Abstract
Purpose
The aim of this study is to assess how anti-mullerian hormone (AMH) is used worldwide to test ovarian reserve and guide in vitro fertilization (IVF) cycle management.
Methods
An internet-based survey was sent electronically to registered IVF providers within the IVF-Worldwide.com network. This survey consisted of nine questions which assessed the clinics’ use of AMH. The questionnaire was completed online through the IVF-Worldwide.com website, and quality assurance tools were used to verify that only one survey was completed per clinical IVF center. Results are reported as the proportion of IVF cycles represented by a particular answer choice.
Results
Survey responses were completed from 796 globally distributed IVF clinics, representing 593,200 IVF cycles worldwide. Sixty percent of the respondent-IVF cycles reported to use AMH as a first line test, and 54 % reported it as the best test for evaluating ovarian reserve. Eighty-nine percent reported that AMH results were extremely relevant or relevant to clinical practice. However in contrast, for predicting live birth rate, 81 % reported age as the best predictor.
Conclusions
AMH is currently considered a first line test for evaluating ovarian reserve and is considered relevant to clinical practice by the majority of IVF providers.
Keywords: Anti-mullerian hormone, Survey, IVF-Worldwide, Ovarian reserve, IVF
Introduction
Since the advent of in vitro fertilization (IVF) and the adjunct use of gonadotropins, the need for tests predictive of patient response to controlled ovarian hyperstimulation (COH) has become essential. Currently, a variety of tests can be used to estimate ovarian reserve, or the reproductive potential of the oocytes remaining within the ovary. Ovarian reserve testing modalities include antral follicle count (AFC), ovarian volume, early follicular phase follicle stimulation hormone (FSH) and estradiol (E2) levels, inhibin B, the clomiphene citrate challenge test, and anti-mullerian hormone (AMH) [1]. However, identifying which of these tests are most accurate in predicting the ovarian response to COH and the potential for predicting pregnancy has yet to be definitively established. As a result, practitioners currently rely on a variety of different tests as part of their infertility evaluation and to predict ovarian response to COH [1–3].
AMH is one of the most recent tests developed to measure ovarian reserve. AMH is a glycoprotein belonging to the transforming growth factor super family secreted from the granulosa cells of small ovarian follicles [4]. In the developing female human embryo, the absence of AMH allows the mullerian ducts to differentiate into the upper portion of the vagina, cervix, uterus, and fallopian tubes [5]. However, as early as 36 weeks gestation, female fetuses begin producing AMH, which steadily increases in production until follicles reach the antral phase [6]. Following an incremental increase in AMH levels throughout a women’s reproductive time span, there is a steady decline in levels until it becomes undetectable, which correlates with the onset of menopause [7]. This rise and fall of the AMH level corresponds with the number of oocytes remaining in the ovary [8].
Given that AMH represents the pool of oocytes remaining in the ovary, as a metric, it results in both consistent inter- and intra-menstrual cycle measurements [9]. Numerous studies have attempted to establish the efficacy of AMH in the fertility evaluation and treatment with measures of sensitivity, specificity, and predictive values. In a recent committee opinion, the American Society for Reproductive Medicine (ASRM) concluded that AMH is useful for assessing ovarian reserve in the general IVF patient population and for those patients at risk for decreased ovarian reserve. Additionally, ASRM and the European Society of Human Reproduction and Embryology (ESHRE) state in a “best practices” publication that AFC and AMH appear to have the best sensitivity and specificity for measuring poor ovarian response to COH [3, 10, 11]. Given the large constituency that ASRM and ESHRE address, these committee opinions likely reflect an increase in interest and routine use of AMH by IVF providers.
Although committee opinions from major organizations support the routine use of AMH, it is unclear what proportion of IVF providers in the world are using AMH and how it is currently used to guide IVF cycle management in relation to other measures of ovarian reserve. No prior studies have attempted to assess IVF providers’ perceptions and practice patterns with regard to the use of AMH on a global level. Only two previously published studies [12, 13] have attempted to assess how IVF providers use tests to manage IVF cycles and assess ovarian reserve; however, these studies used a small sample size of IVF providers, were not globally distributed, and likely do not reflect the current use of AMH given the time lapse since they were published. Currently, there is a paucity of data regarding how AMH is currently used by IVF practitioners.
