Abstract
Purpose
The aim of this study is to evaluate the outcomes of in vitro fertilization (IVF), including cumulative live birth rate, among women <25 years, 25 to <30 years, and 30 to <35 years.
Methods
A retrospective cohort study of all women 18 to <35 years of age at their first fresh-embryo, non-donor IVF cycle from January 1995 through December 2012 at a single center was conducted. A competing-risk regression model was used to estimate the cumulative probability and 95 % confidence interval (CI) of the first live birth in up to 6 cycles during the study period with IVF cycle number as the time metric.
Results
Among 7243 women who underwent 16,792 cycles, there were 163 (2.3 %) women <25 years, 1691 (23.3 %) women 25 to <30 years, and 5389 (74.4 %) women 30 to <35 years. Women <25 years had the lowest cumulative live birth rate after each cycle, followed by women 30 to <35 years. In both groups, the cumulative live birth rate after 6 cycles was significantly lower than that of women 25 to <30 years; these rates were 58 % (95 % CI 0.51–0.66) among women <25 years, 69 % (95 % CI 0.67–0.71) among women 25 to <30 years, and 64 % (95 % CI 0.63–0.65) among women 30 to <35 years.
Conclusions
Our findings are consistent with other reports of less favorable IVF treatment outcomes in women <25 years of age following their first IVF cycle. This indicates that there are underlying factors in couples with a female <25 years of age that should lead to different treatment counseling when they attempt IVF.
Keywords: In vitro fertilization, IVF, Cumulative live birth rate, Young women
Introduction
There is little dispute that advancing maternal age results in decreased fertility. While several large studies have reported age-specific outcomes of in vitro fertilization (IVF), the primary interest is generally on outcomes among older women. Thus, narrower age ranges are used for older women, and no further stratification is presented for women less than 35 years of age. This is consistent with the way data are presented by the Society for Assisted Reproductive Technologies (SART).
Malizia et al. reported cumulative live birth rates among 6164 women undergoing IVF stratified into the following age groups: <35 years, 35 to <38 years, 38 to <40 years, and ≥40 years. As expected, cumulative live birth rates decreased with increasing maternal age [1]. Similar results were reported by Luke et al. in a more recent study [2].
While advanced maternal age has clearly been associated with a decline in cumulative live birth rates in patients older than 35, the reproductive performance of women younger than 35 has been less well investigated. Based on the available data, most physicians assume that young women should produce the healthiest oocytes and have the best treatment outcomes. Interestingly, some small studies have suggested that this may not be the case [3, 4]. Nazemian et al. compared IVF treatment outcomes among young infertile patients 19–25 years of age to outcomes in infertile patients 30–35 years of age. Patients age 25 and younger had a lower fertilization rate, a reduced number of good quality embryos, and a higher miscarriage rate, though their clinical pregnancy rates were similar to their older counterparts [3]. Although this study only included 89 patients 25 years or younger and only reported outcomes after the first cycle, it raised an interesting question about the reproductive performance of very young women. An intriguing report by Franasiak et al. also showed that even though aneuploidy increased predictably in the embryos of women after 26 years of age, a slightly increased prevalence was noted at younger ages, with >40 % aneuploidy in women 23 years and under [5]. Additionally, 2009 national data from Australia and New Zealand revealed that women aged 25–32 years had the highest live birth rates, while women in their early 20s had a lower live birth rate [4].
In this study, we report outcomes following IVF at a single center, including the cumulative live birth rate, among women less than 35 stratified into the following three age groups: <25 years, 25 to <30 years, and 30 to <35 years.
Materials and methods
We performed a retrospective cohort study of all patients who were 18 to <35 years of age at the start of their first fresh-embryo, non-donor IVF cycle from January 1995 through December 2012. Data were collected prospectively for clinical purposes. Subsequent frozen-embryo transfer cycles were included as distinct cycles [1, 6]. We included up to six fresh- and frozen-embryo transfer cycles for all eligible women. Although numerous clinical (e.g., type of follicle stimulating hormone, luteal support) and laboratory (e.g., embryo culture media, day of freezing) changes occurred during the study period, there was no indication that the age cohorts were treated differently over time.
We collected baseline demographic characteristics, cycle characteristics, and cycle outcomes, including clinical pregnancy, spontaneous abortion, and live birth from the medical record. Pregnancy was confirmed by rising serum ß-human chorionic gonadotropin and fetal heartbeat seen on ultrasound.
Patients underwent treatment protocols for ovarian stimulation, monitoring, and oocyte retrieval as previously described [7]. The number of embryos transferred into the uterus was consistent with national guidelines [8] and decreased in the <35 age groups from a mean of approximately 2.5 to 1.7 during the study period (unpublished data). Patients received luteal phase support until 8 weeks of gestation [7]. Intracytoplasmic sperm injection (ICSI) and assisted hatching were performed when indicated.
Prior to the transfer of cryopreserved embryos, patients received exogenous estradiol with or without a GnRH agonist as previously described [1]. Transvaginal ultrasound was used to evaluate the endometrial lining. Once the lining was noted to be ≥7 mm, luteal phase support was started either 4 days prior to a day-3 embryo transfer or 6 days prior to a blastocyst transfer. Patients received luteal phase support until 10 weeks of gestation.
Descriptive data are presented as mean ± standard deviation or proportion and were compared using a t test or chi-square test. All tests were two-sided, and a p value <0.05 was considered statistically significant. All statistical analyses were conducted with SAS 9.3 (SAS Institute, Cary, NC) and Stata12 (StataCorp, College Station, TX).
