Abstract
Introduction
Melanocytes produce pigment granules that color both skin and hair. In the hair follicles melanocytes are derived from stem cells (MelSC) that are present in hair bulges or sub-bulge regions and function as melanocyte reservoirs. Quiescence, maintenance, activation, and proliferation of MelSC are controlled by specific activities in the microenvironment that can influence the differentiation and regeneration of melanocytes. Therefore, understanding MelSC and their niche may lead to use of MelSC in new treatments for various pigmentation disorders.
Areas covered
We describe here pathophysiological mechanisms by which melanocyte defects lead to skin pigmentation disorders such as vitiligo and hair graying. The development, migration, and proliferation of melanocytes and factors involved in the survival, maintenance, and regeneration of MelSC are reviewed with regard to the biological roles and potential therapeutic applications in skin pigmentation diseases.
Expert Opinion
MelSC biology and niche factors have been studied mainly in murine experimental models. Human MelSC markers or methods to isolate them are much less well understood. Identification, isolation and culturing of human MelSC would represent a major step toward new biological therapeutic options for patients with recalcitrant pigmentary disorders or hair graying. By modulating the niche factors for MelSC it may one day be possible to control skin pigmentary disorders and prevent or reverse hair graying.
Keywords: melanocyte, melanocyte stem cell, pigmentation, vitiligo, graying hair
1. INTRODUCTION
Melanocytes that are located in the epidermis and hair follicles of the skin play a major role in pigmentation of the skin or hair. Pigment producing cells are also distributed in the eyes, ears, brain, heart, lung, and bone [1–3]. The functions of melanocytes in these other locations are not known in detail, although a role in scavenging reactive oxygen species has been reported [4,5]. In the skin, melanin pigment is taken up by skin keratinocytes and organized into a shield around the nucleus where it is thought to protect genomic DNA from the harmful effects of ultraviolet light. Pigment produced by the melanocytes in hair follicles is incorporated into the growing hair and therefore determines the coat color in mammals. : The maintenance of the melanocyte is dependent on a population of melanocyte stem cells (MelSCs), a quiescent population that is present in the bulge region of the hair follicle and acts as a melanocyte reservoir. After migration into epidermis, MelSCs give rise to differentiated, pigment-producing melanocyte. Also, as many local and systemic factors are thought to participate in the pathogenesis of skin diseases such as vitiligo and hair growth disorders, it is important to understand the environmental effects on melanocytes including serotoninergic/melatoninergic system in the skin response to stresses as well as cytochrome-dependent and proopiomelanocortin (POMC) systems [6–10].
Skin and hair melanocytes are derived from neural crest cells early in development [11–14]. The cranial and trunk-located neural crest stem cells differentiate during migration. Melanoblasts are precursor cells with properties similar to Schwann cell precursors and they share various signaling pathways with neurons [15–18]. After migration into the epidermis, melanocyte precursor cells are positioned in the lower permanent portion (LPP) during formation of the hair placode. The melanocytic lineage population in this region is thought to include melanocyte stem cells (MelSCs), previously known as amelanotic melanocytes in human follicles. These melanocyte precursor cells can remain and persist in the dermis of the skin, and have been suggested to have overlapping characteristics with cells of the nervous system [19]. For example, cultured melanoblasts can be differentiated towards neurons, glial cells, or smooth muscle cells [20–22]. This indicates that neural crest stem cell-derived melanoblasts in the skin retain reprogramming ability and show multipotency related to signals from the microenvironment. Also, melanocytes are sensory and regulatory cells for the maintenance of the cutaneous homeostasis and have been defined as neuroendocrine cells that could efficiently regulate local and systemic homeostasis [7,14,23].
During the hair cycle, MelSCs differentiate into terminally differentiated melanocytes that produce mature pigment containing melanosomes, and that are incorporated into the growing hair shaft (anagen coupled melanogenesis) resulting in pigmented hair [24–30]. In catagen, melanin formation is switched off and is absent throughout telogen [26]. A key factor during melanocyte differentiation is microphthalmia associated transcription factor (MITF) [31–34]. MITF controls the expression of key pigment synthetic genes including TRP-1, DCT, and tyrosinase [32]. Various extracelluar signaling pathways converge on MITF to control both migration and survival of melanoblasts [35–40].
2. MELANOCYTE STEM CELLS IN THE HAIR BULGE
Differentiated melanocytes in the hair bulb and melanocyte precursor cells (transient amplifying (TA) cells) in the outer root sheath originate from MelSCs in the hair follicle bulge region (Figure 1). In mice, MelSCs can be identified by use of dopachrometautomerase (Dct) promoter. Specifically in Dct promoter-LacZ reporter engineered mice, non-pigmented small oval-shaped cells with scant cytoplasm that share similarity with amelanotic melanocytes localized in the lower permanent portion of the hair follicle stain LacZ-positive and therefore are identified as MelSCs [41]. In contrast to bulb melanocytes, these MelSCs exhibit low expression of pigmentation-related genes [42–45]. Osawa et al. isolated murine MelSCs and analyzed their gene expression patterns, finding that multiple key genes such as Dct and Pax3, potential candidate MelSC markers, were also expressed in transient amplifying (TA) cells. Other important melanocytic genes such as Kit, Si, Tyr, Tyrp1, Mki67, Lef1, Sox10, and Mitf were expressed at higher levels only in TA cells, not in MelSC [42].
Figure 1.
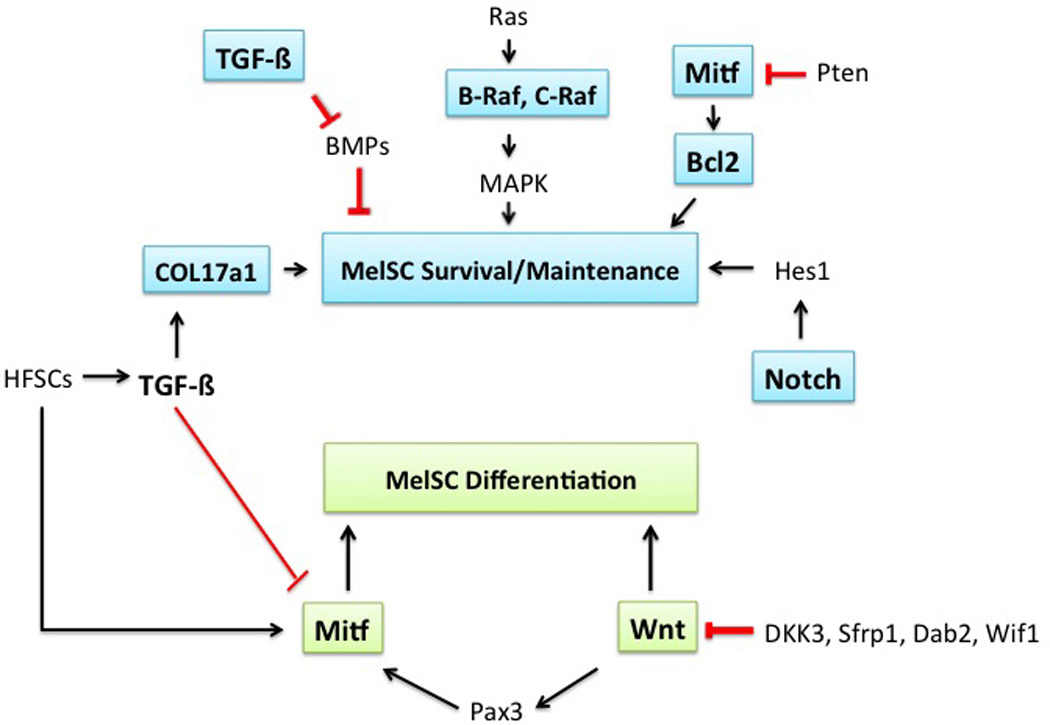
Possible related pathways in MelSC survival, maintenance, and differentiation. MITF, Bcl-2, B-Raf, C-Raf, TGF-b, Notch pathways are involved in MelSC survival and maintenance. PAX-3 and Wnt pathways are related to MelSC differentiation.