The objective of this study is to use a web-based survey from a large global distribution of IVF providers within the IVF-Worldwide.com network to provide insight into how AMH is currently applied to IVF cycle management and the measure of ovarian reserve.
Materials and methods
Survey content
The authors developed a 14-item questionnaire which collected the demographics of the responding provider’s clinic (5 questions) and assessed the clinical applications of AMH (9 questions) to IVF cycle management and the evaluation of ovarian reserve. Table 1 lists the clinical AMH questions and results.
Table 1.
This table presents the survey question stems which assessed how the respondents’ clinics valued and used anti-mullerian hormone as part of assessing ovarian reserve and managing IVF cycles
| 1) Have the majority of patients visiting your IVF clinic already undergone ovarian function testing? | |||||
| Yes | No | I do not know | |||
| % of Clinics | 71.4 | 27.9 | 0.8 | ||
| % of Cycles | 75.0 | 23.4 | 1.6 | ||
| 2) Would you consider AMH testing a first- or second-line test? | |||||
| First | Second | I do not know | |||
| % of Clinics | 59.2 | 39.1 | 1.8 | ||
| % of Cycles | 59.8 | 38.8 | 1.4 | ||
| 3) Would you change your stimulation protocol based on AMH results? | |||||
| Yes | No | I do not know | |||
| % of Clinics | 87.1 | 10.2 | 2.8 | ||
| % of Cycles | 86.4 | 10.7 | 2.9 | ||
| 4) How relevant do you consider the use of AMH testing in you routine/practice? | |||||
| Extremely relevant | Relevant | Not so relevant | Uninteresting | ||
| % of Clinics | 37.3 | 50.3 | 11.6 | 0.9 | |
| % of Cycles | 36.0 | 51.9 | 11.1 | 1.0 | |
| 5) If the AMH test was available in your clinic at no cost, would you use it routinely? | |||||
| Yes | No | I have not thought about this | |||
| % of Clinics | 86.1 | 9.0 | 4.9 | ||
| % of Cycles | 87.2 | 7.5 | 5.3 | ||
| 6) If you had to choose one of the factors listed below, which would serve you best in assigning the starting gonadotropin dose? | |||||
| Age | FSH | AFC | AMH | ||
| % of Clinics | 17.5 | 7.2 | 43.6 | 31.8 | |
| % of Cycles | 18.9 | 4.5 | 47.0 | 29.6 | |
| 7) Which test do you think is best for evaluating ovarian reserve? | |||||
| AMH | AFC | Basal hormone tests (FSH, LH, and E2) | None of the above | ||
| % of Clinics | 54.3 | 35.9 | 7.4 | 2.4 | |
| % of Cycles | 50.5 | 39.5 | 6.4 | 3.5 | |
| 8) In your opinion, what is the best use of AMH? | |||||
| To predict low ovarian reserve and response to stimulation | To predict high ovarian reserve and response to stimulation | To predict both low and high ovarian reserve and response to stimulation | To predict pregnancy and live birth rates | None of the above | |
| % of Clinics | 22.9 | 3.5 | 71.4 | 0.6 | 1.6 |
| % of Cycles | 21.8 | 3.3 | 73.3 | 0.3 | 1.3 |
| 9) What do you think is the best predictor of ongoing pregnancy rate? | |||||
| Age | FSH | AFC | AMH | Other | |
| % of Clinics | 81.7 | 3.0 | 2.6 | 3.7 | 8.9 |
| % of Cycles | 80.3 | 2.9 | 2.8 | 6.2 | 7.9 |
AMH anti-mullerian hormone, FSH follicle stimulating hormone, E2 estradiol, LH luteinizing hormone, AFC antral follicle count
For each question, multiple choice answers were provided from which only a single answer could be selected. This web-based questionnaire was made available on the IVF-Worldwide website from August 2014 to September 2014. All registered members of the IVF-Worldwide network were invited by an e-mail message to participate in completing the survey. The survey contained a demographic section which collected the name of the IVF clinic, the name of the clinic’s medical director, e-mail address, country, and the number of IVF cycles completed at the clinic in the most recent year. The medical section of the survey evaluated the practice patterns and opinions of respondents with a series of multiple choice questions, presented in Table 1 and discussed within the results section.
Quality assurance methods used
In order to minimize duplicate reports from a clinic and possible data errors, four demographic parameters were used to crosscheck the demographic information submitted by the survey respondent with the existing registration information for that clinic on the IVF-Worldwide website. These parameters included the name of the clinic, the name of the clinic director, country, and e-mail address. If at least three of these parameters matched the information previously registered through the IVF-Worldwide website, then the results from the reporting site were included in the statistical analyses.