Each woman in the study population experienced one of three possible outcomes—achieving a live birth, completing six IVF cycles without having a live birth, or not returning to care before either of the first two outcomes occurred. The women who did not have a live birth and did not return for further treatment included those who opted to pursue oocyte donation and/or gestational carrier, transferred care to another treatment center, had a spontaneous pregnancy, or discontinued all IVF treatments before completing six IVF cycles. Not returning to care was treated as a competing risk in the analysis. A competing-risk regression model was used to compute the cumulative incidence function to estimate the cumulative probability and 95 % confidence interval (CI) of the first live birth during the study period with IVF cycle number as the time metric [9]. This provides a more conservative estimate of cumulative live birth rates than the Kaplan-Meier approach, in which women who do not return to care are censored at the time of their last IVF cycle. When the only competing risk is not returning to care and all of the censored observations are women who did not return to care, as is the case with the dataset used here, the competing risk approach is mathematically equivalent to the “conservative” estimate we and others presented previously [1, 2]. Cumulative live birth rates were calculated for the full cohort and for the following age strata: <25 years, 25 to <30 years, and 30 to <35 years. When the cumulative incidence function curves were stratified by age, the Pepe-Mori test was used to compare the curves [10]. As we have done elsewhere, we used the term cumulative live birth rate despite it being a proportion rather than a rate, to remain consistent with terminology used throughout the literature [1, 6].
The Committee on Clinical Investigations at Beth Israel Deaconess Medical Center approved this study.
Results
Baseline and cycle characteristics
This study included 7243 women <35 years at the start of their first cycle who underwent 16,792 non-donor IVF cycles at a single center. There were 163 (2.3 %) women <25 years; 1691 (23.3 %) women 25 to <30 years; and 5389 (74.4 %) women 30 to <35 years. There were no differences in BMI or year in which the first cycle was performed across the three age groups. Women <25 years were more likely to be nulligravid and nulliparous and had a lower level of cycle-day-3 follicle stimulating hormone than women in the two older age groups, though these differences were statistically significant only when compared with women 30 to <35 years (all p ≤ 0.02). Baseline characteristics are shown in Table 1.
Table 1.
Participant characteristics at the start of the first non-donor IVF cycle for women under the age of 35
| Characteristic | <25 years N = 163 | 25 to <30 years N = 1691 | P | 30 to <35 years N = 5389 | P a |
|---|---|---|---|---|---|
| Maternal age (years) | 23.7 ± 1.2 | 28.3 ± 1.3 | <0.001 | 32.7 ± 1.4 | <0.001 |
| Paternal age (years) | 29.8 ± 6.2 | 32.2 ± 4.6 | 0.001 | 35.1 ± 4.4 | <0.001 |
| Body mass index | 25.8 ± 6.0 | 25.8 ± 5.9 | 0.99 | 25.1 ± 5.4 | 0.19 |
| Gravidity | 0.11 | <0.001 | |||
| 0 | 115 (74.2) | 1111 (68.0) | 3074 (58.5) | ||
| ≥1 | 40 (25.8) | 523 (32.0) | 2181 (41.5) | ||
| Parity | 0.17 | <0.001 | |||
| 0 | 117 (90.7) | 1189 (86.4) | 3474 (77.5) | ||
| ≥1 | 12 (9.3) | 187 (13.6) | 1011 (22.5) | ||
| Cycle day 3 FSH | 5.2 ± 2.6 | 5.6 ± 2.7 | 0.37 | 6.2 ± 3.1 | 0.02 |
| Infertility diagnosisb | |||||
| Tubal factor | 31 (19.0) | 300 (17.7) | 0.68 | 871 (16.2) | 0.33 |
| Ovulatory dysfunction | 26 (16.0) | 264 (15.6) | 0.91 | 630 (11.7) | 0.10 |
| Diminished ovarian reserve | 0 (0.0) | 4 (0.2) | 1.00 | 53 (1.0) | 0.41 |
| Endometriosis | 6 (3.7) | 116 (6.9) | 0.12 | 494 (9.2) | 0.01 |
| Uterine factor | 2 (1.2) | 25 (1.5) | 1.00 | 108 (2.0) | 0.77 |
| Male factor | 69 (42.3) | 537 (31.8) | 0.01 | 1393 (25.9) | <0.001 |
| Other factor | 12 (7.4) | 188 (11.1) | 0.14 | 558 (10.4) | 0.21 |
| Unexplained | 30 (18.4) | 345 (20.4) | 0.54 | 1520 (28.2) | 0.01 |
| Cycle year | 0.32 | 0.10 | |||
| 1995–2000 | 41 (25.2) | 521 (30.8) | 1772 (32.9) | ||
| 2001–2006 | 60 (36.8) | 575 (34.0) | 1873 (34.8) | ||
| 2007–2012 | 62 (38.0) | 595 (35.2) | 1744 (32.4) | ||
Data are presented as mean ± standard deviation or N (%)
FSH follicle stimulating hormone
a P compares <25 years with 30 to <35 years
bMultiple diagnoses may be reported
Infertility diagnoses as reported to SART are also shown in Table 1. Male factor infertility and unexplained infertility were common diagnoses across all age groups in this study. Compared with women 30 to <35 years, women <25 years were more likely to have a diagnosis of male factor infertility and less likely to have unexplained infertility and endometriosis (all p ≤ 0.01). Interestingly, there were no notable differences in the prevalence of tubal factor infertility, ovulatory dysfunction, diminished ovarian reserve, or uterine factor infertility when comparing the youngest age group with each of the two older age groups (all p ≥ 0.10).
