Abstract
Collembolans are common soil arthropods that may be exposed to insecticidal proteins produced in genetically engineered (GE) plants by ingestion of crop residues or root exudates. In the present study, a dietary exposure assay was validated and used to assess the lethal and sublethal effects of two Bacillus thuringiensis (Bt) insecticidal proteins, Cry1C and Cry2A, on Folsomia candida. Using the insecticidal compounds potassium arsenate (PA), protease inhibitor (E-64), and Galanthus nivalis agglutinin (GNA) mixed into Baker’s yeast, we show that the assay used can detect adverse effects on F. candida. Survival and development were significantly reduced when F. candida was fed a diet containing PA, E-64, and GNA at 9, 75, and 100 μg/g diet, respectively, but not when fed a diet containing 300 μg/g Cry1C or 600 μg/g Cry2A. The activities of test antioxidant-, detoxification-, and digestion-related enzymes in F. candida were unaltered by a diet containing 300 μg/g Cry1C or 600 μg/g Cry2A, but were significantly increased by a diet containing 75 μg/g E-64. The results confirm that Cry1C and Cry2A are not toxic to F. candida at concentrations that are much higher than those encountered under field conditions.
Since the appearance of genetically engineered (GE) crops, their potential to cause adverse effects on the environment has drawn much attention. This concern has triggered a regulation requiring that novel GE crop varieties are subjected to rigorous environmental risk assessment before commercialization. One of the risks associated with the planting of GE crops is their potential to harm valued non-target organisms. This is especially relevant for insect-resistant genetically engineered (IRGE) plants including plants expressing cry genes derived from various subspecies of Bacillus thuringiensis (Bt).
When Bt crops are grown, the Cry proteins produced by these plants can enter the soil system as a consequence of rhizodeposition before harvest or plant residue decomposition after harvest1,2,3. For example, Li et al. monitored the degradation of Cry1Ac in rice stalks after harvest and found that Cry1Ac protein concentration decreased rapidly to 50% during the first month but then more slowly so that the residue rice stalks still contained 21% of the original Cry1Ac concentration after 7 months2. A number of studies have indicated that Bt proteins released from plant residues or secreted from the roots can be readily adsorbed and bound to soil particles and thus persist in soil4,5 and maintain their insecticidal activity5. Thus, soil organisms have the potential to be exposed to plant-produced Bt proteins, therefore assessing the impact of Bt proteins or Bt plant tissue is part of the non-target risk assessment of GE crops6,7.
Collembolans, such as Folsomia candida (Isotomidae), are commonly found in plant rhizospheres, including those of IRGE rice plants, where they can be exposed to transgene-derived proteins exuded from roots. Furthermore, collembolans play a role in the decomposition of organic matter and may therefore be exposed to transgene-derived proteins that remain in crop residues. Because of their contribution to the decomposition of plant litter and in the formation of soil microstructure, and because they are relatively sensitive to soil quality, collembolans are recognized as suitable indicator species of soil quality and health8,9. For example, collembolans have been used to assess the quality of polluted soils and of forest and agricultural soil as bio-indicators10,11,12.
The common soil collembolan F. candida can be easily maintained in the laboratory13,14 and is often used to assess the effect of chemical insecticides and insecticidal proteins as part of regulatory risk assessments7. Thus, the effects of GE plants on F. candida have been assessed under controlled laboratory conditions in many studies15,16,17,18. While most previous studies have used GE plant tissue or artificial soil treated with plant tissue in order to expose the test insects to the insecticidal proteins, in the current study we used baker’s yeast to expose F. candida to known concentrations of purified Cry proteins. Such dietary exposure assays (often referred to as Tier-1 assays) are regarded as more conservative (i.e., more likely to detect toxic effects) than assays in which test species are exposed to insecticidal compounds by feeding on GE plant tissue or are exposed in other ways19,20,21.
