Abstract
The gut microbiota is suspected to promote colorectal cancer (CRC). Escherichia coli are more frequently found in CCR biopsies than in healthy mucosa; furthermore, the majority of mucosa-associated E. coli isolated from CCR harbors the pks genomic island (pks+ E. coli) that is responsible for the synthesis of colibactin, a genotoxic compound. We have recently reported that transient contact of a few malignant cells with colibactin-producing E. coli increases tumor growth in a xenograft mouse model. Growth is sustained by cellular senescence that is accompanied by the production of growth factors. We demonstrated that cellular senescence is a consequence of the pks+ E. coli-induced alteration of p53 SUMOylation, an essential post-translational modification in eukaryotic cells. The underlying mechanisms for this process involve the induction of miR-20a-5p expression, which targets SENP1, a key protein in the regulation of the SUMOylation process. These results are consistent with the expression of SENP1, miR-20a-5p and growth factors that are observed in a CRC mouse model and in human CCR biopsies colonized by pks+ E. coli. Overall, the data reveal a new paradigm for carcinogenesis in which pks+ E. coli infection induces cellular senescence characterized by the production of growth factors that promote the proliferation of uninfected cells and, subsequently, tumor growth.
Keywords: colibactin; colorectal cancer; Escherichia coli, microbiota; toxin, microRNA; SENP1; SUMO
Abbreviations
- CRC
colorectal cancer
- pks+ E. coli
colibactin-producing E. coli
- pks- E. coli
isogenic mutant of pks+ E. coli deficient for colibactin production
- miR
microRNA
- MOI
multiplicity of infection
- CM
conditioned medium
- SA-β-gal
senescence-associated β-galactosidase
- SASP
senescence-associated secretory phenotype
- AOM
azoxymethane
- DSS
dextran sodium sulfate
Approximately 20% of cancers are considered to be a consequence of infection by bacteria and/or viruses typically classified as pathogens. However, many cancers occur in tissues highly exposed to microbiota (commensal microbes), suggesting that microbes that are not typically thought of as pathogens might be involved in promoting carcinogenesis.1 Through the action of various microbial structural components and microbial gene products/metabolites, the intestinal microbiota, which mainly consists of bacteria,2 plays an essential role in gut homeostasis.3 However, the chronic carriage of commensal bacteria producing metabolites or toxins that directly insult the host DNA and/or are responsible for chronic inflammatory stress represents an important factor for chronic damage of epithelial cells and constitutes a potential etiologic component of sporadic colorectal cancer (CRC).
The bacterial species Escherichia coli is a normal component of the gut microbiota. However, various strains of E. coli, referred as pathobionts, have acquired pathogen-like features, such that those pathobionts are able to down-regulate the expression of DNA mismatch repair proteins4 or to produce various toxins exhibiting carcinogenic features.5,6 Certain strains producing the colibactin toxin are frequently associated with human colorectal tumors (55–66.7% vs 19–21% associated with intestinal control tissue).7,8 These E. coli strains induce double-strand DNA breaks, mutations, chromosomal rearrangements, and cell cycle arrest, and they have been reported to have carcinogenic effects in mice,8-11 suggesting that colibactin-producing E. coli may impact colorectal carcinogenesis. Our group extended this paradigm by demonstrating that the means by which colibactin-producing E. coli (pks+ E. coli) can promote cancer goes beyond merely driving inflammation or DNA damage.
