Abstract
Purpose
Only 50–60 % of immature human oocytes attain the mature stage in vitro. Such a deficiency may be a reflection of inadequate conditions of in vitro maturation (IVM) or a manifestation of intrinsic oocyte defects. In the present study, we explored the possibility that the DNA of immature oocytes may be damaged and that such a condition, or inability to trigger a repair action, is associated to germinal vesicle (GV) arrest.
Methods
Immature oocytes (GV-stage oocytes) were obtained from women undergoing stimulated (Stim-C) or IVM (IVM-C) cycles. GV oocytes obtained from stimulated cycles were fixed for successive analysis either after recovery (T0) or following 30 h (T30) of culture if still arrested at the GV stage. Oocytes retrieved in IVM cycles were used only if they were found arrested at the GV stage after 30 h (T30) of culture. All oocytes were fixed and stained to detect chromatin and actin. They were also assessed for positivity to γH2AX and Rad51, markers revealing the presence of double-strand DNA breaks and the activation of a DNA repair response, respectively. Labelled oocytes were analysed using a Leica TCS SP2 laser scanning confocal microscope.
Results
In Stim-C oocytes, γH2AX positivity was 47.5 and 81.5 % in the T0 and T30 groups, respectively (P = 0.003), while γH2AX-positive oocytes were 58.3 % in the IVM-C T30 group (Stim-C T0 vs. IVM-C T30, P = 0.178; Stim-C T30 vs. IVM-C T30, P = 0.035). Positivity for nuclear staining to Rad51 occurred in 42.1 and 74.1 % of Stim-C in the T0 and T30 subgroups, respectively (T = 0.006), while 66.7 % of IVM-C T30 oocytes resulted positive for a DNA repair response (Stim-C T0 vs. IVM-C T30, P = 0.010; Stim-C T30 vs. IVM-C T30, P = 0.345).
Conclusions
The present data document the existence of double-strand DNA breaks (DSBs) in human immature oocytes. Also, they are consistent with the hypothesis that insults to DNA integrity may be an important factor affecting meiotic resumption.
Keywords: Oocytes, DNA damage, DNA repair, In vitro maturation, Germinal vesicle
Introduction
In animal models, especially in the cow and the mouse, meiotic resumption and progression to metaphase II (MII) can be achieved in vitro very efficiently by fully grown oocytes, with rates of maturation exceeding 80–90 % [1]. On the contrary, in the human model, only 50–60 % of immature oocytes attain the mature stage in vitro [2, 3], often as a consequence of an inability to undergo germinal vesicle breakdown (GVBD). The reduced rate by which oocyte maturation is achieved in our species could be a reflection of inadequate conditions of in vitro maturation (IVM) systems [4]. Alternatively, it could implicate that a fraction of germinal vesicle (GV)-stage oocytes are intrinsically incompetent to support meiotic resumption and progression as a result, for example, of activation of cell cycle checkpoints [5] or inadequate chromatin constitution [6]. Oocyte meiotic failure has significant clinical implications not only for IVM treatments but also for conventionally stimulated IVF cycles, in which up to 30 % of all retrieved oocytes do not mature in vivo despite full exposure to gonadotropins. At least part of this phenomenon is likely to involve directly the oocyte, as suggested by the fact that in stimulated IVF cycles immature oocytes are almost invariably associated to expanded cumulus masses, a sign that the somatic component is generally competent to respond to the maturation stimulus represented by ovulatory doses of hCG.
In the present study, we explored the possibility that the DNA of immature oocytes may be damaged and that such a condition, or inability to trigger a repair action, is associated to GV arrest. DNA damage is a very common insult to which all cells are exposed during their lifetime following exposure to exogenous mutagens, the occurrence of physiological processes or as a consequence of pathologies or aging [7]. Mammalian oocytes are particularly exposed to DNA damage. In fact, at the time of primordial follicle formation, double-strand DNA breaks (DSBs) have physiological relevance in the context of meiotic recombination, by allowing the exchange of portions of DNA between homologous non-sister chromatids [8–10]. In addition, DNA damage can potentially accumulate during the long period of oocyte dormancy (lasting up to more than 40 years in the human) that precedes growth and maturation. To test the above hypothesis, we assessed the presence of sites of DSBs and the occurrence of a repair response by detecting γH2AX and Rad51, respectively, by adopting immunofluorescence confocal microscopy as an investigation tool. γH2AX is an effector that modifies and makes accessible the structure of chromatin domains at the lesion sites [11, 12], ultimately acting as a catalyst for the recruitment of the necessary cell cycle checkpoint and DNA repair factors [13]. Rad51 is the central enzymatic component of the mechanism that ensures homologous recombination and error-free repair of DSBs [14]. Collectively, the present data reveal the occurrence of DSBs and the activation of a repair response in human oocytes, suggesting novel aspects of the context that dictate success or failure of meiotic resumption.
Materials and methods
This study, exclusively including fully grown GV-stage oocytes, was carried out using material donated by patients after signing of informed consent and with permission from the local institutional review board. GV oocytes obtained from stimulated cycles were fixed for successive analysis either 2–3 h after recovery or following 30 h of culture if still arrested at the GV stage. Oocytes retrieved in IVM cycles were used only if they were found arrested at the GV stage after 30 h of culture, because surplus freshly recovered GV oocytes are rarely available for research.
