Abstract
Upon intensive, exhaustive exercise, exercise-induced reactive oxygen species may exceed the antioxidant defence threshold, consequently resulting in muscular damage or late-onset chronic inflammation. Recently, the therapeutic antioxidant and anti-inflammatory effects of molecular hydrogen (H2) for human rheumatoid arthritis have been demonstrated. However, it is also important to clarify the effects of administrating H2 in large animals other than humans, as H2 is thought to reach the target organ by passive diffusion upon delivery from the blood flow, indicating that the distance from the administration point to the target is critical. However, data on the effects of H2 on oxidative stress in real-life exhaustive exercise in large animals are currently lacking. We here investigated 13 Thoroughbred horses administered intravenous 2-L saline with or without 0.6-ppm H2 (placebo, N = 6; H2, N = 7) before participating in a high-intensity simulation race. Intravenous H2-saline significantly suppressed oxidative stress immediately, 3 h, and 24 h after the race, although the antioxidant capability was not affected throughout the study. The serum creatine kinase, lactate, and uric acid levels were increased in both groups. Taken together, these results indicate that intravenous H2-saline can significantly and specifically suppress oxidative stress induced after exhaustive racing in Thoroughbred horses.
Muscular damage or late-onset chronic inflammation can result from exercise-induced reactive oxygen species (ROS). The balance between these exercise-induced ROS and the defence mechanisms by intrinsic or extrinsic antioxidants plays an important role in maintaining energy homeostasis and mitochondrial functions. These effects of exercise-induced ROS are largely regulated by the peroxisome proliferator-activated receptor-γ co-activator 1α (PGC-1α)-related pathways, including AMP-activated protein kinase and sirtuin 1, in combination with various energy metabolic products1,2. PGC-1α directs the exercise-related mitochondrial biogenesis, resulting in increase in oxygen supply to the muscle3 and over-generation of ROS accompanied by electron leakage from the mitochondrial respiratory chain4. Despite the increase in metabolic sources for ROS during intensive and exhaustive exercises, the deleterious effects by ROS are suppressed by the adaptive induction of numerous intrinsic ROS-elimination mechanisms, including superoxide dismutase, glutathione peroxidase, and non-enzymatic glutathione5. Endurance exercise can stimulate the release of anti-inflammatory cytokines6,7, and, therefore, it is considered to prevent the morbidity of age-related chronic inflammatory diseases such as diabetes, cancer, atherosclerosis, and cardiovascular disorders.
In contrast to endurance training, intensive exercise, including for example human athletics and horse races, requires fast and high-power contraction of white muscles rich in fast type IIx fibres, in which the majority of energy is supplied through glycolytic and anaerobic reactions8. Although the role of ROS in anaerobic exercise, in which the energy supply is quickly exhausted, is not fully understood, the majority of such anaerobic strenuous exercises continue over 30 seconds up to several minutes, thereby subsequently or simultaneously requiring aerobic energy metabolism9,10. Under such circumstances, oxidative stress is thought to largely regulate the muscle conditions after exercise11,12,13, and it is thus important to balance the oxidative state with that of the antioxidant defence capabilities.
On the other hand, during such exhaustive exercises, the threshold for maintaining architectural or stoichiometric strength of the skeletal muscle is frequently exceeded, resulting in muscle damage, accompanied by elevation of various serum biomarkers, including creatine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase, and lactate dehydrogenase (LDH), which are released through the affected muscle cell membrane14,15,16. It has been hypothesised that chemical stress, as represented by ROS, during strenuous exercise increases the permeability of muscle cells by peroxidation of the cell membranes17,18, thereby promoting leakage of the intracellular enzymes19. Furthermore, the rapid depletion of anaerobic energy sources such as free ATP, phosphocreatine, and glycogen results in the degradation of AMP to generate inosine 5′-monophosphate and ammonia20,21; therefore, the final metabolite of purine nucleotide metabolism, uric acid (UA), is a useful marker for the exhaustion of energy sources in addition to serum lactate, which also increases upon depletion of the glycolytic energy supply22,23,24.
These deleterious influences by exercise are not restricted to strenuous exercise. For example, in elderly people25 and individuals with sarcopoenia26,27 or chronic inflammatory diseases28, non-intensive exercise can easily exceed the resistance limitations of the muscles. In such conditions, muscle atrophy is common, thereby impairing the antioxidant defence capabilities, as well as the anti-inflammatory systems. Therefore, it is important to determine methods to prevent the excess ROS from overwhelming the antioxidant defence potential; this would not only be useful for protecting skeletal muscle from injury, which is hard to avoid in high-intensity exercises such as horse racing, as well as in human athletic exercises, but also for successfully designing rehabilitation strategies for morbid or wasted muscles.
Among numerous antioxidants that are exogenously absorbable, molecular hydrogen (H2) has shown promising anti-inflammatory potential, as well as high bio-safety29,30,31. In addition to its anti-inflammatory properties, H2 also appears to show protective effects on the muscle against the detrimental complications accompanied by excessive exercise-related oxidative stress. Recently, the reducing potential of H2 against oxidative stress in atrophic muscle in rodents32 and a possibility that H2 may work as an antioxidant during treadmill exercise in Thoroughbred horses33 have been reported. However, direct evidence regarding whether H2 possesses antioxidant properties or protective effects for skeletal muscle exposed to strenuous exercise is still lacking.