In their first IVF cycle, women <25 years utilized ICSI more frequently, indicating a higher prevalence of male factor infertility, and used lower total doses of gonadotropins than women in each of the older age groups (both p ≤ 0.02), but peak estradiol levels were similar (both p ≥ 0.19). Although the number of oocytes retrieved was not significantly different, the fertilization rate was substantially lower for women <25 years than for their older counterparts (both p < 0.001). Compared with each of the older age groups, the mean number of embryos transferred was lower for women <25 years (both p ≤ 0.02), though embryo transfer day did not differ between any of the groups (both p ≥ 0.87). In addition, women <25 years were more likely to undergo a single embryo transfer with their first IVF cycle than older women, though this difference was significant only when compared with women 25 to <30 years. In all age groups, fewer than 2 % of cycles underwent preimplantation genetic screening or diagnosis, and these proportions did not differ between the groups at the first cycle (both p ≥ 0.56). Use among the youngest age group remained below 2 % in subsequent cycles, while use among women 25 to <30 years and 30 to <35 years peaked at 4.8 % in the fifth cycle and 6.0 % in the sixth cycle, respectively. Clinical characteristics of the first IVF cycle stratified by age are reported in Table 2.
Table 2.
Clinical characteristics of the first IVF cycle for all participants stratified by age
| Characteristic | <25 years N = 163 | 25 to <30 years N = 1691 | P | 30 to <35 years N = 5389 | P a |
|---|---|---|---|---|---|
| Manipulation | |||||
| Intracytoplasmic sperm injection | 77 (47.2) | 563 (33.3) | <0.001 | 1516 (28.1) | <0.001 |
| Assisted hatching | 0 (0.0) | 9 (0.5) | 1.00 | 42 (0.8) | 0.64 |
| Total gonadotropin dose (IU) | 1682 ± 837 | 1890 ± 1007 | 0.02 | 2240 ± 1219 | <0.001 |
| Peak estradiol (pg/ml) | 1804 ± 1193 | 1821 ± 1407 | 0.90 | 1641 ± 1207 | 0.19 |
| Oocytes retrieved | 12.8 ± 8.1 | 12.7 ± 7.3 | 0.88 | 11.7 ± 6.7 | 0.08 |
| Fertilization rateb | 62.7 ± 30.1 | 71.9 ± 30.4 | <0.001 | 72.4 ± 32.1 | <0.001 |
| Embryos cryopreserved | 0.26 | 0.32 | |||
| 0 | 81 (55.5) | 832 (54.2) | 2776 (57.5) | ||
| 1–3 | 42 (28.8) | 380 (24.8) | 1145 (23.7) | ||
| ≥4 | 23 (15.8) | 323 (21.0) | 906 (18.8) | ||
| Embryos transferred | 1.8 ± 0.8 | 1.9 ± 0.8 | 0.02 | 1.9 ± 0.9 | 0.01 |
| Single embryo transferc | 29 (21.0) | 211 (14.7) | 0.049 | 744 (16.5) | 0.16 |
| Day of embryo transferd | 0.95 | 0.77 | |||
| 3 | 118 (85.5) | 1224 (85.2) | 3934 (87.1) | ||
| 5 | 18 (13.0) | 186 (13.0) | 506 (11.2) | ||
| 2/4/6/missing | 2 (1.4) | 26 (1.9) | 79 (1.5) | ||
Data are presented as mean ± standard deviation or N (%)
a P compares women <25 years with women 30 to <35 years
bThe number of normally fertilized oocytes divided by the number of mature oocytes
cCalculated among cycles with an embryo transfer
dCalculated among fresh cycles with an embryo transfer
The utilization of ICSI and assisted hatching, as well as the total dose of gonadotropin medications used, the number of embryos transferred, and the proportion of fresh cycles in which no embryos were cryopreserved tended to increase with subsequent cycles in each of the age groups. The peak estradiol level and the number of oocytes retrieved remained relatively stable across all 6 cycles. Clinical characteristics stratified by IVF cycle for women <25 years, 25 to <30 years, and 30 to <35 years are reported in Table 3.
Table 3.
