis both showed that upon conditioned medium treatment, miR-663 inhibitor transfection led to a greater DNA damage effect than those cells that were transfected with miR-663 mimics (Figs. 2B and 2C), indicating a reversed relationship between miR-663 level in bystander cells and the radiation-induced bystander effect. Such a case was also observed in co-culture experiments, in which bystander cells were situated in the same medium together with the directly irradiated cells (Fig. 2D). Besides, we also detected the effects of miR-663 on DNA damage in directly hit cells and obtained the similar results (Fig. S5).
Figure 2.
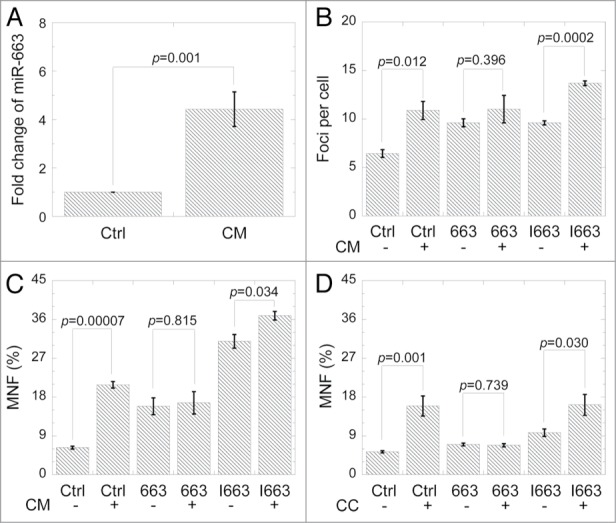
MiR-663 upregulation was observed in bystander cells. (A) MiR-663 expression in bystander HeLa cells 12 h after receiving conditioned medium from HeLa cells subjected to 4 Gy X-ray irradiation. (B) 24 h after transfection with miR-663 mimics or inhibitors, yields of 53BP1 foci in bystander HeLa cells cultured for 2 h in medium conditioned by HeLa cells for 24 h after 4 Gy X-ray irradiation. (C) 24 h after transfection with miR-663 mimics or inhibitors, MNF in bystander HeLa cells cultured in medium conditioned by HeLa cells for 24 h after treatment with 4 Gy X-ray irradiation. (D) 24 h after transfection with miR-663 mimics or inhibitors, MNF in bystander HeLa cells co-cultured with HeLa cells exposed to 4 Gy X-rays. Data were obtained from at least three independent experiments, and presented as means ± SE P values between the indicated samples and sham control are also presented.
TGF-β1 is a mediator of radiation-induced bystander effects
As mentioned previously, TGF-β1 acts as a signal molecule in RIBE. Experiments were performed with HeLa cells to confirm the role of TGF-β1 in RIBE. TGFB1 transcript level was increased more than 2-folds in HeLa cells exposed to 4 Gy X-rays. However, similar as that of miR-663, TGFB1 level was unchanged upon 8 Gy irradiation (Fig. 3A). The upregulation of TGFB1 was time-dependent, peaked at 2 h and reduced back to sham control level 24 h post-irradiation (Fig. 3B). TGF-β1 protein level was also induced evidently after exposure to the indicated X-ray doses (Fig. 3C). For cells treated with 4 Gy X-rays, TGF-β1 increased marginally till 12 h after irradiation and declined back to non-irradiated control level by 48 h. TGF-β1 concentration in the CM was increased from 19.4 ± 4.8 pg/mL to 61.2 ± 5.6 pg/mL two hours after 4 Gy irradiation (P < 0.01) and remained unchanged till 48 h post-irradiation. It was suppressed by miR-663 expression while increased by miR-663 inhibition (Fig. 3D). We further measured CM-induced DNA damage in bystander cells with 53BP1 foci and micronucleus formation assays. The number of 53BP1 foci increased significantly in bystander cells (P < 0.001) but was greatly suppressed by TGF-β1 neutralization (Fig. 3E). Additionally, MNF increased dramatically in bystander cells after CM transfer (P < 0.001). Again, addition of TGF-β1 neutralization antibody could reduce such an increment (P < 0.01) (Fig. 3F).
Figure 3.
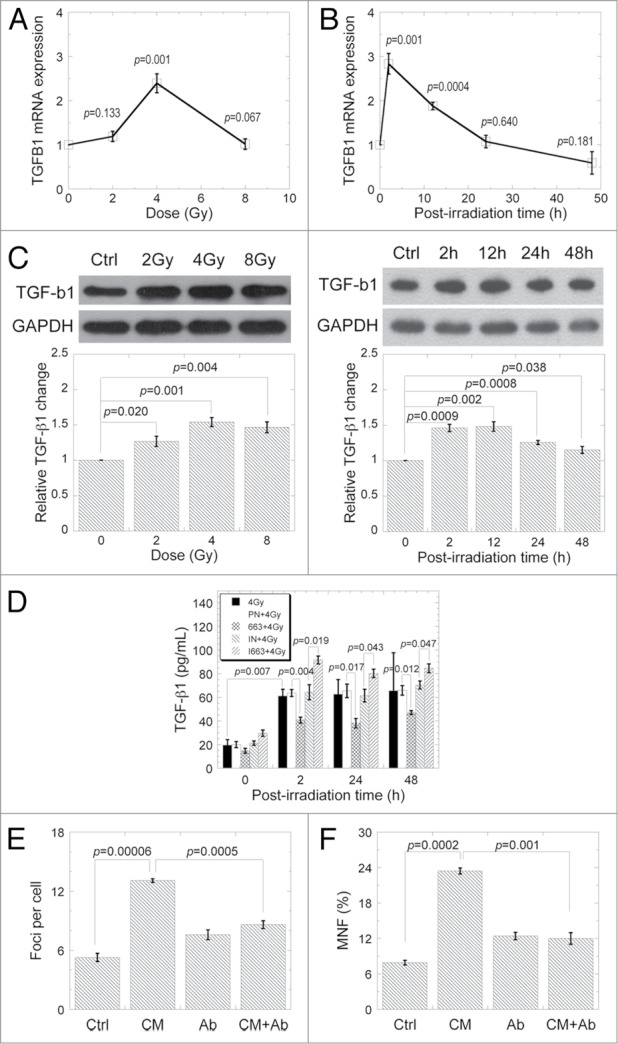
TGF-β1 is a mediator of radiation-induced bystander effects. TGFB1 mRNA levels were detected using qRT-PCR in HeLa cells 2 h after exposure to different doses of X-ray irradiation. (B) TGFB1 mRNA levels were detected in HeLa cells exposed to 4 Gy X-ray irradiation at different time-points. (C) TGF-β1 protein levels were examined by western blot in HeLa cells 2 h after exposure to different doses of X-ray irradiation, or in HeLa cells exposed to 4 Gy X-ray irradiation at different time-points. Western blot results from three independent experiments were quantified using ImageJ and a representative result is shown. (D) TGF-β1 protein level in the culture medium. After exposure to 4 Gy X-rays, HeLa cell culture medium was harvested at the indicated time-points for measurement of TGF-β1 protein with an ELISA kit. (E) Yields of 53BP1 foci in bystander HeLa cells cultured for 2 h in medium harvested from HeLa cells 24 h after exposuse to 4 Gy X-rays. (F) Frequency of micronuclei (MNF) in bystander HeLa cells cultured in medium harvested from HeLa cells 24 h after exposure to 4 Gy X-rays. Data from at least three independent experiments are presented as means ± SE.