Further, it should be noted that the body size of the experimental model used is also important. Because H2 is thought to distribute throughout the body by passive diffusion upon delivery from the blood flow, the H2 molecules that reach the target organ is in inverse proportion to the cube of the distance from the area of administration. Most of the investigations concerning H2 therapy are performed using small animals such as rodents, and there is a large discrepancy of the distances from the administration point to the target organ between rodents and humans and other large mammals. To assess the accessibility of H2, we tested the efficacy of intravenous infusion of approximately 0.6-ppm H2 dissolved saline (H2-saline) in thoroughbred horses exposed to extremely high levels of oxidative stress by high-intensity competitive races. Further, to clarify the protective and/or preventable capability of H2 against oxidative stress or muscle damages caused by high-intensity exercise, we investigated the oxidative state and several metabolic/injury biomarkers before and after simulation races in which placebo-controlled intravenous infusion of H2-saline was administered. To evaluate the oxidative or reducing potential present in the sera of the racing horses, we performed the diacron-Reactive Oxygen Metabolite (d-ROM) and Biological Antioxidant Potential (BAP) tests, respectively34,35. Moreover, among the biomarkers for oxidative stress, we particularly focused on the alteration of serum 8-hydroxydeoxyguanosine (8-OHdG), which directly reflects the consequences of oxidative stress, as well as the generation of mutagenic damages on DNA, which in turn may induce secondary, chronic morbidity.
Results
Analysis of oxidative stress and antioxidant potentials in the serum
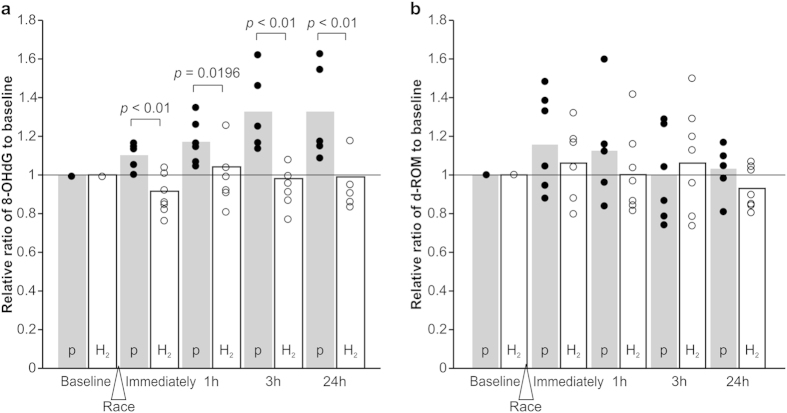
At baseline, the serum 8-OHdG levels were 1.29 ± 0.104 ng/mL in the placebo group and 1.45 ± 0.077 ng/mL in the H2 group. The relative ratios of 8-OHdG at each time point after the race to the baseline are presented in Fig. 1a. The 8-OHdG levels in the H2 group were significantly suppressed immediately, 3 h, and 24 h after the race compared to in the placebo group. On the other hand, the mean values of the d-ROMs, which is an indirect method to reflect the free radicals in the serum, were 149 ± 24.6 U.CARR in the placebo group and 154 ± 24.8 U.CARR in the H2 group at baseline, and no significant increases were observed during the study in either group (Fig. 1b).
Figure 1. Variations in the relative ratios to baseline of (a) serum 8-hydroxydeoxyguanosine (8-OHdG) and (b) serum diacron-Reactive Oxygen Metabolites (d-ROM) immediately, and 1, 3, and 24 h after the race.
The scatter plots indicate the value for each individual horse (closed circles: placebo [P] group, open circles: H2 group). The mean values of the relative ratios to baseline of serum 8-OHdG and d-ROM are shown as grey columns for the placebo group and white columns for the H2 group.
The BAP test was also performed to measure the reducing potential in the serum. The mean values of the BAP test, which reflects the reduction potential in the serum, were 3850 ± 251 μmol/L in the placebo group and 3560 ± 1100 μmol/L in the H2 group at baseline. The relative ratios of the scores at each time point after the race to the baseline are shown in Fig. 2. Although there was no significant difference between the H2 and placebo groups, the ratio tended to be elevated in the H2 group (36.8 ± 20.6%), as compared to in the placebo group (8.50 ± 12.6%; p = 0.0212) immediately after the race.
Figure 2. Variations in the relative ratio to baseline of the Biological Antioxidant Potential (BAP) scores in the serum immediately, and 1, 3, and 24 h after the race.