Oocytes derived from controlled ovarian stimulation cycles
In controlled ovarian stimulation (COS) cycles, pituitary downregulation was achieved by a gonadotropin-releasing hormone (GnRH) agonist (Decapeptyl 3.75 or 0.1 mg, Ipsen, Italy) while stimulation of follicle growth was carried out with rFSH (Merck Serono, Rome, Italy, or MSD, Milan, Italy), with doses and duration varying on the basis of patient typology [15]. Ten thousand IU of hCG were administered 36 h prior to oocyte collection. After retrieval, oocytes were cultured in IVF medium (Origio, Maløv, Denmark). Within 2 h from collection, cumulus cells were removed by brief exposure to culture medium containing cumulase (80 U/ml; Origio, Maløv, Denmark), followed by mechanical action achieved by passage through a fine bore pipette. GV-stage oocytes were identified and allocated to two groups. In one case, oocytes were fixed and stained to detect chromatin, actin, γH2AX and Rad51 by fluorescence confocal microscopy. Alternatively, GV oocytes were placed in microdrops of IVM medium (Origio, Maløv, Denmark) at 37 °C in a 6 % CO2/5 % O2 humidified atmosphere to achieve in vitro maturation. After 30 h, oocytes that were unable to mature and showing a well-defined GV were fixed and stained for confocal analysis.
Oocytes obtained from IVM cycles
In IVM cycles, patients were primed with 150 IU/day FSH for 3 days from day 3 of the cycle [2]. Women were monitored for follicular growth until a leading follicle of 10–12 mm in diameter and an endometrial thickness >6 mm were observed. Under such conditions, oocyte retrieval was scheduled to occur after 38 h from hCG administration (10,000 IU). Follicle aspiration was performed under transvaginal ultrasound guidance using a single-lumen aspiration needle (code 4551-E2 ∅17, gauge 35 cm; Gynetics, Lommel, Belgium) connected to a vacuum pump (pressure 80–100 mmHg; Craft Pump, Rocket Medical, Washington, UK). Follicular aspirates containing cumulus cell-oocyte complexes (COCs) were collected in a single 50-ml tissue culture flask containing 15 ml of pre-warmed flushing medium with heparin (Origio, Maløv, Denmark). Follicular aspirates were filtered through a 70-μm cell strainer (Becton-Dickinson, Buccinasco, Italy) and washed with flushing medium. COCs were detected, examined and classified according to cumulus oophorus morphology and stage of oocyte maturation [3]. Surplus donated COCs with a GV-stage oocyte surrounded by multiple layers of cubical and tightly compacted cumulus cells were selected for in vitro maturation. To this end, immature COCs were transferred to a single-well petri dish containing 0.5 ml of IVM medium (Origio, Maløv, Denmark) supplemented with 75 mIU/ml recombinant FSH (Merck Serono, Rome, Italy) and 100 mIU/ml hCG (Merck Serono, Rome, Italy). Culture was carried out at 37 °C in a 6 % CO2 humidified atmosphere for 30 h [3]. Afterwards, COCs were treated enzymatically and mechanically to remove cumulus cells, as described above. Oocytes arrested at GV stage without any signs of degeneration (IVM-arrested GV) were fixed and stored at 4 °C for subsequent staining and confocal analysis.
Oocyte fixation and immunostaining
Unless otherwise specified, all chemicals were purchased from Sigma (St. Louis, MO). Oocytes were fixed in microtubule-stabilising buffer (100 mM PIPES, 5 mM MgCl2, 2.5 mM EGTA, 2 % formaldehyde, 0.1 % Triton X-100, 1 mM taxol, 10 U/ml aprotinin and 50 % deuterium oxide) for 30 min at 37 °C and stored in blocking solution (0.2 % sodium azide, 2 % normal goat serum, 1 % BSA, 0.1 M glycine and 0.1 % Triton X-100 in PBS) at 4 °C until processing (for a few weeks maximum). Oocytes were further processed for immunostaining, through serial incubations with primary and secondary antibodies (2 h per antibody at 37 °C with shaking) followed by washing (three washes of 15 min) in blocking solution after each antibody incubation [16]. A mix of primary antibodies was used: a mouse monoclonal anti-γH2AX (phosphoS139) antibody (Millipore, Darmstadt, Germany) plus a rabbit monoclonal anti-Rad51 antibody (Millipore, Darmstadt, Germany) diluted 1:100 in wash solution. Then, oocytes were incubated in a mix containing Alexa 488 goat anti-mouse IgG (Molecular Probes, Eugene, OR; 1:500 dilution), Alexa 568 goat anti-rabbit IgG (1:500; Molecular Probes), rhodamine-phalloidin (Molecular Probes, Eugene, OR; 1:500 dilution) and 1 μg/ml Hoechst 33258 (Molecular Probes, Eugene, OR) to detect chromatin. Finally, samples were mounted in medium containing 50 % glycerol, 25 mg/ml sodium azide and 1 μg/ml Hoechst 33258 using wax cushions to avoid compression of samples.