In the present study, we validate a dietary exposure assay for evaluating the potential effects of oral insecticidal compounds on F. candida. We then use the assay to assess the potential effects of Cry1C or Cry2A on F. candida survival and development because relatively little is known regarding the sensitivity of species belonging to the Collembola compared to other arthropod taxa22. In addition, the activity of antioxidant-, detoxification-, and digestion-related enzymes in F. candida was measured after the collembolan was fed Cry1C or Cry2A protein because changes in enzyme activities would indicate potential sublethal effects of the Bt proteins.
Results
Response of F. candida to PA, E-64, or GNA
Potassium arsenate (PA), the protease inhibitor E-64, and Galanthus nivalis agglutinin (GNA) were used to validate the test system because their toxicity to F. candida were established in a preliminary experiment. In the pure diet control treatment, the survival rate of F. candida after 28 days of feeding was >85%. With increasing concentrations of PA, E-64, or GNA in the diet, the survival rate of F. candida steadily declined (Fig. 1). Relative to survival on the control diet, survival was significantly reduced on diets containing PA at ≥9.0 μg/g FW diet, E-64 at ≥75 μg/g FW diet, or GNA at ≥100 μg/g FW diet (Dunnett’s test: all P < 0.03) (Fig. 1).
Figure 1. Survival of Folsomia candida when fed artificial diets containing different concentrations of PA, E-64, or GNA for 28 d.
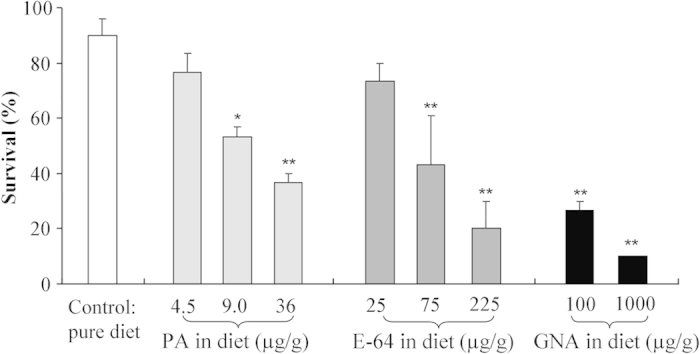
Values are means + SE, n = 3. Asterisks indicate significant differences between the toxin treatment and the control (*P < 0.05, **P < 0.01).
Independent from the treatment, F. candida increased in size during the duration of the experiment (till day 28) (Table 1). However, growth was significantly reduced in the PA, E-64and GNA treatments when compared to the untreated control. When compared to the control, the body length of F. candida was significantly reduced by day 7 at the highest concentration of PA (36 μg/g FW diet), while at day 14, 21, and 28, significant reductions were caused by PA at 9.0 μg/g FW diet (P < 0.05). The head width of F. candida was significantly reduced by PA at ≥9.0 μg/g FW diet from day 7 onwards (all P < 0.01). For the diets containing E-64, significant reductions in body length were recorded with 75 μg/g FW diet from day 21 onwards and from day 7 onwards at the highest concentration of 225 μg/g FW diet (P < 0.05). For diets containing GNA, 100 μg/g FW diet significantly reduced both body length and head width by day 7 (Table 1).
Table 1. Body length and head width of Folsomia candida when fed artificial diets containing different concentrations of PA, E-64, or GNA for 7, 14, 21, or 28 days. Values are means ± SE, n = 3.
