Abstract
Background
Infectious diseases (IDs) are an important cause of infant mortality in the United States. This study describes maternal and infant characteristics associated with infant ID deaths in the United States.
Methods
Infant deaths with an ID underlying cause of death occurring in the United States were examined using the 2008–2009 Period Linked Birth/Infant Death public use data files. Average annual ID infant mortality rates (IMRs) for singleton infants were calculated. A retrospective case-control study was conducted to determine infant and maternal risk factors for infant ID death among low (LBW) and normal (NBW) birth weight groups. Controls were defined as infants surviving to the end of their birth year. Risk factors for infant ID deaths were determined through multivariable logistic regression.
Results
An estimated 3,843 infant ID deaths occurred in the United Sates during 2008–2009, an overall ID IMR of 47.5 deaths per 100,000 live births. The mortality rates for LBW and NBW infants were 514.8 and 15.5, respectively. Male sex, younger maternal age (<25 years), a live birth order of fourth or more, and low 5-minute Apgar score were associated with increased ID death among LBW and NBW infants. Additionally, black maternal race was associated with increased ID death among LBW infants, and having an unmarried mother was associated with increased ID death among NBW infants.
Conclusions
Awareness of associations with infant ID death should help in development of further strategic measures to reduce infant ID morbidity and mortality.
Keywords: Infant, Infectious Disease, Mortality, birth weight
INTRODUCTION
Emerging, reemerging, and endemic infectious diseases (IDs) are a significant concern in the United States as they contribute to substantial morbidity, mortality, and financial burdens. 1,2 The burden of ID hospitalizations, particularly among infants (<1 year of age), is substantial; a recent report indicated that in 2003, 42.8% of infant hospitalizations were due to IDs and the in-hospital fatality rate for infant ID hospitalizations was 0.16%.1
Although IDs, when aggregated, have been shown to be an important cause of infant mortality in the United States,3 recent studies of infant and maternal risk factors for infant ID death have focused on specific IDs, such as diarrhea, lower respiratory tract infections, pertussis, and bronchiolitis.4–7 These studies have identified associations of infant mortality with race and birth weight. Infants of black race and low birth weight (LBW) infants were shown to have higher noncongenital ID death rates in the 1980s; noncongenital infections accounted for almost 90% of infant ID deaths.3 Postneonates (infants at least 28 days old) of black race or LBW were at increased risk for a noncongenital ID death.3 With increasing survival of LBW preterm infants8 and continued racial disparity in rates of LBW and LBW-related infant mortality,9–13 we expect continued importance of infant ID deaths.
The objective of the present study is to describe maternal and infant characteristics associated with infant deaths due to IDs in the United States and to further explore the role that birth weight and race have on infant ID mortality.
MATERIALS AND METHODS
Data source and inclusion criteria
Infant ID deaths occurring in the United States were examined using the 2008 and 2009 Period Linked Birth/Infant Death public use data files from the National Center for Health Statistics, Centers for Disease Control and Prevention.14,15 Infant ID deaths were defined as singleton infants (<1 year of age) born in the United States to U.S. resident mothers with an ID International Classification of Diseases, 10th Revision, (ICD-10) code listed as the underlying cause of death (UCOD) on the death record. 16 The ID ICD-10 codes used in this study were adapted from a previous classification of ID deaths using ICD-9 codes.17 Results are reported among all infant ID deaths unless the neonatal or postneonatal periods are specified.
ID Infant Mortality Rates
In 2008 and 2009, 1.3% and 1.4%, respectively, of all infant death records could not be linked to the corresponding birth certificate.14,15 To account for the unlinked death records, the weighted number of infant ID deaths was estimated using weights provided by the National Center for Health Statistics.12–15 Maternal and infant characteristics that were comparable between the 1989 and 2003 birth certificate revisions were examined and included sex, birth weight (very low birth weight [VLBW, <1500 grams], moderately low birth weight [1500–2499 grams], LBW [<2500 grams] and normal birth weight [NBW, ≥2500 grams]), gestational age (<37 weeks and ≥37 weeks), 5-minute Apgar score (<7 and ≥7), maternal age (≤19, 20–24, 25–29, and ≥30 years), maternal marital status, maternal race (white, black, American Indian/Alaska Native [AI/AN], and Asian/Pacific Islander [A/PI]), maternal Hispanic origin, live birth order (first, second, third, and fourth or more), and number of prenatal visits (0, 1–2, 3–5, and ≥6).14,15 Some characteristics had missing data and therefore did not sum to the total number of infant ID deaths; percentages calculated exclude missing data. Average annual ID infant mortality rates (IMRs) were calculated overall and by each characteristic as the weighted number of deaths per 100,000 live births of the corresponding group during 2008–2009. In order to compare age periods at death—since the neonatal (<28 days of age) period is shorter than the postneonatal period—neonatal and postneonatal IMRs were calculated as the weighted number of deaths in the age period per 100,000 person-years. Person-years were calculated from the total number of person-days that contributed to each age period. Poisson regression was used to calculate risk ratios (RRs) and 95% confidence intervals (CIs) within each characteristic.