MiR-663 directly targets TGFB1 and suppresses its expression
The findings that miR-663 level was altered both in directly irradiated and bystander cells suggested that this miRNA may play an important role in cellular response to X-ray irradiation. According to online software such as TargetScan, miRanda, etc., TGFB1 was universally predicted to be a possible target of miR-663. In addition, our results presented above seemed to indicate that expression levels of TGFB1 and miR-663 were negatively correlated. Therefore, we further examined whether the alteration in miR-663 expression indeed had an effect on the expression of TGFB1. As shown in Figures 4A and B, overexpression of miR-663 led to suppression of TGFB1 at both mRNA and protein levels. On the other hand, its inhibition resulted in upregulation of both TGFB1 mRNA and protein. Furthermore, overexpression of miR-663 in HeLa cells markedly abolished the induction of TGF-β1 with 4 Gy irradiation (Fig. 4C).
Figure 4.
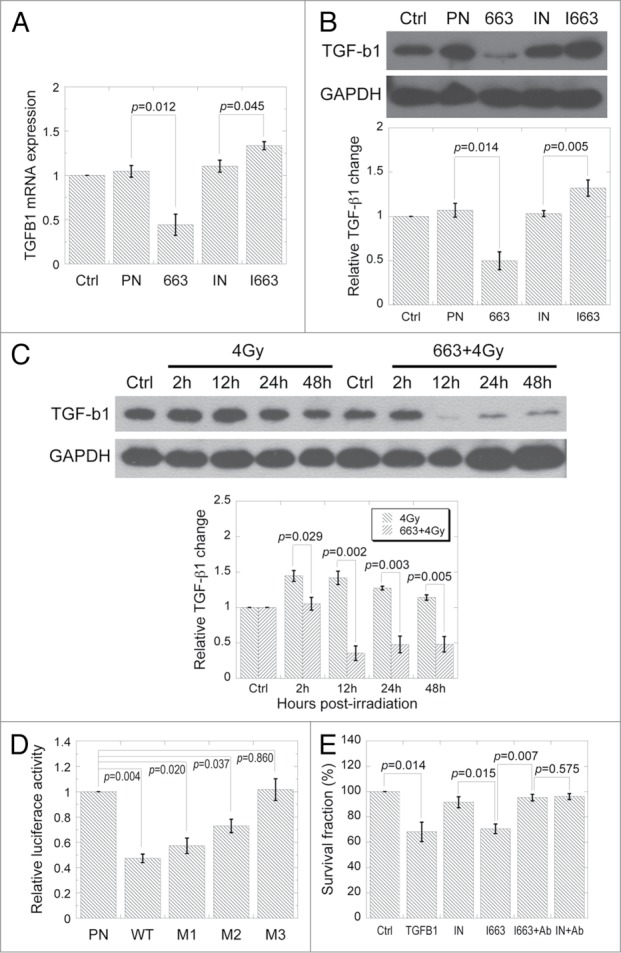
MiR-663 directly targets TGFB1 and suppresses its expression. (A) TGFB1 mRNA levels were detected using qRT-PCR in HeLa cells transfected with either miR-663 mimics (663) or inhibitors (I663). PN, negative control for miR-663 mimics; IN, negative control for miR-663 inhibitors. (B) TGF-β1 protein levels in HeLa cells transfected by either miR-663 mimics or inhibitors were examined by western blot. Western blot results from three independent experiments were quantified using ImageJ and a representative result is shown. (C) TGF-β1 protein levels in HeLa cells exposed to 4 Gy X-ray irradiation only or irradiation plus transfection with either miR-663 mimics or negative control (PN) were examined with western blot. Western blot results from three independent experiments were quantified using ImageJ and a representative result is shown. (D) Luciferase reporter assay. HeLa cells were co-transfected with pMIR-Report-Luciferase vectors containing either wild-type (WT) or mutated TGFB1 3′-UTR (M1 for mutant-1, M2 for mutant-2, M3 for mutant-3), the renilla luciferase vectors pGL4.74 (as an internal control), and either miR-663 mimics or negative control (PN). Firefly luciferase activity was normalized to that of Renilla. (E) Colony forming assay was performed on HeLa cells subjected to different treatments, after which cells were allowed to grow for 13 d. Ab, antibody against TGF-β1. Data from at least three independent experiments are presented as means ± SE.