Imaging acquisition and analysis
The GV of labelled oocytes was analysed at multiple focal planes using a Leica TCS SP2 Laser Scanning Confocal Microscope using a 63X objective and KrArg (488 nm exCitation) and HeNe (543 nm exCitation) lasers for collection of complete three channel sections. To make signal intensity and patterns comparable between samples at different times during the period of the study, the confocal microscopy apparatus was subject to regular checks by the staff of the microscopy facility (Alembic, Milan, Italy) to confirm repeatability of signal emission and image acquisition was carried out under identical instrumental conditions in each confocal microscopy session.
Statistical analysis
Rad51 and γH2AX were recorded as absolute and percentage frequencies in stimulated cycle (Stim-C) and IVM cycle (IVM-C) classes of oocytes. DNA damage frequencies were analysed for each group compared to the others according to Fisher’s exact test. Logistic regression was used to evaluate the association of female age with DNA damage both in a univariate model and in a multifactorial model including immature oocyte classes.
A level of P < 0.05 was adopted for significance. Stata software 9.0 (Stata Corporation, College Station, TX) was used for performing statistical analysis.
Results
Overall, 84 GV-stage oocytes obtained from Stim-C and 48 GV-stage oocytes derived from IVM-C were examined in this study. Stim-C oocytes were fixed for confocal microscopy analysis either within 2 h after retrieval (T0, N = 57) or following culture for 30 h (T30, N = 27). Stim-C oocytes observed at T0 were donated by 32 women whose age (mean ± SD) was 37.1 ± 3.2 years (range 31–43), while Stim-C oocytes fixed at T30 were obtained from 22 women aged 36.4 ± 4.2 years (range 30–42). IVM-C GV-stage oocytes were donated by 25 women with a mean age of 32.3 ± 4.4 years (range 25–37) and were fixed for analysis after 30 h (T30) of culture. The mean age of women contributing their oocytes to the IVM-C group was significantly younger (P = 0.0001). This was expected because selection criteria for IVM treatment involve a younger female age range [17]. No IVM-C GV-stage oocytes fixed at T0 were included in this study because such material is very valuable for IVM treatments and therefore rarely available for research. A maximum of six oocytes was donated from each woman.