Age at Death Analysis
The proportion of unweighted neonatal and postneonatal ID deaths and age at death in months were examined overall and by birth weight group. The median age at death in weeks and the interquartile range (IQR) were calculated overall and by birth weight group; the Wilcoxon rank-sum test18 was used to statistically compare age at death in weeks between LBW and NBW infants.
Cause of Death
The proportion of weighted infant deaths due to ID was calculated. The most frequently listed ID UCODs on the death certificate were examined overall and by birth weight group, and the proportion of neonatal deaths among LBW infants with a most frequently listed ID death was calculated. The number and percent of ID death records with bacterial sepsis of newborn (ICD-10 code P36) as the UCOD or with prematurity (P07) listed anywhere on the death record were examined overall and by birth weight group. The proportion of neonatal deaths among all deaths due to bacterial sepsis was calculated.
Case-control analysis
A retrospective case-control study was conducted to determine infant and maternal characteristics associated with infant mortality due to IDs among LBW and NBW infants. The cases for each birth weight group were the unweighted infant ID deaths. Controls for each birth weight group were selected from singleton infants of the corresponding birth weight group who survived to the end of their birth year and were born in the United States to U.S. resident mothers using a simple random sample to obtain a 1:1 ratio of cases to controls.
Univariate analysis was conducted for each of the birth weight groups using logistic regression.19 Odds ratios (ORs) and 95% CIs were calculated for sex, 5-minute Apgar score, maternal age (years), race, maternal marital status, Hispanic origin, and live birth order. Significant characteristics (P<0.10) in univariate logistic regression, along with the interaction of these characteristics with maternal race, were included as potential predictors in the initial LBW and NBW multivariable logistic regression models. The interaction terms were included to further examine maternal race in relationship with these characteristics. Hierarchical backward elimination was then used to determine significant characteristics and interactions within the multivariable models for each birth weight group. Characteristics and interaction terms were retained in the LBW and NBW multivariable models at the P<0.05 significance level. Adjusted ORs and 95% CIs were calculated for the significant characteristics within each of the final LBW and NBW multivariable regression models.
RESULTS
Infant Infectious Disease Deaths and Mortality Rates
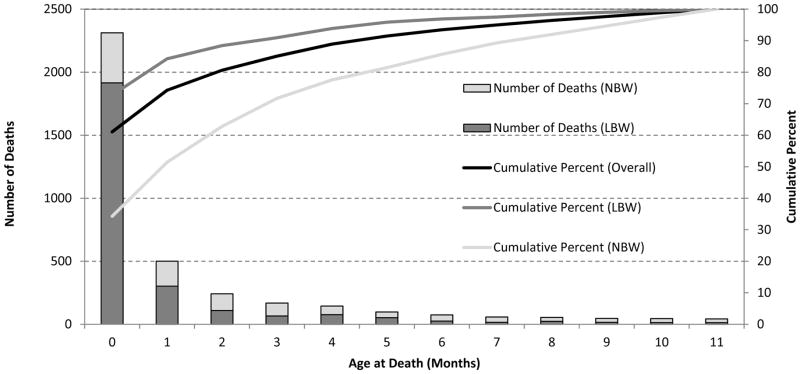
An estimated 3,843 infant ID deaths occurred in the United States during 2008 and 2009, accounting for 8.3% of all infant deaths. Among these deaths, 2,665 (69.5%) were LBW infants and 1,170 (30.5%) were NBW infants (Table 1). More than 60% of the infant ID deaths occurred during the neonatal period; this percentage was higher for LBW infants (72.7%) compared to NBW infants (34.3%, P<0.01; Figure 1). The overall median age of ID death among infants was two weeks (IQR: 0–8 weeks). LBW infants had a lower age of death (median: 1 week, IQR: 0–4 weeks) than NBW infants (median: 7 weeks, IQR: 2–20 weeks; P<0.0001).
Table 1.