MelSCs remain in a quiescent state during the telogen phase of the hair cycle without transcription of pigmentation-related genes [29,42,44,45]. However, once activated in the anagen phase of the hair cycle, pigmentation-related genes are upregulated and the cells proliferate with dendrite formation [41]. After the mid-anagen phase, the pigmentation-related genes are downregulated and MelSCs become inactivated again [46–48]. Repeated hair cycles result in melanocyte differentiation from the MelSCs, and eventually differentiated melanocytes undergo apoptosis during the catagen stage of the hair cycle [49,50], although MelSCs still remain in the LPP of the hair follicle. The terminal differentiation of melanocytes within the hair follicle is coupled with hair cycle progression [51]. During the catagen and telogen stages, MelSCs reside in the LPP region of the hair, indicating that MelSC activation is related to the niche factors during the hair follicle cycle [24,41,52]. In humans, a selective MelSC marker has not been elucidated, in part because of the limited availability of genetic studies of these human amelanotic melanocytes [53]. Human amelanotic melanocytes are considered equivalent to MelSCs in mice because they show similar morphology and location in the LPP [46,53–55].
3. MELANOCYTE STEM CELL NICHE
The specific niche environment that surrounds MelSC plays a major role in regulating quiescence, differentiation, proliferation, and survival of MelSC [41,43,46,56]. For example, certain environmental conditions can maintain quiescent characteristics of MelSCs by downregulating basal transcription and translational levels of some housekeeping genes [42,57].
Although many factors, including genes, transcription factors, and signaling pathways are implicated in embryonic development, differentiation, and proliferation of the melanoblast and melanocyte (e.g. c-Kit, SCF, Ednrb, Wnt, Mitf, Pax-3, Sox-10, and c-myc), the niche factors that directly regulate the MelSCs are incompletely understood [58–61]. Some niche factors have been proposed to affect MelSC maintenance and quiescence in mice, and have been summarized in Table 1. For example, MITF, known as the master regulator of melanocyte development, differentiation, and pigmentation, also plays an important role in MelSC maintenance [46,59,62]. Bcl2, a survival gene which is a downstream target of MITF, plays a central role in MelSCs [46,63]. Bcl2 protects against apoptosis of melanocytes and promotes the survival of MelSCs, thus mutation of Bcl2 causes melanocyte loss [63–65]. More specifically, it was observed that germ line BCL2 knockout results in complete loss of melanocyte stem cells at post-natal day 8, but this death did not occur for bulb melanocytes. As a consequence, fur remained fully pigmented through the initial hair follicle cycle, but became white starting at the second hair follicle cycle. Additional analysis revealed that TGF-β signaling is related to MelSC quiescence or MelSC depletion (in the absence of Bcl2) [41,46,63–65].
Table 1.
Niche factors that affect melanocyte stem cell maintenance and quiescence in mice
| Protein | Function | Mouse model | Result | Reference |
|---|---|---|---|---|
| Bcl-2 | MelSC maintenance | Bcl-2 null mice | Pigmentation loss | Veis et al. [111] Kamada et al.[112] Yamamura et al. [64] Mak et al. [63] |
| Mitf | MelSC survival MelSC maintenance |
Bcl-2 deficient mice |
Pigmentation loss LPP colonization |
Nishimura et al. [46] |
| C-Kit | Bcl activation Not required for MelSC |
c-Kit KO | No hair color change | McGill et al. [62] |
| BRAF, CRAF | MelSC maintenance | Raf KO mouse | Graying hair | Valluet et al [68] |
| TGF-β | MelSC maintenance | TGFbRII-deficient mice |
Loss of MSC | Nishimura et al. [47] |
| Pax3 | MelSC differentiation MelSC development |
Mitf activation Coexpression with Mitf and Sox 10 |
Lang et al. [72] | |
| Wnt | MelSC differentiation MelSC maintenance |
Control Pax3, Mitf function |
Kubic et al. [71] Lang et al. [72] Moriyama et al. [74] |
|
| Notch | MelSC survival MelSC maintenance |
RBP-J KO mice | Premature hair graying | Osawa et al. [76] Moriyama et al. [74] Schouwey et al. [75] |
B-Raf and C-Raf protein kinases are important effectors of the MAPK pathway downstream of RAS [66,67]. Recently, a double-knockout of B-raf and C-raf in mice showed marked abnormality in coat color although single mutation of B-raf or C-raf did not show this phenotypic change, demonstrating a key function for these kinases (and likely for the MAPK pathway) in the self-maintenance of MelSCs [68].
MelSCs reside in the hair follicle bulge area where epidermal stem cells are located [48]. It is likely that factors related to epidermal stem cells may also affect MelSCs in the niche because of the proximity of these stem cell populations within the bulge. Wnt ligands are responsible for the activation of MelSCs to proliferate into melanocyte precursor cells whereas transforming growth factor-beta (TGF-β) is vital for the quiescence maintenance of MelSCs, a vital aspect of stemness [47,69,70]. Wnt signaling is upstream of Mitf and Pax3, which are also related to MelSC maintenance [71]. These Wnt pathway targets-- especially Pax3, Sox10, and Mitf--are likely to regulate MelSC maintenance. In particular Pax3 prevents terminal differentiation of MelSCs into melanocytes, a process which is antagonized by β-catenin [21,71,72]. Activation of the Wnt signaling pathway results in MelSC differentiation into melanocytes, whereas inhibition maintains the MelSC phenotype. The niche expresses Wnt inhibitors such as DKK3, Sfrp1, and Dab2 [71,73]. Also, MelSCs themselves express DKK5, Sfrp1, Dab2, or Wif1 [42,44,71] which may help to maintain MelSC progeny in the niche.
Notch signaling is also involved in MelSC survival and maintenance [74–76]. Genetic ablation of notch signaling resulted in premature hair graying in mice [74,75]. Moriyama et al. demonstrated that notch signaling acts through the Hes1 downstream transcription factor. This finding suggests that, as with melanoblast development and differentiation, interactions and collaborations between the melanocyte lineage cells and hair follicle stem cells (HFSCs) plays an important role in the regulation of MelSC maintenance [77]. It has also been shown that Col17a1-mediated HFSC depletion results in MelSC defects [47]. Tanimura et al. [70] demonstrated that MelSC maintenance was rescued via TGF-β in Col17a1-knockout mice expressing COL17A1 under control of the Keratin promoter, which targets epidermal keratinocytes.
Chang et al. [78] also showed that the transcription factor NFIB that controls endothelin 2 (Edn2) expression, plays the role of a coordinator of HFSC-MelSC behaviors within the niche. The uncoupling of stem cell synchrony by HFSC-specific conditional targeting of Nfib occurs by promoting MelSCs to produce premature melanin pigmentation and results in precocious MelSC differentiation and HFSC apoptosis. This finding suggests the importance of cooperation between stem cells within the niche in skin injury, stress, and disease states, including skin cancer development involving the NFIB pathway.