Statistical analyses
The analysis was based on the number of IVF cycles reported by the unit and not on the number of clinics in the study. For each question, the survey provided multiple choices from which only a single answer could be chosen (“radio buttons”). The results of the multiple choice questions are reported as both the proportion of the total number of IVF cycles represented by that particular answer choice (respondent-IVF cycles) and as the proportion of clinics which selected that answer choice (Table 1) [14].
Results
Completed electronic survey forms were received from 796 IVF clinics which performed a total of 593,200 IVF cycles annually, from 96 countries representing all 5 continents. The crude response rate to the electronically sent invitation for survey completion was 21 % of the 3764 clinics registered to the IVF-Worldwide website network. The geographic location and the percent contribution to the total survey response was divided into 6 world regions: USA and Canada (64,800 cycles [11 %], 121 clinics [15 %]), South America (39,100 cycles [6.6 %], 93 clinics [12 %]), Australia and New Zealand (37,800 cycles [6.4 %], 28 clinics [4 %]), Asia (140,300 cycles [24 %], 174 clinics [22 %]), Europe (275,900 cycles [47 %], 330 clinics [41 %]), and Africa (35,300 [6 %], 50 clinics [6 %]).
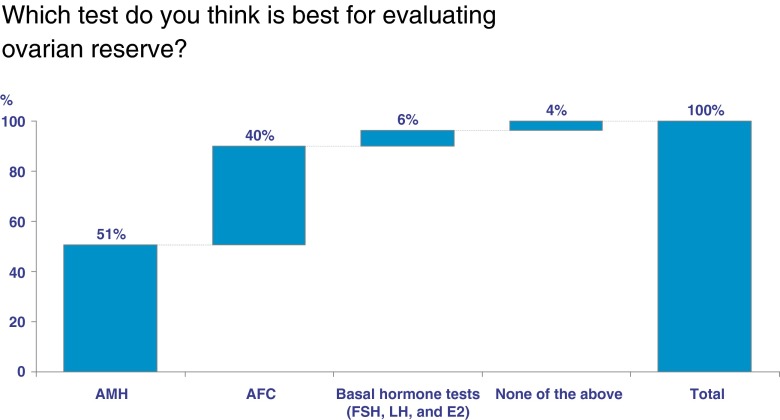
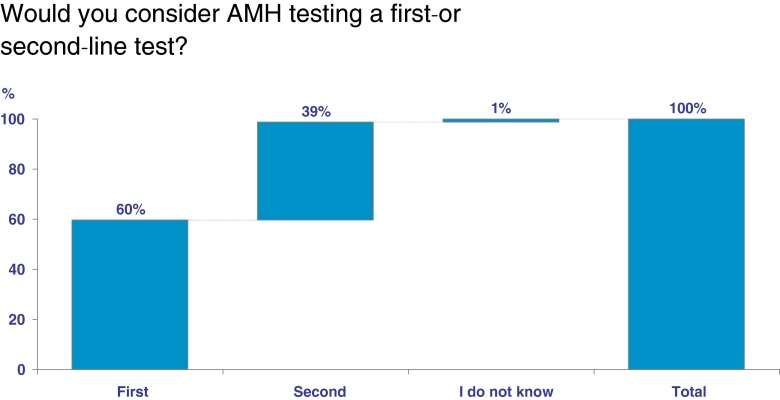
Overall, 75 % (444,900 cycles) of respondent-IVF cycles reported that the majority of patients visiting their IVF clinic underwent ovarian reserve testing. Fifty-one percent (299,566 cycles) reported that AMH is the best test for evaluating ovarian reserve, while 36 % (212,959 cycles) reported that AFC is the best test (Fig. 1). Only 6 % (37,965 cycles) reported that basal hormone testing is the best test for evaluating ovarian reserve. When asked which factor or test best predicts ongoing pregnancy rate, 82 % (484,700 cycles) reported age, 4 % (22,200) reported AMH, and 3 % (15,300) reported AFC. When asked if respondents consider AMH as a first- or second-line test of ovarian reserve, 60 % (354,900 cycles) of respondent-IVF cycles reported it as a first line test (Fig. 2). Finally, respondents were asked how often they would draw an AMH level: 32 % (189,824 cycles) reported only once, 39 % (231,348 cycles) yearly, 18 % (106,776 cycles) twice yearly, 8 % (47,456 cycles) every 3 to 4 months, and 4 % (23,728 cycles) not at all.
