SUMMARY
SETTING
In the Philippines, programmatic treatment of drug-resistant tuberculosis (TB) was initiated by the Tropical Disease Foundation in 1999 and transitioned to the National TB Program in 2006.
OBJECTIVE
To determine patient and socio-demographic characteristics associated with default, and the impact of patient support measures on default.
DESIGN
Retrospective cohort analysis of 583 MDR-TB patients treated from 1999 to 2006.
RESULTS
A total of 88 (15%) patients defaulted from treatment. The median follow-up time for patients who defaulted was 289 days (range 1–846). In multivariate analysis adjusted for age, sex and previous TB treatment, receiving a greater number of treatment drugs (≥5 vs. 2–3 drugs, HR 7.2, 95%CI 3.3–16.0, P < 0.001) was significantly associated with an increased risk of default, while decentralization reduced the risk of default (HR 0.3, 95%CI 0.2–0.7, P < 0.001).
CONCLUSION
Improving access to treatment for MDR-TB through decentralization of care to centers near the patient’s residence reduced the risk of default. Further research is needed to evaluate the feasibility, impact and cost-effectiveness of decentralized care models for MDR-TB treatment.
Keywords: drug-resistant tuberculosis, treatment adherence, patient incentives, risk factors, program interventions
MULTIDRUG-RESISTANT tuberculosis (MDR-TB) is a global problem, with an estimated 440 000 new cases and 150 000 deaths in 2008.1 In the Philippines, approximately 5000 new patients with MDR-TB occur annually, representing about 4% of all new and 21% of retreatment cases.2 Treatment for MDR-TB is complex, involving extended treatment with toxic and less effective second-line drugs. Although most MDR-TB patients can be cured, many default, defined as missing at least 2 consecutive months of treatment during treatment.3 Patients who default or fail treatment are at increased risk for amplified drug resistance, leaving few treatment options. These patients are also prone to increased morbidity and mortality from TB, and contribute to transmission of drug-resistant TB in the community.
In 2000, the Tropical Disease Foundation (TDF), a non-government organization (NGO) in collaboration with the Department of Health (DOH), initiated a program to treat patients with MDR-TB, based at the Makati Medical Center (MMC) in Metro Manila, capital of the Philippines. During the first 3 years, default rates were high, averaging 11.4%.4 In response, starting in 2003, TDF implemented several measures to increase treatment adherence. Support included provision of food baskets, housing and transportation allowances, ancillary drugs to manage adverse events and hospitalization support when necessary. A clinic staff member was assigned to monitor patients for treatment interruptions and conduct home visits to optimize treatment adherence. Patient care was decentralized to community facilities, including faith-based organizations, public health centers and NGOs, once a patient had an initial conversion to a negative sputum culture.
In this study, we evaluated the association between patients’ socio-demographic and clinical characteristics and the impact of programmatic patient support measures on risk for treatment default among patients treated for MDR-TB between 1999 and 2006.
MATERIALS AND METHODS
Population and program description
The present study was conducted in the three MDR-TB treatment centers established by TDF in Metro Manila. The first center was a small clinic at the Makati Medical Center, a tertiary care hospital. Patient data were reviewed individually and a Category IV treatment regimen, based on drug resistance profile and treatment history, was determined by an expert committee of MDR-TB clinicians. With the exception of critically ill patients, review of current drug susceptibility testing (DST) results was required before treatment initiation. Prescribed regimens included at least four drugs, including a second-line injectable drug in the first 6 months or until the patient was culture-negative for 4 months (intensive phase), a fluoroquinolone that the patient had not previously received and at least two of the Group 4 drugs (cycloserine, prothionamide, para-aminosalicylic acid). Dosages were based on guidelines initially provided by Partners in Health5 and later by the World Health Organization (WHO).6
All patients were given directly observed treatment (DOT) at an out-patient clinic 6 days a week. All doses were recorded on a patient treatment card. Patients who lived outside Metro Manila or more than 10 km from the treatment center were asked to transfer to a residence within 5 km of the center. Treatment was monitored by monthly smears throughout therapy, and monthly sputum cultures during the intensive phase and every other month during the last 12 months of treatment. Smears, cultures and DST were performed by the TDF laboratory, which was quality assured by the Korean Institute of Tuberculosis. Final treatment outcomes were determined by consensus after review among a committee of MDR-TB clinicians using standard WHO definitions.3,6
The first patients enrolled were those who failed a retreatment regimen in the TDF clinic and were diagnosed with MDR-TB. Initial patients were provided with free first- and second-line anti-tuberculosis drugs with DOT support; no other form of support could be offered due to limited funds. The default rate for the first cohort of 117 MDR-TB patients treated during 1999–2003 was 9%.7 In 2004 and 2005, the program expanded to include two additional centers in nearby Quezon City with housing facilities, out-patient services and patient support measures, including food baskets, housing and transportation allowances, and free ancillary drugs for the management of adverse drug reactions (ADRs). Support measures or enablers were given on an as-needed basis as determined by the facility social worker. The additional centers facilitated access to physicians and nurses, and staffing at each center was increased to include a social worker, a pharmacist, a psychiatrist and a psychologist. In 2006, the TDF-initiated program started integration with the Philippines Department of Health National TB Program.