| Treatment | Body length (mm) |
Head width (mm) |
||||||
|---|---|---|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | Day 28 | Day 7 | Day 14 | Day 21 | Day 28 | |
| Control: pure diet | 0.901 ± 0.008 | 1.169 ± 0.024 | 1.272 ± 0.054 | 1.329 ± 0.036 | 0.231 ± 0.005 | 0.241 ± 0.005 | 0.251 ± 0.004 | 0.272 ± 0.007 |
| PA 4.5 μg/g diet | 0.897 ± 0.029 | 1.108 ± 0.031 | 1.224 ± 0.025 | 1.283 ± 0.016 | 0.229 ± 0.008 | 0.235 ± 0.004 | 0.247 ± 0.003 | 0.265 ± 0.008 |
| PA 9.0 μg/g diet | 0.823 ± 0.020 | 1.029 ± 0.040* | 1.084 ± 0.017** | 1.085 ± 0.029** | 0.196 ± 0.003** | 0.196 ± 0.005** | 0.215 ± 0.003** | 0.221 ± 0.007** |
| PA 36 μg/g diet | 0.798 ± 0.015* | 0.960 ± 0.012** | – | – | 0.186 ± 0.005** | 0.197 ± 0.004** | – | – |
| E-64 25 μg/g diet | 0.924 ± 0.032 | 1.135 ± 0.022 | 1.191 ± 0.012 | 1.257 ± 0.027 | 0.228 ± 0.002 | 0.237 ± 0.008 | 0.250 ± 0.002 | 0.261 ± 0.005 |
| E-64 75 μg/g diet | 0.832 ± 0.006 | 1.108 ± 0.023 | 1.078 ± 0.046* | 1.156 ± 0.013** | 0.200 ± 0.003* | 0.211 ± 0.005** | 0.214 ± 0.006** | 0.216 ± 0.009** |
| E-64 225 μg/g diet | 0.791 ± 0.005** | 0.917 ± 0.039** | – | – | 0.187 ± 0.005** | 0.206 ± 0.002** | – | – |
| GNA 100 μg/g diet | 0.836 ± 0.006** | 1.074 ± 0.011* | 1.096 ± 0.023* | – | 0.194 ± 0.004** | 0.205 ± 0.006** | – | – |
| GNA 1000 μg/g diet | 0.805 ± 0.012** | 1.015 ± 0.007** | 1.019 ± 0.038** | – | 0.187 ± 0.003** | 0.199 ± 0.004** | – | – |
“–” indicates that either no insects remained alive or that the number of alive individuals was insufficient for measurement.
Each insecticidal protein treatments was statistically compared with the control at each sampling date. Asterisks indicate significant differences between the toxin treatment and the control (*P < 0.05, **P < 0.01).
Toxicity of Cry proteins to F. candida
Effects on life-table parameters
The survival rates of F. candida were >85% when fed a pure diet or a diet containing Cry1C or Cry2A at 300 or 600 μg/g diet, respectively, and pair-wise comparisons revealed no significant difference between survival with Cry1C or Cry2A and the untreated control (χ2 = 0.00, P = 0.984 for Cry1C; χ2 = 0.08, P = 0.774 for Cry2A) (Fig. 2). In contrast, F. candida survival was significantly reduced by feeding on a diet containing E-64 at 75 μg/g diet (χ2 = 31.526, P < 0.001). Similarly, F. candida body length and head width were not affected by Cry1C and Cry2A (Dunnett’s test; all P > 0.1) but were significantly reduced by E-64 feeding when compared to the control (P < 0.05) (Table 2). In addition, the mean number of offspring produced per insect during the 28 days of feeding was not significantly affected by Cry1C or Cry2A (Dunnett’s test; P > 0.05) (Fig. 3) but was significantly reduced by E-64 (P < 0.001).
Figure 2. Survival of Folsomia candida when fed a pure artificial diet (negative control) or diets containing Cry1C, Cry2A, or E-64 (positive control).
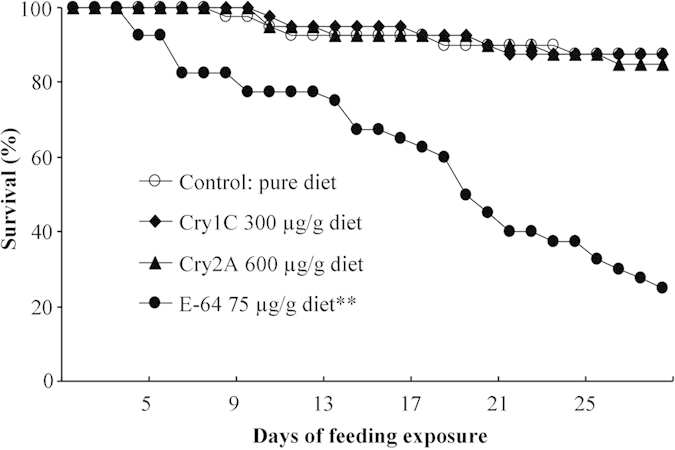
Asterisks indicate a significant difference between the treatment and the negative control (P < 0.01) (n = 40).