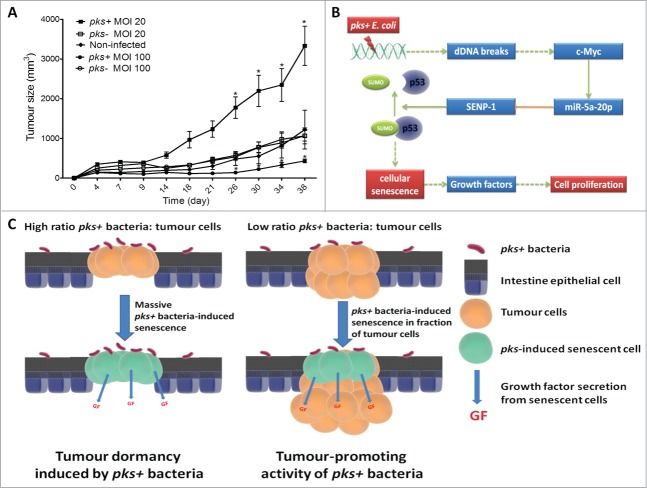
To explore the mechanisms involved in the tumor growth associated with colonization by colibactin-producing bacteria, we compared the behaviors of intestinal epithelial cell xenografts infected by pks+ E. coli or by pks- E. coli (the isogenic pks+ E. coli mutant is defective for colibactin production) in nude mice. In this model, a single and transient exposure of xenografts to pks+ E. coli at a multiplicity of infection (MOI) of 20 was associated with an increase in tumor growth and Ki67-positive cell numbers, suggesting that pks+ E. coli enhance cell proliferation (Fig. 1A). However, as previously reported,9 pks+ E. coli induced cell cycle arrest of intestinal epithelial cells in vitro, and accordingly, xenografts infected by pks+ E. coli at a high MOI (i.e. 100) resulted in a decrease in tumor growth (unpublished data, Fig. 1A). These results were consistent with a tumor dormancy resulting from the induction of a massive cell cycle arrest by pks+ E. coli in implanted cells. They also suggested a tumor growth at low MOI (i.e., Twenty) sustained by the stimulation of uninfected cell proliferation via an indirect effect mediated by colibactin-producing E. coli. We therefore assessed the ability of conditioned medium (CM) derived from intestinal epithelial cells infected by pks+ E. coli or by pks- E. coli to promote the proliferation of uninfected cells. Only CM derived from pks+ E. coli-infected cells increased the proliferation of uninfected cells, and the enhancement was similar to that obtained with medium containing 10% fetal bovine serum. Similar results were obtained using various intestinal epithelial cells lines (Int-407, HCT8, Caco-2, HT-29 and HCT116) and non-transformed IEC-6 cells. These results demonstrated an indirect effect of colibactin-producing E. coli on tumor growth and cell proliferation.
Figure 1.
Tumor growth in response to infection with pks+ E. coli. (A) HCT116 cells (2 × 106) were subcutaneously injected into nude mice and immediately infected for 3 hours with pks+ E. coli or pks- E. coli. The infection was performed at low (20) and high (100) multiplicity of infections (MOIs). Tumor size was monitored during the indicated period (n = 5/group; *P < 0 .05). (B) Cell signaling associated with pks+ E. coli-induced cellular senescence and tumor growth. (C) Model outlining the impact of pks+ bacteria on CRC according to the ratio of pks+ bacteria:tumor cells.
To identify the potential mechanisms by which the bacteria accomplished this pro-proliferative effect, we explored the physiological state of the infected cells. pks+ E. coli are known to induce megalocytosis and cell cycle arrest,9 which are features of cellular senescence. Senescence-associated β-galactosidase (SA-β-gal) activity, cell signaling and the senescence-associated secretory phenotype (SASP) were therefore assessed. pks+ E. coli, in contrast to pks- E. coli, induced a time-dependent and dose-dependent increase in SA-β-gal activity. pks+ E. coli-infected cells exhibited an accumulation of phospho-p53, p21Cip, and Retinoblastoma protein as well as a decrease in E2F-1 expression at day 5 post-infection, which correspond to a protein expression profile characteristic of senescent cells.12 Interestingly, pks+ E. coli-infected cells expressed significantly higher levels of the growth factors HGF, FGF, and GM-CSF than pks- E. coli-infected cells. Increased levels of growth factor expression were observed using 5 different intestinal epithelial cell lines (transformed and non-transformed cells). We next investigated whether pks+ E. coli induced senescence in vivo at early time points in tumor development. Nude mice were subcutaneously injected with intestinal epithelial cells infected with pks+ or pks- E. coli, and the tumors were analyzed at day 10 post-implantation. Although tumor sizes were comparable at this early time, tumors derived from pks+ E. coli-infected cells exhibited an ∼3-fold increase in the percentage of senescent cells compared to tumors derived from pks- E. coli-infected cells. Of note, the expression levels of FGF, GM-CSF and HGF were also increased in xenografts at 10 d post-pks+ E. coli infection compared to xenografts infected with pks- E. coli. All together, these results indicate that pks+ E. coli induce senescence of intestinal epithelial cells, and those senescent cells consequently produced growth factors that might stimulate tumor growth.