Study design
All patients enrolled in the MDR-TB treatment program from 1999 to 2006 who had a final treatment outcome were included in the analysis. Individual patient and programmatic factors were abstracted from patient medical charts, social work and fiscal records. Treatment outcomes were abstracted from patient charts or records of the case management committee. Pharmacy records were abstracted to determine ancillary drugs prescribed to patients during treatment.
Exposure variables
Baseline patient socio-demographic and clinical information abstracted included age, sex, income, marital status, body mass index (BMI), history of smoking, alcohol and substance abuse, the number of previous courses of anti-tuberculosis treatment, duration of illness, history of TB or MDR-TB exposure, history of default from previous anti-tuberculosis treatment and extent of disease. Patient charts were reviewed to establish whether the patient had comorbidities, including diabetes, cancer, lung disease, seizures or human immunodeficiency virus infection. Treatment characteristics, including baseline DST results, treatment drugs, treatment start and stop dates, treatment interruptions in the intensive and continuation phase, DOT adherence and ADRs were noted. ADRs were defined as any documented event reported by the patient to the clinician during the course of treatment. Information about programmatic factors, including treatment delay, and the provision of food baskets, housing and transportation allowance, hospitalization support and ancillary drugs was abstracted.
All information was recorded on standardized forms designed for the study. All data were verified by a member of the research staff independent from the data abstractor.
Definitions used for data analysis
Patients were categorized as defaulters (those who missed at least 2 consecutive months of treatment) or non-defaulters (cured, treatment completed, death, failed) based on standard WHO definitions.3 Duration of illness was defined as the duration of symptoms reported on the screening form. Waiting time to diagnosis was defined as the date DST results were reported to the clinic minus the sputum collection date. Waiting time to start of treatment was defined as the date treatment started minus the date of initial screening. Waiting time from diagnosis to treatment was defined as treatment start date minus the date the DST was released to the clinician. ADRs were considered uncontrolled if the same reaction was reported at least three times prior to treatment outcome, if it was reported continuously for at least 2 weeks or if it required hospitalization. Treatment interruption was defined as missing more than 4 consecutive days of treatment. Decentralization was defined as the transfer of care from a centralized MDR-TB treatment center to a community DOTS facility. Number of drugs used was defined as the number of drugs at the last day of treatment intake. Patients were treated for at least 18 months after stable culture conversion to negative. For this analysis, the intensive phase of treatment was defined as the initial period of treatment until four consecutive cultures were negative. If the patient had frequent treatment interruptions, the end of the intensive phase was defined as completion of 156 doses following the first month of negative culture. The continuation phase was defined as the period after the intensive phase lasting for at least 12 months, or until 312 doses were completed or until 18 consecutive culture-negative months were reached.
For this analysis we defined the pre-support phase as the period from 1999 to 1 August 2003, when the only support provided to patients was free anti-tuberculosis drugs. Support phase refers to the period from 1 August 2003 to 2006, when the program was enhanced with additional staffing, decentralization and patient incentives as described above.