Clinical characteristics stratified by age and IVF cycle number
| Characteristic | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Cycle 6 |
|---|---|---|---|---|---|---|
| <25 years | ||||||
| Overall cohort—N/total N (%)a | 163/163 (100.0) | 91/115 (79.1) | 53/67 (79.1) | 24/38 (63.2) | 17/21 (81.0) | 6/14 (42.9) |
| Type of cycle | ||||||
| Fresh | 163 (100.0) | 66 (72.5) | 33 (62.3) | 17 (70.8) | 14 (82.4) | 4 (66.7) |
| Thaw | 0 (0.0) | 25 (27.5) | 20 (37.7) | 7 (29.2) | 3 (17.7) | 2 (33.3) |
| Manipulation | ||||||
| Intracytoplasmic sperm injection† | 0 (0.0) | 0 (0.0) | 1 (1.9) | 2 (8.3) | 3 (17.7) | 1 (16.7) |
| Assisted hatchingb | 51 (0.7) | 107 (2.5) | 172 (6.6) | 228 (15.7) | 162 (20.1) | 111 (26.5) |
| Total gonadotropin dose (IU)b | 1682 ± 837 | 2205 ± 1011 | 1972 ± 955 | 1773 ± 850 | 2161 ± 1362 | 2488 ± 2137 |
| Peak estradiol (pg/ml)b | 1804 ± 1193 | 1864 ± 1731 | 1684 ± 1335 | 1298 ± 701 | 1818 ± 1947 | 2726 ± 0.0 |
| Oocytes retrievedb | 12.8 ± 8.1 | 13.9 ± 8.1 | 12.3 ± 5.5 | 10.9 ± 4.4 | 12.4 ± 6.7 | 12.8 ± 10.2 |
| Embryos cryopreservedb | ||||||
| 0 | 81 (55.5) | 41 (64.1) | 19 (59.4) | 10 (62.5) | 9 (75.0) | 1 (25.0) |
| 1 to 3 | 42 (28.8) | 12 (18.8) | 10 (31.3) | 4 (25.0) | 1 (8.3) | 2 (50.0) |
| ≥4 | 23 (15.8) | 11 (17.2) | 3 (9.4) | 2 (12.5) | 2 (16.7) | 1 (25.0) |
| Embryos transferredb | 1.8 ± 0.0 | 1.9 ± 0.8 | 1.9 ± 1.3 | 2.1 ± 1.0 | 2.1 ± 1.1 | 2.5 ± 2.8 |
| Day of embryo transferc | ||||||
| 3 | 118 (85.5) | 48 (85.7) | 20 (71.4) | 13 (86.7) | 9 (81.8) | 3 (75.0) |
| 5 | 18 (13.0) | 8 (14.3) | 7 (25.0) | 2 (13.3) | 2 (18.2) | 1 (25.0) |
| 2/4/6/missing | 2 (1.4) | 0 (0.0) | 1 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 25–<30 years | ||||||
| Overall cohort—N/total N (%)a | 1691/1691 (100.0) | 987/1128 (87.5) | 591/712 (83.0) | 326/415 (78.6) | 168/244 (68.9) | 80/117 (68.4) |
| Type of cycle | ||||||
| Fresh | 1691 (100.0) | 727 (73.7) | 438 (74.1) | 261 (80.1) | 124 (73.8) | 68 (85.0) |
| Thaw | 0 (0.0) | 260 (26.3) | 153 (25.9) | 65 (19.9) | 44 (26.2) | 12 (15.0) |
| Manipulation | ||||||
| Intracytoplasmic sperm injectionb | 563 (33.3) | 332 (33.6) | 216 (36.6) | 128 (39.3) | 62 (36.9) | 39 (48.8) |
| Assisted hatchingb | 9 (0.5) | 18 (1.8) | 31 (5.3) | 49 (15.0) | 34 (20.2) | 19 (23.8) |
| Total gonadotropin dose (IU)b | 1890 ± 1007 | 2234 ± 1212 | 2397 ± 1469 | 2633 ± 1444 | 2804 ± 1450 | 2938 ± 1354 |
| Peak estradiol (pg/ml)b | 1821 ± 1407 | 1637 ± 1100 | 1500 ± 1054 | 1609 ± 1469 | 1562 ± 1096 | 1379 ± 920 |
| Oocytes retrieved† | 12.7 ± 7.3 | 11.9 ± 6.3 | 12.4 ± 6.5 | 12.6 ± 6.4 | 12.8 ± 6.8 | 12.6 ± 8.0 |
| Embryos cryopreservedb | ||||||
| 0 | 832 (54.2) | 429 (63.4) | 262 (66.7) | 149 (62.3) | 79 (68.7) | 42 (65.6) |
| 1 to 3 | 380 (24.8) | 160 (23.6) | 83 (21.1) | 56 (23.4) | 22 (19.1) | 13 (20.3) |
| ≥4 | 323 (21.0) | 88 (13.0) | 48 (12.2) | 34 (14.2) | 14 (12.2) | 9 (14.1) |
| Embryos transferredb | 1.9 ± 0.8 | 2.1 ± 0.9 | 2.3 ± 0.9 | 2.5 ± 1.0 | 2.5 ± 1.3 | 2.6 ± 1.3 |
| Day of embryo transferc | ||||||
| 3 | 1224 (85.2) | 573 (89.3) | 329 (86.4) | 204 (86.8) | 91 (83.5) | 49 (84.5) |
| 5 | 186 (13.0) | 59 (9.2) | 48 (12.6) | 29 (12.3) | 16 (14.7) | 9 (15.5) |
| 2/4/6/missing | 26 (1.8) | 10 (1.6) | 4 (1.0) | 2 (0.9) | 2 (1.8) | 0 (0.0) |
| 30–<35 years | ||||||
| Overall cohort—N/total N (%)a | 5389/5389 (100.0) | 3201/3696 (86.6) | 1945/2391 (81.3) | 1106/1464 (75.5) | 621/850 (73.0) | 333/479 (69.5) |
| Type of cycle | ||||||
| Fresh | 5389 (100.0) | 2419 (75.6) | 1522 (78.3) | 846 (76.5) | 488 (78.6) | 257 (77.2) |
| Thaw | 0 (0.0) | 782 (24.4) | 423 (21.8) | 260 (23.5) | 133 (21.4) | 76 (22.8) |
