Dear Editor
Cas9 protein of the Type II CRISPR-Cas (Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR associated)1 bacterial adaptive immune system emerged recently as a promising tool for genome editing in human and other eukaryotic cells.2,3 Cas9 binds a dual crRNA (CRISPR RNA)-tracrRNA (trans-activating RNA) molecule, or an artificial single-guide RNA (sgRNA) into a functional complex that acts as an RNA-directed DNA endonuclease. It locates and binds to the target site guided by crRNA (sgRNA) while the Cas9 protein cuts DNA generating a double strand break (DSB) within the target sequence.4,5 In eukaryotic cells, DSB is repaired by “error prone” non-homologous end joining (NHEJ)6 or by homology directed repair (HDR)7 mechanisms resulting in the genome modification or insertion of new genetic information.8-10
Cas9 of Streptococcus pyogenes5 (SpCas9) is currently used as a model system for genome editing applications. Typically, the DNA expression cassettes encoding nucleus-targeted codon-optimized Cas9 protein and sgRNAs are transfected into the cells.11-13 The efficiency of DNA cleavage by plasmid-delivered Cas9 in eukaryotic cells depends on multiple factors, including expression vector design, transfection efficiency, cell type, recovery yield of functional Cas9 complex,14 and usually requires optimization of a set of experimental conditions. Cas9 delivery by plasmid transfection is still difficult to achieve for some hard-to-transform cell lines including human primary cells and pluripotent stem cells.15-17 Moreover, plasmid transfection occasionally results in undesirable integration of vector plasmid into the genome and is often inefficient and stressful to cells.18 Here we report an alternative way for the Cas9-mediated genome modification in eukaryotic cells (Fig. 1A) by chemical transfection of in vitro reconstituted functionally active Cas9-crRNA-tracrRNA complex of Streptococcus thermophilus (StCas9) CRISPR3-Cas system.
Figure 1.
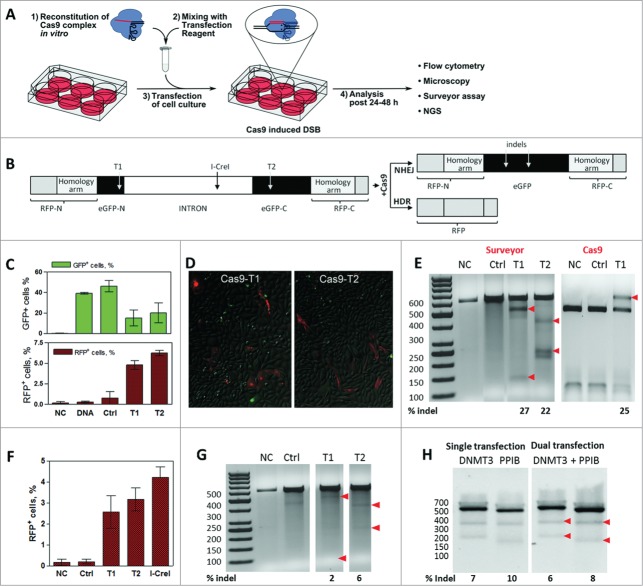
Gene editing by in vitro reconstituted S. thermophilus Cas9-crRNA-tracrRNA complex. (A) Workflow of the cell transfection experiment: 1) In vitro reconstitution of the Cas9-crRNA-tracrRNA complex; 2) Transfection mix preparation; 3) Transfection of cells; 4) Analysis of Cas9-mediated genome modification 24–48 h post transfection. (B) CMV-promoter driven dual reporter gene cassette for the Cas9 cleavage analysis and expected NHEJ or HDR repair products. T1 and T2 designate 2 different Cas9 target sites. Insertion of the reporter gene cassette into a plasmid vector generates a reporter plasmid while insertion into the CHO-K1 cell genome generates a stable reporter cell line. (C) Percentage of GFP+ and RFP+ cells (in T1 and T2-targeted samples) estimated by a flow cytometry. (D) Detection of RFP+ cells (in T1 and T2-targeted samples) using fluorescent microscope imaging. E) Cleavage analysis of the reporter plasmid. Regions surrounding Cas9 target sites in the reporter plasmid were PCR amplified and reannealed PCR amplicons (592 bp) were digested with Surveyor nuclease or T1 targeting Cas9 complex. Red arrows indicate 100+492 bp and 363+229 bp hydrolysis products for T1 and T2, respectively, and undigested 592 bp fragment. Numbers below indicate indel % calculated by densitometric analysis of corresponding bands. (F) FACS analysis of RFP+ cells in the reporter CHO-K1 cell line transfected by Cas9 or I-CreI nucleases. (G) Cleavage of the chromosomal Cas9 target sites in the CHO-K1 cell line. Regions surrounding chromosomal Cas9 target sites were PCR amplified and reannealed PCR amplicons (592 bp) were digested with Surveyor nuclease to produce 100+492 bp fragments (T1) and 363+229 bp fragments (T2), respectively. Arrowheads indicate nuclease digestion products, numbers below indicate indel % calculated by densitometric analysis of corresponding bands. (H) HEK293T cells were transfected with Cas9 complexes specific for DNMT3B and/or PPIB gene loci. Surveyor digestion was performed on reannealed PCR amplicons - 544 bp (DNMT3B) or 505 bp (PPIB) yielding hydrolysis products of 335+209 bp or 330+174 bp, respectively (red arrowheads). Calculated indel percentages indicate each gene modification extent from single or dual transfection experiments (C and F graphs shows mean values ± SD, n = 3).