Stim-C and IVM-C oocytes found arrested at the GV stage after 30 h of culture were obtained from pools of oocytes whose overall maturation rate (% MII oocytes after culture) was 33.3 % (n = 39) and 34.2 % (n = 19), respectively. Such low rates of maturation were expected, because oocyte pools included in the study were positively selected for the presence of at least one GV-stage oocyte after 30 h of culture.
Double-strand DNA breaks and repair response
Multiple focal planes of the GV of each oocyte were observed in order to monitor the entire nuclear compartment. Table 1 describes the percentages of positivity of the three classes of oocytes to DSBs and DNA repair response, as assessed by γH2AX and Rad51 staining, respectively.
Table 1.
Percentages of immature oocytes obtained from stimulated (Stim-C) or IVM (IVM-C) cycles assessed for positivity to γH2AX or Rad51 staining immediately after recovery (T0) or after culture for 30 h (T30)
| Stim-C T0 | Stim-C T30 | IVM-C T30 | P value | |
|---|---|---|---|---|
| Age (mean ± SD) | 37.05 ± 3.20 | 36.44 ± 4.20 | 32.27 ± 4.41 | 1 vs. 2, 0.233 |
| 1 vs. 3, <0.0001 | ||||
| 2 vs. 3, 0.0001 | ||||
| γH2AX | 27/57 (47.5 %) | 22/27 (81.5 %) | 28/48 (58.3 %) | 1 vs. 2, 0.003 |
| 1 vs. 3, 0.178 | ||||
| 2 vs. 3, 0.035 | ||||
| RAD51 | 24/57 (42.1 %) | 20/27 (74.1 %) | 32/48 (66.7 %) | 1 vs. 2, 0.006 |
| 1 vs. 3, 0.010 | ||||
| 2 vs. 3, 0.345 |
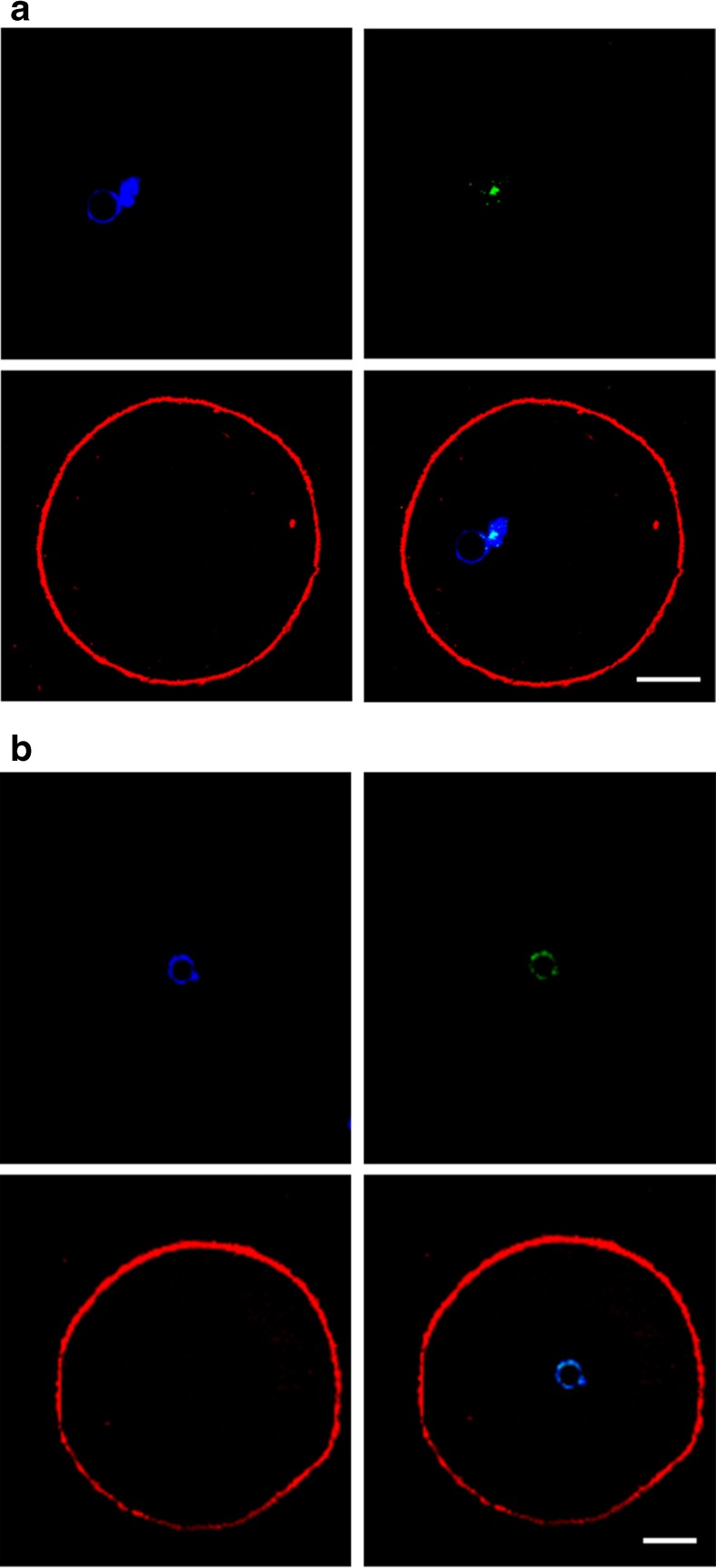
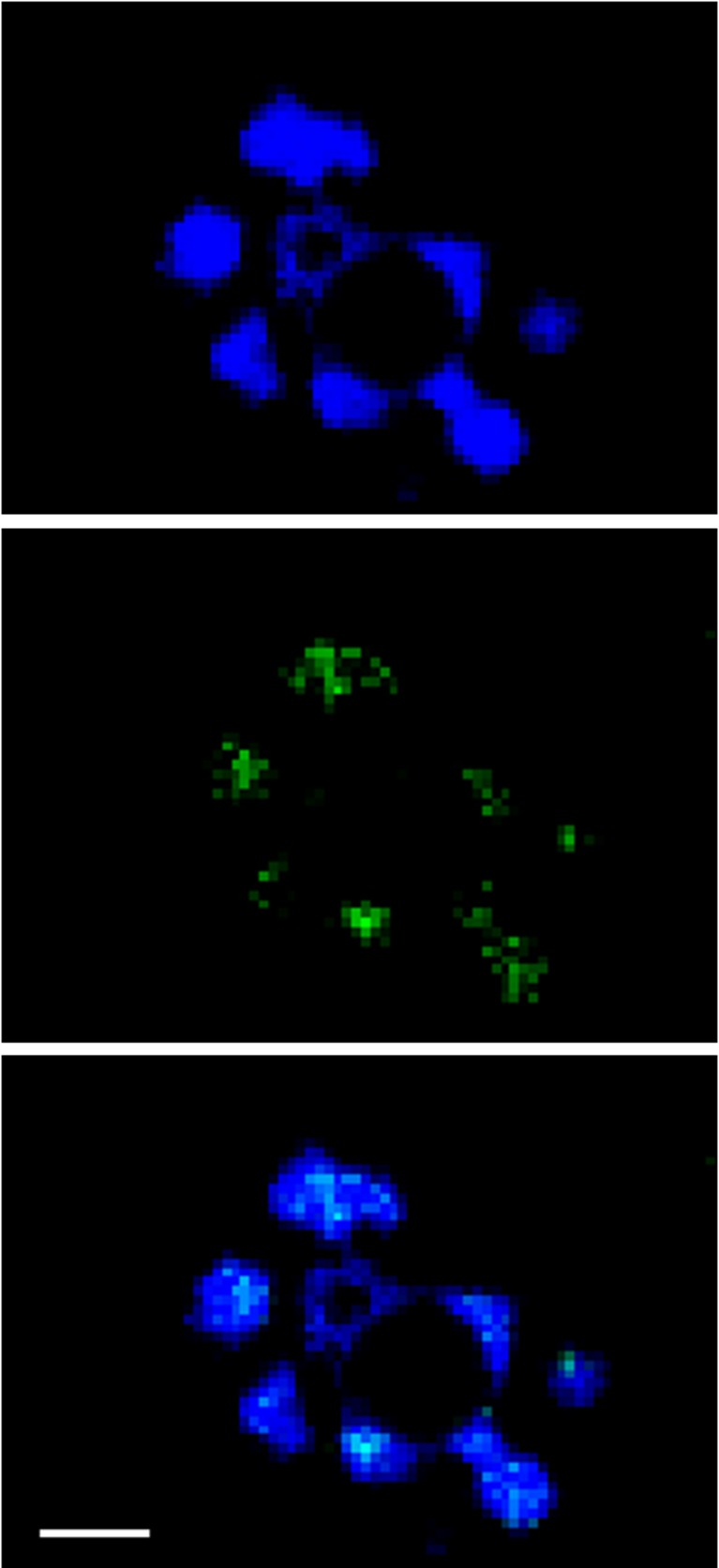
Positivity for γH2AX appeared as a single spot or as multiple staining spots colocalised with chromatin material (Figs. 1a, b and 2). In Stim-C oocytes, γH2AX positivity was 47.5 and 81.5 % in the T0 and T30 groups, respectively (P = 0.003), while γH2AX-positive oocytes were 58.3 % in the IVM-C T30 group (Stim-C T0 vs. IVM-C T30, P = 0.178; Stim-C T30 vs. IVM-C T30, P = 0.035).