Since online tools predicted 5 overlapping miR-663 binding sites distributed within two clusters in the 3′-UTR of TGFB1 mRNA (Fig. S6), we postulated that there may be a direct interaction between miR-663 and TGFB1. HeLa cells were co-transfected with miR-663 mimics and pMIR-TGFB1–3′-UTR that contained the 3′-UTR of TGFB1 transcript downstream of the luciferase gene. As shown in Figure 4D, luciferase activity was decreased by more than 50% compared with cells transfected with mimics negative control and pMIR-TGFB1–3′-UTR. The introduction of seed sequence mutations in the second or fourth miR-663 binding sites in TGFB1 transcript 3′-UTR, of which also resulted in mutations in other binding sites, rescued luciferase activity to various degrees (M1 and M2 in Figure 4D). Mutation of both seed sequences totally rescued luciferase activity up to the control level (M3 in Figure 4D). Moreover, though addition of TGF-β1 as well as transfection of miR-663 inhibitors reduced cell survival rate, the combination of miR-663 inhibitor transfection and TGF-β1 neutralizing antibody treatment (a double inhibition effect) led to a cell survival fraction that was comparable to that of the non-treated control (Fig. 4E), suggesting an interplay exists between miR-663 and TGF-β1.
MiR-663 regulates RIBE mediated by TGF-β1
Since TGFB1 is a direct target of miR-663, we aimed to determine whether miR-663 plays a role in TGF-β1-mediated RIBE. The stably inducible cell line HeLa-TetR-663 was used to increase miR-663 expression in directly irradiated cells. As shown in Figures 5A and B, upon miR-663 induction by tetracycline treatment in directly irradiated cells from which CM was generated, a significant suppression of 53BP1 foci and MNF was observed in bystander HeLa cells whose miR-663 expression level was not artificially changed, suggesting a role of miR-663 in mediating RIBE.
Figure 5.
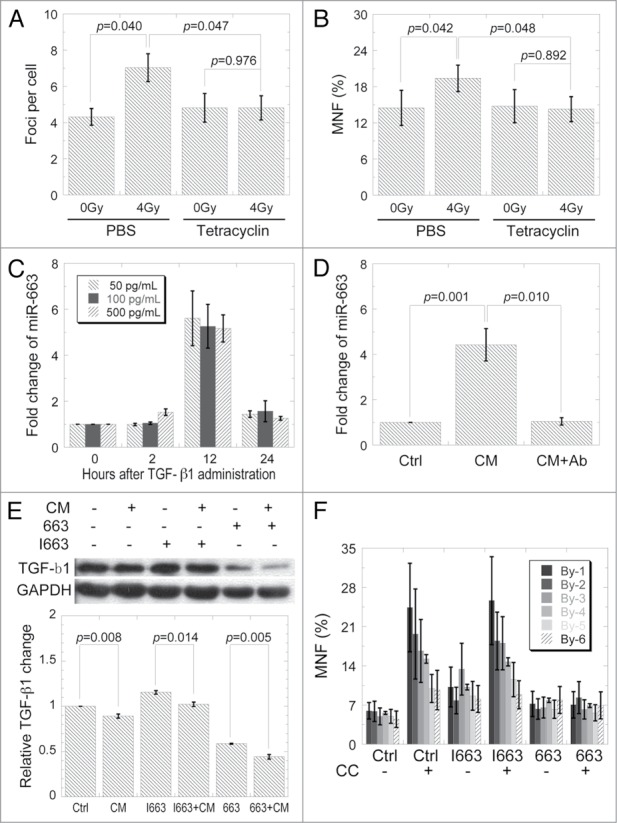
MiR-663 regulates RIBE mediated by TGF-β1. (A) Yields of 53BP1 foci in bystander HeLa cells cultured for 2 h in the medium harvested from HeLa cells with inducible miR-663 treated with/without tetracycline for 24 h and post-incubated for 24 h after exposure to 4 Gy X-rays. (B) MNF in bystander cells receiving conditioned medium harvested from cells, as indicated in panel A. (C) Induction of miR-663 in HeLa cells by TGF-β1. Indicated amounts of recombinant human TGF-β1 were added directly into the culture medium and expression levels of miR-663 measured with qRT-PCR at the indicated time-points. (D) MiR-663 expression in bystander HeLa cells 12 h after receiving conditioned medium from HeLa cells subjected to 4 Gy X-ray irradiation with or without TGF-β1 neutralizing antibody. (E) 12 h after CM transfer, TGF-β1 protein levels in HeLa cells transfected with either miR-663 mimics or inhibitors were examined with western blot. Western blot results from three independent experiments were quantified using ImageJ and a representative result is shown. (F) 24 h after transfection with miR-663 mimics or inhibitors, MNF in bystander HeLa cell populations co-cultured with HeLa cells exposed to 4 Gy X-ray irradiation. Three 12 × 12 mm coverslips were put in a line and divided equally into 6 parts according to different distances from directly irradiated cells, which were named By-1 to By-6. By-1 is the closest (3 mm) while By-6 is the farthest (33 mm). Data were obtained from at least three independent experiments and presented as means ± SE.
It has been demonstrated that TGF-β1 regulates expression of several miRNAs.32-35 Therefore, we want to investigate whether TGF-β1 also exhibits a regulatory effect on miR-663. MiR-663 expression was significantly enhanced 12 h after TGF-β1 administration (Fig. 5C), but was reduced back to untreated control level 24 h post-treatment. As shown in Figure 2A, conditioned medium significantly induced miR-663 expression in bystander cells. However, when TGF-β1 neutralizing antibody was added into the conditioned medium, miR-663 induction in bystander cells was completely abolished (Fig. 5D). These results implicated that TGF-β1 may regulate miR-663 in a feed-back mode. The TGF-β1 signaling strength was also evaluated in both directly hit cells and bystander cells by analysis of Smad2 phosphorylation, the results showed that CM-induced miR-663 upregulation did attenuate the TGF-β1 signaling in bystander cells (Fig. S7).