The StCas9 protein bearing the nuclear localization signal (NLS) and 6xHis tag was purified from E.coli, and the StCas9 complex was reconstituted in vitro as described by Karvelis et al.19,20 To enable the delivery of reconstituted StCas919 complex into CHO-K1 cells, transfection experiments were performed using a protein delivery agent TurboFect™. Alternatively, other transfection reagents like Lipofectamine® 2000 or 3000 can be used to transfect Cas9 complexes into cells (data not shown). StCas9 localization was monitored using mouse polyclonal anti-Cas9 antibodies along with FITC-labeled secondary antibodies; crRNA-tracrRNA duplex was detected via 3′-biotin labeled tracrRNA using streptavidin-coupled Qdots® 585 nm. Both StCas9 and tracrRNA were observed in the perinuclear region and within the nucleus indicating that in vitro pre-assembled StCas9 complexes can be efficiently delivered into mammalian cells for targeted genome modification (Fig. S1).
To monitor the DNA cleavage activity of transfected Cas9 complexes in mammalian cells, we constructed a dual reporter cassette bearing Red Fluorescent Protein (RFP) and enhanced Green Fluorescent Protein (eGFP) genes (Fig. 1B). eGFP gene contains 2 sites, T1 and T2, targeted by 2 different StCas9 complexes. The I-CreI nuclease21 target site was also engineered into the cassette. In the absence of Cas9, eGFP fluorescence should be observed following intron processing in vivo. Cas9 facilitated DSB at T1 or T2 target site should trigger DNA repair either through NHEJ or HDR. In case of NHEJ, mutations within the eGFP gene would result in lost or diminished eGFP fluorescence. HDR, on the other hand, should result in the RFP expression due to reassembly of the RFP gene via the engineered homology arms, and enable quantification of HDR efficiency within the population of transfected cells. Integration of the dual reporter cassette into a plasmid vector generated a reporter plasmid while integration into the CHO-K1 cell genome produced a stable reporter cell line.
Functional activity of pre-assembled Cas9 complexes was first confirmed in vitro (Fig. S2). To demonstrate StCas9 functional activity in vivo, the dual reporter plasmid was co-transfected together with pre-assembled StCas9 complexes. StCas9 and reporter DNA transfection mixtures were prepared in separate tubes and added to the cell culture at the same time; the percentage of eGFP+ cells was estimated 48 h later by flow cytometry. When cells were transfected with the reporter plasmid alone, the percentage of eGFP+ cells was ∼40%, indicative of overall transfection efficiency (Fig. 1C). Upon the StCas9 complex transfection, the percentage of eGFP+ cells was reduced to 5–15%. Cell transfection by StCas9 complexes bearing non-targeting crRNA had no affect on GFP fluorescence. Surveyor nuclease assay22 revealed ∼27% and ∼22% of insertions/deletions (indels) at the T1 and T2 sites, respectively, indicating that Cas9 cleavage at these sites triggered DSB repair via NHEJ. Importantly, transfection of the pre-assembled StCas9 complex (Fig. 1E) or cotransfection of StCas9 and sgRNA encoding plasmids (Fig. S3), resulted in similar indel percentage in the target gene. These values were further verified by an independent in vitro cleavage assay. In this assay amplified eGFP gene fragments were digested in vitro with the Cas9 complex targeting T1 site. Assuming that NHEJ introduces indels, we expected that PCR fragments with indels will remain intact while unmodified fragments will be cut by Cas9. It turned out that the percentage of the Cas9-resistant DNA (25%) is very similar to that established by the Surveyor assay. Thus, in vitro digestion of PCR amplicons with Cas9 provides an alternative to Surveyor assay for indel quantification in Cas9-mediated genome modification in vivo (Fig. 1E). In this case the indel detection is faster compared to Surveyor assay since no additional steps of amplicon reannealing is required. Taken together these results demonstrate that in vitro reconstituted Cas9 complex delivered by transfection promotes generation of DSB at the target site; subsequent repair by NHEJ produces indels and inactivates the eGFP gene.