Fig 1.
Demonstrates the proportion of respondent-IVF cycles represented by selecting a specific test to evaluate ovarian reserve in response to the question “Which test do you think is best for evaluating ovarian reserve,” anti-mullerian hormone (AMH), antral follicle count (AFC), basal hormone tests (follicle stimulating hormone [FSH], luteinizing hormone [LH], and estradiol [E2]), none of the above
Fig 2.
The proportion of respondent-IVF cycles represented by selecting anti-mullerian hormone (AMH) as either a first line test, second line test, or selecting “I don’t know”
Regarding the clinical application of AMH, 88 % (522,016 cycles) of respondent-IVF cycles reported it as either extremely relevant or relevant, while 12 % (71,184 cycles) considered it not relevant. The same results where noted when the influence of the cost to perform an AMH test was removed as a consideration for performing the test.
The majority of respondent-IVF cycles (86 %; 510,152 cycles) reported that they would stimulate patients based on the AMH levels. However, when asked what single test is best for choosing a gonadotropin dose, AFC was selected over AMH, 44 % (261,008 cycles) versus 32 % (189,824 cycles) respectively. Finally, respondents were asked how best to use AMH, and the majority (73 %; 433,036 cycles) reported to use it to predict both low and high ovarian reserve.
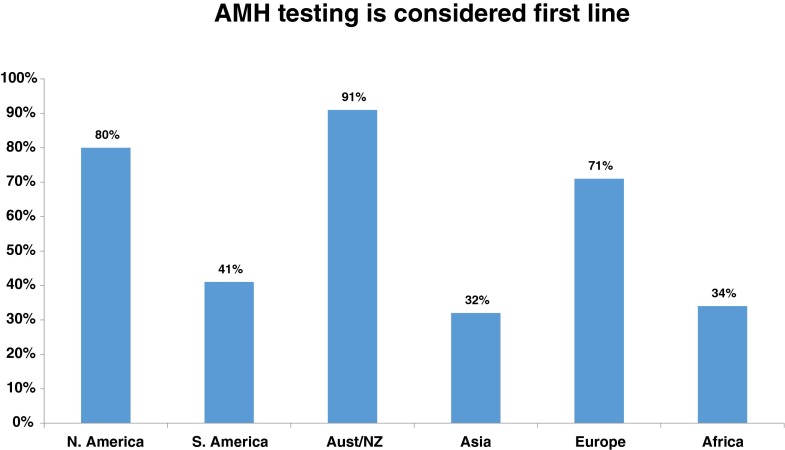
As resource availability and practice patterns may vary between socio-geographic areas of the world, the above listed survey responses were also separated into the six different geographic areas: USA/Canada, South America, Australia and New Zealand, Asia, Europe, and Africa (Table 2). When asked if AMH is considered a first line test, 91 % of respondent-IVF cycles from Australia/New Zealand reported yes and only 32 and 34 % reported yes from Asia and Africa respectively (Fig. 3). Similarly, only 16 and 13 % of respondent-IVF cycles from Africa and Asia would choose AMH for assigning gonadotropin dosages above other ovarian reserve test choices (age, FSH, AFC) as compared to 72 % in Australia/New Zealand. Finally, the question with the highest similarity between geographic regions was the low selection for “AMH is the best predictor of ongoing pregnancy rates” with a range from 2.4 to 4.6 %, demonstrating that all regions essentially agreed that AMH was not the best predictor of ongoing pregnancy rates (Table 2).
Table 2.