Since miR-663 showed inhibitory effect on TGF-β1 expression and an induction of miR-663 was observed in bystander cells, we measured TGF-β1 level in bystander cells and found that TGF-β1 level was marginally decreased upon CM treatment. Addition of miR-663 mimics seemed to enhance such an effect while miR-663 inhibitors attenuated the reduction (Fig. 5E). Further, we wanted to see if such a reduced level of TGF-β1 was related with the limited transmitted distance of RIBE. As shown in Figure 5F, a distance dependency of bystander effects was observed in co-cultured cells. Cells that were transfected with miR-663 inhibitors displayed similar effects as those observed in the un-transfected control; while no such phenomenon was observed in cells that were transfected with miR-663 mimics.
Discussion
In this study, we showed that miR-663 is radiosensitive and significantly downregulated upon irradiation, which is consistent with earlier study with fibroblasts.36 This implicated that miR-663 may play an important role in cellular response toward radiation. Further studies revealed that miR-663 directly regulates level of TGFB1 in HeLa cells, an effect that was shown before in human SW480 colon cancer cells.37 In addition, we demonstrated for the first time that miR-663 functions both in directly irradiated cells and bystander cells through its direct interaction with TGFB1.
In the current study, we showed that TGF-β1 is a bona fide target of miR-663. MiR-663 appears to suppress the TGFB1 transcript by binding to two groups of overlapping miRNA responsive elements (MRE) located in the 3′-UTR of TGFB1 mRNA. Such an observation is consistent with a previous report related with colon cancer cells.37 Overexpression of miR-663 was found to attenuate the reduction of cell survival after X-ray treatment, suggesting a protective role of this microRNA toward irradiation. Its suppressive effect on TGF-β1 seems to support such a role because TGF-β1 induces cytostatic and apoptotic responses.38 These observations concur with the finding that TGFB1(+/−), particularly TGFB1(−/−) cells, are significantly more radio-resistant than TGFB1(+/+) cells, indicating that the TGF-β1 level may positively correlate with radio sensitivity.39 Based on our results, we speculated that the overexpression of miR-663 inhibits TGF-β1 production after X-ray irradiation, which in turn results in reduced transportation of TGF-β1 precursor out of the directly irradiated cells and further leads to lower level of existing TGF-β1 precursors in extracellular medium40 and thus limits the cytostatic effect of radiation. These findings were confirmed in NOD/SCID mice with xenografts derived from HeLa cells. Overexpression of miR-663 in xenografts also significantly suppressed the cytostatic effect of radiation. This may be due to the fact that the ectopic expression of miR-663 rescued radiation-induced miR-663 downregulation and inhibited TGF-β1 expression, and then counteracted its cytostatic effects in xenografts. In addition, we notice that the 3′-UTRs of Smad3 and Smad4 also contain potential miR-663 binding sites, implying that the miRNA simultaneously suppresses more than one component in the TGF-β1 signaling pathway. Taken together, miR-663 is an important modulator of the TGF-β1 signaling pathway.
Besides its involvement in the direct radiation response, TGF-β1 was also identified as a critical signal molecule in radiation-induced bystander effects. Compared with ROS or NOS, TGF-β1 is a relatively stable factor that can diffuse freely in the medium and induce RIBE in non-targeted cells. The downregulation of miR-663 in directly irradiated HeLa cells may contribute to the increased level of TGF-β1 in the culture medium (conditioned medium), of which subsequently participates in the bystander effects. In the present study, it was demonstrated that bystander effects generated by conditioned medium were abrogated by TGF-β1 neutralizing antibody. This result, together with the increased concentration of TGF-β1 detected in conditioned medium, suggested that TGF-β1 plays an important role in the transmission of RIBE. An interesting finding of our current study was that overexpression of miR-663 in directly irradiated cells diminished RIBE, possibly through its inhibitory effect upon TGF-β1 expression. Furthermore, the effects of miR-663 on cell migration, invasion, and EMT, which are all biological effects mediated by TGF-β1 signaling, were also studied in both directly hit cells and bystander cells. It is interesting that both irradiation and CM treatment induced migration, invasion, and EMT in HeLa cells, while overexpression of miR-663 inhibited these effects in both irradiated and bystander cells, and its inhibition aggravated them (Figs. S8–10).
In addition to the finding that miR-663 directly inhibits TGF-β1, we found out that TGF-β1 also has a regulatory effect on miR-663 expression. TGF-β1 has been shown to modulate the expression of several miRNAs.32-35 Our results showed that miR-663 expression was upregulated in HeLa cells by conditioned medium that contained higher level of TGF-β1 released from the directly hit cells and that addition of TGF-β1 neutralizing antibody completely abrogated such an induction. Inhibition of miR-663 in bystander cells strongly aggravates the DNA damage caused by transfer of conditioned medium, indicating that loss of miR-663 enhances RIBE damage caused by TGF-β1 signaling. However, miR-663 overexpression in bystander cells abrogates RIBE damage. Co-culture experiments performed to confirm the results revealed that RIBE damage is dependent on the distance from directly irradiated cells. Since TGF-β1 signals are inhibited by miR-663, bystander cells barely secrete TGF-β1, leading to loss of signals. A distance-dependent concentration gradient is formed by TGF-β1 secreted mainly by directly irradiated cells, which provides an explanation for the distance-dependency of bystander effects.
Based on our results, a model for the interplay between miR-663 and TGF-β1 in directly irradiated and bystander cells was proposed (Fig. 6). In irradiated cells, miR-663 downregulation upon irradiation leads to de-repression of TGFB1 transcript, partially results in increased TGF-β1 level in the conditioned medium. TGF-β1 in culture medium diffuses to bystander cells to trigger intracellular signaling, including induction of ROS and NO production, which cause DNA damage.10,11 Theoretically, these increased NO and ROS should contribute to either the production or activation of TGF-β1 in the bystander cell population11,40 and lead to infinite transmission of RIBE. However, the increased level of TGF-β1 in conditioned medium upregulates miR-663, which leads to inhibition of TGFB1 expression in bystander cells. This negative feedback loop adds an important negative regulation element to the TGF-β1 signaling pathway and exerts suppressive effects on transmission of RIBE mediated by TGF-β1, resulting in a distance-dependent effect of bystander effects.
Figure 6.