Weighted Number of Infant Infectious Disease (ID) Deaths and Average Annual Infant Mortality Rates by Selected Maternal and Infant Characteristics, United States, 2008–2009+
| Characteristics | Weighted No. (%)‡ | Rate* | Risk Ratio (95% CI) |
|---|---|---|---|
| Total | 3,843 | 47.5 | - |
| Age Period at Death | |||
| Neonatal | 2,349 (61.1) | 394.0 | 19.7 (18.4–21.0) |
| Postneonatal | 1,494 (38.9) | 20.0 | Reference |
| Sex | |||
| Male | 2,102 (54.7) | 50.7 | 1.2 (1.1–1.2) |
| Female | 1,741 (45.3) | 44.1 | Reference |
| Birth Weight (grams) | |||
| <2500 | 2,665 (69.5) | 514.8 | 33.3 (31.1–35.7) |
| <1500 | 2,256 (58.8) | 2,478.0 | 160.3 (149.4–172.1) |
| 1500–2499 | 410 (10.7) | 96.0 | 6.2 (5.5–6.9) |
| ≥2500 | 1,170 (30.5) | 15.5 | Reference |
| Gestational Age (weeks) | |||
| <37 | 2,679 (70.1) | 314.7 | 19.9 (18.5–21.3) |
| ≥37 | 1,145 (29.9) | 15.8 | Reference |
| 5-Minute Apgar Score | |||
| <7 | 1,682 (44.9) | 1,278.8 | 49.0 (46.0–52.3) |
| ≥7 | 2,062 (55.1) | 26.1 | Reference |
| Maternal Age (years) | |||
| ≤19 | 628 (16.3) | 74.6 | 1.8 (1.7–2.0) |
| 20–24 | 1,159 (30.2) | 57.7 | 1.4 (1.3–1.6) |
| 25–29 | 926 (24.1) | 40.5 | Reference |
| ≥30 | 1,129 (29.4) | 38.3 | 0.9 (0.9–1.0) |
| Race | |||
| White | 2,360 (61.4) | 37.9 | Reference |
| Black | 1,267 (33.0) | 99.1 | 2.6 (2.4–2.8) |
| AI/AN | 60 (1.6) | 62.4 | 1.6 (1.3–2.1) |
| A/PI | 155 (4.0) | 31.8 | 0.8 (0.7–1.0)† |
| Maternal Marital Status | |||
| Unmarried | 2,289 (59.6) | 68.7 | 2.1 (2.0–2.2) |
| Married | 1,554 (40.4) | 32.6 | Reference |
| Hispanic Origin | |||
| Hispanic | 811 (21.4) | 40.7 | 0.8 (0.8–0.9) |
| Non-Hispanic | 2,986 (78.6) | 49.5 | Reference |
| Live Birth Order | |||
| First | 1,691 (44.5) | 51.3 | 1.3 (1.2–1.4) |
| Second | 985 (26.0) | 39.1 | Reference |
| Third | 586 (15.4) | 44.3 | 1.1 (1.0–1.3)† |
| Fourth or more | 534 (14.1) | 59.2 | 1.5 (1.4–1.7) |
| Number of Prenatal Visits | |||
| 0 | 284 (8.0) | 233.9 | 7.8 (6.9–8.8) |
| 1–2 | 212 (6.0) | 255.5 | 8.5 (7.4–9.8) |
| 3–5 | 871 (24.7) | 246.8 | 8.2 (7.6–8.9) |
| ≥6 | 2,166 (61.3) | 30.0 | Reference |
Infant ID deaths were restricted to singleton infants born in the United States to U.S. residents with an infectious disease underlying cause of death on the death record. Maternal race is used to represent infant race and ethnicity in this study. AI/AN: American Indian and Alaska Native, A/PI: Asian/Pacific Islander. Infant: <1 year old.
Neonatal: infants <28 days old, Postneonatal: infants ≥28 days old. CI: Confidence Interval. The number of deaths is weighted to adjust for unlinked death records and is estimated using weights provided by the National Center for Health Statistics12–15
Maternal and infant characteristics may not add up to the total number of infant infectious disease deaths due to missing data or rounding due to weights. The percentages calculated exclude missing data.
Average annual rate per 100,000 live births of corresponding group except for age period which is the average annual rate per 100,000 person-years.
p<0.05
Figure 1.
Number of Infant Infectious Disease (ID) Deaths and Cumulative Percent by Age at Death in Months, Overall and by Birth Weight Group*, United States, 2008–2009+
* LBW: Low Birth Weight (<2500g), NBW: Normal Birth Weight (≥2500g)
+Infant ID deaths were restricted to singleton infants born in the United States to U.S. residents and defined as an ID underlying cause of death on the death record. Seven infant death records were missing birth weight and were not included in this analysis. There were no death records missing age at death in months.