| Manipulation | ||||||
| Intracytoplasmic sperm injectionb | 1516 (28.1) | 1005 (31.4) | 635 (32.7) | 370 (33.5) | 224 (36.1) | 130 (39.0) |
| Assisted hatchingb | 42 (0.8) | 89 (2.8) | 140 (7.2) | 177 (16.0) | 125 (20.1) | 91 (27.3) |
| Total gonadotropin dose (IU)b | 2240 ± 1219 | 2958 ± 1634 | 3200 ± 1858 | 3383 ± 1884 | 3493 ± 1836 | 3468 ± 1707 |
| Peak estradiol (pg/ml)b | 1641 ± 1207 | 1438 ± 1023 | 1499 ± 1085 | 1575 ± 1137 | 1425 ± 1096 | 1555 ± 1066 |
| Oocytes retrievedb | 11.7 ± 6.7 | 10.7 ± 6.2 | 11.3 ± 6.3 | 11.4 ± 6.6 | 12.3 ± 7.1 | 12.6 ± 7.7 |
| Embryos cryopreservedb | ||||||
| 0 | 2776 (57.5) | 1488 (68.6) | 937 (68.7) | 517 (68.3) | 285 (65.1) | 169 (70.4) |
| 1 to 3 | 1145 (23.7) | 420 (19.4) | 260 (19.1) | 143 (18.9) | 91 (20.8) | 44 (18.3) |
| ≥4 | 906 (18.8) | 260 (12.0) | 167 (12.2) | 97 (12.8) | 62 (14.2) | 27 (11.3) |
| Embryos transferred† | 1.9 ± 0.9 | 2.1 ± 1.0 | 2.3 ± 1.0 | 2.4 ± 1.2 | 2.5 ± 1.2 | 2.7 ± 1.3 |
| Day of embryo transfer | ||||||
| 3 | 3934 (87.1) | 1826 (89.3) | 1150 (88.6) | 621 (87.1) | 344 (81.9) | 187 (81.7) |
| 5 | 506 (11.2) | 177 (8.7) | 121 (9.3) | 81 (11.4) | 68 (16.2) | 40 (17.5) |
| 2/4/6/missingc | 79 (1.7) | 43 (2.1) | 27 (2.1) | 11 (1.5) | 8 (1.9) | 2 (0.9) |
Data are presented as mean ± standard deviation or N (%)
aDenominator is the number of women eligible to return for that cycle (the number of women in the previous cycle minus the number of women with a pregnancy resulting in a live birth)
bCalculated only for fresh transfer cycles
cCalculated among fresh cycles with an embryo transfer
Cycles without ICSI
In order to assess whether the higher prevalence of male factor infertility explained the lower fertilization rate in women <25 years, we restricted the analysis of fertilization rate in the first IVF cycle to women without a diagnosis of male factor infertility and for whom routine IVF insemination was used. For the 75 cycles among women <25 years, the fertilization rate was 65.7 ± 33.2, while it was 74.8 ± 32.3 for the 941 cycles among women 25 to <30 years and 75.8 ± 33.3 for the 3220 cycles among women 30 to <35 years. The fertilization rate was significantly lower for the youngest age group compared with women 25 to <30 years (p = 0.02) and women 30 to <35 years (p = 0.01).
Pregnancy and live birth outcomes
In this cohort of women <35 years, 5445 (75.2 %) achieved a clinical pregnancy, 4720 (65.2 %) experienced a live birth, and 2202 (30.4 %) did not return for treatment before achieving a live birth or completing 6 cycles. Among the live births, there were 3160 (66.9 %) singletons, 1442 (30.6 %) sets of twins, 117 (2.5 %) sets of triplets, and 1 (0.02 %) set of quadruplets.
Outcomes of the first IVF cycle were similar across the three age groups. There were no significant differences with regard to the likelihood of cycle cancelation before oocyte retrieval, embryo transfer, or implantation rate. Likewise, in the first IVF cycle, the incidence of clinical pregnancy, spontaneous abortion, and live birth per cycle start and per transfer were not significantly different among the age groups. Of the women who conceived in the first IVF cycle, the incidence of multiple live born infants was similar across the three age groups (both p ≥ 0.69). Table 4 presents outcomes of the first IVF cycle stratified by age. Table 5 shows the number of women undergoing an oocyte retrieval and embryo transfer in each cycle, as well as the incidence of clinical pregnancy and live birth for each cycle among women <25 years, 25 to <30 years, and 30 to <35 years.
Table 4.