Compares the proportion of respondent answers between geographic regions. Answers are reported in percentages. Far right hand column lists the overall mean and standard deviation, demonstrating the variation between geographic regions
| NA | SA | Aust/NZ | Asia | Europe | Africa | Overall Mean (std) | |
|---|---|---|---|---|---|---|---|
| Majority of patients undergo ovarian reserve testing | 61 | 69 | 69 | 83 | 74 | 88 | 74 (10) |
| AMH testing considered first line | 80 | 41 | 91 | 32 | 71 | 34 | 58 (26) |
| AMH results change stimulation protocol | 92 | 73 | 92 | 74 | 93 | 87 | 85 (9) |
| AMH is considered extremely relevant or relevant | 94 | 80 | 92 | 83 | 90 | 92 | 89 (6) |
| If the AMH test was available in your clinic at no cost, would you use it routinely? | 96 | 79 | 90 | 82 | 89 | 82 | 86 (6) |
| Would you choose AMH above other tests (Age, FSH, AFC, baseline hormones) to assign dosage of gonadotropin? | 35 | 19 | 72 | 16 | 33 | 13 | 31 (22) |
| AMH is the best test for evaluating ovarian reserve | 62 | 43 | 79 | 36 | 53 | 50 | 54 (15) |
| AMH is best used to predict low and high ovarian reserve | 82 | 58 | 80 | 63 | 78 | 68 | 72 (10) |
| AMH is the best predictor of ongoing pregnancy ratesa | 3.2 | 4.6 | 2.4 | 4.4 | 3.6 | 3.7 | 4 (1) |
NA North America (USA and Canada), SA South America, Aust/NZ Australia and New Zealand, AMH anti-mullerian hormone, FSH follicle stimulating hormone, AFC antral follicle count, std standard deviation
aOther options were age, FSH, AFC, Other
Fig 3.
The different proportions of respondent-IVF cycles by geographic region which considered anti-mullerian hormone (AMH) a first line test. Regions included North America (USA and Canada), South America (includes all countries south of the United States), Australia and New Zealand (Aust/NZ), Asia, Europe and Africa
Discussion
This report represents the largest, most globally distributed survey of IVF providers evaluating the use of AMH and its application to IVF. Currently, IVF providers have multiple tests for evaluating ovarian reserve and estimating the potential response to COH. Based on our survey results, AMH appears to be a highly valued test with an important role in both evaluating ovarian reserve and attempting to predict ovarian response to stimulation with 60 % of respondent-IVF cycles reporting it as a first line test and 51 % reporting that it is the best test for evaluating ovarian reserve.
As compared to our study, previously conducted studies examining the use of AMH by IVF providers were considerably smaller. In 2008, Maheshwari et al. reported on survey results from 72 IVF providers within the UK’s National Health Service with the goal of ascertaining how IVF was offered to women of advanced maternal age and how ovarian reserve was assessed [13]. They identified that 95 % of clinics used baseline FSH to evaluate ovarian reserve and only 4.5 % used AMH. Of note, this study’s data was collected from 2006 to 2007, and clinical practice patterns within the IVF community continually change. Given the time lapse from 2006 until 2014 (the year of our study), this study likely does not accurately reflect the current use of AMH within the UK. In our study, when the data for just the UK is reviewed, which includes 37 IVF centers and represents 37,000 IVF cycles annually, 81 % (30/37) considered AMH as a first line test for evaluating ovarian reserve. Thus, as illustrated by this contrast, AMH use has dramatically increased from 2006 to the present.
A second study with aims similar to our study, published in 2010 by Van Voorhis et al., surveyed clinics within the USA with the highest reported IVF live birth rates to assess for specific practice patterns that contributed to the IVF success. They identified specific practices common to high-performing clinics which included ovarian reserve screening for all patients and the use of cycle day 3 FSH and AFC. With specific regard to AMH, they reported that only 30 % of high-performing clinics used AMH to measure ovarian reserve [12]. In contrast, when the data collected in our study from the USA is separated, representing 104 centers and 52,500 IVF cycles, 82 % of centers reported AMH has a first line test of ovarian reserve. The discrepancies between the findings from our study and the data published by Maheshwari et al. and Van Voorhis et al. is likely due to the time lapse of greater than 5 years since the collection of this data and may not reflect the current actual use of AMH. Additionally, our study collected a more comprehensive sampling of both the UK and the USA. Based on the 174,962 IVF cycles reported by the Society of Assisted Reproductive Technology (SART) for 2013, our study represented approximately 30 % of the total IVF cycles reported by SART.