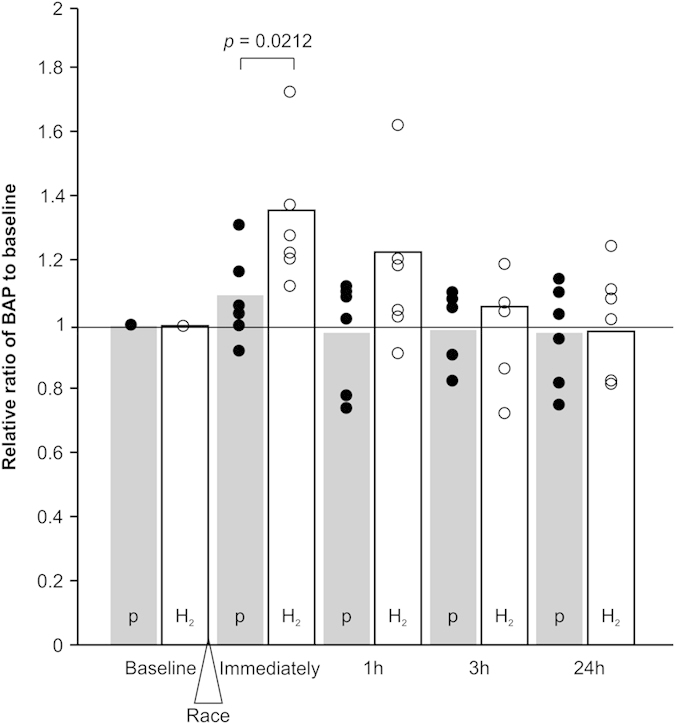
The scatter plots indicate the value for each individual horse (closed circles: placebo [P] group, open circles: H2 group). The mean values of the relative ratios to baseline are shown as grey columns for the placebo group and white columns for the H2 group.
Biomarkers for muscular damage and anaerobic metabolism during the race
As biomarkers for exercise-induced muscle damages, CK, AST, and LDH were measured (Table 1). In both groups, the CK levels were significantly increased 3 h after the race, but were restored to near the baseline values within 24 h after the race. There were no significant differences between the placebo and H2 groups. In both groups, the AST and LDH levels did not significantly change during the 24-h period after the race.
Table 1. Mean values of CK, AST, and LDH before and after racing.
|
Placebo (N = 6) |
H2 (N = 7) |
|||
|---|---|---|---|---|
| CK (U/L) | p value | CK (U/L) | p value | |
| Baseline | 231 ± 74.0 | — | 186 ± 38.0 | — |
| Immediately post-racing | 290 ± 83.0 | 0.7186 | 252 ± 43.0 | 0.2802 |
| 1 h | 306 ± 71.0 | 0.529 | 270 ± 78.0 | 0.124 |
| 3 h | 385 ± 142 | 0. 0493* | 354 ± 106 | 0.0006* |
| 24 h | 228 ± 77.0 | >0.99 | 211 ± 48.0 | 0.916 |
| AST (U/L) | p value | AST (U/L) | p value | |
| Baseline | 318 ± 64.0 | — | 283 ± 45.0 | — |
| Immediately post-racing | 382 ± 62.0 | 0.2508 | 348 ± 70.0 | 0.1519 |
| 1 h | 342 ± 31.0 | 0.9022 | 295 ± 58.0 | 0.9883 |
| 3 h | 369 ± 63.0 | 0.435 | 330 ± 59.0 | 0.4066 |
| 24 h | 339 ± 58.0 | 0.9372 | 295 ± 36.0 | 0.9888 |
| LDH (U/L) | p value | LDH (U/L) | p value | |
| Baseline | 478 ± 118 | — | 417 ± 100 | — |
| Immediately post-racing | 569 ± 125 | 0.5782 | 500 ± 96.0 | 0.386 |
| 1 h | 555 ± 74.0 | 0.7042 | 449 ± 102 | 0.9422 |
| 3 h | 523 ± 138 | 0.9352 | 450 ± 116 | 0.9331 |
| 24 h | 397 ± 134 | 0.6669 | 345 ± 48.0 | 0.493 |
Abbreviations: AST, aspartate aminotransferase; CK, creatine kinase; LDH, lactate dehydrogenase.
Baseline indicates the values just prior to injection.
Values are presented as mean ± standard deviation.
*Significant increase vs. baseline.
Next, to estimate the alterations induced by anaerobic metabolism, the serum lactate and UA levels were measured (Table 2). The serum lactate levels were significantly increased in both groups 1 h after the race. They reached their maximum levels (28.8 and 38.1-fold increases in the placebo and H2 groups, respectively) immediately after the race and reduced rapidly within 3 h, remaining 1.7 and 1.8-fold elevated compared to the baseline in the placebo and H2 groups, respectively, after 24 h. However, there were no significant differences in the lactate levels between the groups at any time point. On the other hand, the UA level was significantly increased immediately after the race only in the H2 group; at 1 h after the race, significant increases in the UA levels were observed in both groups. However, there were no significant differences between the groups. At 3 h after the race, the UA levels gradually decreased and were restored to near the baseline values within 24 h after the race.