Outcomes of the first IVF cycle stratified by age
| Cycle outcome | <25 years N = 163 | 25 to <30 years N = 1691 | P | 30 to <35 years N = 5389 | P a |
|---|---|---|---|---|---|
| Cancelation | 8 (4.9) | 94 (5.6) | 0.73 | 369 (6.9) | 0.33 |
| Oocyte retrieval | 155 (95.1) | 1597 (94.4) | 0.73 | 5020 (93.2) | 0.33 |
| Embryo transferb | 138 (89.0) | 1436 (89.9) | 0.73 | 4519 (90.0) | 0.69 |
| Implantation ratec | 28.2 ± 40.3 | 29.7 ± 40.7 | 0.76 | 29.2 ± 40.5 | 0.84 |
| Outcomes per cycle start | |||||
| Clinical pregnancy | 52 (31.9) | 630 (37.3) | 0.18 | 1947 (36.1) | 0.27 |
| Spontaneous abortion | 3 (1.8) | 61 (3.6) | 0.36 | 225 (4.2) | 0.14 |
| Termination | 0 (0.0) | 2 (0.12) | 1.0 | 11 (0.20) | 1.0 |
| Live birth | 48 (29.5) | 563 (33.3) | 0.32 | 1693 (31.4) | 0.60 |
| Singleton | 30 (62.5) | 377 (67.0) | 0.69d | 1126 (66.5) | 0.70d |
| Twin | 17 (35.4) | 174 (30.9) | 530 (31.3) | ||
| Triplet | 1 (2.1) | 12 (2.1) | 37 (2.2) | ||
| Stillbirth | 0 (0.0) | 1 (0.06) | 1.0 | 9 (0.17) | 1.0 |
| Unknown | 1 (0.61) | 3 (0.18) | 0.31 | 9 (0.17) | 0.26 |
| Outcomes per transfer | |||||
| Clinical pregnancy | 52 (37.7) | 630 (43.9) | 0.16 | 1947 (43.1) | 0.21 |
| Spontaneous abortion | 3 (2.2) | 61 (4.3) | 0.36 | 225 (5.0) | 0.16 |
| Live birth | 48 (34.8) | 563 (39.2) | 0.31 | 1693 (37.5) | 0.52 |
| Singleton | 30 (62.5) | 377 (67.0) | 0.69d | 1126 (66.5) | 0.70d |
| Twin | 17 (35.4) | 174 (30.9) | 530 (31.3) | ||
| Triplet | 1 (2.1) | 12 (2.1) | 37 (2.2) | ||
a P compares women <25 years with women 30 to <35 years
bDenominator is the number of cycles that were not canceled before oocyte retrieval
cThe number of fetal heartbeats divided by the number of transferred embryos among cycles with an embryo transfer
dP is for the 3 × 2 comparison for number of live births, not a pairwise comparison for singletons
Table 5.
Outcomes stratified by age and IVF cycle number
| Cycle | Cycle cohort | Patients who did not return for treatmenta N/Total N (%) | Oocyte retrievalsb | Embryo transfersb | Clinical pregnanciesb | Live birthsb |
|---|---|---|---|---|---|---|
| <25 years | ||||||
| 1 | 163 | Not applicable | 155 (95.1) | 138 (84.7) | 52 (31.9) | 48 (29.5) |
| 2 | 91 | 24/115 (20.9) | 89 (97.8) | 80 (87.9) | 29 (31.9) | 24 (26.4) |
| 3 | 53 | 14/67 (20.9) | 53 (100.0) | 44 (83.0) | 19 (35.9) | 15 (28.3) |
| 4 | 24 | 14/38 (36.8) | 23 (95.8) | 22 (91.7) | 4 (16.7) | 3 (12.5) |
| 5 | 17 | 4/21 (19.0) | 16 (94.1) | 14 (82.4) | 4 (23.5) | 3 (17.7) |
| 6 | 6 | 8/14 (57.1) | 6 (100.0) | 5 (83.3) | 3 (50.0) | 2 (33.3) |
| 25<30 years | ||||||
| 1 | 1691 | Not applicable | 1597 (94.4) | 1436 (84.9) | 630 (37.3) | 563 (33.3) |
| 2 | 987 | 141/1128 (12.5) | 956 (96.9) | 886 (89.8) | 319 (32.3) | 275 (27.9) |
| 3 | 591 | 121/712 (17.0) | 562 (95.1) | 520 (88.0) | 201 (34.0) | 176 (29.8) |
| 4 | 326 | 89/415 (21.4) | 309 (94.8) | 294 (90.2) | 97 (29.8) | 82 (25.2) |
| 5 | 168 | 76/244 (31.1) | 163 (97.0) | 149 (88.7) | 59 (35.1) | 51 (30.4) |
| 6 | 80 | 37/117 (31.6) | 78 (97.5) | 69 (86.3) | 23 (28.8) | 19 (23.8) |
| 30–<35 years | ||||||
| 1 | 5389 | Not applicable | 5020 (93.2) | 4519 (83.9) | 1947 (36.1) | 1693 (31.4) |
| 2 | 3201 | 495/3696 (13.4) | 3025 (94.5) | 2767 (86.4) | 933 (29.2) | 810 (25.3) |
| 3 | 1945 | 446/2391 (18.7) | 1838 (94.4) | 1695 (87.2) | 550 (28.3) | 481 (24.7) |
| 4 | 1106 | 358/1464 (24.5) | 1051 (95.0) | 953 (86.2) | 311 (28.1) | 256 (23.2) |
| 5 | 621 | 229/850 (26.9) | 595 (95.8) | 539 (86.8) | 173 (27.9) | 142 (22.9) |
| 6 | 333 | 146/479 (30.5) | 322 (96.7) | 300 (90.1) | 91 (27.3) | 77 (23.1) |
aDenominator is the number of women eligible to return for that cycle (the number of women in the previous cycle minus the number of women with a pregnancy resulting in a live birth)
bDenominator is the number of women in the cycle cohort
Women <25 years consistently had the lowest cumulative live birth rate after each cycle, followed by women 30 to <35 years (Table 6). For both the youngest and oldest age groups, the cumulative live birth rate after 5 cycles and after 6 cycles was significantly lower than the cumulative live birth rate for women 25 to <30 years. Among women <25 years, the cumulative live birth rate after 6 cycles of IVF was 58 % (95 % CI 0.51–0.66). The cumulative live birth rate after 6 cycles of IVF was 69 % (95 % CI 0.67–0.71) among women 25 to <30 years and 64 % (95 % CI 0.63–0.65) among women 30 to <35 years. The mean number of cycles was similar for women <25 years (2.3 ± 1.6), women 25 to <30 years (2.1 ± 1.5), and women 30 to <35 years (2.2 ± 1.5). Table 6 reports the cumulative live birth rates for the full cohort and for each age strata.