In review of the most recent literature, we sought to identify evidence that could potentially underlie the respondents’ opinions. In our study, 75 % of respondent-IVF cycles reported testing all IVF patients for ovarian reserve. However, it remains unanswered whether all patients require ovarian reserve testing prior to IVF. Although fertility predictably declines with age, the outcomes of infertility treatment vary greatly among patients within the same age cohorts. Ovarian reserve testing may assist IVF providers in identifying those patients at risk for suboptimal outcomes and provide information to more effectively counsel and select a gonadotropin dose for the patient undergoing COH. However, when ovarian reserve testing is applied broadly, especially to young women, the potential for a false positive result identifying low ovarian reserve increases and lowers the predictive power of the test, and may be misleading [1, 15]. In a recent ASRM committee opinion, the authors concluded that evidence of decreased ovarian reserve does not necessarily equate with inability to conceive, which has a significant amount of literature to support it [15–17]. However, the ASRM committee opinion does not discourage the broad use of AMH as there is likely benefit and conclude by stating “there is insufficient evidence to recommend that any ovarian reserve test now available should be used as a sole criterion for the use of assisted reproductive technologies” [1]. Additionally, a consensus opinion from ASRM and ESHRE stated that “ovarian reserve testing has moderate accuracy in predicting quantitative responses but low accuracy for qualitative predictions, unless very high thresholds are used” [3]. At this juncture, no definitive statement that all IVF patients should undergo ovarian reserve testing has been made from the two largest reproductive societies in the world.
When the respondents were asked if AMH is considered a first line test and if they believed AMH was the best test for evaluating ovarian reserve, the response was 60 and 51 % respectively. Within the literature, multiple analyses comparing the various ovarian reserve tests have been completed investigating outcomes. Again, no clear consensus was reached [16, 18]; however, AMH and AFC appear to be favored within the literature [18–20]. Prior researchers have attempted to address the precision, accuracy, and predictive value of AMH in addressing those outcomes, comparing AMH both individually and in combination with other tests. In the past, the use of early follicular FSH serum levels was considered the gold standard for assessing ovarian reserve; however, multiple other metrics have since emerged with robust studies supporting their use. Using baseline FSH levels, linear correlations demonstrate that rising FSH values correspond with decreasing AMH values; however, predictive superiority for actual IVF outcomes has yet to be coordinated [21]. When a single marker is selected, AMH appears to outperform other markers of poor ovarian reserve in predicting oocytes retrieved and the number of canceled cycles [20]; however, findings throughout the literature are mixed [22]. This is especially seen with analyzing pregnancy rates and live births. In several series, AMH levels are associated with live births [18, 23]; however, in others, no association was identified [24]. In a recent 2015 meta-analysis conducted by Tal et al., 19 studies were analyzed by specific subpopulations comparing the predictive value of AMH with implantation and pregnancy rates, and they concluded that AMH has some association, but its predictive value is weak [25]. In another recent meta-analysis, which included 13 studies, they also conclude that AMH does have some association with predicting live birth; however, its predictive accuracy is poor with low calculated diagnostic odds ratios [26].
Despite the confounding results identified within the literature regarding the predictive value for pregnancy outcomes, AMH as a measure of ovarian reserve is generally reported as uniformly accurate. However, the hesitance to fully adopt AMH into clinical practice demonstrated by our study may have a historical basis based on the development of the AMH assays integrated into clinical use [27]. Confounding the clinical use and study of AMH from 2002 to 2010 was the availability of different commercial assays, which resulted in substantially different quantitative values [28, 29]. Prior to 2011, the two different tests available were the Diagnostic Systems Lab (DSL) and the Immunotech (IOT) AMH assays. The use of two different assays created challenges to the readership of the infertility literature in applying the findings from the studies. One challenge has been that the IOT assays produced AMH concentrations up to 40 % higher than DSL, making interpretation of the absolute values difficult [28, 30]. Studies that produced cut-off values and nomograms must be scrutinized for the assay used prior to applying the information, regardless of how compelling the data appears, to the reader’s own clinical situation [1, 31]. Currently, the above listed labs DSL and IOT have been acquired by Beckman Coulter, and a single patented assay for testing AMH (AMH Gen II) is now available. Since approximately 2011, studies reporting on AMH values should be measured by the AMH Gen II assay and thus lead to an improvement in the homogeneity of the AMH literature. However, when reviewing an AMH study published since 2011, the generation of the assay should be specifically noted by the authors and the reader should be aware of the assay used by their clinical laboratory prior to attempting to apply AMH values derived from the published literature. Nelson and La Marca provide in great detail an excellent review on the previously used AMH assays the new generation of AMH assay and its implications on future clinical applications and research using AMH [29].