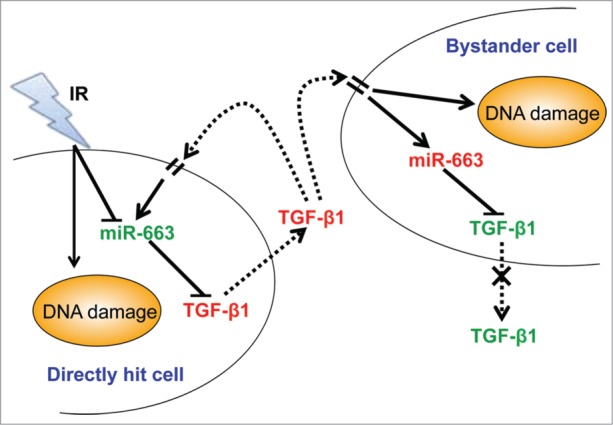
Proposed model for the function of miR-663 in TGF-β1-mediated RIBE. In irradiated cells, miR-663 downregulation leads to derepression of the TGFB1 transcript, partially resulting in increased TGF-β1 protein levels in the culture medium. TGF-β1 in culture medium diffuses to bystander cells to trigger intracellular signaling, including induction of DNA damage. In bystander cells, miR-663 is upregulated via TGF-β1 signaling, and in turn, inhibits expression of TGF-β1, resulting in lower TGF-β1 production and secretion. Thus, bystander effects are observed in a distance-dependent manner.
Conclusion
Radiosensitive miR-663 has been identified as an important participant in both direct irradiation effects and RIBE in HeLa cells by targeting TGFB1. Secretion of TGF-β1 signals by bystander cells is inhibited via a feedback regulation by overexpressed miR-663, which may explain the limited distance transmission of bystander effects. Although the precise mechanism underlying the modulation of miR-663 by TGF-β1 remains unclear at present, the current findings pave the way for further research on miRNA functions in RIBE. Moreover, this study may provide new insights to the development of radioprotectants or radiosensitizers based on miR-663 in clinical radiotherapy.
Materials and Methods
Cell culture
Human cervical cancer cells (HeLa) were grown in RMPI-1640 culture medium (Gibco) supplemented with 10% fetal bovine serum (Hyclone), 100 units/mL penicillin and 100 mg/mL streptomycin and maintained at 37°C in 5% CO2 in a humidified incubator (Thermo Scientific).
Irradiation
HeLa cells plated in 35 mm diameter tissue culture dishes (Corning) were irradiated at a dose rate of 1.2 Gy/min using a cabinet X-ray generator (Faxitron) at room temperature. Non-irradiated cells were used as the sham control. Radiation doses were measured with a calibrated semiconductor detector (Diados). The NOD/SCID mice bearing xenografts of HeLa cells were subjected to half-body irradiation at room temperature at a dose rate of 1 Gy/min using the Varian CL2100 medical linear accelerator (Varian) at the Radiotherapy Center of Lanzhou General Hospital.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Cells were harvested with TRIzol reagent (Invitrogen). RNA was isolated following the manufacturer's protocol. Total RNA was reverse-transcribed using the GoScript Reverse Transcription System (Promega) and amplified with qPCR master mix from Promega. Primers for both reverse transcription and PCR were listed in Table S1. The miR-663 PCR was performed with a Chromo4 system (Bio-Rad) under the following conditions: initiation for 2 min at 95°C, followed by 40 thermal cycles each at 95°C for 30 s and at 62°C for 40 s. PCR program for TGFB1 was set as follows: 2 min at 95°C, followed by 40 thermal cycles at 95°C for 15 s, 57°C for 20 s, and 72°C for 30 s. All data were analyzed with the C(t) value comparison method.
Clonogenic survival assay
HeLa cells were plated in 12-well plates at a density of 2 × 105 cells per well. Cells were transfected with 30 nM miR-663 mimics or inhibitors (Ambion) using Lipofectamine 2000 (Invitrogen) on the next day. Irradiation with 4 Gy X-rays was performed 6 h after the transfection. Immediately after irradiation, cells were harvested, counted, plated into φ60 mm dishes, and returned to the incubator. Plated cell numbers varied, depending on the different treatments. In terms of TGF-β1 or anti-TGF-β1 treatment, 10 ng/mL recombinant human TGF-β1 (Sigma-Aldrich) or 200 pg/mL anti-TGF-β1 antibody (Merck) was introduced into the culture. Cells were grown for 13 d, fixed with 70% ethanol for 5 min and stained with crystal violet. Colonies containing >50 cells were counted as survivors. At least 3 parallel dishes were scored for each treatment.
Luciferase reporter assay
The 3′-untranslated region (3′-UTR) of the human TGFB1 transcript was cloned downstream of the luciferase gene between the SpeI and HindIII sites of pMIR-Report-Luciferase vector (Ambion). We additionally generated a construction that contained a mutated seed sequence of miR-663. HeLa cells (2 × 105) on a 12-well plate were co-transfected with 300 ng DNA (pMIR-Reporter-Luciferase-TGFB1–3′-UTR constructs or derived mutants) and 30 nM miR-663 mimics or miR-663 inhibitors and 300 ng renilla luciferase control vector pGL4.74 (Promega) using Lipofectamine 2000. Luciferase activity was measured 48 h later using the Dual Luciferase Reporter Assay System (Promega) with a Tecan Infinite M200 Pro microplate reader (Tecan).
Enzyme-linked immunosorbent assay (ELISA)
Human TGF-β1 ELISA kits were purchased from Boster Corporation (Boster). Cell culture medium was harvested at the indicated time-points for ELISA according to the manufacturer's instruction. Absorbance at 450 nm was measured using a Tecan Infinite M200 Pro microplate reader. According to the ELISA kit manual, all samples were acidulated before ELISA by the addition of 1 N HCl (0.2 volume of sample) to the samples for 10 min and neutralized with 1.2 N NaOH containing 0.5 M HEPES (0.2 volume of sample). This step facilitates TGF-β1 dissociation from the latency-associated peptide (LAP) and activation.