Table 2. Mean values of serum lactate and UA before and after racing.
|
Placebo (N = 6) |
H2 (N = 7) |
|||
|---|---|---|---|---|
| Lactate (mmol/L) | p value | Lactate (mmol/L) | p value | |
| Baseline | 0.52 ± 0.16 | — | 0.43 ± 0.21 | — |
| Immediately post-racing | 15.0 ± 2.22 | <0.0001* | 16.4 ± 2.61 | <0.0001* |
| 1 h | 7.38 ± 1.27 | <0.0001* | 7.89 ± 2.97 | <0.0001* |
| 3 h | 0.88 ± 0.12 | 0.942 | 0.84 ± 0.20 | 0.9742 |
| UA (mg/dL) | p value | UA (mg/dL) | p value | |
| Baseline | 0.17 ± 0.05 | — | 0.20 ± 0.06 | — |
| Immediately post-racing | 1.10 ± 0.29 | 0.1151 | 1.06 ± 0.36 | 0.0095* |
| 1 h | 3.50 ± 1.42 | <0.0001* | 3.53 ± 0.86 | <0.0001* |
| 3 h | 0.83 ± 0.29 | 0.3476 | 0.81 ± 0.39 | 0.0872 |
| 24 h | 0.13 ± 0.01 | 0.999 | 0.14 ± 0.05 | 0.9983 |
Abbreviations: UA, uric acid.
Baseline indicates the values just prior to injection.
Values are presented as mean ± standard deviation.
*Significant increase vs. baseline.
Discussion
In this study, intravenous infusion of H2-saline showed significant antioxidant effects in Thoroughbred horses after high-intensity racing exercise, as demonstrated by the prevention of increases in the formation of 8-OHdG in the H2 group compared to in the placebo group. This result strongly indicates protective effects of H2 against exercise-induced ROS-mediated detrimental tissue damage in racing horses. However, it should be noted that another assay to measure oxidative stress, namely d-ROM, showed no significant differences between the two groups. We speculate that this discrepancy between the 8-OHdG and d-ROM assays is likely caused by the indirect and non-specific metabolites detected by d-ROMs. For example, superoxide and H2O2, two major ROS, are balanced by the corresponding scavengers, superoxide dismutase and catalase, respectively. The oxidative stress measured by d-ROM may reflect that of the ROS after they are reduced by their specific scavengers. On the other hand, serum 8-OHdG is a direct and reliable marker for elevated oxidative stress, as it reflects oxidised DNA. Further, while 8-OHdG reflects the intracellular oxidation or oxidants that reach the DNA, d-ROM may reflect the extracellular oxidants, which may be scavenged in the serum and may not reach the intracellular components, such as the mitochondrial or genomic DNA. Therefore, we believe that the results obtained by serum 8-OHdG are reliable and accurate.
On the other hand, although a tendency of an elevation of the antioxidant potential in the serum measured by the BAP test was observed immediately after the race in the H2-saline infused group, no significant differences in the elevation of antioxidant potential was observed in either group throughout the study. Recently, it was demonstrated that, in humans in rest position, intravenously infused H2 emerged relatively slowly from the skin, and that it took more than 30 min after administration for it to be excreted36. In the present study, although there is a possibility that the H2 molecules administered 2 h before the race may have been partially retained in the body of the horses during and after the race, the H2 would have been discharged or consumed early during the race due to the high intensity of horse races, and therefore, elevation of the BAP value may not have been observed throughout the race except for immediately after the race. Alternatively, the discrepancy between the BAP and 8-OHdG values seems to suggest that the serum BAP value does not reflect the intracellular antioxidant potential, unlike 8-OHdG. These results suggest that the intravenously administered H2 molecules have reached the muscles cells and sustained the anti-oxidant potential even after the race. However, there remain the possibilities that the H2 molecules in blood were insufficient to reach the muscle cells for the racing exercise and instead, other unknown mechanisms which had worked on the cells or molecules in the circulating blood, may have indirectly suppressed 8-OHdG in the muscle cells.
There are numerous previous studies regarding muscular damage due to anaerobic exercise37,38,39. In the present study, although the AST and LDH levels did not significantly change in either group, the CK level was elevated equally in both groups upon racing, indicating the occurrence of muscular damage during horse racing (Table 1). Additionally, these results also indicate that infused H2 did not completely protect the muscular cells from damage caused by the exhaustive racing exercise. In addition, neutrophils, which may infiltrate into the muscle tissue upon strenuous muscle contractions, can damage the muscle cells via nicotinamide adenine dinucleotide phosphate oxidase-generated superoxide, thus resulting in lysis of the muscular cells40,41. The finding that muscle cell damage was not influenced by the infused H2-saline, however, suggests that the occurrence of muscular damages upon exercise in the present study was independent from the exercise-induced ROS generation, as indicated by the suppression of the elevated oxidative stress in H2-infused horses. Apart from chemical stress, including ROS, the muscle cell membranes are thought to be exposed to mechanical stress due to strenuous loading and intensive contraction of the muscle fibres during high-intensity exercise14. When mechanical stress overwhelms the muscular resistance, the sarcomere units, which are assembled from myofibrils and connective fibrils and fixed by structural proteins such as actin, dystrophin, and titin, become overstretched and disrupted, resulting in membrane failure and leakage of the sarcoplasmic components. In the present study, we observed a significant influx of CK into the serum after the race, regardless of the pre-treatment. In this case, the muscle damage seems to originate from mechanical stress; in spite of the increased oxidative stress and absence of responsive antioxidant potentials, as indicated by the serum 8-OHdG and BAP test results, the recovery from muscular damage, demonstrated by the CK values, was completed within 24 h after the race in both groups. In other words, the muscle damage seemed to be transient, suggesting prompt recovery of the muscle membrane and absence of ischemic tissue necrosis.