Data analysis
The distribution of patient and program factors among defaulters was compared to non-defaulters using the χ2 test. Time from initiation of treatment to treatment outcome (default or non-default) was calculated. Cox proportional hazards analysis was used to determine the association between patient and program characteristics and default. All variables that occurred at a specific time after treatment initiation were considered as time-varying. Unadjusted models were fit using each risk factor of interest as the only variable in the model, and variables with P < 0.20 were considered for the multivariate model. Tests for collinearity were performed to identify correlation between factors. The final model included age and factors identified as significantly associated with treatment default based on an alpha level of 0.05. A stratified analysis was also conducted to examine factors associated with default in the pre-support and support phases of the program, with patient cohorts (strata) determined by the time of treatment initiation. All data were analyzed using Stata 9.0 (Stata Corp, College Station, TX, USA).
Ethical review
The study was approved by the TDF Institutional Review Board.
RESULTS
A total of 610 patients were treated for MDR-TB by our centers from 1999 to 2006. Medical records and treatment outcomes were available for 583 treatment episodes for 577 patients (six patients had two episodes); of these, 88 (15%) defaulted from treatment. The median follow-up time for all patients was 584 days, while the median follow-up time for patients who defaulted was 289 days (range 1–846). The majority (n = 55, 63%) of patients who defaulted from treatment did so during the intensive phase of treatment (Figure). The average age was 39 years (range 6–80), and the majority were male (Table 1). Most patients were college graduates (52%), and most were also unemployed (66%). Almost half (49%) of the patients were underweight (BMI < 18.5 kg/m2). A history of alcohol abuse was recorded for 63%; 98% of the patients had a history of prior anti-tuberculosis treatment, with 86% having had at least two previous courses of treatment (Table 2). The majority of the patients had pulmonary TB, with bilateral lung involvement. Of 194 patients tested for susceptibility to both first- and second-line drugs (ofloxacin and kanamycin) with available results, 22 (11%) had extensively drug-resistant TB. At the time of presentation, almost half of the patients reported having TB symptoms for more than 1 year, and 48% had a waiting time of 6 months to more than 1 year from the time of screening to treatment initiation. Most patients (53%) were treated with a four-drug regimen, and most defaulters were treated with five or more drugs. Patients who defaulted were more likely to have treatment interruptions during both the intensive and continuation phases.
Figure.
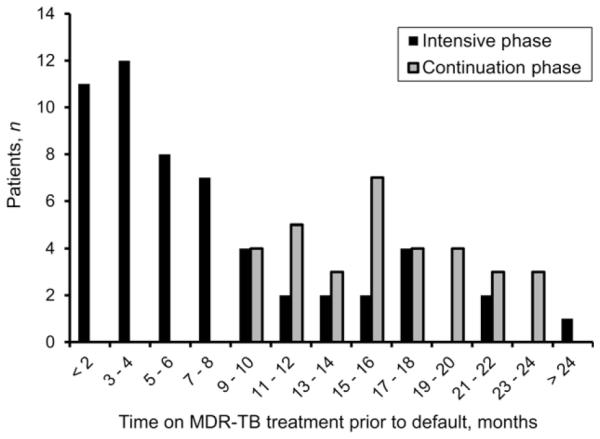
Number of patients who defaulted from treatment during the intensive and continuation phases of MDR-TB treatment, by duration of MDR-TB treatment follow-up time prior to default, in months (n = 88). MDR-TB = multidrug-resistant tuberculosis.
Table 1.