Fig. 1.
Representative images of GV-stage oocytes displaying a single site (a) or multiple sites (b) of DNA damage. Oocytes were stained for chromatin (blue, top left), γH2AX (green, top right) and actin (red, bottom left). Merged images (bottom right) show co-localisation of γH2AX with chromatin. Scale bar equals 15 μm
Fig. 2.
GV of an immature oocyte stained for chromatin (blue, top) and γH2AX (green, middle). The merged image shows total co-localisation of γH2AX with chromatin. Scale bar equals 5 μm
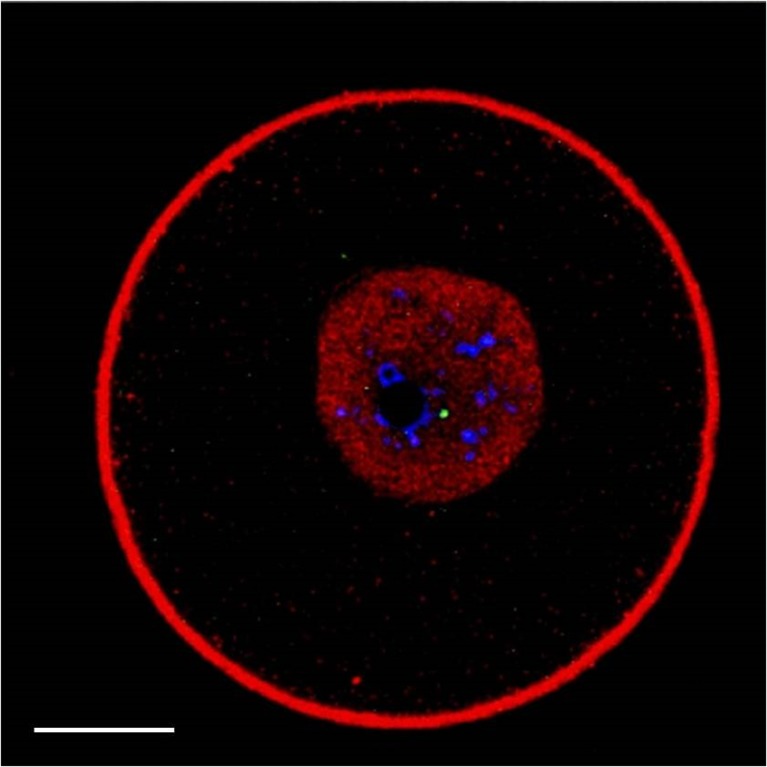
Rad51 staining occurred as an intranuclear homogeneous fine granular pattern, without focal sites of accumulation (Fig. 3). Notably, in numerous previous observations [18], we never detected actin within the nuclear compartment of hundreds of human oocytes, allowing us to conclude that the red staining confined in the GV was specific for Rad51.
Fig. 3.
Representative image of a GV-stage oocyte stained for γH2AX (green), chromatin (blue), actin (red) and Rad51 (red). In addition to showing a site of damaged DNA and the chromatin organised around the nucleolus or partly dispersed, the nucleus displays an intense and homogeneous red staining. Under the conditions applied in this study, actin is never observed in the GV (see also the “Results” section). Therefore, intranuclear red staining corresponds to Rad51 localisation. Scale bar equals 15 μm
Positivity for nuclear staining to Rad51 occurred in 42.1 and 74.1 % of Stim-C in the T0 and T30 subgroups, respectively (T = 0.006), while 66.7 % of IVM-C T30 oocytes resulted positive for a DNA repair response (Stim-C T0 vs. IVM-C T30, P = 0.010; Stim-C T30 vs. IVM-C T30, P = 0.345).