Table 6.
Cumulative live birth rates and 95 % confidence intervals for all participants and stratified by age group
| Cycle | All women N = 7243 | <25 years N = 163 | 25 to <30 years N = 1691 | 30 to <35 years N = 5389 |
|---|---|---|---|---|
| 1 | 0.32 (0.31–0.33) | 0.29 (0.23–0.37) | 0.33 (0.31–0.36) | 0.31 (0.30–0.33) |
| 2 | 0.47 (0.46–0.48) | 0.44 (0.37–0.52) | 0.50 (0.47–0.52) | 0.46 (0.45–0.48) |
| 3 | 0.56 (0.55–0.58) | 0.53 (0.46–0.61) | 0.60 (0.58–0.62) | 0.55 (0.54–0.57) |
| 4 | 0.61 (0.60–0.62) | 0.55 (0.48–0.63) | 0.65 (0.63–0.67) | 0.60 (0.59–0.61) |
| 5 | 0.64 (0.63–0.65) | 0.57 (0.50–0.65) | 0.68 (0.66–0.70) | 0.63 (0.61–0.64) |
| 6 | 0.65 (0.64–0.66) | 0.58 (0.51–0.66) | 0.69 (0.67–0.71) | 0.64 (0.63–0.65) |
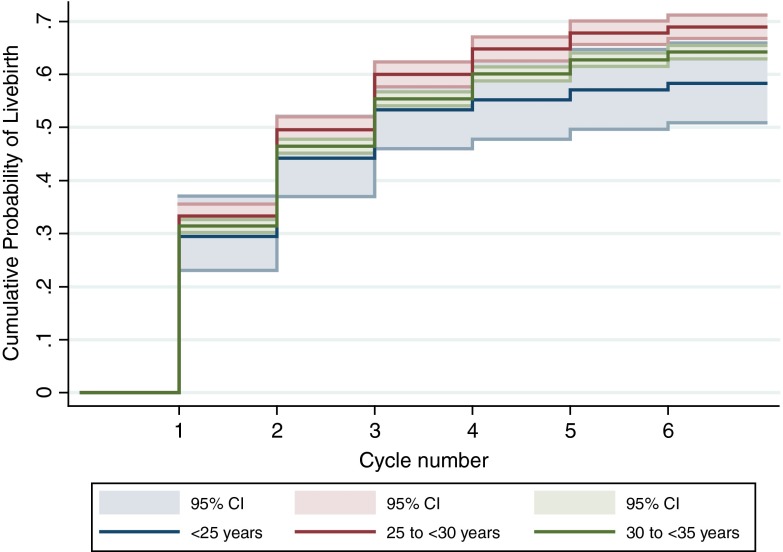
The age-specific cumulative live birth rates are graphically represented in Fig. 1 and demonstrate a significantly higher likelihood of live birth among women 25 to <30 years compared with women 30 to <35 years (p = 0.007). Although women <25 years experienced an even lower cumulative live birth rate than women in the two older age groups, the overall curve for women <25 years was not significantly different from the curves for women 25 to <30 years (p = 0.10) and women 30 to <35 years (p = 0.45).
Fig. 1.
Cumulative incidence curves for live birth stratified by age at the start of the first IVF cycle. The figure shows the cumulative probability of live birth stratified by age at the start of the first fresh, non-donor IVF cycle. The solid lines are the cumulative incidence curves, and the shaded bands are the 95 % confidence intervals
Discussion
In women <35 years who underwent IVF, we found that the cumulative live birth rate after 6 cycles was significantly lower for women <25 years than for women 25 to <30 years. Women 25 to <30 years also had a significantly higher cumulative live birth rate after 6 cycles than women 30 to <35 years. When comparing the overall cumulative live birth rate curves in the two older age groups, women 25 to <30 had significantly better outcomes. Despite the larger discrepancy in live birth rates between women <25 years and those 25 to <30 years, the sample size in the youngest age group was relatively small, and thus the comparison did not reach statistical significance—despite statistically significant differences in cumulative live birth rates after 5 and 6 cycles.
Our findings are consistent with reports of less favorable IVF treatment outcomes in women <25 years undergoing their first IVF cycle [3, 4]. This finding challenges our assumption that young women produce the healthiest embryos. Here, women <25 years had a significantly lower fertilization rate in the first IVF cycle compared with women in the older age groups. This raises the question of whether there is an inherent issue with either the oocytes from these very young women and/or is it the male component that is driving the poorer outcomes.
Nazemian et al. speculated that ovarian stimulation in very young women may result in the retrieval of oocytes that have already activated a cell death program, which could explain the lower fertilization rates, increased fragmentation rates, and poorer reproductive outcomes seen among women <25 years [3]. Munne et al. reported that only 43 % of embryos from young oocyte donors were chromosomally normal, with almost one third of patients having less than 30 % normal embryos, therefore offering some explanation as to why very young women may have less favorable outcomes [11]. Interestingly, our results in the <25 age group coincide with the report by Franasiak et al., who reported a slightly increased aneuploidy prevalence in embryos from young women, with >40 % aneuploidy in women ≤23 years, while women aged 26 to 37 were the least likely to have no euploid embryos [5]. However, we cannot assume that the poorer outcomes seen in younger women are due solely to their younger age, as couples with women <25 years were significantly more likely to be diagnosed with male factor infertility. We attempted to account for this by restricting the analysis of fertilization rate to those couples without apparent male factor infertility and for whom routine IVF insemination was used. Even in this restricted sample, women <25 years still had a significantly lower fertilization rate compared to each of the two older age groups. This still could suggest that oocytes in the youngest age group may have poorer reproductive outcomes, though this contradicts findings from a recent study [12].