The resistance to the prolonged muscle damages observed in this study regardless of the administration of H2 could be explained by the adaptive response to the habitual endurance training of the horses. It has been demonstrated that endurance training induces mitochondrial biogenesis, represented by muscle fibre switching, which can improve energy metabolism and increase the resistance to fatigue as well as enhance the antioxidant potential42,43,44. It should be noted that the Thoroughbred horses included in the present study undergo daily training; this endurance training is thought to induce, at least in part, metabolic and antioxidant changes of the cellular defence mechanisms, which are believed to be modulated by PGC-1α1. It has been reported that PGC-1α is up-regulated in Thoroughbred horses undergoing high-intensity training for 18 weeks45. In addition, it is known that the muscles of Thoroughbred horses have a high proportion of type IIa fibres, which is consistent with the responsiveness to daily training46. In this study, the muscle fibres, particularly the type IIa fibres, may have been accustomed by the habitual endurance training, thus resulting in resistance to the increase of d-ROMs upon racing and in rapid improvement of the CK levels after the race.
Highly intensive exercises, which exhaust the anaerobic energy supply, require aerobic generation of ATP. Bioenergy in such strenuous exercises, including horse racing and human sprint athletics, is therefore provided by mixed and interlinked metabolic pathways composed of both anaerobic and aerobic reactions47. The present data obtained from Thoroughbred horses clearly demonstrated the influences of anaerobic metabolism as well as the presence of muscular damages. In the present study, a rapid and significant increase of serum lactate was observed (Table 1); the serum lactate levels peaked immediately after the race, clearly demonstrating the breakdown of free ATP and phosphocreatine, followed by the activation of anaerobic glycolysis. The notion of depletion of the anaerobic supply of ATP during racing is also consistent with the subsequent peak observed in the serum UA at 1 h after the race in both groups, which indicated insufficient re-phosphorylation of AMP during the race. Although a significant increase of UA immediately after the race compared to the baseline was observed only in the H2 group, no difference in the manner of energy supply was observed between the placebo and H2 groups. It should be noted here that, under such exhaustive circumstances, in which ischemia and hypoxia in the muscle are also induced, xanthine oxidase, which is converted from xanthine dehydrogenase, could generate superoxide via conversion of hypoxanthine to xanthine, and of xanthine to UA48. Further, apart from electron leakage through the mitochondrial membrane, another source of ROS may have appeared and contributed to the continuous oxidative state observed in the horses, which in turn may have been scavenged in the H2 group. The elevations of the UA and lactate levels in both groups are suggestive of post-exercise fatigue8,13. Although H2 may not improve such primary symptoms, the suppression of oxidative stress suggests a reduction of the secondary deleterious effects of racing exercise.
Finally, as mentioned, the horses used in the present study seemed to be equipped with adaptive antioxidant responses in the serum, as demonstrated by the suppression of the elevation of d-ROMs after high-intensity, exhaustive racing in both groups (Fig. 1b). The inveterate oxidative stress associated with high-intensity racing may induce late-onset or masked inflammation, which in turn is associated with increased risks of injuries, morbidity, and early mortality; this prolonged inflammation is accompanied and modulated by the elevated ROS via coupling with pro-inflammatory cytokines and NF-κB positive feedback loops1,31. Importantly, it should be noted that, unlike in the placebo group, significant suppression of the serum 8-OHdG was observed in the H2 group even 24 h after the race (Fig. 1a). This finding suggests that the administration of H2 may be applicable to many other situations in which oxidative stress is excessive, regardless of the status of the antioxidant defence. The possibility that oral daily consumption of water containing 5–7 ppm of H2 may counteract such oxidative risks has been hypothesised31,49. In addition, as demonstrated herein, it seems important to prepare for unexpected or dangerous oxidative stress increases that may overwhelm the effects of hydrogen-enriched water. Our findings suggest that one potential solution is infusion of H2-saline, and recent progress in the treatment of rheumatoid arthritis in humans using H2-saline supports this conclusion50.
There were some limitations to this study. Most importantly, collecting real-life data from habitually trained horses before and after racing under serious, strenuous conditions is an extensive task, and therefore, the number of horses studied was relatively small (N = 13) and a cross-over study design was not possible. However, although the present study was focused on the acute oxidative or antioxidative responses of Thoroughbred horses after racing, and although the data were restricted to within 24 h of the race, the remarkable reduction of serum 8-OHdG observed in the H2 group indicates a preventable potential of pre-treatment with H2-saline against the prolonged and detrimental effects resulting from exhaustive exercise. To confirm our findings, the effects of H2-saline injections on anaerobic metabolism should be further investigated, both in large-scale animal studies and in humans, in the future.