Socio-demographic characteristics of patients treated for multidrug-resistant tuberculosis, 1999–2006
| Characteristic | Non-defaulters (n = 495) n (%) |
Defaulters (n = 88) n (%) |
Total (N = 583) n (%) |
P value* |
|---|---|---|---|---|
| Age group, years | ||||
| 1–24 | 62 (13) | 14 (16) | 73 (13) | |
| 25–44 | 279 (56) | 45 (51) | 324 (56) | |
| 45–64 | 145 (29) | 27 (31) | 172 (30) | |
| ≥65 | 9 (2) | 2 (2) | 11 (2) | 0.76 |
| Male sex | 293 (59) | 58 (66) | 351 (60) | 0.24 |
| Highest level of education | ||||
| <High school | 36 (13) | 6 (13) | 42 (13) | |
| High school | 95 (33) | 13 (29) | 108 (33) | |
| College or graduate | 153 (54) | 26 (58) | 179 (54) | 0.83 |
| Married | 294 (60) | 58 (66) | 352 (60) | 0.26 |
| Unemployed | 316 (67) | 54 (62) | 370 (66) | 0.41 |
| Income ≤5000 Ph pesos†/month | 109 (22) | 22 (25) | 131 (22) | 0.54 |
| Underweight (BMI <18.5 kg/m2) | 212 (49) | 37 (49) | 249 (49) | 0.98 |
| History of smoking | 198 (40) | 42 (48) | 240 (41) | 0.18 |
| History of alcohol use | 207 (42) | 45 (63) | 252 (43) | 0.10 |
| History of substance abuse | 60 (12) | 15 (17) | 75 (13) | 0.20 |
| Diabetes mellitus | 129 (26) | 15 (17) | 144 (25) | 0.07 |
| Lung disease | 63 (13) | 5 (6) | 68 (12) | 0.06 |
Using χ2 test comparing defaulters and non-defaulters.
1 USD = 42.67 Ph pesos.
BMI = body mass index.
Table 2.
Disease severity and treatment characteristics of patients treated for multidrug-resistant-tuberculosis, 1999–2006
| Characteristic | Non-defaulters (n = 495) n (%) |
Defaulters (n = 88) n (%) |
Total (N = 583) n (%) |
P value* |
|---|---|---|---|---|
| Number of previous anti-tuberculosis treatment episodes | ||||
| None | 7 (1) | 5 (6) | 12 (2) | |
| 1 | 60 (12) | 8 (9) | 68 (12) | |
| ≥2 | 428 (86) | 75 (85) | 503 (86) | 0.03 |
| Baseline disease characteristics | ||||
| Culture-positive† | 473 (96) | 84 (95) | 557 (96) | 0.90 |
| Bilateral disease† | 363 (80) | 59 (75) | 422 (79) | 0.29 |
| Cavitary disease† | 190 (42) | 29 (37) | 219 (41) | 0.37 |
| DST pattern‡ | ||||
| First-line drugs only (H, R, Z, E, S) | 300 (63) | 50 (58) | 350 (62) | 0.43 |
| HR + 1 second-line drug class (± other first-line drug; not XDR-TB) |
154 (79) | 32 (84) | 186 (80) | 0.47 |
| XDR-TB (HR + second-line injection + FQ) | 18 (11) | 4 (15) | 22 (11) | 0.49 |
| Diagnosis, treatment, clinical course | ||||
| Duration of current illness at time of screening | ||||
| <2 weeks | 10 (2) | 1 (1) | 11 (2) | |
| 2 weeks–1 month | 43 (9) | 6 (7) | 49 (9) | |
| >1 month–1 year | 184 (40) | 32 (40) | 216 (40) | |
| >1 year | 223 (48) | 42 (52) | 265 (49) | 0.86 |
| Total waiting time from screening to treatment start | ||||
| <1 month | 74 (15) | 5 (6) | 79 (14) | |
| 1–5 months | 188 (38) | 33 (38) | 221 (38) | |
| 6 months–1 year | 176 (36) | 41 (47) | 217 (37) | |
| >1 year | 57 (12) | 9 (10) | 66 (11) | 0.06 |
| Total number of drugs used during treatment | ||||
| 2–3 | 133 (27) | 9 (10) | 142 (24) | |
| 4 | 285 (58) | 26 (30) | 311 (53) | |
| ≥5 | 77 (16) | 53 (60) | 130 (22) | <0.001 |
| Uncontrolled ADRs | 270 (55) | 43 (49) | 313 (54) | 0.35 |
| Hospitalized while on treatment | 109 (22) | 17 (19) | 126 (22) | 0.55 |
| Treatment interruption (>4 missed doses) during intensive phase |
143 (29) | 58 (66) | 201 (35) | <0.001 |
| Treatment interruption (>4 missed doses) during continuation phase |
253 (57) | 30 (73) | 283 (58) | 0.04 |
Using χ2 test comparing defaulters and non-defaulters.