Western blot
Cells were collected and lysed using RIPA buffer. Samples were sonicated (120 W, 3 s followed by 8 s cooling) and centrifuged at 12 000 g for 15 min at 4°C. The total protein concentration was determined by using DC Protein Assay Kit I (Bio-Rad, Richmond). Samples were denatured at 100°C for 5 min, resolved using SDS-PAGE, and transferred to polyvinylidene difluoride (PVDF) membranes (GE Healthcare, Piscataway, NJ, USA). After blockage with PBST (PBS with 0.1% Tween-20) containing 5% skimmed milk for 1 h at room temperature, the membrane was incubated with primary antibody for 2 h at room temperature, washed three times with PBST for 5 min each, and then incubated with HRP-conjugated secondary antibody for 1 h, washed three times with PBST. Protein bands were visualized using the chemiluminescence system (Millipore) and exposed to X-ray medical films (Kodak/Carestream Health). GAPDH was used as a loading control. Anti-TGF-β1 was purchased from Cell Signaling Technology (Beverly) and anti-GAPDH was from Santa Cruz Biotechnology.
Apoptosis assay
HeLa cells were transfected with 30 nM miR-663 mimics or miRNA precursor negative control 24 h after plated in φ35 mm dishes (4 × 105). Subsequently, cells were subjected to 4 Gy X-ray irradiation. Following a 24 h period, cells were trypsinized and collected via centrifugation at 130 g for 5 min. After washing once with PBS, cells were stained with Hoechst33342 (10 μg/mL) and propidium iodide (PI) (50 μg/mL) at 37°C for 15 min. Cells were then observed under a Leica AF6000 microscope (Leica). At least 500 cells were scored for each sample.
MiR-663 construct and cell line generation
A 300 bp sequence containing miR-663 precursor or negative control sequence was cloned into the pcDNATM6.2-GW/EmGFP-miR (Invitrogen), and the expression cassette transferred into pT-REx-DEST30 (Invitrogen) containing a tetracycline operator through gateway reaction. After verified via sequencing, the construct was transfected into HeLa cells stably expressing the tetracyclin repressor (TetR). The stable cell lines, designated HeLa-TetR-663, and the negative control HeLa-TetR-Neg were established via selection with G418 (Invitrogen) for 14 d. Inducible miR-663 expression was verified using qRT-PCR.
In vivo experiments
HeLa-TetR-663 or HeLa-TetR-Neg cells (1.6 × 106) were injected subcutaneously into the flanks of 5-wk-old NOD/SCID mice (n = 10 flanks). One month later, all mice developed solid tumors. Half of the mice were exposed to 5 Gy X-ray irradiation. Subsequently, miR-663 expression was induced continuously for 7 d by injecting tetracycline (Sigma-Aldrich) intraperitoneally at a daily dose of 4 mg/mouse, and tumor volumes measured every 5 d using a caliper for 1 mo. All tumor volume data were normalized to those obtained just before irradiation. Mice were housed in the Institute of Tumor, Gansu Academy of Medical Sciences, under the approval of the Institutional Review Board or Animal Care and Use Committee.
Conditioned medium (CM) transfer, co-culture, immunocytochemistry, and micronucleus assay
For CM transfer, HeLa cells (8 × 104) were plated in φ35 mm dishes for direct irradiation, while bystander HeLa cells (4 × 104) were plated on 12 × 12 mm coverslips placed in 12-well plates. After irradiation, cell medium was immediately removed and substituted with fresh RPMI-1640 medium. The conditioned medium was harvested 24 h after irradiation, spun at 2500 rpm for 5 min to exclude cells and cellular fragments, and transferred to bystander cells. Two hours after CM transfer, the bystander population on coverslips was fixed for 10 min in 4% paraformaldehyde for immunostaining or incubated with 2.5 μg/mL cytochalasin B (Sigma-Aldrich) for 30 h for the micronucleus (MN) assay. Immunostaining was conducted according to a previous report by our group.41 Cells were incubated with 53BP1 (Cell Signaling Technology) and subjected to Alexa Fluor® 546 anti-rabbit antibody or Alexa Fluor® 488 anti-mouse antibody (Molecular Probes). 53BP1 foci were counted using an Olympus BX51 fluorescence microscope (Olympus). At least 100 cells were scored for each sample. As for the MN assay, cells were fixed in Carnoy's solution for 15 min. Air-dried cells were stained with acridine orange (30 μg/mL) before observation. At least 500 binucleated cells were counted for each sample. In some experiments, cells were treated with TGF-β1 mouse monoclonal antibody to neutralize the TGF-β1 induced by irradiation. For co-culture, HeLa cells for direct irradiation were plated on 12 × 12 mm coverslips placed in φ35 mm dishes at a concentration of 1 × 105 cells/dish. HeLa cells (3 × 105) were plated in φ60 mm dishes as bystander populations in which six 6 × 12 mm coverslips were pre-located. At 18 h after plating, cells were transfected with either miR-663 mimics (663) or miR-663 inhibitors (I663). Twenty-four hours after transfection, cells in φ35 mm dishes were irradiated and placed into the predetermined sites in the φ60 mm dishes that contained bystander cells. Subsequently, co-culture cells were incubated with 2.5 μg/mL cytochalasin B for 30 h for the MN assay as described above. Bystander cells were divided into six populations, based on distance from directly irradiated cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Ms. Hongyun Guo for taking care of mice, Mr. Qingxiang Gao for experimental help, and Mr. Zhitong Bing for data processing.