To identify relevant secreted mediators involved in cell proliferation, we explored the impact of specific inhibitors and neutralizing antibodies on the pro-proliferative effect of CM derived from pks+ E. coli-infected cells. An HGF pathway inhibitor, in contrast to vehicle or other growth factor inhibitors abrogated the pro-proliferative activity of CM in vitro. Similar results were obtained using neutralizing antibodies. As observed in vitro, HGF inhibitor significantly blocked the growth of xenografts obtained from cells infected with pks+ E. coli. All together, the data show that the pks+ E. coli-associated promotion of xenograft growth is dependent on the SASP and more particularly on HGF, which is a key determinant of colon cancer progression, a marker of poor prognosis and a target for CRC treatment.13,14
To identify the potential mechanisms by which pks+ E. coli induce cell senescence, we investigated protein SUMOylation, which has recently emerged as a key regulator of cellular senescence.15 Interestingly, pks+ E. coli-infected cells displayed a modified pattern of SUMO-conjugated proteins compared with pks- E. coli-infected cells or uninfected cells (unpublished data). Furthermore, the use of anacardic acid, an inhibitor of protein SUMOylation,16 abrogated pks+ E. coli-induced senescence (unpublished data). We therefore hypothesized that the senescence triggered by pks+ E. coli might involve deregulation of the control of the protein SUMOylation process. Accordingly, we observed an accumulation of SUMO1-conjugated p53, which is known to drive cellular senescence.17 This accumulation was associated with a decrease in SENP1 expression, a key enzyme involved in the control of the SUMOylation process.17 Interestingly, over-expression of SENP1, unlike overexpression of an inactive SENP1, significantly decreased the number of senescent cells induced by pks+ E. coli infection, confirming the role of SENP1 in pks+ E. coli-induced senescence. In addition, over-expression of SENP1 blocked the modification of the SUMO-conjugated protein patterns that was observed in response to pks+ E. coli infection (unpublished data). Of note, CM derived from pks+ E. coli-infected cells over-expressing a functional SENP1 did not promote cell proliferation. All together, these data show that SENP1 down-expression and the subsequent protein SUMOylation modifications are key features in pks+ E. coli-induced senescence.
Among the microRNAs (miRs) reported to be deregulated during senescence,18 in silico predictions revealed that miR-20a-5p potentially targets SENP1. Interestingly, miR-20a-5p expression was significantly up-regulated in pks+ E. coli-infected cells, unlike in pks- E. coli-infected cells. Furthermore, transfection of cells with mature miR-20a-5p decreased SENP1 expression at both the mRNA and protein levels. In addition, using a reporter assay, we demonstrated that miR-20a-5p binds to the SENP1 mRNA 3’-UTR. These results show that pks+ E. coli up-regulate miR-20a-5p expression, which in turn down-regulates SENP1 expression.
We next investigated the role of miR-20a-5p in senescence. In cells transfected with miR-20a-5p, a significant increase in the number of senescent cells was observed compared to cells transfected with a miR-control. Furthermore, an anti-miR-20a-5p abrogated pks+ E. coli-induced accumulation of SUMO1-conjugated p53 and, consequently, cellular senescence. As observed upon pks+ E. coli infection, cells transfected with miR-20a-5p produced significant levels of HGF, and their CM induced the proliferation of untransfected cells. In contrast, anti-miR-20a-5p abolished the effect of pks+ E. coli on HGF expression and cell proliferation. These results show that pks+ E. coli induce senescence via miR-20a-5p and, subsequently, a SASP that is responsible for cell proliferation.