Because of the convenience of genetic manipulation and identification of phenotypic changes of coat color, experimental results regarding MelSC maintenance are almost all derived from mouse models. The exact mechanisms controlling human MelSC biology are poorly understood and may have differences from what we have learned in mouse models. Evidence from the clinical the setting can provide precious clues to melanocyte stem cell biology and potential therapeutic applications for the future.
4. MELANOCYTE STEM CELLS IN HUMAN SKIN DISORDERS
4.1 VITILIGO
Vitiligo is a condition that causes skin depigmentation and occurs in 0.5–1% of the population [79,80]. Autoimmune, genetic, viral, or oxidative stresses have been proposed as the pathogenic mechanism(s) of melanocyte loss although the most common subtypes are likely to be autoimmune-based [81]. There is still debate over the complete versus partial absence of melanocytes in the vitiligo lesions, but it is generally accepted that melanocyte number is reduced and some patients show complete melanocyte loss in severe cases.
After therapy for vitiligo, such as immune-suppressive modalities, repigmentation frequently begins in the peri-follicular area. This likely arises from the reservoir of MelSCs in the hair follicle bulge [82]. Previously described amelanotic melanocytes from the outer root sheath are thought to be a reservoir for this migration [41,83]. Although it is difficult to identify the presence of melanoblasts or MelSCs in the clinical setting, non-pigmented melanocytes have been identified microscopically in chronic recalcitrant vitiligo [84], which suggests that MelSCs can remain in the niche and potentially provide a chance of repigmentation. It is also possible that the undifferentiated state of melanocyte stem cells prevents autoimmune recognition if such recognition would have required expression of melanocytic differentiation factors/antigens. Seleit et al. also showed that 54% and 63% of melanocyte precursor cells/MelSCs remain at the interfollicular and follicular areas of vitiligo lesional skin respectively [85]. Another clinical study demonstrated that 65.5% of 352 vitiligo patches showed a perifollicular repigmentation pattern on systemic PUVA (psoralin UVA) treatment [86]. Another vitiligo treatment option, narrow band UVB (NB-UVB (311-nm)), is a relatively effective therapy and has been substituted for conventional PUVA therapy [87]. NB-UVB treatment induces Sox10, Kit, and MC1R and enhances differentiation of melanocytes, possibly from the MelSCs [88]. The mechanism of repigmentation after UV has been also studied. UVB irradiation induces wnt7a activation, which triggers MelSC differentiation through the activation of β-catenin and migration of melanocyte precursor cells to the epidermis with Kit induction [89]. The distribution of MelSCs and melanocytes in vitiliginous lesions is schematically illustrated in Figure 1.
Several areas are known to resist repigmentation during vitiligo treatment, such as the hands and feet [79] or leucotrichia-associated lesions [90]. The most relevant reasons for recalcitrance to photochemotherapy in acral vitiligo lesions seem to be inherent lower melanocyte density, lower melanocyte stem cell reservoirs, and lower baseline levels of epidermal stem cell requiring factors [91].
Becasue MelSCs reside in the outer root sheath of hair follicles, suspensions of outer root sheath cells have functioned as a source of MelSC when transplanted into patients with vitiligo or leukoderma [92,93]. Vanscheidt and Hunziker [92] have used single-cell suspensions of plucked hair follicles in the treatment of vitiligo with good results. Mohanty et al. [93] reported that application of non-cultured autologous outer root sheath cell suspensions resulted in repigmentation in 65.7% of vitiligo patches. Although a larger long-term study is essential for validation of the efficacy, these studies demonstrated a therapeutic potential of using MelSCs in pigmentary disorders. This approach is a more focused strategy than the use of epidermal suction blister transplants for treatment of vitiligo, which are used quite widely throughout the world. Considerable research is still required in order to refine methods for use of melanocyte stem cells in vitiligo treatment. These will include methods for isolation, culture, supplementation, protection, and reactivation to repopulate epidermal melanocytes within hypopigmented lesions.
Epidermal melanocytes originate from follicular MelSCs after skin injury by a mechanism dependent on the melanocortin 1 receptor (MC1R) pathway. Thus, it is plausible that modulating the MC1R pathway might contribute to improvement to pigmentary disorders such as vitiligo, melasma, or postinflammatory hyper/hypopigmentation [94]. Furthermore, other clinical and cosmetic implications of MelSCs have been postulated in a review by Stanley [95]. Considering stem cell anatomy and biology, permanent hair removal by electrolysis or laser treatment should be focused on eliminating the hair bulge, even though there is some difficulty in targeting selectively the bulge areas. Because MelSC are unpigmented, they present challenges as targets of selective photothermolysis.
4.2 GRAYING HAIR
To study the mechanism by which MelSCs differentiation into hair follicle melanocytes, observational experiments of the coat color phenotype in genetically manipulated or mutant mice have been used. MelSCs reside in the lower permanent portion (LPP) of the follicle, also called the bulge region due to the insertion of the arector pili muscle at that location. Nishimura et al. [46] identified a mechanism for age-related hair graying in mice as MelSC depletion in the bulge and sub-bulge area. Loss of the same melanocyte population was also observed in an age-correlated fashion in human hair follicles (Figure 2). MelSC depletion was accompanied by ectopic pigmentation of bulge melanocytes -- a phenomenon which is predicted to be inconsistent with maintenance of the non-differentiated state required for maintenance of “stemness”. Indeed the presence of pigmented bulge melanocytes inversely correlated with melanocyte stem cell loss, and was also associated with apoptosis of these cells, suggesting that this ectopic pigmentation event represents a mode of melanocyte stem cell attrition during aging. Importantly, Nishimura and colleagues went on to demonstrate that MelSC depletion and subsequent graying hair is induced by genotoxic stresses (e.g., ionizing radiation, H2O2 treatment, DNA damaging drugs, or DNA repair deficiencies) in mouse models, and these stresses also induce premature ectopic pigmentation in the bulge–sub-bulge area where the MelSCs are located [96] (Figure 2).
Figure 2.
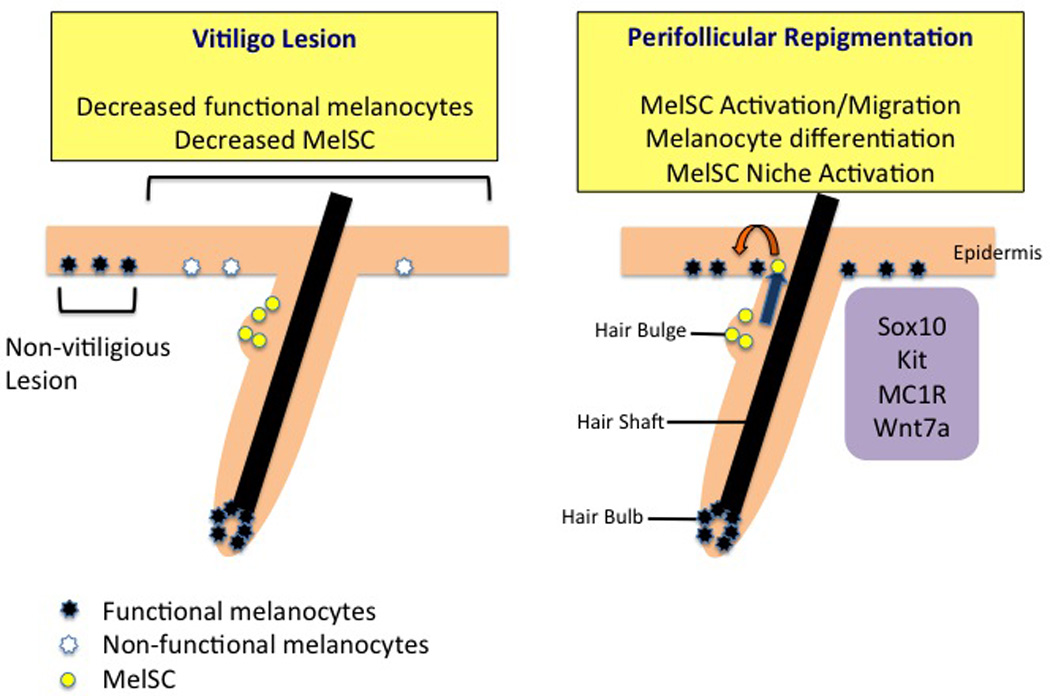
MelSC in vitiligo. In the vitiligious lesion, the number of functional melanocytes and MelSC is decreased compared with the adjacent non-vitiligious lesion. MelSC or melanocyte precursor cells can remain in the hair bulge and provide the chance of repigmentation. During the process of repigmentation after the treatment of vitiligo, repigmentation frequently starts in the perifollicular area from the hair bulge MelSC. Sox10, Kit, MC1R, and wnt7a are related to MelSC activation, migration, and differentiation.