Other non-biological factors may play into the observation that women <25 years had a lower cumulative pregnancy rate. Perhaps, very young women have less favorable outcomes because providers take a less aggressive approach due to concerns regarding an over response to injectable gonadotropins, as well as multiple gestation. In this study, in the first IVF cycle, women <25 years did receive a significantly lower dose of injectable gonadotropins compared with both of the older age groups, although peak estradiol and the number of oocytes retrieved were similar across the three groups, making medication dosing an unlikely explanation for poorer outcomes in women <25 years. In the first IVF cycle, women <25 years were significantly more likely than women 25 to <30 years to have a single embryo transfer. This may play a role in the lower clinical pregnancy rates seen among women in the youngest age group; however, these women were also noted to have a lower, albeit not statistically significant, implantation rate when compared with the older two age groups.
The strengths of this study include the relatively large cohort of younger women undergoing their first fresh IVF cycle, prospectively collected clinical data, and the calculation of cumulative rather than per-cycle live birth rates. Despite 18 years of data and more than 7000 women 25 to <35 years, we had only 163 women in the youngest age group. Although women <25 years have a lower cumulative live birth rate at the end of 5 and 6 cycles, the cumulative incidence curves are not significantly different owing to the smaller discrepancy in live birth rates at earlier IVF cycles and limited power due to the small number of women <25 years who utilize IVF. Finally, while clinical practices have changed during the 18-year study period, we do not have evidence that practices were altered differentially across the age groups in the study. This is supported by our finding that the day of embryo transfer and the use of preimplantation genetic diagnosis did not differ between the groups. Additionally, the year during which women initiated their first IVF cycle was similar across the age groups.
This study suggests that the optimal age for couples with young women using their own oocytes is from 25 to <30 years. It is most likely that the poorer IVF outcomes in very young women (<25 years) may be complicated by the increased presence of male factor infertility and also may indicate an unmeasured paternal component [13]. In addition, our findings of significantly lower fertilization rates among women <25 years with no diagnosis of male factor infertility may contradict the belief that young oocytes can overcome other inherent problems, especially related to the male [12].
This study highlights the need for additional investigation of the risk factors for poorer IVF outcomes in very young women, in particular when a male component is involved. It also suggests that we may want to reassess how IVF outcomes are reported to SART and consider finer age stratification for data presented in women less than 35 years of age. Finally, it is important that providers are able to appropriately counsel couples in which the woman is very young in order to establish reasonable expectations regarding treatment outcomes.
Acknowledgments
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. This work was supported by training grant T32 ES 07069, which supported LED.
Competing interests
None of the authors have any competing financial interests.
Footnotes
Capsule The cumulative live birth rate after 6 cycles was significantly lower among women <25 years than among women 25 to <30 years.
The authors consider that the first two authors should be regarded as joint First Authors.
References
- 1.Malizia BA, Hacker MR, Penzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360:236–243. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 2.Luke B, Brown MB, Wantman E, et al. Cumulative birth rates with linked assisted reproductive technology cycles. N Engl J Med. 2012;366:2483–2491. doi: 10.1056/NEJMoa1110238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nazemian Z, Esfandiari N, Javed M, Casper RF. The effect of age on in vitro fertilization outcome: is too young possible? J Assist Reprod Genet. 2011;28:101–106. doi: 10.1007/s10815-010-9499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Macaldowie A, Hayward I, Chambers G, Sullivan E. Assisted reproductive technology in Australia and New Zealand 2009. Canberra: Australia Institute of Health and Welfare.2011
- 5.Franasiak JM, Forman EJ, Hong KH, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101:656–663. doi: 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Malizia BA, Dodge LE, Penzias AS, Hacker MR. The cumulative probability of liveborn multiples after in vitro fertilization: a cohort study of more than 10,000 women. Fertil Steril. 2013;99:393–399. doi: 10.1016/j.fertnstert.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Eaton JL, Hacker MR, Harris D, Thornton KL, Penzias AS. Assessment of day-3 morphology and euploidy for individual chromosomes in embryos that develop to the blastocyst stage. Fertil Steril. 2009;91:2432–2436. doi: 10.1016/j.fertnstert.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 8.American Society for Reproductive Medicine Practice Committee Documents. Criteria for number of embryos to transfer: A committee opinion. 2013. Ref Type: Report [DOI] [PubMed]
- 9.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 10.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12:737–751. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 11.Munne S, Ary J, Zouves C, et al. Wide range of chromosome abnormalities in the embryos of young egg donors. Reprod Biomed Online. 2006;12:340–346. doi: 10.1016/S1472-6483(10)61007-3. [DOI] [PubMed] [Google Scholar]
- 12.Begueria R, Garcia D, Obradors A, Poisot F, Vassena R, Vernaeve V. Paternal age and assisted reproductive outcomes in ICSI donor oocytes: is there an effect of older fathers? Hum Reprod. 2014;29:2114–2122. doi: 10.1093/humrep/deu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humm KC, Sakkas D. Role of increased male age in IVF and egg donation: is sperm DNA fragmentation responsible? Fertil Steril. 2013;99:30–36. doi: 10.1016/j.fertnstert.2012.11.024. [DOI] [PubMed] [Google Scholar]