Among pulmonary TB patients (n = 569).
Of those patients with DST results available: n = 565 for first-line drugs; n = 233 for first + at least 1 class of second-line drugs; n = 194 for XDR-TB. DST = drug susceptibility testing; H = isoniazid; R = rifampin; Z = pyrazinamide; E = ethambutol; S = streptomycin; XDR-TB = extensively drug-resistant tuberculosis; FQ = fluoroquinolone; ADR = adverse drug reaction.
Patients whose treatment was decentralized after culture conversion were less likely to default than those whose treatment was continued at one of the central TDF MDR-TB treatment centers (Table 3). None of the other patient support measures were significantly associated with default. The overall proportion of patients who defaulted on treatment did not differ during the pre-support and support phases.
Table 3.
Treatment support measures provided to patients treated for multidrug-resistant tuberculosis, 1999–2006
| Characteristic | Non-defaulters (n = 495) n (%) |
Defaulters (n = 88) n (%) |
Total (N = 583) n (%) |
P value* |
|---|---|---|---|---|
| Transportation allowance |
80 (16) | 11 (13) | 91 (16) | 0.38 |
| Housing allowance | 15 (4) | 2 (3) | 17 (3) | 0.67 |
| Food baskets | 231 (47) | 34 (39) | 265 (45) | 0.16 |
| Ancillary drugs | 71 (14) | 8 (9) | 79 (14) | 0.19 |
| Hospitalization support (if hospitalized) |
22 (4) | 1 (1) | 23 (4) | 0.14 |
| Decentralization | 158 (32) | 9 (10) | 167 (29) | <0.001 |
Using χ2 test comparing defaulters and non-defaulters.
In multivariate analysis adjusted for age, sex and previous anti-tuberculosis treatment, receiving a greater number of drugs (≥5 vs. 2–3 drugs, hazard ratio [HR] 7.2, 95% confidence interval [CI] 3.3–16.0, P < 0.001) was significantly associated with increased risk for default, while decentralization reduced the risk of default (HR 0.3, 95%CI 0.2–0.7, P < 0.001, Table 4). These results were similar when the analysis was restricted to the cohort of patients who initiated treatment during the support phase.
Table 4.
Association between patient and program characteristics and risk for treatment default among patients treated for multidrug-resistant tuberculosis from 1999–2006
| Characteristic | Multivariate*
HR (95%CI) |
P value |
|---|---|---|
| Number of drugs used during treatment |
||
| 2–3 | Reference | |
| 4 | 1.3 (0.6–2.8) | 0.51 |
| ≥5 | 7.2 (3.3–16.0) | <0.001 |
| Decentralization | 0.3 (0.2–0.7) | 0.006 |
Model also adjusted for age, sex and history of previous anti-tuberculosis treatment.
HR = hazard ratio; CI = confidence interval.
DISCUSSION
Our study confirms other reports8–11 that default during treatment is a persistent problem for MDR-TB programs. Our treatment cohort from 1999 to 2006 had a 15% default rate, similar to rates experienced in other settings, including Korea (39%8 and 28.9%),9 Latvia (13%),10 and Peru (11%6 and 10%).11 The MDR-TB program in the Philippines has implemented interventions aimed at reducing default, including transportation to DOT treatment centers, housing allowance, food baskets and decentralization of treatment to facilities near the patient’s residence. Reducing default rates will improve treatment success rates and reduce the risk of additional acquired drug resistance and the spread of drug-resistant strains in the community.
In this retrospective cohort study among patients with MDR-TB in a high TB burden country, patients receiving treatment regimens containing at least five drugs had an increased risk for treatment default, and decentralization of MDR-TB treatment to DOTS treatment facilities near the patient’s residence was associated with a 70% reduced risk for default. The provision of housing or transport allowances or food baskets was not associated with reduced default.