The relevance of this data set is the insight it provides to fertility providers regarding the prevalence and influence of AMH on infertility treatment patterns on a global, diversely distributed population of IVF providers. As listed above, the predictive value of AMH on IVF outcomes is still yet to be definitively established and multiple reviews addressing the use of AMH have outlined both actual and theoretical shortcomings of its application to fertility treatments. With newer, more reliable AMH assays available, the need for relevant nomograms and cut-off values related to treatment outcomes should be forthcoming, but currently are still lacking. Given that the exact role of AMH has yet to be definitively established, our data provides an interesting perspective that despite the potential shortcomings of AMH and the current evolution of the assays used, it is still considered a first line test of ovarian reserve and is reported by the majority of respondents to have significant direct value in the management of IVF cycles.
Despite the encouraging recent reports of AMH use and the widespread use demonstrated by our study, providers must be cognoscente of the limitations; especially if it is used as the primary test to evaluate ovarian reserve. First, the AMH values produced by the specific assay should be considered unique to the laboratory completing the assay. As discussed above, Gen II AMH assays should be used making comparing values obtained with current literature more relevant. However, even with the same Gen II assay, a wide range of average values were still reported by Zuvela et al. [32]. One of the principle concerns is the effects of storage conditions varying from laboratory to laboratory resulted in the variation. Additionally, caution must be used if results from a single ovarian reserve marker will lead to specific, definitive IVF treatment alteration. This is especially important that IVF providers use caution to not deny patients autologous oocyte use based on a single AMH value. AMH has demonstrated benefits with assigning gonadotropin dosing, avoiding cycle cancelation and OHSS. The predictive value relating live birth rates with AMH levels has resulted in varying levels of association depending on the study.
Although our study represents the largest of its kind to-date, a specific weakness with this study is the potential for respondent bias or incorrectly classifying their clinical IVF volume and/or clinical practice patterns. As with all survey data, selection and reporter bias must be considered when reviewing the results. Additionally, as these findings represent the opinions of IVF providers, our findings do not necessarily represent best clinical practices or evidence-based medicine. As outlined above, we attempted to verify if the findings of the majority of respondents correlated with evidence from the literature; however, providers must use their own interpretation of the literature, professional committee guidelines, and their own IVF centers’ capabilities to guide patient care. Future studies of interests could potentially query respondents regarding how specific values of AMH guide decision making in the management of fertility care. Specific values were not elicited in this study due to the absence of data within the literature that would establish the utility of specific values. Once more definitive data demonstrating the value of specific AMH levels is available, querying IVF providers regarding how they integrate these values will be insightful.
In conclusion, this study provides insight into the use of AMH and its value relative to other metrics of ovarian reserve from a very large population of IVF providers. At this time, it appears that the vast majority of IVF providers is using AMH regularly in clinical practice and place a high value on its ability to evaluate ovarian reserve and its predictive abilities for COH.
Acknowledgments
The authors would like to thank the IVF-Worldwide.com network of providers for their participation.
Footnotes
Capsule
The majority of respondents from a globally distributed survey representing 796 IVF clinics and 593,200 IVF cycles considered antimullerian hormone as a first line metric for evaluating ovarian reserve and managing ovarian hyperstimulation protocols for IVF.