MiR-20a-5p is known to be regulated by the transcription factor c-Myc,19 which is involved in the DNA damage response.20 Accordingly, we observed that pks+ E. coli infection, which is known to induce DNA damage,9,10 induced c-Myc expression and its binding to the miR-20a-5p promoter. Transfection of pks+ E. coli-infected cells with c-Myc siRNA strongly reduced the expression of miR20a-5p, increased the expression of SENP1, reduced the level of SUMO-conjugated p53, decreased the number of pks+ E. coli-induced senescent cells and consequently abolished the pro-proliferative effect of the CM derived from those cells. These findings suggest that c-Myc plays an upstream role in the cellular signaling cascade induced by pks+ E. coli. Finally, time-course experiments showed that pks+ E. coli induced c-Myc expression 3 d after infection, followed by modifications of miR-20a-5p and SENP1 expression levels 2 d later. Altogether, these results revealed the mechanism underlying pks+ E. coli-induced senescence (Fig. 1B). The expression of c-Myc is increased in response to pks+ E. coli-induced DNA damage. Consequently, c-Myc stimulates the expression of miR-20a-5p, which binds to the SENP1 mRNA 3’UTR, resulting in its translational silencing. SENP1 down-regulation induced by colibactin-producing E. coli triggers an accumulation of SUMO-conjugated p53, which is a well-known enhancer of cellular senescence. However, it should be noted that pks+ E. coli-induced senescence was also observed in p53-/- cells, suggesting that pathways other than p53 signaling could be involved in the senescence process.
To confirm the reliability of our findings, we explored the impact of a clinical pks+ E. coli strain in an AOM/DSS (azoxymethane/dextran sodium sulfate) CRC mouse model. The colonization of AOM/DSS-treated mice gut significantly increased the number of colon tumors in comparison to mice infected with the clinical strain mutated in the pks island (isogenic mutant). pks+ E. coli did not affect the inflammatory score or the size of the tumors. The neoplastic grade was not significantly affected but tended to increase in the presence of pks+ E. coli. The DNA damage marker γH2Ax, the miR-20a-5p expression level and senescence markers (SA-β-gal activity and p21cip expression) were significantly increased in tumors isolated from mice colonized by the clinical pks+ E. coli strain compared with those isolated from mice colonized by the corresponding isogenic mutant. Finally, a clinical pks+ E. coli strain induced tumor activation of the HGF pathway, which was characterized by high levels of HGF mRNA and phosphorylation of HGF receptors. To substantiate these results, we investigated human colon adenocarcinomas colonized by pks+ E. coli or by pks- E. coli. As observed in the AOM/DSS mouse model, human biopsies colonized by pks+ E. coli harbored high expression levels of γH2Ax, miR-20a-5p, senescence markers (SA-β-gal, p21cip), and HGF mRNA and activated HGF receptors, as well as a decrease in the number of SENP1-expressing cells. These biological stigmas significantly differentiated human CCR colonized by pks+ E. coli from those colonized by pks- E. coli, as shown by redundancy analyses. Overall, these data indicate that pks+ E. coli impact the physiopathology of CRC. pks+ E. coli activate the miR-20a-5p/SENP1/senescence/HGF pathway (Fig. 1B) in human and murine colon adenocarcinomas and therefore may contribute to CCR development. Of note, in our study, we focused on E. coli strains producing colibactin as the sole toxin affecting the cell cycle. In addition to colibactin, clinical pks+ E. coli frequently produce the CNF1 toxin, which is a pro-inflammatory toxin that targets Rho GTPases and consequently induces cell-cycle deregulation.5 The impact of such strains on CRC remains to be explored.
The tissue microenvironment of tumors is defined by the phenotypes of the cells in the immediate area and by the properties of the soluble and insoluble factors surrounding the tumor cells. These properties include various molecules that may be produced locally, such as growth factors and cytokines. Tumor cells form a dynamic network that contributes to their microenvironment, and at the same time, the tissue microenvironment regulates tumor cell behavior. Our findings suggest that colibactin-producing E. coli modulate the tumor microenvironment by inducing the secretion of several growth factors from senescent cells, which can promote tumor growth. The promotion of tumor growth was clearly observed in the subcutaneous xenograft model using immunodeficient mice. The toxicity mediated by colibactin requires live bacteria and direct contact between the bacteria and cells.9 Colibactin produced by pks+ E. coli is not a diffusible toxin, in contrast to anticancer drugs, which can induce massive senescence in tumors. Due to their partial accessibility to tumor cells and/or a limited number of bacteria, pks+ E. coli may induce senescence in only a fraction of the tumor tissue. pks+ E. coli may thereby support a permissive, senescent microenvironment that has been reported to relax the control over cell behavior21 and promote tumourigenesis by inducing the production of growth factors that fuel the uninfected tumor cells (Fig. 1C).