A large proportion of ID deaths were due to bacterial sepsis of newborn (28.3% overall, Table 2) with 96% of these deaths occurring in the neonatal period. The proportion of ID deaths due to bacterial sepsis of newborn was higher in LBW infants (34.1%) than NBW infants (15.3%; P<0.01). Bacterial sepsis of newborn, unspecified was the most frequently listed UCOD among LBW (27.5%) and NBW (10.8%) infants. Among LBW infants, the other most frequently listed UCODs were newborn affected by chorioamnionitis (27.0%); septicemia, unspecified (9.2%); infection specific to the perinatal period, unspecified (3.1%); and congenital pneumonia, unspecified (2.1%). The top five UCODs represent 68.9% of LBW infant deaths; 84.7% of these were for neonates. The other most frequently listed UCODs among NBW infants were pneumonia, unspecified (7.4%); bronchopneumonia, unspecified (6.9%); septicemia, unspecified (5.8%); and congenital herpes viral [herpes simplex] infection (5.6%). Overall, 2,294 (59.7%) of the infant ID deaths had prematurity (ICD-10 code P07) listed on their death record. Among LBW infant ID deaths, 84.8% had prematurity listed on their death record while 2.3% of NBW infant ID deaths had prematurity listed on their death record.
Table 2.
Weighted Number of Infant Infectious Disease (ID) Deaths due to Bacterial Sepsis of the Newborn, Overall and by Birth Weight Group, United States, 2008–2009+
| Underlying Cause of Death (ICD-10 Code) | Overall N (%) |
LBW* N (%) |
NBW* N (%) |
|---|---|---|---|
| Bacterial sepsis of newborn (P36) | 1088 (28.3) | 909 (34.1) | 180 (15.3) |
| Bacterial sepsis of newborn, unspecified (P36.9) | 860 (22.4) | 733 (27.5) | 127 (10.8) |
| Sepsis of newborn due to Escherichia coli (P36.4) | 64 (1.7) | 53 (2.0) | 10 (0.9) |
| Other bacterial sepsis of newborn (P36.8) | 60 (1.6) | 53 (2.0) | 6 (0.5) |
| Sepsis of newborn due to streptococcus, group B (P36.0) | 53 (1.4) | 24 (0.9) | 28 (2.4) |
| Sepsis of newborn due to Staphylococcus aureus (P36.2) | 25 (0.7) | 25 (0.9) | 0 (0.0) |
| Sepsis of newborn due to other and unspecified staphylococci (P36.3) | 15 (0.4) | 14 (0.5) | 1 (0.1) |
| Sepsis of newborn due to other and unspecified streptococci (P36.1) | 11 (0.3) | 5 (0.2) | 6 (0.5) |
| Sepsis of newborn due to anaerobes (P36.5) | 1 (0.03) | 0 (0.0) | 1 (0.1) |
Infant ID deaths were restricted to singleton infants born in the United States to U.S. residents with an infectious disease underlying cause of death on the death record. Infant: <1 year old. The number of deaths is weighted to adjust for unlinked death records and is estimated using weights provided by the National Center for Health Statistics.12–15 The percent is calculated among all infant ID deaths.
LBW: Low Birth Weight (<2500 g), NBW: Normal Birth Weight (≥2500 g). LBW and NBW deaths may not add up to overall deaths because of rounding due to weights.
The overall ID IMR was 47.5 deaths per 100,000 live births (Table 1). The neonatal mortality rate was higher than the postneonatal mortality rate (RR: 19.7, 95% CI: 18.4–21.0). The IMR was higher for male infants, infants with a 5-minute Apgar score <7, infants with a gestational age <37 weeks, infants born to an unmarried mother, infants born to young mothers (<19 and 20–24 compared to 25–29 years), infants with a live birth order of first, third, or fourth or more compared to second, infants of black race or of AI/AN race compared to white race, and infants whose mothers had fewer prenatal visits (0, 1–2, and 3–5 compared to ≥6). The IMR for LBW infants was much higher (RR: 33.3, 95% CI: 31.1–35.7) than that for NBW infants and was highest for VLBW infants (RR: 160.3, 95% CI: 149.4–172.1). The IMR was lower for Hispanic infants and infants of A/PI race compared to white race.
Infant and Maternal Risk Factors for Infant Infectious Disease Mortality
The case-control study included infants whose 2008–2009 ID death record was linked to the corresponding birth certificate (2,633 LBW infants and 1,158 NBW; Table 3). In the univariate analyses, all characteristics were significantly associated with ID death in logistic regression for NBW infants, and all except for Hispanic origin were significant in logistic regression for LBW infants. Male sex, 5-minute Apgar score <7, young maternal age (≤19 and 20–24 years), black race, unmarried maternal marital status, and live birth order of fourth or more were positively associated with ID death in both LBW and NBW infants (Table 3). For NBW infants, AI/AN race was positively associated with ID death whereas having Hispanic origin and maternal age ≥30 years were negatively associated with ID death. For LBW infants, A/PI race was negatively associated with ID death compared to white race.
Table 3.