To find out whether HDR contributes to the DSB repair in the reporter plasmid, we looked at the appearance of RFP+ cells: RFP was expressed in 5–8% of cells transfected with T1 or T2 targeting StCas9 complexes, whereas no red cells were detected in cultures transfected with non-targeting Cas9 complexes (Fig 1C). The presence of RFP positive cells was subsequently confirmed by fluorescence microscopy (Fig 1D).
For further analysis of StCas9 cleavage activity on the chromosomal DNA we used the CHO-K1 cell line with a dual reporter cassette (Fig. 1B) integrated into the genome. The weak eGFP fluorescence signal in the engineered cell line hindered a reliable quantification of the Cas9 cleavage efficiency by monitoring the decrease of the eGFP signal by flow cytometry. The Surveyor assay, however, revealed that indel values reached 2–6% for respective chromosomal target sites (Fig. 1G). Importantly, RFP+ cells were also identified by the fluorescent microscopy indicating that DSBs are also repaired by HDR (Fig. S4). FACS analysis confirmed that about 3–4% of cells expressed RFP in reporter cell cultures (Fig. 1F). Transfection of recombinant I-CreI nuclease yielded ∼4.5% of RFP+ cells, indicating that similar cleavage efficiencies can be achieved by different recombinant nucleases delivered using chemical transfection (Fig. 1G). StCas9 cleavage specificity was further verified by deep sequencing using MiSeq system (Illumina). NHEJ-mediated indels were centered about the target site validating the cleavage specificity (Fig. S5).
To extend the study of recombinant Cas9 potential in multiplex genome modulation, a series of experiments were carried out using HEK293T cells and StCas9 complexes specific for endogenous genes DNMT3B or PPIB. The cells were transfected with each gene targeting complexes separately (single transfection) or together (dual transfection). Surveyor assay revealed that for StCas9 complex indel values for genes DNMT3B or PPIB reached 7 and 10% in single transfections or 6 and 8% in dual transfections, respectively (Fig. 1H). Transfections with the pre-assembled SpCas9 complex yielded comparable indel (%) values (Fig. S6). Overall, this data validated that in vitro pre-assembled Cas9 complexes could be successfully used for genome modulation at different genomic loci and in addition demonstrated the potential of targeting multiple loci at the same time.
In conclusion, this study demonstrates for the first time the gene editing potential of in vitro reconstituted Cas9-crRNA-tracrRNA complex delivered by a chemical transfection agent. This finding expands the arsenal of available tools for direct delivery of Cas9 nuclease into cells23,24 and opens new avenues for targeted genome modification.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. A. Pivoriunas (Center of Innovative Medicine, Vilnius) for his help with laser confocal imaging and A. Berezniakovas and T. Radzvilavicius (Thermo Fisher Scientific Baltics, Vilnius) for technical assistance.