Although the mechanism of hair graying is generally accepted to be incomplete MelSC maintenance and MelSC depletion, the ability to utilize MelSC for expansion and/or transplantation as a treatment for graying hair or leukotrichia remains a significant technical challenge. The bulge area of hair follicles shows a decrease in MelSCs and ectopic differentiation in the bulge–sub-bulge area upon aging [46]—findings which are seen in different mammalian species, suggesting that MelSC maintenance is incomplete with aging and results in stem cell depletion as well as hair graying. Two broad strategies which may conceptually improve the hair graying phenotype—but which carry technical challenges at this time—are to 1) identify a means of expanding and transplanting the melanocyte stem cells, or 2) identify a means of preventing the process by which melanocyte stem cells become ectopically pigmented and lose their stemness capabilities (Figure 2).
4.3 WOUND HEALING
Stem cells are activated for differentiation during tissue regeneration to provide functional mature cells [97]. Apart from the conventional function of melanocytes in pigmentation, a role for melanocytes in epithelial regeneration has recently been proposed. Chou et al. [94] performed an experiment that demonstrated migration and proliferation of MelSCs out of the niche after skin wounding or UVB irradiation (Figure 3). They further showed that this phenomenon is associated with the MC1R–ACTH-α-MSH signaling pathway and that MelSC migration preceded melanocyte proliferation. These findings imply that MelSCs play a role in wound healing after skin injury rather than simply maintaining quiescence, and suggest that follicular MelSCs may indeed be a source of epidermal melanocytes.
Figure 3.
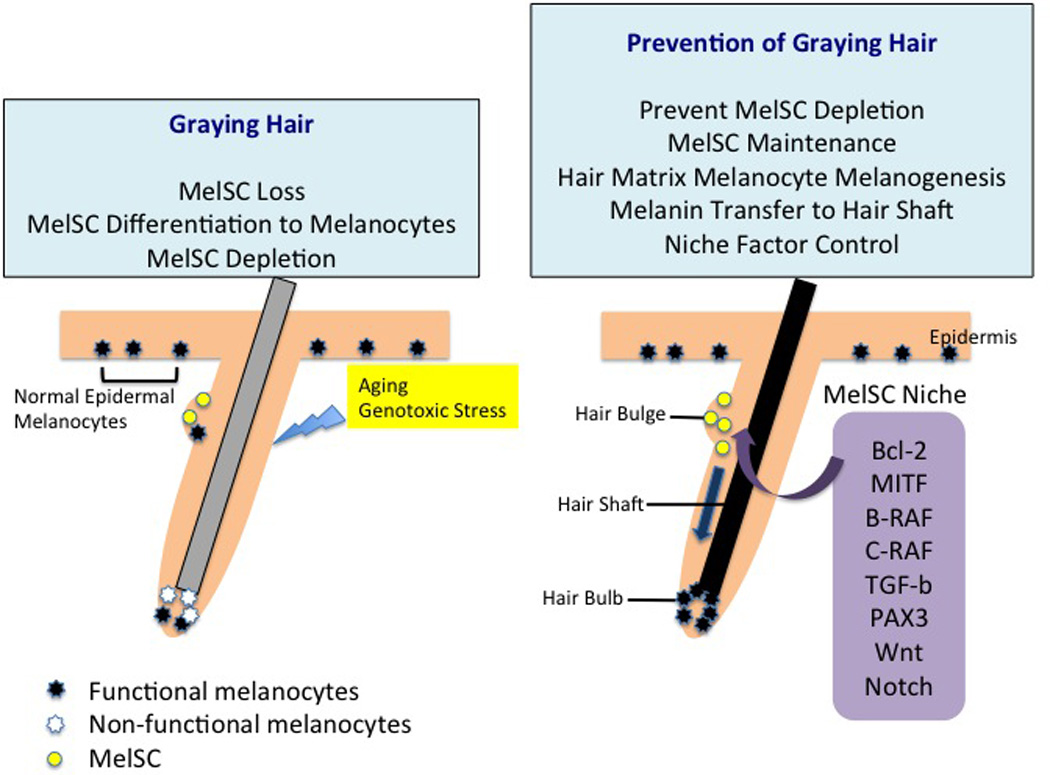
MelSC in graying hair. Factors such as aging and genotoxic stress can induce hair graying through MelSC loss or MelSC differentiation into melanocytes. Repeated MelSC loss induces MelSC depletion and leads to hair graying. For hair pigmentation, not only MelSC maintenance but also melanogenesis of hair matrix melanocytes and melanin transfer to the hair shaft should occur. MelSC maintenance and activation are regulated by niche factors including Bcl-2, MITF, B-raf, C-raf, TGF-β, PAX3, Wnt, and Notch.
5. EXPERT OPINION: POTENTIAL CLINICAL APPLICATIONS OF MELANOCYTE STEM CELLS
Development of MelSCs as a biologic therapeutic method will require a better understanding of MelSC characteristics and identifying markers. So far, studies on MelSCs have been performed in murine models rather than in humans. Modeling of the interactions of stem cells and niche components observed using in vivo systems by isolation and culture of MelSCs has been attempted. Using keratinocyte XB2 cells as feeder cells, stem cell factor, and basic fibroblast growth factor (bFGF), Nishikawa-Torikai et al. [98] tried to culture MelSCs in Dct(tm1(Cre)Bee)/CAG-CAT-GFP mice and demonstrated replication/proliferation and differentiation of the MelSCs although there was no evidence of dormant MelSCs. Furthermore, MelSCs could be isolated by a fluorescent activated cell sorter using the markers of c-Kitlow, side scatterlow, suggesting an undifferentiated state of MelSCs in mice. These cells could differentiate into melanocytes and application of this technology to human MelSCs will allow further investigation of human MelSC isolation.
For repigmentation in vitiligo, various medical therapies may be employed including corticosteroids, immunomodulators, phototherapy, 308-nm Excimer laser, or other adjuvant therapies, whereas for stable hypopigmentary lesions, surgical therapies using cells or grafts may be utilized [99]. Although isolated or cultured MelSCs have not yet been used in the clinic, mature melanocytes have been transplanted into vitiligo lesions [100,101]. To date, many studies have used cultured/non-cultured melanocytes with/without keratinocytes or epidermal grafts containing melanocytes as treatment for depigmented lesions. Compared with epidermal grafting, transplantation of a non-cultured epidermal suspension showed better results, suggesting that keratinocytes and niche factors are crucial for repigmentation [102].