In the AOM/DSS colon cancer model, we observed an increase in tumor number, as previously observed in AOM-treated ILl0−/- mice.8 This result is in line of pks+ E. coli inducing cancer via DNA damage. The classical model of carcinogenesis comprises an initiation step characterized by genomic changes within the “cancer cell,” such as point mutations or chromosomal rearrangements creating the potential for neoplastic development. Although the protumoural activity of pks+ E. coli observed in mice requires the administration of the mutagenic agent azoxymethane,8 we cannot exclude that pks+ E. coli is involved in this process.10 The subsequent changes of an initiated cell leading to neoplastic transformation also require exposures to non-mutagenic stimuli promoting the clonal expansion of these “initiated” cells, a key step allowing the fixation of mutations in daughter cells. The results obtained in human xenografts growing in immunodeficient mice support that pks+ E. coli is also involved in this CRC promotion step. However, we did not observe an increase in tumor size in response to pks+ E. coli infection in the AOM/DSS colon cancer model using immunocompetent mice. This disagreement may be linked to constraints, making the promoting effect of pks+ E. coli on the size of intestinal tumors difficult to observe. There is evidence that senescent tumors generate an immune response that recognizes and eliminates tumor cells.22 This process may constrain the expansion of a tumor in the context of immunocompetent mice.
After initation and promotion, cancerous cells invade nearby tissues as well as migrate to other tissues. In AOM-treated IL10-/- mice, the pks+ feature of E. coli favored carcinoma invasion at 18 weeks of evolution.8 Similarly, in the AOM/DSS murine colon cancer model, the neoplastic grade tended to increase after 7 weeks of colonization by pks+ E. coli. Finally, a correlation was observed between the neoplastic grade of human CRC and colonization by pks+ E. coli.23 pks+ E. coli may therefore also support a permissive microenvironment that favors cancer cell invasion and aggressiveness. This activity might be supported by the secretion of HGF from pks+ E. coli-induced senescent cells, which is known to enhance cancer cell invasiveness.24,25
Surgery is the first line treatment for CCR, but with later-stage cancers, chemotherapy is commonly used as an adjunct to surgery. Many new cancer drugs target specific molecular aberrations or cell-signaling pathways, but the response to these drugs can be highly variable between individuals due to the molecular differences between tumors. Consequently, a ‘one-size-fits-all’ approach treatment is suboptimal, and so, there has been increasing interest in a more personalized approach to treatment. Increasing numbers of molecular biomarkers of the tumor and the pharmacogenomic profile of the patient are therefore taken into account when selecting the appropriate chemotherapy.26 This paradigm of personalized treatment for anticancer chemotherapy may be extended in the future by taking into account the contribution of the microbiota to colorectal carcinogenesis and using adjuvant therapy targeting major determinants of microbiota-associated carcinogenesis. Colibactin-producing bacteria might be such targets, and therefore, colibactin-inhibiting drugs may be a strategy to restrain the production of pro-tumourigenic factors without giving rise to alterations of the cellular processes that are responsible for side effects.
Cellular senescence is known to have opposing effects on carcinogenesis. If SASP promotes tumor growth, then cellular senescence is also perceived as an irreversible or prolonged growth arrested state that induces a definitive or prolonged reproductive “dead”27,28 that is used by senescence-inducing anticancer drugs.22,29,30 When xenografts were infected by pks+ E. coli at a MOI of 100, we did not observe any pro-proliferative effect and even observed a decrease in tumor growth (Fig. 1A). Under such conditions (accessible cells and high ratios of pks+ E. coli:tumor cells), we can speculate that almost all cells in the xenograft are senescent, resulting in a form of tumor dormancy. Consequently, pks+ E. coli can have a tumor-suppressing activity or a tumor dormancy-formatting activity under favorable conditions (Fig. 1C). However, the role played by this type of interaction in the context of intestinal tumors remains to be assessed.