Univariate Analysis of Selected Maternal and Infant Characteristics by Infectious Disease (ID) Infant Deaths and Infant Survivors, United States, 2008–2009+
| Characteristics | LBW* | NBW* | ||||
|---|---|---|---|---|---|---|
| Deaths No. (%) | Survivors No. (%) | OR (95% CI) | Deaths No. (%) | Survivors No. (%) | OR (95% CI) | |
| Total | 2,633 | 2,633 | - | 1,158 | 1,158 | - |
| Sex | ||||||
| Male | 1,425 (54.1) | 1,273 (48.3) | 1.3 (1.1–1.4) | 650 (56.1) | 574 (49.6) | 1.3 (1.1–1.5) |
| Female | 1,208 (45.9) | 1,360 (51.7) | Reference | 508 (43.9) | 584 (50.4) | Reference |
| 5-Minute Apgar Score | ||||||
| < 7 | 1,557 (60.9) | 150 (5.7) | 25.6 (21.4–30.8) | 98 (8.6) | 12 (1.0) | 8.9 (5.1–17.2) |
| ≥ 7 | 998 (39.1) | 2,459 (94.3) | Reference | 1,043 (91.4) | 1,138 (99.0) | Reference |
| Maternal Age (years) | ||||||
| ≤ 19 | 424 (16.1) | 359 (13.6) | 1.3 (1.1–1.6) | 197 (17.0) | 128 (11.1) | 1.7 (1.3–2.3) |
| 20–24 | 726 (27.6) | 665 (25.3) | 1.2 (1.1–1.4) | 419 (36.2) | 312 (26.9) | 1.5 (1.2–1.9) |
| 25–29 | 628 (23.9) | 705 (26.8) | Reference | 285 (24.6) | 321 (27.7) | Reference |
| ≥ 30 | 855 (32.5) | 904 (34.3) | 1.1 (0.9–1.2) | 257 (22.2) | 397 (34.3) | 0.7 (0.6–0.9) |
| Race | ||||||
| White | 1,523 (57.8) | 1,667 (63.3) | Reference | 804 (69.4) | 883 (76.3) | Reference |
| Black | 986 (37.5) | 741 (28.1) | 1.5 (1.3–1.6) | 266 (23.0) | 184 (15.9) | 1.6 (1.3–2.0) |
| AI/AN | 23 (0.9) | 41 (1.6) | 0.6 (0.4–1.0) | 36 (3.1) | 18 (1.6) | 2.2 (1.3–4.0) |
| A/PI | 101 (3.8) | 184 (7.0) | 0.6 (0.5–0.8) | 52 (4.5) | 73 (6.3) | 0.8 (0.5–1.1) |
| Maternal Marital Status | ||||||
| Married | 1,067 (40.5) | 1,253 (47.6) | Reference | 464 (40.1) | 671 (57.9) | Reference |
| Unmarried | 1,566 (59.5) | 1,380 (52.4) | 1.3 (1.2–1.5) | 694 (59.9) | 487 (42.1) | 2.1 (1.7–2.4) |
| Hispanic Origin | ||||||
| Hispanic | 564 (21.7) | 561 (21.5) | 1.0 (0.9–1.2) | 234 (20.3) | 291 (25.4) | 0.8 (0.6–0.9) |
| Non-Hispanic | 2,033 (78.3) | 2,045 (78.5) | Reference | 917 (79.7) | 855 (74.6) | Reference |
| Live Birth Order | ||||||
| First | 1,220 (47.0) | 1,203 (46.2) | 1.1 (1.0–1.3) | 449 (39.1) | 486 (42.3) | 1.0 (0.8–1.2) |
| Second | 641 (24.7) | 692 (26.6) | Reference | 332 (28.9) | 343 (29.9) | Reference |
| Third | 384 (14.8) | 431 (16.5) | 1.0 (0.8–1.1) | 194 (16.9) | 187 (16.3) | 1.1 (0.8–1.4) |
| Fourth or more | 353 (13.6) | 279 (10.7) | 1.4 (1.1–1.7) | 174 (15.1) | 133 (11.6) | 1.4 (1.0–1.8)† |
Infant ID deaths and infant survivors were restricted to singleton infants born in the United States to U.S. residents. An ID death is defined as an ID underlying cause of death on the death record. Maternal race is used to represent infant race and ethnicity in this study. AI/AN: American Indian and Alaska Native, A/PI: Asian/Pacific Islander
LBW: Low Birth Weight (<2500 g), NBW: Normal Birth Weight (≥2500 g), OR: Odds Ratio, CI: Confidence Interval Maternal and Infant Characteristics may not add up to the total number of infant infectious disease deaths due to missing data. The percentages calculated exclude missing data.
p<0.05
In the multivariable analyses, all characteristics that were included in the initial logistic regression models were significant except for maternal marital status in the model for LBW infants and maternal race in the model for NBW infants (Table 4). Maternal race did not interact with any of the characteristics included in the initial LBW and NBW multivariable regression models. Male sex, live birth order of fourth or more, young maternal age (<19 and 20–24 years), and low 5-minute Apgar score were positively associated with ID death in both LBW and NBW infants (Table 4); however, the effect of 5-minute Apgar score was greater for LBW infants than NBW infants. For LBW infants, black race was associated with increased odds of ID death. Among NBW infants, maternal age of ≥30 years, a live birth order of first, and Hispanic origin were associated with decreased odds of ID death whereas being born to an unmarried mother was associated with increased odds of ID death.