In addition, non-cultured epidermal suspension and outer root sheath (ORS) cells from extracted hairs showed statistically similar clinical repigmentation results in patients with stable vitiligo, indicating that epidermal melanocytes and follicular MelSCs may have similar effects [103]. However these modalities are mixtures of various cells, thus we cannot define the effect of MelSCs or melanocytes only. Although transplantation of cultured melanocyte suspensions showed excellent results in stable localized vitiligo with 84–94% repigmentation [101,104,105], in theory MelSCs may have the greater therapeutic potential of a more long-term benefit. The superiority of transplantation of MelSCs with renewal factors or terminally differentiated melanocytes should be determined after MelSCs can be isolated.
In addition, there has been an attempt to culture ORS cells from the hair follicles to regenerate melanocytes because the bulge area of ORS contains MelSCs as well as other stem cells. As ORS cells include pluripotent neural crest stem cell (NCSC)-like stem cells and quiescent stem cells that have potential to be differentiated into various cell types, ORS cells are a valuable resource in regenerative medicine [106]. Dieckmann et al. demonstrated methods to isolate melanocytes from ORS cells in human anagen hair and propagated them in vivo; moreover, the yields of the melanocytes could be improved by a sequential method [107,108]. Although this method is not yet used in the clinical setting, the source of the melanocytes could be the MelSCs in ORS and this represents a promising method for biologic therapeutics in the near future. Moreover, with narrow band-UVB (NB-UVB) treatment, hair follicle NCSCs were directly affected and differentiated into a melanocyte lineage that produced pigmentation in vitro [88]. Such studies suggest that these MelSC reservoirs are important for repigmentation of the skin. Thus, NB-UVB treatment together with biologic therapeutics using MelSCs is expected to have efficacy for pigment production in hypopigmentary disorders. The use of induced pluripotent stem (iPS) cells to generate melanocytes has also have been explored in several experiments, but has not yet been proved in vivo [109].
MelSCs are also responsible for hair graying; however, effective therapeutic methods to prevent or reverse hair graying have not been reported. Considering the pathogenesis of the graying hair, prevention of MelSC depletion, enhancement of MelSC renewal or maintenance, and control of the MelSC niche should be therapeutic directions for hair graying. For hyperpigmentary skin disorders, few studies involving MelSCs have been reported so far. Although congenital melanocytic nevi were revealed to be derived from skin stem cells that have melanocytic differentiation in immunohistochemical studies, further studies of MelSC biology in recalcitrant pigmentary disorders such as melasma and melanocytic nevi should be performed [110].
In summary, methods for identification, isolation, and culturing of murine MelSCs have been developed and provide the biomolecular basis for research on the characterization and function of MelSCs. These findings will facilitate updated research in humans and further clinical applications of MelSCs can be expected. With the identification of specific markers for human MelSCs and in vivo tracing, we may be enable to elucidate the biology of MelSCs and melanocyte lineages. Furthermore, such efforts will provide novel therapeutics using MelSCs for various pigmentary disorders and hair graying, as well as a valuable model pertinent to other stem cell research.
Figure 4.
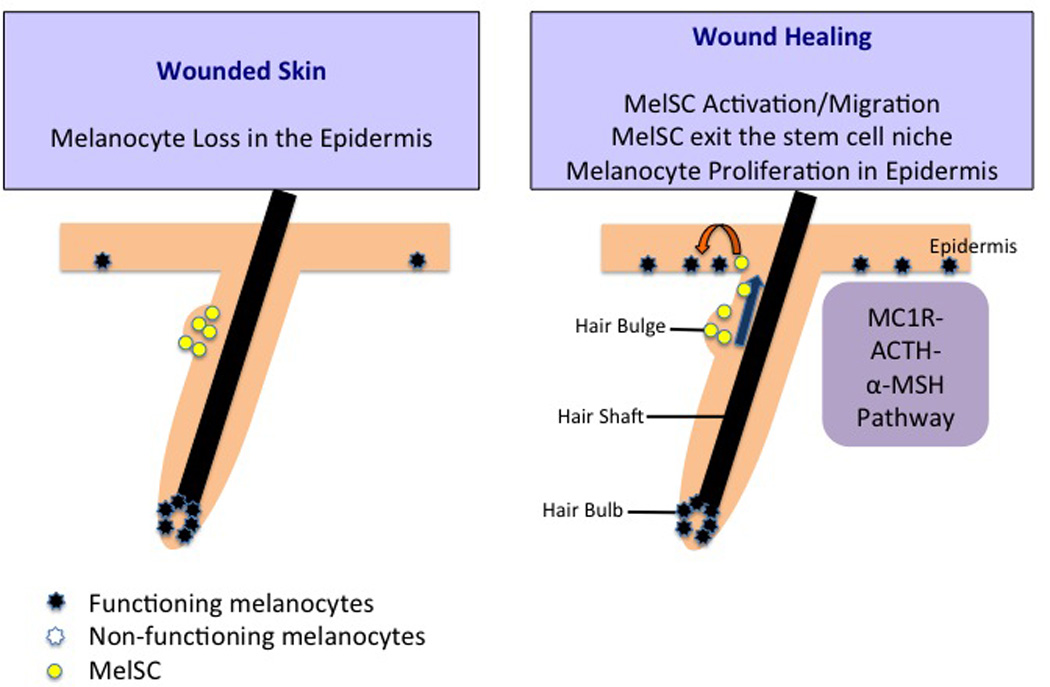
MelSC in wound healing. Wounded skin lacks melanocytes on the basal layer of the epidermis. As wound healing progresses, activated MelSC migrate to the epidermis from the hair bulge area rather than proliferating. The MelSC then proliferate into melanocytes to be the source of epidermal melanocytes and maintain the cutaneous epithelium biology. This migration and proliferation of MelSC after skin wounding is dependent on the MC1R–ACTH-α-MSH signaling pathway.
Abbreviations
- MelSC
melanocyte stem cell
- LPP
lower permanent portion
- MITF
microphthalmia associated transcription factor
- SCF
stem cell factor
- TGF-β
transforming growth factor-beta
- NB-UVB
narrow band UVB
- ORS
outer root sheath.