In conclusion, our data support the hypothesis that colibactin-producing bacteria can play an important role in CRC. Previous works have shown that pks+ E. coli can induce DNA damage leading to mutations and chromosomal instability, which may be involved in cancer development. They also induce senescence by subverting the SUMOylation process via SENP1 downregulation under the control of a miRNA. Consequently, colibactin-producing bacteria can modulate the tumor microenvironment to favor the emergence of senescent cells, which may also affects tumor promotion and cancer progression via the secretion of growth factors. Targeting colibactin production may therefore be a strategy to restrain the production of pro-tumourigenic factors from the tumor microenvironment. However, our understanding of the cancer-promoting potential of pks+ E. coli remains limited, and the clinical consequences should be clarified.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Thanks to the many colleagues and collaborators at CHU de Clermont-Ferrand and CNRS UMR 5234, Université de Bordeaux who contributed to these studies. Special thanks to Harald Wodrich for his support and hospitality.
Funding
The work described in this addendum was funded by the Ministère de la Recherche et de la Technologie, the Institut National de la Santé et de la Recherche Médicale (UMR Inserm U1071), the l’Institut National de la Recherche Agronomique (USC-2018), and the Ligue Contre le Cancer and the Center Hospitalier Régional Universitaire de Clermont-Ferrand, France.
References
- 1. Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893-917; PMID:21351269; http://dx.doi.org/ 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2. The Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature 2012; 486:207-14; PMID:22699609; http://dx.doi.org/ 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clements A, Young JC, Constantinou N, Frankel G. Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes 2012; 3:71-87; PMID:22555463; http://dx.doi.org/ 10.4161/gmic.19182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maddocks ODK, Short AJ, Donnenberg MS, Bader S, Harrison DJ. Attaching and effacing Escherichia coli downregulate DNA mismatch repair protein in vitro and are associated with colorectal adenocarcinomas in humans. PLoS ONE 2009; 4:e5517; PMID:19436735; http://dx.doi.org/ 10.1371/journal.pone.0005517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nougayrède J-P, Taieb F, De Rycke J, Oswald E. Cyclomodulins: bacterial effectors that modulate the eukaryotic cell cycle. Trends Microbiol 2005; 13:103-10; http://dx.doi.org/ 10.1016/j.tim.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 6. Lax AJ. Opinion: bacterial toxins and cancer–a case to answer? Nat Rev Microbiol 2005; 3:343-9; PMID:15806096; http://dx.doi.org/ 10.1038/nrmicro1130 [DOI] [PubMed] [Google Scholar]
- 7. Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, Pezet D, Bonnet R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 2013; 8:e56964; PMID:23457644; http://dx.doi.org/ 10.1371/journal.pone.0056964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan T-J, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012; 338:120-3; PMID:22903521; http://dx.doi.org/ 10.1126/science.1224820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nougayrède J-P, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006; 313:848-51; http://dx.doi.org/ 10.1126/science.1127059 [DOI] [PubMed] [Google Scholar]
- 10. Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrède J-P. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci USA 2010; 107:11537-42; PMID:20534522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, Gibold L, Sauvanet P, Darcha C, Dechelotte P, Bonnet M, et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut [Internet] 2014. [cited 2014 Jul 18]; Available from: http://gut.bmj.com/cgi/doi/ 10.1136/gutjnl-2013-305257 [DOI] [PubMed] [Google Scholar]
- 12. Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol 2005; 37:961-76; PMID:15743671; http://dx.doi.org/ 10.1016/j.biocel.2004.10.013 [DOI] [PubMed] [Google Scholar]
- 13. Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, Birchmeier W, Schlag PM. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med 2009; 15:59-67; PMID:19098908; http://dx.doi.org/ 10.1038/nm.1889 [DOI] [PubMed] [Google Scholar]