MDR-TB treatment programs have employed several service models since the WHO DOTS-Plus strategy was started.7 Most countries have adopted an approach that involves hospitalization for the initial phase of treatment, aiming to protect the greater community from ongoing transmission of drug-resistant TB strains. However, it is important to balance the need to protect the public with patient rights and autonomy. In the Philippines, treatment for MDR-TB is centralized, and the individual is therefore often required to move away from home to access treatment. When a patient is no longer sputum culture-positive, care is decentralized to a local health center near the patient’s residence after health care workers in the center have been trained to administer and monitor treatment with second-line drugs. Studies in other countries have demonstrated that decentralized or community-based models of MDR-TB treatment promote favorable patient outcomes, and our findings provide further evidence in support of this approach.12,13 Furthermore, a recent review on the cost-effectiveness of MDR-TB treatment showed that the out-patient care model was more cost-effective than that for in-patients.14
While decentralization was associated with a lower risk of default, 88 patients still defaulted during treatment. Patients default during treatment for multiple reasons, including competing health priorities associated with comorbidities,15,16 financial and work constraints, and lack of social support.11 A previous study conducted among 240 patients in the TDF MDR-TB treatment program from 2003 to 2005 identified that patients who missed more than 20% of treatment days, weighed <47 kg, or had TB strains resistant to second-line drugs were more likely to default.17
Our study has several limitations. As this study was retrospective, we were unable to assess reasons why patients interrupted treatment or defaulted, and we had limited information on programmatic factors. The data for this study were abstracted from medical charts that were not standardized or intended for research, and data on some variables were missing for some patients. Patients who were decentralized had to take their treatment continually for the first 6 months to establish culture conversion and be eligible for decentralization, potentially introducing a survival bias. However, this potential bias was minimized by considering decentralization as a time-varying covariate in the analysis. During the support phase, only patients considered in need of additional treatment support were provided with program incentives. Our inability to identify an association between transport, housing and other support measures and default may have been due to a pre-selection of patients at greater risk for poor outcomes. Patients who had a greater drug burden during treatment were also identified as having a greater risk of default. However, the drug burden was classified according to the number of drugs received at the last dose of treatment; if patients were also more likely to default during the intensive phase, this could potentially inflate the association between the drug burden and default risk. It is also possible that these patients were provided with more drugs due to a history of poor adherence or because they had a TB strain resistant to several first-line drugs, both risk factors for treatment default and poor outcomes. Moreover, there may have been a competing risk of death in the 2–3 drug referent group. The increased risk of default among patients who received ≥5 drugs could be due, in part, to increased risk of death in patients receiving 2–3 drugs, a substandard regimen by global recommendations. Despite these limitations, this study provides a comprehensive assessment of patient, disease, treatment and program factors for a cohort of over 500 patients treated for MDR-TB. We were able to assess the impact of several patient treatment support interventions on treatment default, which will be used to inform our treatment program and others on the best use of limited resources.
In conclusion, decentralization of treatment to facilities near a patient’s residence reduced default during treatment for MDR-TB in a high-burden country. Additional research is needed to further identify programmatic and patient factors that may contribute to risk of default. As the global burden of MDR-TB continues to expand and exceeds the capacity of countries to hospitalize or provide centralized treatment, it will be critical to assess the impact of different strategies on treatment outcomes and community transmission, and the cost-effectiveness of implementing these programs.
Acknowledgements
The authors thank R Guilatco for his assistance with the cleaning and management of the data, and for the valuable input from other participants and colleagues in the Tropical Disease Foundation–Centers for Disease Control Philippines operational research workshop. The Tropical Disease Foundation multidrug-resistant tuberculosis program and this study were made possible through the financial support of the Global Fund to Fight AIDS, Tuberculosis and Malaria.