Footnotes
Financial and competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Randhawa M, Huff T, Valencia JC, et al. Evidence for the ectopic synthesis of melanin in human adipose tissue. FASEB J. 2009;23(3):835–843. doi: 10.1096/fj.08-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dupin E, Sommer L. Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol. 2012;366(1):83–95. doi: 10.1016/j.ydbio.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 3.Yaar M, Park HY. Melanocytes: a window into the nervous system. J Invest Dermatol. 2012;132(3 Pt 2):835–845. doi: 10.1038/jid.2011.386. [DOI] [PubMed] [Google Scholar]
- 4.Wittgen HG, van Kempen LC. Reactive oxygen species in melanoma and its therapeutic implications. Melanoma Res. 2007;17(6):400–409. doi: 10.1097/CMR.0b013e3282f1d312. [DOI] [PubMed] [Google Scholar]
- 5.Funasaka Y, Komoto M, Ichihashi M. Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res. 2000;13(Suppl 8):170–174. doi: 10.1111/j.0893-5785.2000.130830.x. [DOI] [PubMed] [Google Scholar]
- 6.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19(2):176–194. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 7. Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v–vii. 1–115. doi: 10.1007/978-3-642-19683-6_1. * This article emphasize the skin is an important peripheral neuro-endocrine-immune organ in sensing the environment with subsequent regulation of local and global homeostasis
- 8.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34(6):827–884. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80(3):979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 10.Slominski A, Zbytek B, Nikolakis G, et al. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol. 2013;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131(19):4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- 12.Weston JA. The migration and differentiation of neural crest cells. Adv Morphog. 1970;8:41–114. doi: 10.1016/b978-0-12-028608-9.50006-5. [DOI] [PubMed] [Google Scholar]
- 13.Ernfors P. Cellular origin and developmental mechanisms during the formation of skin melanocytes. Exp Cell Res. 2010;316(8):1397–1407. doi: 10.1016/j.yexcr.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 14.Slominski A, Paus R, Schadendorf D. Melanocytes as "sensory" and regulatory cells in the epidermis. J Theor Biol. 1993;164(1):103–120. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- 15.Dupin E, Le Douarin NM. Development of melanocyte precursors from the vertebrate neural crest. Oncogene. 2003;22(20):3016–3023. doi: 10.1038/sj.onc.1206460. [DOI] [PubMed] [Google Scholar]
- 16.Dupin E, Real C, Glavieux-Pardanaud C, Vaigot P, Le Douarin NM. Reversal of developmental restrictions in neural crest lineages: transition from Schwann cells to glial-melanocytic precursors in vitro. Proc Natl Acad Sci U S A. 2003;100(9):5229–5233. doi: 10.1073/pnas.0831229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adameyko I, Lallemend F, Aquino JB, et al. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009;139(2):366–379. doi: 10.1016/j.cell.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami T, Kumasaka M, Kato M, Mizoguchi M, Soma Y. BMP-4 down-regulates the expression of Ret in murine melanocyte precursors. J Dermatol Sci. 2011;63(1):66–69. doi: 10.1016/j.jdermsci.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Gleason BC, Crum CP, Murphy GF. Expression patterns of MITF during human cutaneous embryogenesis: evidence for bulge epithelial expression and persistence of dermal melanoblasts. J Cutan Pathol. 2008;35(7):615–622. doi: 10.1111/j.1600-0560.2007.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motohashi T, Yamanaka K, Chiba K, Aoki H, Kunisada T. Unexpected multipotency of melanoblasts isolated from murine skin. Stem Cells. 2009;27(4):888–897. doi: 10.1634/stemcells.2008-0678. [DOI] [PubMed] [Google Scholar]
- 21.Sommer L. Checkpoints of melanocyte stem cell development. Sci STKE. 2005;2005(298):pe42. doi: 10.1126/stke.2982005pe42. [DOI] [PubMed] [Google Scholar]
- 22.Trentin A, Glavieux-Pardanaud C, Le Douarin NM, Dupin E. Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proc Natl Acad Sci U S A. 2004;101(13):4495–4500. doi: 10.1073/pnas.0400629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21(5):457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 24.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124(1):13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Den Bossche K, Naeyaert JM, Lambert J. The quest for the mechanism of melanin transfer. Traffic. 2006;7(7):769–778. doi: 10.1111/j.1600-0854.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 26.Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 Suppl):90S–97S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- 27.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 28.Slominski A, Paus R, Costantino R. Differential expression and activity of melanogenesis-related proteins during induced hair growth in mice. J Invest Dermatol. 1991;96(2):172–179. doi: 10.1111/1523-1747.ep12460956. [DOI] [PubMed] [Google Scholar]
- 29.Slominski A, Paus R, Plonka P, et al. Melanogenesis during the anagen-catagen-telogen transformation of the murine hair cycle. J Invest Dermatol. 1994;102(6):862–869. doi: 10.1111/1523-1747.ep12382606. [DOI] [PubMed] [Google Scholar]
- 30.Slominski A, Paus R, Plonka P, et al. Pharmacological disruption of hair follicle pigmentation by cyclophosphamide as a model for studying the melanocyte response to and recovery from cytotoxic drug damage in situ. J Invest Dermatol. 1996;106(6):1203–1211. doi: 10.1111/1523-1747.ep12348479. [DOI] [PubMed] [Google Scholar]
- 31.Widlund HR, Fisher DE. Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene. 2003;22(20):3035–3041. doi: 10.1038/sj.onc.1206443. [DOI] [PubMed] [Google Scholar]
- 32.Lin CB, Babiarz L, Liebel F, et al. Modulation of microphthalmia-associated transcription factor gene expression alters skin pigmentation. J Invest Dermatol. 2002;119(6):1330–1340. doi: 10.1046/j.1523-1747.2002.19615.x. [DOI] [PubMed] [Google Scholar]
- 33. Fisher DE. Microphthalmia: a signal responsive transcriptional regulator in development. Pigment Cell Res. 2000;13(Suppl 8):145–149. doi: 10.1034/j.1600-0749.13.s8.26.x. * This review provides microphthalmia may regulate pigment enzyme gene expression and may also serve as a target for the pigmenting hormone melanocyte stimulating hormone.
- 34.Hemesath TJ, Steingrimsson E, McGill G, et al. microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 1994;8(22):2770–2780. doi: 10.1101/gad.8.22.2770. [DOI] [PubMed] [Google Scholar]
- 35.Tabone-Eglinger S, Wehrle-Haller M, Aebischer N, Jacquier MC, Wehrle-Haller B. Membrane-bound Kit ligand regulates melanocyte adhesion and survival, providing physical interaction with an intraepithelial niche. FASEB J. 2012;26(9):3738–3753. doi: 10.1096/fj.12-206045. [DOI] [PubMed] [Google Scholar]
- 36.Peters EM, Tobin DJ, Botchkareva N, Maurer M, Paus R. Migration of melanoblasts into the developing murine hair follicle is accompanied by transient c-Kit expression. J Histochem Cytochem. 2002;50(6):751–766. doi: 10.1177/002215540205000602. [DOI] [PubMed] [Google Scholar]
- 37.Mackenzie MA, Jordan SA, Budd PS, Jackson IJ. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev Biol. 1997;192(1):99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- 38.Opdecamp K, Nakayama A, Nguyen MT, Hodgkinson CA, Pavan WJ, Arnheiter H. Melanocyte development in vivo and in neural crest cell cultures: crucial dependence on the Mitf basic-helix-loop-helix-zipper transcription factor. Development. 1997;124(12):2377–2386. doi: 10.1242/dev.124.12.2377. [DOI] [PubMed] [Google Scholar]
- 39.Steel KP, Davidson DR, Jackson IJ. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992;115(4):1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- 40.Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25(1):14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nishimura EK, Jordan SA, Oshima H, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416(6883):854–860. doi: 10.1038/416854a. ** This study provides the niche has a dominant role in the fate determination of melanocyte stem-cell progeny.
- 42.Osawa M, Egawa G, Mak SS, et al. Molecular characterization of melanocyte stem cells in their niche. Development. 2005;132(24):5589–5599. doi: 10.1242/dev.02161. [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa SI, Osawa M, Yonetani S, Torikai-Nishikawa S, Freter R. Niche required for inducing quiescent stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:67–71. doi: 10.1101/sqb.2008.73.024. [DOI] [PubMed] [Google Scholar]
- 44.Nishikawa S, Osawa M. Generating quiescent stem cells. Pigment Cell Res. 2007;20(4):263–270. doi: 10.1111/j.1600-0749.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 45.Freter R, Osawa M, Nishikawa S. Adult stem cells exhibit global suppression of RNA polymerase II serine-2 phosphorylation. Stem Cells. 2010;28(9):1571–1580. doi: 10.1002/stem.476. [DOI] [PubMed] [Google Scholar]
- 46. Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307(5710):720–724. doi: 10.1126/science.1099593. * This study demonstrate that hair graying is caused by defective self-maintenance of melanocyte stem cells using melanocyte-tagged transgenic mice and aging human hair follicles.