Table 4.
Selected Maternal and Infant Characteristics associated with Infant Infectious Disease (ID) Death in Multivariable Logistic Regression Analysis by Birth Weight Group, United States, 2008–2009+
| Characteristics | LBW OR (95% CI)* |
NBW OR (95% CI)* |
|---|---|---|
| Hispanic Origin† | ||
| Hispanic | - | 0.67 (0.54–0.82) |
| Non-Hispanic | - | Reference |
| Maternal Marital Status | ||
| Unmarried | - | 1.69 (1.39–2.05) |
| Married | - | Reference |
| Live Birth Order | ||
| First | 0.92 (0.78–1.10) | 0.71 (0.57–0.89) |
| Second | Reference | Reference |
| Third | 0.95 (0.76–1.18) | 1.15 (0.88–1.50) |
| Fourth or more | 1.38 (1.08–1.75) | 1.75 (1.30–2.35) |
| Sex | ||
| Male | 1.24 (1.08–1.42) | 1.23 (1.03–1.46) |
| Female | Reference | Reference |
| Maternal Age (years) | ||
| ≤ 19 | 1.69 (1.34–2.13) | 1.83 (1.33–2.52) |
| 20–24 | 1.39 (1.15–1.69) | 1.47 (1.16–1.86) |
| 25–29 | Reference | Reference |
| ≥30 | 1.02 (0.85–1.23) | 0.65 (0.51–0.83) |
| 5-Minute Apgar Score | ||
| <7 | 25.96 (21.62–31.38) | 11.30 (6.31–22.11) |
| ≥7 | Reference | Reference |
| Race | ||
| White | Reference | - |
| Black | 1.35 (1.16–1.57) | - |
| AI/AN | 0.60 (0.31–1.12) | - |
| A/PI | 0.77 (0.56–1.06) | - |
Infant ID deaths and infant survivors were restricted to singleton infants born in the United States to U.S. residents. An ID death is defined as an ID underlying cause of death on the death record. Maternal race is used to represent infant race and ethnicity in this study. AI/AN: American Indian and Alaska Native, A/PI: Asian/Pacific Islander
LBW: Low Birth Weight (<2500 g), NBW: Normal Birth Weight (≥2500 g), OR: Odds Ratio, CI: Confidence Interval
Hispanic Origin was not significant in univariate logistic regression for LBW infants.
DISCUSSION
The overall average annual ID IMR for 2008–2009 was 47.5 deaths per 100,000 live births, nearly half of that reported for 1983–1987 (89 deaths per 100,000 live births);3 however, the proportion of infant deaths due to ID remained similar (8% in 2008–2009 compared to 9% in 1983–1987).3 The present study found higher ID IMRs in neonates, male infants, LBW and preterm infants (gestational age <37), infants with a 5-minute Apgar score <7, infants of black or AI/AN race, and non-Hispanic infants. A maternal age of ≤19 and 20–24 years, unmarried maternal marital status, a live birth order of first or fourth or more, and 0, 1–2, or 3–5 prenatal visits also had higher ID IMRs. Noncongenital ID deaths from 1983–1987 also showed higher infant death rates for male infants, LBW infants, infants of black race, infants born to young mothers, infants born to unmarried mothers, and infants whose mothers had few or no prenatal visits.3
Infants with an ID UCOD are dying early and many of these deaths are potentially preventable; a majority of these deaths are occurring in the first month of life and a large proportion of infants are dying from bacterial sepsis, specifically from an unspecified bacteria. Prevention and intervention should focus on neonatal ID deaths with emphasis on neonatal sepsis. Attention should be paid to appropriate screening for infections in pregnant women. The Centers for Disease Control and Prevention (CDC) along with the American College of OB/GYN and American Academy of Pediatrics recommend that all pregnant women be screened for colonization with Group B streptococcus (GBS) and that colonized women receive intrapartum antibiotic prophylaxis to prevent vertical transmission of GBS to the newborn.20–22 This national strategy has proven effective in substantially decreasing the incidence of early-onset neonatal bacterial sepsis due to GBS.21–25 However, GBS is still the bacteria most frequently isolated from early onset neonatal sepsis cultures, particularly among term infants.20–22,26,27 Missed opportunities for prevention still exist, particularly among women with no or limited prenatal care who are not screened for GBS colonization, women who deliver preterm, and those with suspected penicillin allergy, underscoring the importance of strictly following national prevention guidelines.26–32 Availability of accurate point of care diagnostics to identify women with colonization at delivery would help identify women without prior screening or those who have become colonized late in pregnancy. In the future, both early- and late-onset neonatal bacterial sepsis due to GBS could potentially be prevented by a safe and effective maternal GBS vaccine.30,31 Esherichia coli is another important early-onset neonatal sepsis pathogen, especially among preterm and LBW infants, but there are no recommended strategies for preventing bacterial sepsis due to E. coli.26,27
Racial disparities persist in infant ID mortality. In the present study, infants of black race had a higher ID IMR than that for infants of white race, and black race was associated with increased odds for ID death among LBW infants. Studies of infants in California and North Carolina also found that the mortality rate due to overall ID was highest in infants born to black mothers.10,11 In the present study, AI/AN race had a higher ID IMR than white race and was a risk factor in univariate analysis for NBW infants. However, there was no association between AI/AN race and ID death when controlling for other maternal and infant characteristics in both LBW and NBW infants; this finding may be due to the small number of AI/AN infant ID deaths and needs to be confirmed by future studies. It is also of interest to note that Hispanic origin is protective against NBW infant ID deaths in multivariable analysis.