References
- 1.World Health Organization . Global tuberculosis control 2010. WHO; Geneva, Switzerland: 2010. WHO/HTM/TB/2010.7. [Google Scholar]
- 2.Phillippines Nationwide Tuberculosis Drug Resistance Survey Team Nationwide drug resistance survey of tuberculosis in the Philippines. Int J Tuberc Lung Dis. 2009;13:500–507. [PubMed] [Google Scholar]
- 3.World Health Organization . Guidelines for the programmatic management of drug-resistant tuberculosis, emergency update 2008. WHO; Geneva, Switzerland: 2008. WHO/HTM/TB/2008.402. [PubMed] [Google Scholar]
- 4.Tupasi TE, Quelapio MI, Orillaza RB, et al. DOTS-Plus for multidrug-resistant tuberculosis in the Philippines: global assistance urgently needed. Tuberculosis. 2003;83:53–58. doi: 10.1016/s1472-9792(02)00072-0. [DOI] [PubMed] [Google Scholar]
- 5.Partners in Health, Harvard Medical School, Bill and Melinda Gates Foundation. A DOTS-Plus handbook . Guide to the community-based treatment of MDR TB. Partners in Health; Boston, MA, USA: 2002. [Google Scholar]
- 6.World Health Organization . Guidelines for the programmatic management of drug-resistant tuberculosis. WHO; Geneva, Switzerland: 2006. p. 361. WHO/HTM/2006. [PubMed] [Google Scholar]
- 7.World Health Organization . Proceedings of the Meeting of the Stop TB Working Group on DOTS-Plus for MDR TB, Talinn, Estonia, 1–12 April 2002. WHO; Geneva, Switzerland: 2002. DOTS-PLUS: preliminary results and emerging issues. WHO/CDS/TB/2002.307. [Google Scholar]
- 8.Kim HJ, Hong YP, Kim SJ, Lew WJ, Lee EG. Ambulatory treatment of multidrug-resistant pulmonary tuberculosis patients at a chest clinic. Int J Tuberc Lung Dis. 2001;5:1129–1136. [PubMed] [Google Scholar]
- 9.Park SK, Lee WC, Lee DH, Mitnick CD, Han L, Seung KJ. Self-administered, standardized regimens for multidrug-resistant tuberculosis in South Korea. Int J Tuberc Lung Dis. 2004;8:361–368. [PubMed] [Google Scholar]
- 10.Leimane V, Reikststina V, Holtz TH, et al. Clinical outcomes of individualized treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–326. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 11.Franke MF, Applleton SC, Bayona J, Arteaga F, Palacios E. Risk factors and mortality associated with default from multidrug resistant tuberculosis treatment. Clin Infect Dis. 2008;46:1845–1851. doi: 10.1086/588292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin S, Furin J, Bayona J, Mate K, Kim JY, Farmer P. Community-based treatment of multidrug-resistant tuberculosis in Lima, Peru: 7 years of experience. Soc Sci Med. 2004;59:1529–1539. doi: 10.1016/j.socscimed.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Keyshavee S, Gelmanova IY, Pasechnikov AD, et al. Treating multidrug-resistant tuberculosis in Tomsk, Russia: developing programs that address the linkage between poverty and disease. Ann NY Acad Sci. 2008;1136:1–11. doi: 10.1196/annals.1425.009. [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick C, Floyd K. A systematic review of the cost and cost-effectiveness of treatment for multidrug-resistant tuberculosis. Pharmaeconomics. 2012;30:63–80. doi: 10.2165/11595340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Chan Yeung M, Noertjojo K, Leung CC, Chan SL, Tam CM. Prevalence and predictors of default from tuberculosis treatment in Hong Kong. Hong Kong Med J. 2003;9:263–268. [PubMed] [Google Scholar]
- 16.Daniel OJ, Oladapo OT, Alausa OK. Default from tuberculosis treatment program in Sagamu, Nigeria. Niger J Med. 2006;15:63–67. doi: 10.4314/njm.v15i1.37119. [DOI] [PubMed] [Google Scholar]
- 17.Auer C, Jost M, Gler MT, Quelapio MI, Tupasi TE, Peterhans B. Risk factors and reasons for poor treatment outcome of MDR-TB in Manila, Philippines. Int J Tuberc Lung Dis; 41st Union World Conference on Lung Health; Berlin, Germany. Nov 11–15, 2010. p. S332. Abstract PS-100735-15. 2010. [Google Scholar]