- 47.Nishimura EK, Suzuki M, Igras V, et al. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6(2):130–140. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanpain C, Sotiropoulou PA. A dominant role of the hair follicle stem cell niche in regulating melanocyte stemness. Cell Stem Cell. 2010;6(2):95–96. doi: 10.1016/j.stem.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Sharov A, Tobin DJ, Sharova TY, Atoyan R, Botchkarev VA. Changes in different melanocyte populations during hair follicle involution (catagen) J Invest Dermatol. 2005;125(6):1259–1267. doi: 10.1111/j.0022-202X.2005.23959.x. [DOI] [PubMed] [Google Scholar]
- 50.Tobin DJ, Hagen E, Botchkarev VA, Paus R. Do hair bulb melanocytes undergo apoptosis during hair follicle regression (catagen)? J Invest Dermatol. 1998;111(6):941–947. doi: 10.1046/j.1523-1747.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- 51.Myung P, Ito M. Dissecting the bulge in hair regeneration. J Clin Invest. 2012;122(2):448–454. doi: 10.1172/JCI57414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tobin DJ, Slominski A, Botchkarev V, Paus R. The fate of hair follicle melanocytes during the hair growth cycle. J Investig Dermatol Symp Proc. 1999;4(3):323–332. doi: 10.1038/sj.jidsp.5640239. [DOI] [PubMed] [Google Scholar]
- 53.Horikawa T, Norris DA, Johnson TW, et al. DOPA-negative melanocytes in the outer root sheath of human hair follicles express premelanosomal antigens but not a melanosomal antigen or the melanosome-associated glycoproteins tyrosinase, TRP-1, and TRP-2. J Invest Dermatol. 1996;106(1):28–35. doi: 10.1111/1523-1747.ep12326989. [DOI] [PubMed] [Google Scholar]
- 54.Narisawa Y, Kohda H, Tanaka T. Three-dimensional demonstration of melanocyte distribution of human hair follicles: special reference to the bulge area. Acta Derm Venereol. 1997;77(2):97–101. doi: 10.2340/000155557797101. [DOI] [PubMed] [Google Scholar]
- 55.Commo S, Bernard BA. Melanocyte subpopulation turnover during the human hair cycle: an immunohistochemical study. Pigment Cell Res. 2000;13(4):253–259. doi: 10.1034/j.1600-0749.2000.130407.x. [DOI] [PubMed] [Google Scholar]
- 56.Nishikawa-Torikai S, Nishikawa S. Stem cell niche: from concept to reality. Pigment Cell Melanoma Res. 2012;25(2):122–123. doi: 10.1111/j.1755-148X.2011.00967.x. [DOI] [PubMed] [Google Scholar]
- 57.Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24(7):380–386. doi: 10.1016/s1471-4906(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 58.Hou L, Pavan WJ. Transcriptional and signaling regulation in neural crest stem cell-derived melanocyte development: do all roads lead to Mitf? Cell Res. 2008;18(12):1163–1176. doi: 10.1038/cr.2008.303. [DOI] [PubMed] [Google Scholar]
- 59.Robinson KC, Fisher DE. Specification and loss of melanocyte stem cells. Semin Cell Dev Biol. 2009;20(1):111–116. doi: 10.1016/j.semcdb.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Uong A, Zon LI. Melanocytes in development and cancer. J Cell Physiol. 2010;222(1):38–41. doi: 10.1002/jcp.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pshenichnaya I, Schouwey K, Armaro M, et al. Constitutive gray hair in mice induced by melanocyte-specific deletion of c-Myc. Pigment Cell Melanoma Res. 2012;25(3):312–325. doi: 10.1111/j.1755-148X.2012.00998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGill GG, Horstmann M, Widlund HR, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109(6):707–718. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- 63.Mak SS, Moriyama M, Nishioka E, Osawa M, Nishikawa S. Indispensable role of Bcl2 in the development of the melanocyte stem cell. Dev Biol. 2006;291(1):144–153. doi: 10.1016/j.ydbio.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 64.Yamamura K, Kamada S, Ito S, Nakagawa K, Ichihashi M, Tsujimoto Y. Accelerated disappearance of melanocytes in bcl-2-deficient mice. Cancer Res. 1996;56(15):3546–3550. [PubMed] [Google Scholar]
- 65. Steingrimsson E, Copeland NG, Jenkins NA. Melanocyte stem cell maintenance and hair graying. Cell. 2005;121(1):9–12. doi: 10.1016/j.cell.2005.03.021. * This article reviewws that hair graying is due to incomplete melanocyte stem cell maintenance and identify Pax3 and Mitf as key molecules.
- 66.Wellbrock C, Ogilvie L, Hedley D, et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64(7):2338–2342. doi: 10.1158/0008-5472.can-03-3433. [DOI] [PubMed] [Google Scholar]
- 67.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valluet A, Druillennec S, Barbotin C, et al. B-Raf and C-Raf are required for melanocyte stem cell self-maintenance. Cell Rep. 2012;2(4):774–780. doi: 10.1016/j.celrep.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 69.Rabbani P, Takeo M, Chou W, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145(6):941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanimura S, Tadokoro Y, Inomata K, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8(2):177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 71.Kubic JD, Young KP, Plummer RS, Ludvik AE, Lang D. Pigmentation PAX-ways: the role of Pax3 in melanogenesis, melanocyte stem cell maintenance, and disease. Pigment Cell Melanoma Res. 2008;21(6):627–645. doi: 10.1111/j.1755-148X.2008.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lang D, Lu MM, Huang L, et al. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433(7028):884–887. doi: 10.1038/nature03292. * This article describe the molecular details of a nodal point in adult melanocyte stem cell differentiation in which Pax3 simultaneously functions to initiate a melanogenic cascade while acting downstream to prevent terminal differentiation.