Conclusions
Intravenous infusion of H2-saline significantly and integrally suppressed exercise-induced oxidative stress in Thoroughbred horses after exhaustive racing. Further, pre-exercise treatment also resulted in enhanced antioxidant potential. On the other hand, the transient muscle damages induced by the racing exercise, during which the anaerobic energy supply is depleted, were not affected by H2-saline injection. Taken together, the results of the present study clearly demonstrated that the direct injury caused by rapid and strong muscle contractions occurred independently from the generation of ROS. The antioxidant capabilities of H2 during exercise, in which inveterate oxidative stress significantly elevates the 8-OHdG levels, may aid the beneficial properties of exercise by enhancing the antioxidant potentials as well as the anti-inflammatory effects.
Methods
Subjects
Thirteen retired Thoroughbred racehorses, belonging to the Horseracing School of Japan Racing Association and regularly used by training jockeys, were randomly divided into a placebo group (N = 6; age: 4–11 years; 4 males, 2 females; body weight: 496 ± 35.1 kg) and H2 group (N = 7; age: 3–8 years; 4 males, 3 females; body weight: 487 ± 35.7 kg). There were no significant differences in the baseline characteristics between the two groups. All of the horses are trained 6 days a week, with each training sessions including 20 min of walking, 1,200 m of trotting, and 1,000 m of canter, followed by 2,000–3,000 m of gallop, and ending with cool down trotting and walking for at least 15 min.
Preparation of H2-saline
To prepare the H2-saline, 1 L of saline in a soft plastic bag was placed for 12 h in a bath to circulate the saline. An electrolysis instrument (hydrogen circulator; Ecomo International Co. Ltd., Iizuka, Fukuoka-city, Japan) was used to generate 1.6 ppm H2. Two bags were placed in the infusion apparatus (INFUSTAT, Meiyu Co. Ltd., Yachio-city, Japan) and the H2 concentration was confirmed by using the methylene blue-platinum colloid regent-based titration method51. Just before the infusion, approximately 0.6 ppm of H2 was dissolved in the saline. Placebo saline was prepared in the same water bath without the addition of H2.
Study design
This study was approved by and conducted in accordance with the guideline of the Animal Welfare and Experiment Management Committee of Miho Training Center, Japan Racing Association. The horses took part in a simulation race in which they ran 1,000 m in a dirt track. Immediately after removing the soft bags from the hydrogen circulator, 2 L of H2-saline was infused into the jugular vein of each horse over 5 min, 2 h before the race. Blood samples were collected from the jugular vein just before the infusion (baseline), and immediately (7–10 min), 1 h, 3 h, and 24 h after racing. All blood samples were put on ice immediately after collection, and the serum was separated by centrifugation and frozen at −80 °C. The blood lactate concentrations were analysed immediately after collection. The jockeys were not informed whether the infusion contained H2 or not, and were instructed to ride the horses as if they were taking part in a real, regular race.
Biochemical analyses
To estimate the oxidative potentials in the serum samples, d-ROMs were measured as previously described34. This method is an indirect detection method of free radicals, including ROS, in which N,N-Diethyl-para-phenylenediamine reacting with hydrogen peroxide in the serum is measured by the change in the colour as the absorbance at 505 nm, using the Free Radical Elective Evaluator (WISMERLL Co. Ltd. Hongo, Tokyo, Japan). The results are presented as Carratelli units (U.CARR) based on the calculated absorption. Using the same apparatus, the BAP test was also performed to evaluate the reducing potential of the serum, in which the reduction of FeCl3 is detected as the disappearance of reddish colour35. Serum 8-OHdG, a marker for oxidative stress that reflects 8-hydroxyguanosine in the DNA, was measured using enzyme-linked immunosorbent assay (ELISA), as previously described52. The assay was performed using the highly sensitive ELISA kit for 8-OHdG (JaICA, NIKKEN SEIL Co., Ltd., Shizuoka, Japan). Serum CK, AST, LDH, and UA were measured at the Miho Training Center of the Japan Racing Association. Blood lactate was analysed using Lactate Pro2 (ARKRAY, Inc., Kyoto, Japan).
Statistical Analysis
The data are shown as the mean ± standard deviation. Dunnett’s test was used for analysis of transition data using continuous measurements and for the baseline pre-dose absolute value in each group. For these analyses, p values < 0.05 were considered significant. Relative changes between the H2 and placebo groups were compared using Student’s t-test, with the level of significance set at p < 0.0125 after Bonferroni correction. All statistical analyses were performed using JMP v.6.0.3a software (SAS Institute, Cary, NC).
Additional Information
How to cite this article: Yamazaki, M. et al. Intravenous infusion of H2-saline suppresses oxidative stress and elevates antioxidant potential in Thoroughbred horses after racing exercise. Sci. Rep. 5, 15514; doi: 10.1038/srep15514 (2015).