Impact of age and oocyte type
In an univariate model (Table 2), female age was not a factor associated to the positivity for γH2AX and Rad51, irrespective of whether data were analysed collectively or separately for the three groups. On the contrary, in a multivariate model (Table 3) Rad51 was positively associated to the Stim-C T0 group in comparison to both Stim-C T30 and IVM-C T30, even after correction for female age, while γH2AX data corrected for age showed significant association only for Stim-C T0 vs. Stim-C T30.
Table 2.
Univariate analysis showing lack of association with positivity for γH2AX and Rad51, irrespective of whether data were analysed collectively or separately for the three groups
| γH2AX | Rad51 | |||
|---|---|---|---|---|
| OR (95 % CI) | P | OR (95 % CI) | P | |
| Age | ||||
| All groups | 1.01 (0.93–1.09) | 0.799 | 0.96 (0.88–1.04) | 0.275 |
| Stim-C T0 | 0.98 (0.83–1.16) | 0.840 | 1.02 (0.87–1.21) | 0.752 |
| Stim-C T30 | 0.90 (0.70–1.16) | 0.422 | 0.85 (0.67–1.10) | 0.218 |
| IVM-C T30 | 1.09 (0.95–1.25) | 0.200 | 1.01 (0.88–1.16) | 0.870 |
Table 3.
Multivariate analysis showing Rad51 positive association with the Stim-C T0 group in comparison to both Stim-C T30 and IVM-C T30, even after correction for female age. In the case of γH2AX, data corrected for age showed significant association only for Stim-C T0 vs. Stim-C T30
| γH2AX | Rad51 | |||
|---|---|---|---|---|
| OR (95 % CI) | P | OR (95 % CI) | P | |
| Age | ||||
| Stim-C T0 vs. Stim-C T30 | 0.96 (0.84–1.10) | 0.529 | 0.97 (0.85–1.10) | 0.609 |
| 4.81 (1.59–14.5) | 0.005 | 4.74 (1.66–13.6) | 0.004 | |
| Stim-C T0 vs. IVM-C T30 | 1.05 (0.94–1.16) | 0.376 | 1.02 (0.92–1.13) | 0.743 |
| 1.40 (0.88–2.22) | 0.158 | 1.73 (1.08–2.78) | 0.024 | |
| Stim-C T30 vs. IVM-C T30 | 1.04 (0.93–1.17) | 0.461 | 0.97 (0.86–1.09) | 0.593 |
| 0.38 (0.11–1.27) | 0.116 | 0.50 (0.15–1.66) | 0.256 | |
Discussion
In vivo, and in many species also in vitro, the large majority of fully grown oocytes are able to support meiotic maturation. Human oocytes diverge from this pattern because in IVM cycles, maturation rates rarely exceed 50 % within an interval of 30–32 h of culture [2, 3]. Factors that affect meiotic maturation have therefore attracted considerable interest, representing a bottleneck for the development of IVM as an established approach in human assisted reproduction technology. Unquestionably, inappropriate culture conditions concur to cause meiotic resumption failure in vitro, almost likely as a result of lack of crucial maturation-promoting factors [19, 20]. However, inability to resume and support meiotic progression to MII could also be secondary to intrinsic oocyte conditions [6]. Therefore, motivated by the practical interest of improving the efficiency of human IVM and the urge to better understand the process of oocyte maturation, in this study, we explored the hypothesis that DNA damage has an influence on the rate by which GV oocytes resume meiosis.
In 47.5 % of GV oocytes recovered from fully stimulated IVF cycles, we observed single or multiple sites of DSB. In oocytes of the same source that remained arrested at the GV stage after 30 h of culture, DSBs were much more represented (81.5 %). In the same oocyte groups, occurrence of a DNA repair response, as suggested by positivity to Rad51, was observed with rates (42.1 and 74.1 %, respectively) mirroring those expressing the presence of DNA damage. In oocytes obtained from IVM cycles and arrested at the GV stage after 30 h of culture, the rate of DNA damage was relatively low (58.3 %) in comparison to the equivalent group obtained from stimulated cycles, while the incidence of positivity to Rad51 was 66.7 %.
This study extends previous data on the occurrence of DSBs and the existence of a signal of DNA repair response in human fully grown immature oocytes [21] and above all suggests for the first time that DNA damage may be a cause of meiotic arrest.