- 73.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303(5656):359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moriyama M, Osawa M, Mak SS, et al. Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol. 2006;173(3):333–339. doi: 10.1083/jcb.200509084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schouwey K, Delmas V, Larue L, et al. Notch1 and Notch2 receptors influence progressive hair graying in a dose-dependent manner. Dev Dyn. 2007;236(1):282–289. doi: 10.1002/dvdy.21000. [DOI] [PubMed] [Google Scholar]
- 76.Osawa M, Fisher DE. Notch and melanocytes: diverse outcomes from a single signal. J Invest Dermatol. 2008;128(11):2571–2574. doi: 10.1038/jid.2008.289. [DOI] [PubMed] [Google Scholar]
- 77.Das AV, James J, Zhao X, Rahnenfuhrer J, Ahmad I. Identification of c-Kit receptor as a regulator of adult neural stem cells in the mammalian eye: interactions with Notch signaling. Dev Biol. 2004;273(1):87–105. doi: 10.1016/j.ydbio.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 78.Chang CY, Pasolli HA, Giannopoulou EG, et al. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature. 2013;495(7439):98–102. doi: 10.1038/nature11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taieb A, Picardo M. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20(1):27–35. doi: 10.1111/j.1600-0749.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 80.Nath SK, Majumder PP, Nordlund JJ. Genetic epidemiology of vitiligo: multilocus recessivity cross-validated. Am J Hum Genet. 1994;55(5):981–990. [PMC free article] [PubMed] [Google Scholar]
- 81.Halder RM, Chappell JL. Vitiligo update. Semin Cutan Med Surg. 2009;28(2):86–92. doi: 10.1016/j.sder.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 82.Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24(3):401–410. doi: 10.1111/j.1755-148X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- 83.Kunisada T, Lu SZ, Yoshida H, et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998;187(10):1565–1573. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tobin DJ, Swanson NN, Pittelkow MR, Peters EM, Schallreuter KU. Melanocytes are not absent in lesional skin of long duration vitiligo. J Pathol. 2000;191(4):407–416. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH659>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 85.Seleit I, Bakry OA, Abdou AG, Dawoud NM. Immunohistochemical Study of Melanocyte-Melanocyte Stem Cell lineage in Vitiligo; A Clue to Interfollicular Melanocyte Stem Cell Reservoir. Ultrastruct Pathol. 2014 doi: 10.3109/01913123.2013.870274. [DOI] [PubMed] [Google Scholar]
- 86.Parsad D, Pandhi R, Dogra S, Kumar B. Clinical study of repigmentation patterns with different treatment modalities and their correlation with speed and stability of repigmentation in 352 vitiliginous patches. J Am Acad Dermatol. 2004;50(1):63–67. doi: 10.1016/s0190-9622(03)00786-2. [DOI] [PubMed] [Google Scholar]
- 87.Westerhof W, Nieuweboer-Krobotova L. Treatment of vitiligo with UV-B radiation vs topical psoralen plus UV-A. Arch Dermatol. 1997;133(12):1525–1528. [PubMed] [Google Scholar]
- 88.Dong D, Jiang M, Xu X, et al. The effects of NB-UVB on the hair follicle-derived neural crest stem cells differentiating into melanocyte lineage in vitro. J Dermatol Sci. 2012;66(1):20–28. doi: 10.1016/j.jdermsci.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 89.Yamada T, Hasegawa S, Inoue Y, et al. Wnt/beta-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol. 2013;133(12):2753–2762. doi: 10.1038/jid.2013.235. [DOI] [PubMed] [Google Scholar]
- 90.Sethi S, Mahajan BB, Gupta RR, Ohri A. Comparative evaluation of the therapeutic efficacy of dermabrasion, dermabrasion combined with topical 5% 5-fluorouracil cream, and dermabrasion combined with topical placentrex gel in localized stable vitiligo. Int J Dermatol. 2007;46(8):875–879. doi: 10.1111/j.1365-4632.2007.03226.x. [DOI] [PubMed] [Google Scholar]
- 91.Esmat SM, El-Tawdy AM, Hafez GA, et al. Acral lesions of vitiligo: why are they resistant to photochemotherapy? J Eur Acad Dermatol Venereol. 2012;26(9):1097–1104. doi: 10.1111/j.1468-3083.2011.04215.x. [DOI] [PubMed] [Google Scholar]
- 92.Vanscheidt W, Hunziker T. Repigmentation by outer-root-sheath-derived melanocytes: proof of concept in vitiligo and leucoderma. Dermatology. 2009;218(4):342–343. doi: 10.1159/000197467. [DOI] [PubMed] [Google Scholar]
- 93.Mohanty S, Kumar A, Dhawan J, Sreenivas V, Gupta S. Noncultured extracted hair follicle outer root sheath cell suspension for transplantation in vitiligo. Br J Dermatol. 2011;164(6):1241–1246. doi: 10.1111/j.1365-2133.2011.10234.x. [DOI] [PubMed] [Google Scholar]
- 94. Chou WC, Takeo M, Rabbani P, et al. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med. 2013;19(7):924–929. doi: 10.1038/nm.3194. * This article focused on epidermal repigmentation, melanocyte stem cell migration, and differentiation into pigment-producing melanocyte.
- 95.Stanley JR. Synergy of understanding dermatologic disease and epidermal biology. J Clin Invest. 2012;122(2):436–439. doi: 10.1172/JCI62237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inomata K, Aoto T, Binh NT, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137(6):1088–1099. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 97.Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180(2):273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nishikawa-Torikai S, Osawa M, Nishikawa S. Functional characterization of melanocyte stem cells in hair follicles. J Invest Dermatol. 2011;131(12):2358–2367. doi: 10.1038/jid.2011.195. [DOI] [PubMed] [Google Scholar]
- 99.Falabella R, Barona MI. Update on skin repigmentation therapies in vitiligo. Pigment Cell Melanoma Res. 2009;22(1):42–65. doi: 10.1111/j.1755-148X.2008.00528.x. [DOI] [PubMed] [Google Scholar]
- 100.Lerner AB, Halaban R, Klaus SN, Moellmann GE. Transplantation of human melanocytes. J Invest Dermatol. 1987;89(3):219–224. doi: 10.1111/1523-1747.ep12470973. [DOI] [PubMed] [Google Scholar]
- 101.Olsson MJ, Juhlin L. Melanocyte transplantation in vitiligo. Lancet. 1992;340(8825):981. doi: 10.1016/0140-6736(92)92875-g. [DOI] [PubMed] [Google Scholar]
- 102.Budania A, Parsad D, Kanwar AJ, Dogra S. Comparison between autologous noncultured epidermal cell suspension and suction blister epidermal grafting in stable vitiligo: a randomized study. Br J Dermatol. 2012;167(6):1295–1301. doi: 10.1111/bjd.12007. [DOI] [PubMed] [Google Scholar]
- 103.Singh C, Parsad D, Kanwar AJ, Dogra S, Kumar R. Comparison between autologous noncultured extracted hair follicle outer root sheath cell suspension and autologous noncultured epidermal cell suspension in the treatment of stable vitiligo: a randomized study. Br J Dermatol. 2013;169(2):287–293. doi: 10.1111/bjd.12325. [DOI] [PubMed] [Google Scholar]
- 104.Chen YF, Yang PY, Hu DN, Kuo FS, Hung CS, Hung CM. Treatment of vitiligo by transplantation of cultured pure melanocyte suspension: analysis of 120 cases. J Am Acad Dermatol. 2004;51(1):68–74. doi: 10.1016/j.jaad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 105.Olsson MJ, Juhlin L. Transplantation of melanocytes in vitiligo. Br J Dermatol. 1995;132(4):587–591. doi: 10.1111/j.1365-2133.1995.tb08715.x. [DOI] [PubMed] [Google Scholar]
- 106.Biernaskie J. Human hair follicles: "bulging" with neural crest-like stem cells. J Invest Dermatol. 2010;130(5):1202–1204. doi: 10.1038/jid.2009.449. [DOI] [PubMed] [Google Scholar]
- 107.Savkovic V, Dieckmann C, Milkova L, Simon JC. Improved method of differentiation, selection and amplification of human melanocytes from the hair follicle cell pool. Exp Dermatol. 2012;21(12):948–950. doi: 10.1111/exd.12038. [DOI] [PubMed] [Google Scholar]
- 108.Dieckmann C, Milkova L, Hunziker T, Emmendorffer A, Simon JC. Human melanocytes can be isolated, propagated and expanded from plucked anagen hair follicles. Exp Dermatol. 2010;19(6):543–545. doi: 10.1111/j.1600-0625.2009.01019.x. [DOI] [PubMed] [Google Scholar]
- 109.Ohta S, Imaizumi Y, Okada Y, et al. Generation of human melanocytes from induced pluripotent stem cells. PLoS One. 2011;6(1):e16182. doi: 10.1371/journal.pone.0016182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kinsler VA, Anderson G, Latimer B, et al. Immunohistochemical and ultrastructural features of congenital melanocytic naevus cells support a stem-cell phenotype. Br J Dermatol. 2013;169(2):374–383. doi: 10.1111/bjd.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75(2):229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 112.Kamada S, Shimono A, Shinto Y, et al. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 1995;55(2):354–359. [PubMed] [Google Scholar]