Acknowledgments
We wish to thank the staff of the Horse Racing School and Miho Training Center of the Japan Racing Association for expert technical assistance throughout the study. We thank S. Hirano for his advice. We are grateful to K. Kiyota and K. Fukuoka for their excellent support regarding the preparation and maintenance of the electrolysis instrument.
Footnotes
Author Contributions Both M.Y. and T.I. are corresponding authors and wrote the main manuscript text. K.K., M.K. and K.K. conducted the statistical analyses and prepared the data for all the figures and tables. All authors reviewed the manuscript.
References
- Eisele P. S. & Handschin C. Functional crosstalk of PGC-1 coactivators and inflammation in skeletal muscle pathophysiology. Semin. Immunopathol. 36, 27–53 (2014). [DOI] [PubMed] [Google Scholar]
- St-Pierre J. et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127, 397–408 (2006). [DOI] [PubMed] [Google Scholar]
- Arany Z. et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451, 1008–1012 (2008). [DOI] [PubMed] [Google Scholar]
- Wu Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999). [DOI] [PubMed] [Google Scholar]
- Ji L. L. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic. Biol. Med. 44, 142–152 (2008). [DOI] [PubMed] [Google Scholar]
- Pedersen B. K. & Febbraio M. A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406 (2008). [DOI] [PubMed] [Google Scholar]
- Keller P. et al. Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. FASEB J. 19, 1181–1183 (2005). [DOI] [PubMed] [Google Scholar]
- Westerblad H., Bruton J. D. & Katz A. Skeletal muscle: energy metabolism, fiber types, fatigue and adaptability. Exp. Cell Res. 316, 3093–3099 (2010). [DOI] [PubMed] [Google Scholar]
- Wu Z. et al. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc. Natl. Acad. Sci. USA 103, 14379–14384 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin B., Hellsten Westing Y. & Apple F. S. Biochemical mechanisms for oxygen free radical formation during exercise. Sports Med. 10, 236–254 (1990). [DOI] [PubMed] [Google Scholar]
- Kinnunen S. et al. Exercise-induced oxidative stress and muscle stress protein responses in trotters. Eur. J. Appl. Physiol. 93, 496–501 (2005). [DOI] [PubMed] [Google Scholar]
- Kinnunen S. et al. Oxygen radical absorbance capacity (ORAC) and exercise-induced oxidative stress in trotters. Eur. J. Appl. Physiol. 95, 550–556 (2005). [DOI] [PubMed] [Google Scholar]
- Powers S. K. & Jackson M. J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 88, 1243–1276 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaccio P., Lippi G. & Maffulli N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 48, 757–767 (2010). [DOI] [PubMed] [Google Scholar]
- Harris P. A., Snow D. H., Greet T. R. & Rossdale P. D. Some factors influencing plasma AST/CK activities in thoroughbred racehorses. Equine Vet. J. Suppl. 9, 66–71 (1990). [DOI] [PubMed] [Google Scholar]
- Totsuka M., Nakaji S., Suzuki K., Sugawara K. & Sato K. Break point of serum creatine kinase release after endurance exercise. J. Appl. Physiol. (1985) 93, 1280–1286 (2002). [DOI] [PubMed] [Google Scholar]
- Bloomer R. J. & Goldfarb A. H. Anaerobic exercise and oxidative stress: a review. Can. J. Appl. Physiol. 29, 245–263 (2004). [DOI] [PubMed] [Google Scholar]
- Chan K. M. & Decker E. A. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 34, 403–426 (1994). [DOI] [PubMed] [Google Scholar]
- Newham D. J., Jones D. A., Tolfree S. E. & Edwards R. H. Skeletal muscle damage: a study of isotope uptake, enzyme efflux and pain after stepping. Eur. J. Appl. Physiol. Occup. Physiol. 55, 106–112 (1986). [DOI] [PubMed] [Google Scholar]
- Sewell D. A. & Harris R. C. Adenine nucleotide degradation in the thoroughbred horse with increasing exercise duration. Eur. J. Appl. Physiol. Occup. Physiol. 65, 271–277 (1992). [DOI] [PubMed] [Google Scholar]
- Harris D. B., Harris R. C., Wilson A. M. & Goodship A. ATP loss with exercise in muscle fibres of the gluteus medius of the thoroughbred horse. Res. Vet. Sci. 63, 231–237 (2007). [DOI] [PubMed] [Google Scholar]
- Starling R. D. et al. Effect of inosine supplementation on aerobic and anaerobic cycling performance. Med. Sci. Sports Exerc. 28, 1193–1198 (1996). [DOI] [PubMed] [Google Scholar]
- Mills P. C., Smith N. C., Harris R. C. & Harris P. Effect of allopurinol on the formation of reactive oxygen species during intense exercise in the horse. Res. Vet. Sci 62, 11–16 (1997). [DOI] [PubMed] [Google Scholar]
- Harris R. C., Marlin D. J. & Snow D. H. Metabolic response to maximal exercise of 800 and 2,000 m in the thoroughbred horse. J. Appl. Physiol. (1985) 63, 12–19 (1987). [DOI] [PubMed] [Google Scholar]
- Bouzid M. A., Hammouda O., Matran R., Robin S. & Fabre C. Changes in oxidative stress markers and biological markers of muscle injury with aging at rest and in response to an exhaustive exercise. PloS One 9, e90420 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbré F., Gratas-Delamarche A., Gómez-Cabrera M. C. & Viña J. Inactivity-induced oxidative stress: a central role in age-related sarcopenia? Eur. J. Sport Sci. 14 Suppl 1, S98–108 (2014). [DOI] [PubMed] [Google Scholar]