Funding
This work was supported by the Major State Basic Research Development Program of China (973 Program, No. 2010CB834201), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA01040411), and the National Natural Science Foundation of China awarded to G.Z. (Nos. 11335011, 31070763) and J.W. (Nos. U1232125, 31270895); the US. National Institutes of Health grant P01 CA 049062–21 awarded to T.K.H.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/rnabiology/article/34345/
References
- 1. Hei TK, Zhou H, Chai Y, Ponnaiya B, Ivanov VN. Radiation induced non-targeted response: mechanism and potential clinical implications. Curr Mol Pharmacol 2011; 4:96-105; PMID:21143185; http://dx.doi.org/ 10.2174/1874467211104020096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belyakov OV, Mitchell SA, Parikh D, Randers-Pehrson G, Marino SA, Amundson SA, Geard CR, Brenner DJ. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proc Natl Acad Sci U S A 2005; 102:14203-8; PMID:16162670; http://dx.doi.org/ 10.1073/pnas.0505020102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu B, Wu L, Han W, Zhang L, Chen S, Xu A, Hei TK, Yu Z. The time and spatial effects of bystander response in mammalian cells induced by low dose radiation. Carcinogenesis 2006; 27:245-51; PMID:16150894; http://dx.doi.org/ 10.1093/carcin/bgi224 [DOI] [PubMed] [Google Scholar]
- 4. Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci U S A 2001; 98:473-8; PMID:11149936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 1997; 57:3963-71; PMID:9307280 [PubMed] [Google Scholar]
- 6. Shao C, Stewart V, Folkard M, Michael BD, Prise KM. Nitric oxide-mediated signaling in the bystander response of individually targeted glioma cells. Cancer Res 2003; 63:8437-42; PMID:14679007 [PubMed] [Google Scholar]
- 7. Lyng FM, Howe OL, McClean B. Reactive oxygen species-induced release of signalling factors in irradiated cells triggers membrane signalling and calcium influx in bystander cells. Int J Radiat Biol 2011; 87:683-95; PMID:21294691; http://dx.doi.org/ 10.3109/09553002.2010.549533 [DOI] [PubMed] [Google Scholar]
- 8. Narayanan PK, LaRue KEA, Goodwin EH, Lehnert BE. Alpha particles induce the production of interleukin-8 by human cells. Radiat Res 1999; 152:57-63; PMID:10381841; http://dx.doi.org/ 10.2307/3580049 [DOI] [PubMed] [Google Scholar]
- 9. Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, Hei TK. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci U S A 2005; 102:14641-6; PMID:16203985; http://dx.doi.org/ 10.1073/pnas.0505473102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iyer R, Lehnert BE, Svensson R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res 2000; 60:1290-8; PMID:10728689 [PubMed] [Google Scholar]
- 11. Shao C, Folkard M, Prise KM. Role of TGF-beta1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene 2008; 27:434-40; PMID:17621264; http://dx.doi.org/ 10.1038/sj.onc.1210653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gow MD, Seymour CB, Ryan LA, Mothersill CE. Induction of bystander response in human glioma cells using high-energy electrons: a role for TGF-beta1. Radiat Res 2010; 173:769-78; PMID:20518656; http://dx.doi.org/ 10.1667/RR1895.1 [DOI] [PubMed] [Google Scholar]
- 13. Hu H, Gatti RA. MicroRNAs: new players in the DNA damage response. J Mol Cell Biol 2011; 3:151-8; PMID:21183529; http://dx.doi.org/ 10.1093/jmcb/mjq042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koturbash I, Boyko A, Rodriguez-Juarez R, McDonald RJ, Tryndyak VP, Kovalchuk I, Pogribny IP, Kovalchuk O. Role of epigenetic effectors in maintenance of the long-term persistent bystander effect in spleen in vivo. Carcinogenesis 2007; 28:1831-8; PMID:17347136; http://dx.doi.org/ 10.1093/carcin/bgm053 [DOI] [PubMed] [Google Scholar]
- 15. Koturbash I, Zemp FJ, Kutanzi K, Luzhna L, Loree J, Kolb B, Kovalchuk O. Sex-specific microRNAome deregulation in the shielded bystander spleen of cranially exposed mice. Cell Cycle 2008; 7:1658-67; PMID:18560276; http://dx.doi.org/ 10.4161/cc.7.11.5981 [DOI] [PubMed] [Google Scholar]
- 16. Kovalchuk O, Zemp FJ, Filkowski JN, Altamirano AM, Dickey JS, Jenkins-Baker G, Marino SA, Brenner DJ, Bonner WM, Sedelnikova OA. microRNAome changes in bystander three-dimensional human tissue models suggest priming of apoptotic pathways. Carcinogenesis 2010; 31:1882-8; PMID:20643754; http://dx.doi.org/ 10.1093/carcin/bgq119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chew WS, Poh KW, Siddiqi NJ, Alhomida AS, Yu LE, Ong WY. Short- and long-term changes in blood miRNA levels after nanogold injection in rats–potential biomarkers of nanoparticle exposure. Biomarkers 2012; 17:750-7; PMID:23030236; http://dx.doi.org/ 10.3109/1354750X.2012.727030 [DOI] [PubMed] [Google Scholar]
- 18. Rixe J, Rolf A, Fichtlscherer S, Moellmann H, Nef HM, Liebetrau C, et al. Plasma Levels of Circulating MicroRNAs Correlate with Coronary Plaque Burden as Assessed by Cardiac Computed Tomography. Circulation 2011;124 [Google Scholar]
- 19. Andarawewa KL, Paupert J, Pal A, Barcellos-Hoff MH. New rationales for using TGFbeta inhibitors in radiotherapy. Int J Radiat Biol 2007; 83:803-11; PMID:18058368; http://dx.doi.org/ 10.1080/09553000701711063 [DOI] [PubMed] [Google Scholar]
- 20. Anscher MS, Crocker IR, Jirtle RL. Transforming growth factor-beta 1 expression in irradiated liver. Radiat Res 1990; 122:77-85; PMID:2181527; http://dx.doi.org/ 10.2307/3577586 [DOI] [PubMed] [Google Scholar]