DNA damage is a very common insult to which all cells, and in particular oocytes as discussed above, are exposed during their lifetime [7]. Cells interpret single-strand DNA breaks (SSBs) or DSBs as a serious threat to their integrity, reacting with a DNA damage response involving an arrest of the cell cycle as a downstream effect of the activation of DNA damage checkpoints (DDCs), in order to give time to repair mechanisms to intervene [22]. SSBs and DSBs trigger a DDC response by activating two major kinases, i.e. ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related (ATR) [23, 24]. At the mitotic/meiotic phase G2, in the presence of DNA injury, these regulators inhibit entry into the M phase via a complex regulatory cascade. Taking advantage of the cell cycle arrest, the cell can then repair the damaged DNA. At the DNA lesion site, ATM gives rise to the phosphorylated form of the H2AX histone (γH2AX), which acts as a catalyst for the recruitment of the necessary checkpoint and repair factors [13], including Rad51. A limitation of this work is that we have only studied two markers of the DNA damage detection and response landscape. Therefore, future studies will be required to better define the specific mechanisms employed by human oocytes in effecting and resolving DSBs. Interesting in this respect is our observation that in the nucleus of human oocytes, Rad51 is distributed in a diffuse fashion in the absence of specific sites of accumulation. This is in sharp contrast with the phenomenology of this protein in somatic cells, where discrete Rad51 foci are discernible in response to a DNA damage event [25]. Our data prompts several considerations on the relevance of DNA damage for oocyte maturation. The fact that in oocytes obtained from stimulated cycles and arrested at the GV stage after 30 h of culture the incidence of DNA damage is higher in comparison to the non-cultured group may be explained by two different hypotheses: (a) the presence of DNA damage triggers meiotic arrest at the GV stage to allow the timely expression of a repair response (in such a case, arrest at the GV stage would coincide with an enrichment in the fraction of oocytes with damaged DNA) and (b) culture conditions, such as exposure to non-physiological oxygen concentrations, induce DSBs per se [26]. On the basis of the present data, the latter hypothesis cannot be ruled out, although there is no evidence indicating that culture conditions normally applied in an IVF system are a source of significant DNA damage. Rather, we reckon the former hypothesis to be more likely, also in consideration of data generated in the mouse model. Indeed, recent experiments show that fully grown mouse oocytes are unable to undergo GVBD and progress to MI after exposure to high concentrations of etoposide, a topoisomerase II inhibitor and DSB inducer [27].
Our data also suggest that, in GV oocytes, the occurrence of DSBs is associated to a repair response that progresses at least until the phase of expression of a factor that mediates single-strand DNA exchange between sister chromatids, a process required by the mechanism of homologous recombination. Why in oocytes recovered from stimulated cycles arrested after 30 h of culture the presence of Rad51 does not seem to attenuate the occurrence of DNA damage is unclear, although it is possible that GV oocytes fail to activate other repair response factors acting downstream Rad51. However, it should be reminded again that our data are preliminary, especially concerning the baseline of DNA damage occurring in immature oocytes obtained from IVM cycles.
Another interesting consideration emerges from the comparison of the two groups of oocytes, obtained from stimulated and IVM cycles, that were found arrested at the GV stage after 30 h of culture. In particular, in the IVM group, the rate of damage from DSBs is relatively lower. Such a difference may derive either from endogenously lower levels of DNA damage or a higher capacity of DNA repair of oocytes recovered from IVM cycles. Concerning possible differences inherently existing between oocytes derived from IVM or stimulated cycles, it should be also taken into consideration that high doses of gonadotropins may be a source of profound perturbations of follicle cells and oocyte function, leading to reduced oocyte developmental competence [28]. Therefore, the hypothesis that in stimulated cycles high doses of gonadotropins may generate an increased rate of oocyte DNA damage will deserve attention in future investigations. Unfortunately, as explained in a previous manuscript section, because GV oocytes freshly recovered from IVM cycles are not normally available for research purposes, this ambiguity is destined to remain unresolved in the present manuscript.
Finally, our data do not allow to ascertain whether a possible meiotic arrest at the GV stage caused by the occurrence of DSBs occurs with absolute or high stringency or, vice versa, a fraction of GV oocytes carrying DNA damage escape the mechanism of meiotic block and progress to MII. The present study involved only immature oocytes, but mature oocytes will be the object of our interest in future investigations. In fact, the possible presence of damaged DNA in mature oocytes bears potentially important implications. A recent study carried out in the mouse model indicates that DNA damage may not be detected or repaired during oocyte maturation and therefore can be passed on to the embryo. In particular, it was shown that immature mouse oocytes exposed to bleomycin or laser microbeam dissection, agents causing DSBs, exhibit some delay in the GVBD kinetics but nevertheless are able to extrude the PBI and progress to MII. If activated, these oocytes can support parthenogenetic development, although the resulting embryos display different anomalies, including multiple pronuclei and multiple micronuclei [29].
In conclusion, the present data document the existence of DSBs in human immature oocytes. Moreover, they are consistent with the hypothesis that insults to DNA integrity may be an important factor affecting meiotic resumption and ultimately oocyte maturation, as suggested by an increase in the rate of γH2AX/Rad51 positivity in GV-arrested oocytes derived from both groups of IVF patients. They also improve our perception of possible margins of progress of oocyte IVM and suggest novel avenues for future research on genetic integrity as a novel biomarker of oocyte quality.
Footnotes
Capsule
Immature human oocytes are affected by double-strand DNA damage and manifest the activation of a DNA repair response. Insults to DNA integrity may be an important factor affecting meiotic resumption.