- Meng S. J. & Yu L. J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 11, 1509–1526 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan J. S. & Reid M. B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 35, 411–429 (2007). [DOI] [PubMed] [Google Scholar]
- Ohno K., Ito M., Ichihara M. & Ito M. Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. Oxid. Med. Cell Longev. 2012, 353152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T. et al. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: an open-label pilot study. Med. Gas Res. 2, 27 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T. Molecular hydrogen: new antioxidant and anti-inflammatory therapy for rheumatoid arthritis and related diseases. Curr. Pharm. Des. 19, 6375–6381 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita R. et al. Effect of molecular hydrogen saturated alkaline electrolyzed water on disuse muscle atrophy in gastrocnemius muscle. J. Physiol. Anthropol. 30, 195–201 (2011). [DOI] [PubMed] [Google Scholar]
- Tsubone H. et al. Effect of treadmill exercise and hydrogen-rich water intake on serum oxidative and anti-oxidative metabolites in serum of thoroughbred horses. J. Equine Sci. 24, 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti R., Carratelli M. & Barbieri M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 44, 37–40 (2002). [PubMed] [Google Scholar]
- Carratelli M. et al. Reactive oxygen metabolites and prooxidant status in children with Down’s syndrome. Int. J. Clin. Pharmacol. Res. 21, 79–84 (2001). [PubMed] [Google Scholar]
- Ono H. et al. Hydrogen (H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers. Med. Gas Res. 2, 14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammouda O. et al. Diurnal variations of plasma homocysteine, total antioxidant status, and biological markers of muscle injury during repeated sprint: effect on performance and muscle fatigue—a pilot study. Chronobiol. Int. 28, 958–967 (2011). [DOI] [PubMed] [Google Scholar]
- Shi M. et al. Effects of anaerobic exercise and aerobic exercise on biomarkers of oxidative stress. Environ. Health Prev. Med. 12, 202–208 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer R. J., Goldfarb A. H., Wideman L., McKenzie M. J. & Consitt. L. A. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J. Strength Cond. Res. 19, 276–285 (2005). [DOI] [PubMed] [Google Scholar]
- Nguyen H. X. & Tidball J. G. Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro. J. Physiol. 547, 125–132 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball J. G. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R345–353 (2005). [DOI] [PubMed] [Google Scholar]
- Eivers S. S. et al. PGC-1α encoded by the PPARGC1A gene regulates oxidative energy metabolism in equine skeletal muscle during exercise. Anim. Gen. 43, 153–162 (2012). [DOI] [PubMed] [Google Scholar]
- Summermatter S. et al. Remodeling of calcium handling in skeletal muscle through PGC-1alpha: impact on force, fatigability, and fiber type. Am. J. Physiol. Cell Physiol. 302, C88–99 (2012). [DOI] [PubMed] [Google Scholar]
- Handschin C. & Spiegelman B. M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 454, 463–469 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka Y. et al. Effects of high-intensity training on lipid metabolism in Thoroughbreds. Am. J. Vet. Res. 73, 1813–1818 (2012). [DOI] [PubMed] [Google Scholar]
- Kawai M. et al. Muscle fiber population and biochemical properties of whole body muscles in Thoroughbred horses. Anat. Rec. (Hoboken) 292, 1663–1669 (2009). [DOI] [PubMed] [Google Scholar]
- Morales-Alamo D. & Calbet J. A. Free radicals and sprint exercise in humans. Free Radic. Res. 48, 30–42 (2014). [DOI] [PubMed] [Google Scholar]
- Nishino T. et al. Conversion of xanthine dehydrogenase into oxidase and its role in reperfusion injury. Biochem. Soc. Trans. 25, 783–786 (1997). [DOI] [PubMed] [Google Scholar]
- Sakai T. et al. Consumption of water containing over 3.5 mg of dissolved hydrogen could improve vascular endothelial function. Vasc. Health Risk Manag. 10, 591–597 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T. et al. Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: A randomized, double-blind, placebo-controlled pilot study. Int. Immunopharmacol. 21, 468–473 (2014). [DOI] [PubMed] [Google Scholar]
- Seo T., Kurokawa R. & Sato B. A convenient method for determining the concentration of hydrogen in water: use of methylene blue with colloidal platinum. Med. Gas Res. 2, 1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito. S. et al. Quantitative determination of urinary 8-hydroxydeoxyguanosine (8-OH-dg) by using ELISA. Res. Commun. Mol. Pathol. Pharmacol. 107, 39–44 (2007). [PubMed] [Google Scholar]