- 21. Randall K, Coggle JE. Expression of transforming growth factor-beta 1 in mouse skin during the acute phase of radiation damage. Int J Radiat Biol 1995; 68:301-9; PMID:7561390; http://dx.doi.org/ 10.1080/09553009514551231 [DOI] [PubMed] [Google Scholar]
- 22. Anscher MS, Kong FM, Marks LB, Bentel GC, Jirtle RL. Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys 1997; 37:253-8; PMID:9069294; http://dx.doi.org/ 10.1016/S0360-3016(96)00529-9 [DOI] [PubMed] [Google Scholar]
- 23. Arnold SF, Tims E, Bluman EM, McGrath BE. Regulation of transforming growth factor beta1 by radiation in cells of two human breast cancer cell lines. Radiat Res 1999; 152:487-92; PMID:10521925; http://dx.doi.org/ 10.2307/3580144 [DOI] [PubMed] [Google Scholar]
- 24. Krüse JJ, Bart CI, Visser A, Wondergem J. Changes in transforming growth factor-beta (TGF-beta 1), procollagen types I and II mRNA in the rat heart after irradiation. Int J Radiat Biol 1999; 75:1429-36; PMID:10597916; http://dx.doi.org/ 10.1080/095530099139296 [DOI] [PubMed] [Google Scholar]
- 25. Seong J, Kim SH, Chung EJ, Lee WJ, Suh CO. Early alteration in TGF-beta mRNA expression in irradiated rat liver. Int J Radiat Oncol Biol Phys 2000; 46:639-43; PMID:10701743; http://dx.doi.org/ 10.1016/S0360-3016(99)00401-0 [DOI] [PubMed] [Google Scholar]
- 26. Ewan KB, Henshall-Powell RL, Ravani SA, Pajares MJ, Arteaga C, Warters R, Akhurst RJ, Barcellos-Hoff MH. Transforming growth factor-beta1 mediates cellular response to DNA damage in situ. Cancer Res 2002; 62:5627-31; PMID:12384514 [PubMed] [Google Scholar]
- 27. Boerma M, Bart CI, Wondergem J. Effects of ionizing radiation on gene expression in cultured rat heart cells. Int J Radiat Biol 2002; 78:219-25; PMID:11869477; http://dx.doi.org/ 10.1080/09553000110094797 [DOI] [PubMed] [Google Scholar]
- 28. Kirshner J, Jobling MF, Pajares MJ, Ravani SA, Glick AB, Lavin MJ, Koslov S, Shiloh Y, Barcellos-Hoff MH. Inhibition of transforming growth factor-beta1 signaling attenuates ataxia telangiectasia mutated activity in response to genotoxic stress. Cancer Res 2006; 66:10861-9; PMID:17090522; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-2565 [DOI] [PubMed] [Google Scholar]
- 29. Ehrhart EJ, Segarini P, Tsang MLS, Carroll AG, Barcellos-Hoff MH. Latent transforming growth factor beta1 activation in situ: quantitative and functional evidence after low-dose gamma-irradiation. FASEB J 1997; 11:991-1002; PMID:9337152 [DOI] [PubMed] [Google Scholar]
- 30. Ding N, Wu X, He J, Chang L, Hu W, Li W, Wang J, Wang T, Zhou G. Detection of novel human MiRNAs responding to X-ray irradiation. J Radiat Res 2011; 52:425-32; PMID:21785231; http://dx.doi.org/ 10.1269/jrr.10158 [DOI] [PubMed] [Google Scholar]
- 31. Wang J, He J, Su F, Ding N, Hu W, Yao B, Wang W, Zhou G. Repression of ATR pathway by miR-185 enhances radiation-induced apoptosis and proliferation inhibition. Cell Death Dis 2013; 4:e699; PMID:23807228; http://dx.doi.org/ 10.1038/cddis.2013.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 2008; 454:56-61; PMID:18548003; http://dx.doi.org/ 10.1038/nature07086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, et al. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 2009; 11:881-9; PMID:19543271; http://dx.doi.org/ 10.1038/ncb1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Winbanks CE, Wang B, Beyer C, Koh P, White L, Kantharidis P, Gregorevic P. TGF-β regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J Biol Chem 2011; 286:13805-14; PMID:21324893; http://dx.doi.org/ 10.1074/jbc.M110.192625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Y, Yu Y, Tsuyada A, Ren X, Wu X, Stubblefield K, Rankin-Gee EK, Wang SE. Transforming growth factor-β regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene 2011; 30:1470-80; PMID:21102523; http://dx.doi.org/ 10.1038/onc.2010.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maes OC, An J, Sarojini H, Wu H, Wang E. Changes in MicroRNA expression patterns in human fibroblasts after low-LET radiation. J Cell Biochem 2008; 105:824-34; PMID:18729083; http://dx.doi.org/ 10.1002/jcb.21878 [DOI] [PubMed] [Google Scholar]
- 37. Tili E, Michaille JJ, Alder H, Volinia S, Delmas D, Latruffe N, Croce CM. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem Pharmacol 2010; 80:2057-65; PMID:20637737; http://dx.doi.org/ 10.1016/j.bcp.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 2003; 3:807-21; PMID:14557817; http://dx.doi.org/ 10.1038/nrc1208 [DOI] [PubMed] [Google Scholar]
- 39. Von Pfeil A, Hakenjos L, Herskind C, Dittmann K, Weller M, Rodemann HP. Irradiated homozygous TGF-beta1 knockout fibroblasts show enhanced clonogenic survival as compared with TGF-beta1 wild-type fibroblasts. Int J Radiat Biol 2002; 78:331-9; PMID:12020424; http://dx.doi.org/ 10.1080/095530002753676200 [DOI] [PubMed] [Google Scholar]
- 40. Barcellos-Hoff MH. How do tissues respond to damage at the cellular level? The role of cytokines in irradiated tissues. Radiat Res 1998; 150(Suppl):S109-20; PMID:9806614; http://dx.doi.org/ 10.2307/3579813 [DOI] [PubMed] [Google Scholar]
- 41. He J, Li J, Ye C, Zhou L, Zhu J, Wang J, Mizota A, Furusawa Y, Zhou G. Cell cycle suspension: a novel process lurking in G2 arrest. Cell Cycle 2011; 10:1468-76; PMID:21455017; http://dx.doi.org/ 10.4161/cc.10.9.15510 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