- 14. Matsui S, Osada S, Tomita H, Komori S, Mori R, Sanada Y, Takahashi T, Yamaguchi K, Yoshida K. Clinical significance of aggressive hepatectomy for colorectal liver metastasis, evaluated from the HGF/c-Met pathway. Int J Oncol 2010; 37:289-97; PMID:20596656 [DOI] [PubMed] [Google Scholar]
- 15. Bischof O, Dejean A. SUMO is growing senescent. Cell Cycle 2007; 6:677-81; PMID:17374992; http://dx.doi.org/ 10.4161/cc.6.6.4021 [DOI] [PubMed] [Google Scholar]
- 16. Fukuda I, Ito A, Hirai G, Nishimura S, Kawasaki H, Saitoh H, Kimura K-I, Sodeoka M, Yoshida M. Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem Biol 2009; 16:133-40; PMID:19246003; http://dx.doi.org/ 10.1016/j.chembiol.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 17. Yates KE, Korbel GA, Shtutman M, Roninson IB, DiMaio D. Repression of the SUMO-specific protease Senp1 induces p53-dependent premature senescence in normal human fibroblasts. Aging Cell 2008; 7:609-21; PMID:18616636; http://dx.doi.org/ 10.1111/j.1474-9726.2008.00411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonifacio LN, Jarstfer MB. MiRNA profile associated with replicative senescence, extended cell culture, and ectopic telomerase expression in human foreskin fibroblasts. PLoS ONE 2010; 5:e12519; PMID:20824140; http://dx.doi.org/ 10.1371/journal.pone.0012519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005; 435:839-43; http://dx.doi.org/ 10.1038/nature03677 [DOI] [PubMed] [Google Scholar]
- 20. Guerra L, Albihn A, Tronnersjö S, Yan Q, Guidi R, Stenerlöw B, Sterzenbach T, Josenhans C, Fox JG, Schauer DB, et al. Myc is required for activation of the ATM-dependent checkpoints in response to DNA damage. PLoS ONE 2010; 5:e8924; PMID:20111719; http://dx.doi.org/ 10.1371/journal.pone.0008924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 2011; 192:547-56; PMID:21321098; http://dx.doi.org/ 10.1083/jcb.201009094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguría A, Zaballos A, Flores JM, Barbacid M, et al. Tumour biology: senescence in premalignant tumours. Nature 2005; 436:642; PMID:16079833; http://dx.doi.org/ 10.1038/436642a [DOI] [PubMed] [Google Scholar]
- 23. Bonnet M, Buc E, Sauvanet P, Darcha C, Dubois D, Pereira B, Déchelotte P, Bonnet R, Pezet D, Darfeuille-Michaud A. Colonization of the human gut by E. coli and colorectal cancer risk. Clin Cancer Res 2014; 20:859-67; PMID:24334760; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-1343 [DOI] [PubMed] [Google Scholar]
- 24. Hecht M, Papoutsi M, Tran HD, Wilting J, Schweigerer L. Hepatocyte growth factor/c-Met signaling promotes the progression of experimental human neuroblastomas. Cancer Res 2004; 64:6109-18; PMID:15342394; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-1014 [DOI] [PubMed] [Google Scholar]
- 25. Kermorgant S, Aparicio T, Dessirier V, Lewin MJ, Lehy T. Hepatocyte growth factor induces colonic cancer cell invasiveness via enhanced motility and protease overproduction. Evidence for PI3 kinase and PKC involvement. Carcinogenesis 2001; 22:1035-42; PMID:11408346; http://dx.doi.org/ 10.1093/carcin/22.7.1035 [DOI] [PubMed] [Google Scholar]
- 26. Binefa G, Rodríguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: From prevention to personalized medicine. World J Gastroenterol 2014; 20:6786-808; PMID:24944469; http://dx.doi.org/ 10.3748/wjg.v20.i22.6786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Di X, Shiu RP, Newsham IF, Gewirtz DA. Apoptosis, autophagy, accelerated senescence and reactive oxygen in the response of human breast tumor cells to adriamycin. Biochem Pharmacol 2009; 77:1139-50; PMID:19185564; http://dx.doi.org/ 10.1016/j.bcp.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 28. Biggers JW, Nguyen T, Di X, Gupton JT, Henderson SC, Emery SM, Alotaibi M, White KL, Jr, Brown R, Almenara J, et al. Autophagy, cell death and sustained senescence arrest in B16/F10 melanoma cells and HCT-116 colon carcinoma cells in response to the novel microtubule poison, JG-03-14. Cancer Chemother Pharmacol 2013; 71:441-55; PMID:23178952; http://dx.doi.org/ 10.1007/s00280-012-2024-6 [DOI] [PubMed] [Google Scholar]
- 29. Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol 2001; 11:S27-31; PMID:11684439; http://dx.doi.org/ 10.1016/S0962-8924(01)02151-1 [DOI] [PubMed] [Google Scholar]
- 30. Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007; 445:656-60; PMID:17251933; http://dx.doi.org/ 10.1038/nature05529 [DOI] [PMC free article] [PubMed] [Google Scholar]