References
- 1.Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril, 2015. 103(3): p. e9-e17. [DOI] [PubMed]
- 2.Ferraretti AP, Gianaroli L. The Bologna criteria for the definition of poor ovarian responders: is there a need for revision? Hum Reprod. 2014;29(9):1842–5. doi: 10.1093/humrep/deu139. [DOI] [PubMed] [Google Scholar]
- 3.Gianaroli L, et al. Best practices of ASRM and ESHRE: a journey through reproductive medicine. Fertil Steril. 2012;98(6):1380–94. doi: 10.1016/j.fertnstert.2012.07.1164. [DOI] [PubMed] [Google Scholar]
- 4.Seifer DB, Maclaughlin DT. Mullerian inhibiting substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88(3):539–46. doi: 10.1016/j.fertnstert.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Lee MM, Donahoe PK. Mullerian inhibiting substance: a gonadal hormone with multiple functions. Endocr Rev. 1993;14(2):152–64. doi: 10.1210/edrv-14-2-152. [DOI] [PubMed] [Google Scholar]
- 6.Rajpert-De Meyts E, et al. Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84(10):3836–44. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 7.Broer SL, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96(8):2532–9. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 8.Hansen KR, et al. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–5. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 9.van Disseldorp J, et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod. 2010;25(1):221–7. doi: 10.1093/humrep/dep366. [DOI] [PubMed] [Google Scholar]
- 10.Hendriks DJ, et al. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril. 2005;83(2):291–301. doi: 10.1016/j.fertnstert.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Broer SL, et al. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91(3):705–14. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Van Voorhis BJ, et al. What do consistently high-performing in vitro fertilization programs in the U.S. do? Fertil Steril. 2010;94(4):1346–9. doi: 10.1016/j.fertnstert.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 13.Maheshwari A, Hamilton M, Bhattacharya S. A survey of clinicians’ views on age and access to IVF and the use of tests of ovarian reserve prior to IVF in the United Kingdom. Hum Fertil (Camb) 2008;11(1):23–7. doi: 10.1080/14647270701541095. [DOI] [PubMed] [Google Scholar]
- 14.Vaisbuch E, Leong M, Shoham Z. Progesterone support in IVF: is evidence-based medicine translated to clinical practice? A worldwide web-based survey. Reprod Biomed Online. 2012;25(2):139–45. doi: 10.1016/j.rbmo.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Barnhart K, Osheroff J. Follicle stimulating hormone as a predictor of fertility. Curr Opin Obstet Gynecol. 1998;10(3):227–32. doi: 10.1097/00001703-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Broekmans FJ, et al. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 17.Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17(1):118–23. doi: 10.1093/humrep/17.1.118. [DOI] [PubMed] [Google Scholar]
- 18.Eldar-Geva T, et al. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum Reprod. 2005;20(11):3178–83. doi: 10.1093/humrep/dei203. [DOI] [PubMed] [Google Scholar]
- 19.Nardo LG, et al. Circulating basal anti-Mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92(5):1586–93. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 20.La Marca A, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16(2):113–30. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 21.Singer T, et al. Correlation of antimullerian hormone and baseline follicle-stimulating hormone levels. Fertil Steril. 2009;91(6):2616–9. doi: 10.1016/j.fertnstert.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 22.Broer SL, et al. The role of anti-Mullerian hormone assessment in assisted reproductive technology outcome. Curr Opin Obstet Gynecol. 2010;22(3):193–201. doi: 10.1097/GCO.0b013e3283384911. [DOI] [PubMed] [Google Scholar]
- 23.Nelson SM, Yates RW, Fleming R. Serum anti-Mullerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22(9):2414–21. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- 24.Smeenk JM, et al. Antimullerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87(1):223–6. doi: 10.1016/j.fertnstert.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Tal R, et al. Antimullerian hormone as predictor of implantation and clinical pregnancy after assisted conception: a systematic review and meta-analysis. Fertil Steril. 2015;103(1):119–30. doi: 10.1016/j.fertnstert.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Iliodromiti S, et al. The predictive accuracy of anti-Mullerian hormone for live birth after assisted conception: a systematic review and meta-analysis of the literature. Hum Reprod Update. 2014;20(4):560–70. doi: 10.1093/humupd/dmu003. [DOI] [PubMed] [Google Scholar]
- 27.Schipper I, et al. Limitations and pitfalls of antimullerian hormone measurements. Fertil Steril. 2012;98(4):823–4. doi: 10.1016/j.fertnstert.2012.07.1105. [DOI] [PubMed] [Google Scholar]
- 28.Broer SL, et al. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688–701. doi: 10.1093/humupd/dmu020. [DOI] [PubMed] [Google Scholar]
- 29.Nelson SM, La Marca A. The journey from the old to the new AMH assay: how to avoid getting lost in the values. Reprod Biomed Online. 2011;23(4):411–20. doi: 10.1016/j.rbmo.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Freour T, et al. Measurement of serum anti-mullerian hormone by Beckman coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART) Clin Chim Acta. 2007;375(1-2):162–4. doi: 10.1016/j.cca.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Rustamov O, et al. Anti-Mullerian hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Reprod. 2012;27(10):3085–91. doi: 10.1093/humrep/des260. [DOI] [PubMed] [Google Scholar]
- 32.Zuvela E, Walls M, Matson P. Within-laboratory and between-laboratory variability in the measurement of anti-mullerian hormone determined within an external quality assurance scheme. Reprod Biol. 2013;13(3):255–7. doi: 10.1016/j.repbio.2013.04.005. [DOI] [PubMed] [Google Scholar]