Funding
Work in V.S. lab was supported by the European Social fund under Global grant Measure Grant R100.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science (80- ) 2007; 315:1709-12; http://dx.doi.org/ 10.1126/science.1138140 [DOI] [PubMed] [Google Scholar]
- 2. Hsu PDD, Lander ESS, Zhang F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014; 157:1262-78; PMID:24906146; http://dx.doi.org/ 10.1016/j.cell.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gasiunas G, Siksnys V. RNA-dependent DNA endonuclease Cas9 of the CRISPR system: Holy Grail of genome editing? Trends Microbiol 2013; 21:562-7; PMID:24095303; http://dx.doi.org/ 10.1016/j.tim.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 4. Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A 2012; 109:E2579-86; PMID:22949671; http://dx.doi.org/ 10.1073/pnas.1208507109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012; 337:816-21; PMID:22745249; http://dx.doi.org/ 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 2010; 79:181-211; PMID:20192759; http://dx.doi.org/ 10.1146/annurev.biochem.052308.093131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moynahan ME, Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat Rev Mol Cell Biol 2010; 11:196-207; PMID:20177395; http://dx.doi.org/ 10.1038/nrm2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perez-Pinera P, Ousterout DG, Gersbach CA. Advances in targeted genome editing. Curr Opin Chem Biol 2012; 16:268-77; PMID:22819644; http://dx.doi.org/ 10.1016/j.cbpa.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet 2010; 11:636-46; PMID:20717154; http://dx.doi.org/ 10.1038/nrg2842 [DOI] [PubMed] [Google Scholar]
- 10. Carroll D. Genome engineering with zinc-finger nucleases. Genetics 2011; 188:773-82; PMID:21828278; http://dx.doi.org/ 10.1534/genetics.111.131433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science 2013; 339:823-6; PMID:23287722; http://dx.doi.org/ 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013; 339:819-23; PMID:23287718; http://dx.doi.org/ 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. Elife 2013; 2:e00471; PMID:23386978; http://dx.doi.org/ 10.7554/eLife.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y,Fine EJ, Wu X, Shalem O, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 2013; :1-8; PMID:23302909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li K, Wang G, Andersen T, Zhou P, Pu WT. Optimization of Genome Engineering Approaches with the CRISPR/Cas9 System. PLoS One 2014; 9:e105779; PMID:25166277; http://dx.doi.org/ 10.1371/journal.pone.0105779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim TK, Eberwine JH. Mammalian cell transfection: the present and the future. Anal Bioanal Chem 2010; 397:3173-8; PMID:20549496; http://dx.doi.org/ 10.1007/s00216-010-3821-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamano S, Dai J, Moursi AM. Comparison of transfection efficiency of nonviral gene transfer reagents. Mol Biotechnol 2010; 46:287-300; PMID:20585901; http://dx.doi.org/ 10.1007/s12033-010-9302-5 [DOI] [PubMed] [Google Scholar]
- 18. Gabriel R, Lombardo A, Arens A, Miller JC, Genovese P, Kaeppel C, Nowrouzi A, Bartholomae CC, Wang J, Friedman G, et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat Biotechnol 2011; 29:816-23; PMID:21822255; http://dx.doi.org/ 10.1038/nbt.1948 [DOI] [PubMed] [Google Scholar]
- 19. Karvelis T, Gasiunas G, Miksys A, Barrangou R, Horvath P, Siksnys V. crRNA and tracrRNA guide Cas9-mediated DNA interference in Streptococcus thermophilus. RNA Biol 2013; 10:841-51; PMID:23535272; http://dx.doi.org/ 10.4161/rna.24203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karvelis T, Gasiunas G, Siksnys V. Programmable DNA cleavage in vitro by Cas9. Biochem Soc Trans 2013; 41:1401-6; PMID:24256227 [DOI] [PubMed] [Google Scholar]
- 21. Jurica MS, Monnat RJ, Stoddard BL. DNA recognition and cleavage by the LAGLIDADG homing endonuclease I-CreI. Mol Cell 1998; 2:469-76; PMID:9809068; http://dx.doi.org/ 10.1016/S1097-2765(00)80146-X [DOI] [PubMed] [Google Scholar]
- 22. Guschin DY, Waite AJ, Katibah GE, Miller JC, Holmes MC, Rebar EJ. A rapid and general assay for monitoring endogenous gene modification. Methods Mol Biol 2010; 649:247-56; PMID:20680839; http://dx.doi.org/ 10.1007/978-1-60761-753-2_15 [DOI] [PubMed] [Google Scholar]
- 23. Kim S, Kim D, Cho SW, Kim J, Kim J-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res 2014; 24:1012-9; PMID:24696461; http://dx.doi.org/ 10.1101/gr.171322.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramakrishna S, Kwaku Dad A-B, Beloor J, Gopalappa R, Lee S-K, Kim H. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res 2014;24:1020-7; PMID:24696462; http://dx.doi.org/ 10.1101/gr.171264.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.