There are some limitations to this study. Some maternal characteristics of interest, such as maternal smoking status, maternal education, and adequacy of prenatal care, are potential predictors for infant ID death, but were not comparable between the 1989 and 2003 U.S. Standard Certificate of Live Birth revisions and were not included in the analysis.12,13 In addition, there were only a small number of AI/AN infant ID deaths in 2008 and 2009 which could explain why there is a disparity in the ID IMR for AI/AN race but it is not a risk factor when adjusting for the other maternal and infant characteristics in the multivariable analysis. The small number of AI/AN ID deaths may be an effect of racial misclassification;33 however, we used maternal race and ethnicity as indicated on the birth certificate which are more reliable than infant race and ethnicity reported on the death certificate14,15 so this should limit racial misclassification. Also, ICD-10 coding is subject to miscoding or misdiagnosis that could affect inclusion or exclusion of ID deaths. The present study analyzed deaths with IDs as the UCOD which is a conservative approach to identification of ID deaths.
Families and healthcare providers of infants should be aware of the characteristics associated with higher risk of infant ID death. Awareness of these associations and development of further strategic measures should lead to the prevention strategies and reduction of ID deaths among infants.
Acknowledgments
Source of Funding:
Funded through the Centers for Disease Control and Prevention.
We thank Arialdi Minino and Rachel Albalak for technical assistance.
Footnotes
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of Interest:
The authors have no other funding or conflicts of interest to disclose.
References
- 1.Yorita KL, Holman RC, Sejvar JJ, Steiner CA, Schonberger LB. Infectious disease hospitalizations among infants in the United States. Pediatrics. 2008;121:244–252. doi: 10.1542/peds.2007-1392. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. [Accessed August 1, 2013];A CDC Framework for Preventing Infectious Diseases: Sustaining the Essentials and Innovating for the Future. 2011 Available at: http://www.cdc.gov/oid/docs/ID-Framework.pdf.
- 3.Read JS, Troendle JF, Klebanoff MA. Infectious disease mortality among infants in the United States, 1983 through 1987. Am J Public Health. 1997;87:192–198. doi: 10.2105/ajph.87.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehal JM, Esposito DH, Holman RC, Tate JE, Callinan LS, Parashar UD. Risk factors for diarrhea-associated infant mortality in the United States, 2005–2007. The Pediatric infectious disease journal. 2012;31:717–721. doi: 10.1097/INF.0b013e318253a78b. [DOI] [PubMed] [Google Scholar]
- 5.Singleton RJ, Wirsing EA, Haberling DL, et al. Risk factors for lower respiratory tract infection death among infants in the United States, 1999–2004. Pediatrics. 2009;124:e768–776. doi: 10.1542/peds.2009-0109. [DOI] [PubMed] [Google Scholar]
- 6.Haberling DL, Holman RC, Paddock CD, Murphy TV. Infant and maternal risk factors for pertussis-related infant mortality in the United States, 1999 to 2004. The Pediatric infectious disease journal. 2009;28:194–198. doi: 10.1097/INF.0b013e31818c9032. [DOI] [PubMed] [Google Scholar]
- 7.Holman RC, Shay DK, Curns AT, Lingappa JR, Anderson LJ. Risk factors for bronchiolitis-associated deaths among infants in the United States. The Pediatric infectious disease journal. 2003;22:483–490. doi: 10.1097/01.inf.0000069765.43405.3b. [DOI] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhuri PK, MacDorman MF, Ezzati-Rice TM. Racial differences in leading causes of infant death in the United States. Paediatr Perinat Epidemiol. 2004;18:51–60. doi: 10.1111/j.1365-3016.2004.00535.x. [DOI] [PubMed] [Google Scholar]
- 10.Hessol NA, Fuentes-Afflick E. Ethnic differences in neonatal and postneonatal mortality. Pediatrics. 2005;115:e44–51. doi: 10.1542/peds.2004-0478. [DOI] [PubMed] [Google Scholar]
- 11.Kitsantas P, Gaffney KF. Racial/ethnic disparities in infant mortality. Journal of perinatal medicine. 2010;38:87–94. doi: 10.1515/jpm.2010.014. [DOI] [PubMed] [Google Scholar]