References
- 1.Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum Reprod. 2010;25(12):2999–3011. doi: 10.1093/humrep/deq246. [DOI] [PubMed] [Google Scholar]
- 2.Fadini R, Dal Canto MB, Mignini Renzini M, Brambillasca F, Comi R, Fumagalli D, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod Biomed Online. 2009;19(3):343–351. doi: 10.1016/S1472-6483(10)60168-X. [DOI] [PubMed] [Google Scholar]
- 3.Dal Canto M, Brambillasca F, Mignini Renzini M, Coticchio G, Merola M, Lain M, et al. Cumulus cell-oocyte complexes retrieved from antral follicles in IVM cycles: relationship between COCs morphology, gonadotropin priming and clinical outcome. J Assist Reprod Genet. 2012;29(6):513–519. doi: 10.1007/s10815-012-9766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilchrist RB. Recent insights into oocyte-follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod Fertil Dev. 2011;23(1):23. doi: 10.1071/RD10225. [DOI] [PubMed] [Google Scholar]
- 5.Jones KT. Turning it on and off: M-phase promoting factor during meiotic maturation and fertilization. Mol Hum Reprod. 2004;10(1):1–5. doi: 10.1093/molehr/gah009. [DOI] [PubMed] [Google Scholar]
- 6.Combelles CMH, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17(4):1006–1016. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]
- 7.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. Nature Publishing Group; 2009 Oct 12;461(7267):1071–8 [DOI] [PMC free article] [PubMed]
- 8.Burgoyne PS, Mahadevaiah SK, Turner JMA. The consequences of asynapsis for mammalian meiosis. Nat Rev Genet. 2009;10(3):207–216. doi: 10.1038/nrg2505. [DOI] [PubMed] [Google Scholar]
- 9.McDougall A, Elliott DJ, Hunter N. Pairing, connecting, exchanging, pausing and pulling chromosomes. 2005;120–5. [DOI] [PMC free article] [PubMed]
- 10.Govindaraj V, Basavaraju RK, Rao AJ. Changes in the expression of DNA double strand break repair genes in primordial follicles from immature and aged rats. Reprod Biomed Online. Reproductive Healthcare Ltd; 2015;30(3):303–10. [DOI] [PubMed]
- 11.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273(10):5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 12.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296(5569):922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgoyne PS, Mahadevaiah SK, Turner JMA. The management of DNA double-strand breaks in mitotic G2, and in mammalian meiosis viewed from a mitotic G2 perspective. Bioessays. 2007;29(10):974–986. doi: 10.1002/bies.20639. [DOI] [PubMed] [Google Scholar]
- 14.Cosnefroy O, Tocco A, Lesbats P, Thierry S, Calmels C, Wiktorowicz T, et al. Stimulation of the human RAD51 nucleofilament restricts HIV-1 integration in vitro and in infected cells. J Virol. 2011;86(1):513–526. doi: 10.1128/JVI.05425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fadini R, Brambillasca F, Renzini MM, Merola M, Comi R, De Ponti E, et al. Human oocyte cryopreservation: comparison between slow and ultrarapid methods. Reprod Biomed Online. 2009;19(2):171–180. doi: 10.1016/S1472-6483(10)60069-7. [DOI] [PubMed] [Google Scholar]
- 16.Coticchio G, Guglielmo M-C, Dal Canto M, Fadini R, Mignini Renzini M, De Ponti E, et al. Mechanistic foundations of the metaphase II spindle of human oocytes matured in vivo and in vitro. Hum Reprod. 2013;28(12):3271–3282. doi: 10.1093/humrep/det381. [DOI] [PubMed] [Google Scholar]
- 17.Fadini R, Dal Canto MB, Renzini MM, Brambillasca F, Comi R, Fumagalli D, et al. Predictive factors in in-vitro maturation in unstimulated women with normal ovaries. Reprod Biomed Online. 2009;18(2):251–261. doi: 10.1016/S1472-6483(10)60263-5. [DOI] [PubMed] [Google Scholar]
- 18.Coticchio G, Guglielmo M-C, Albertini DF, Dal Canto M, Mignini Renzini M, De Ponti E, et al. Contributions of the actin cytoskeleton to the emergence of polarity during maturation in human oocytes. Mol Hum Reprod. 2014;20(3):200–207. doi: 10.1093/molehr/gat085. [DOI] [PubMed] [Google Scholar]
- 19.Smitz JEJ, Thompson JG, Gilchrist RB. The promise of in vitro maturation in assisted reproduction and fertility preservation. Semin Reprod Med. 2011;29(1):24–37. doi: 10.1055/s-0030-1268701. [DOI] [PubMed] [Google Scholar]
- 20.Richani D, Ritter LJ, Thompson JG, Gilchrist RB. Mode of oocyte maturation affects EGF-like peptide function and oocyte competence. Mol Hum Reprod. 2013;19(8):500–509. doi: 10.1093/molehr/gat028. [DOI] [PubMed] [Google Scholar]
- 21.Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5(172):172ra21. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19(2):238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 24.Reinhardt HC, Yaffe MB. Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol. 2009;21(2):245–255. doi: 10.1016/j.ceb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waddell N, Pajic M, Patch A-M, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Combelles CMH, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online. 2009;18(6):864–880. doi: 10.1016/S1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage. Current Biology. Elsevier Ltd; 2012;22(11):989–94. [DOI] [PubMed]
- 28.Eppig JJ, O’Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod. 1998;59(6):1445–1453. doi: 10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- 29.Ma J-Y, Ou-Yang Y-C, Wang Z-W, Wang Z-B, Jiang Z-Z, Luo S-M, et al. The effects of DNA double-strand breaks on mouse oocyte meiotic maturation. Cell Cycle. 2013;12(8):1233–1241. doi: 10.4161/cc.24311. [DOI] [PMC free article] [PubMed] [Google Scholar]