- 12.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2008 period linked birth/infant death data set. Natl Vital Stat Rep. 2012;60:1–27. [PubMed] [Google Scholar]
- 13.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2009 period linked birth/infant death data set. Natl Vital Stat Rep. 2013;61:1–46. [PubMed] [Google Scholar]
- 14.National Center for Health Statistics. User Guide to the 2008 Period Linked Birth/Infant Death Public Use File. Hyattsville, MD: National Center for Health Statistics; 2011. [Google Scholar]
- 15.National Center for Health Statistics. User Guide to the 2009 Period Linked Birth/Infant Death Public Use File. Hyattsville, MD: National Center for Health Statistics; 2012. [Google Scholar]
- 16.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) Geneva: World Health Organization; 1992. [Google Scholar]
- 17.Pinner RW, Teutsch SM, Simonsen L, et al. Trends in infectious diseases mortality in the United States. Jama. 1996;275:189–193. [PubMed] [Google Scholar]
- 18.Lehmann EL. Nonparametrics: statistical methods based on ranks. San Francisco: Holden Day; 1975. [Google Scholar]
- 19.Kleinbaum DG, Klein M. Logistic Regression: A Self-Learning Text. 2. Springer-Verlag New York, Inc; 2002. [Google Scholar]
- 20.Verani JR, McGee L, Schrag SJ Division of Bacterial Diseases, NCIRD, CDC. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 21.The American College of Obstetricians and Gynecologists Committee on Obstetric Practice. . ACOG Committee Opinion No. 485: Prevention of early-onset group B streptococcal disease in newborns. [Accessed December 13, 2013];Obstetrics and gynecology. 2011 117:1019–1027. doi: 10.1097/AOG.0b013e318219229b. Available at: https://www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Obstetric_Practice/Prevention_of_Early-Onset_Group_B_Streptococcal_Disease_in_Newborns. [DOI] [PubMed] [Google Scholar]
- 22.Baker CJ, Byington CL, Polin RA Committee on Infectious Disease and Committee on Fetus and Newborn. Policy statement-Recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics. 2011;128:611–616. doi: 10.1542/peds.2011-1466. [DOI] [PubMed] [Google Scholar]
- 23.Schrag SJ, Zywicki S, Farley MM, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. The New England journal of medicine. 2000;342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Early-onset and late-onset neonatal group B streptococcal disease--United States, 1996–2004. MMWR Morb Mortal Wkly Rep. 2005;54:1205–1208. [PubMed] [Google Scholar]
- 25.Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA. 2008;299:2056–2065. doi: 10.1001/jama.299.17.2056. [DOI] [PubMed] [Google Scholar]
- 26.Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. The Pediatric infectious disease journal. 2011;30:937–941. doi: 10.1097/INF.0b013e318223bad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Dyke MK, Phares CR, Lynfield R, et al. Evaluation of universal antenatal screening for group B streptococcus. The New England journal of medicine. 2009;360:2626–2636. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- 29.Shane AL, Stoll BJ. Neonatal sepsis: Progress towards improved outcomes. J Infect. 2013 doi: 10.1016/j.jinf.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013;31 (Suppl 4):D20–26. doi: 10.1016/j.vaccine.2012.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goins WP, Talbot TR, Schaffner W, et al. Adherence to perinatal group B streptococcal prevention guidelines. Obstetrics and gynecology. 2010;115:1217–1224. doi: 10.1097/AOG.0b013e3181dd916f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paccione KA, Wiesenfeld HC. Guideline adherence for intrapartum group B streptococci prophylaxis in penicillin-allergic patients. Infectious diseases in obstetrics and gynecology. 2013;2013:917304. doi: 10.1155/2013/917304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stehr-Green P, Bettles J, Robertson LD. Effect of racial/ethnic misclassification of American Indians and Alaskan Natives on Washington State death certificates, 1989–1997. Am J Public Health. 2002;92:443–444. doi: 10.2105/ajph.92.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]