Table 2. Body length and head width of Folsomia candida when fed artificial diets containing Cry1C, Cry2A, or E-64 protein for 7, 14, 21 or 28 days. Values are means ± SE, n = 40.
| Treatment | Body length (mm) |
Head width (mm) |
||||||
|---|---|---|---|---|---|---|---|---|
| 7 day | 14 day | 21 day | 28 day | 7 day | 14 day | 21 day | 28 day | |
| Control: pure diet | 0.906 ± 0.012 | 1.124 ± 0.019 | 1.300 ± 0.015 | 1.410±0.014 | 0.226 ± 0.002 | 0.243 ± 0.003 | 0.255 ± 0.003 | 0.275 ± 0.004 |
| Cry1C 300 μg/g diet | 0.883 ± 0.014 | 1.100 ± 0.016 | 1.282 ± 0.015 | 1.394±0.017 | 0.217 ± 0.003 | 0.239 ± 0.003 | 0.258 ± 0.004 | 0.270 ± 0.004 |
| Cry2A 600 μg/g diet | 0.870 ± 0.011 | 1.120 ± 0.013 | 1.258 ± 0.016 | 1.371±0.019 | 0.220 ± 0.003 | 0.237 ± 0.002 | 0.256 ± 0.002 | 0.273 ± 0.003 |
| E-64 75 μg/g diet | 0.849 ± 0.015* | 1.110 ± 0.014 | 1.195 ± 0.012** | − | 0.207 ± 0.003** | 0.225 ± 0.003** | 0.241 ± 0.003* | − |
Statistical comparisons were made for each of the insecticidal protein with the control. Asterisks indicate significant differences between the toxin treatment and the control (*P < 0.05, **P < 0.01).
Figure 3. Numbers of offspring produced per Folsomia candida when fed a pure artificial diet (negative control) or diets containing Cry1C, Cry2A, or E-64 (positive control).
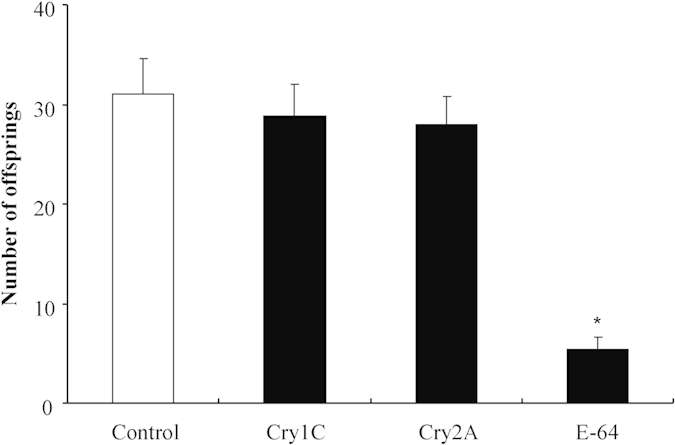
Values are means + SE, n = 40. Asterisks indicate a significant difference between the treatment and the negative control (P < 0.05).
Uptake of Cry proteins by F. candida
ELISA measurements showed that all F. candida fed with Cry protein-incorporated diets contained Cry proteins. The mean concentrations (±SE, n = 3) of Cry1C detected in F. candida on days 14, 21, and 28 were 12.0 ± 2.0, 12 ± 1.0, and 28.0 ± 2.0 ng/g dry weight (DW), respectively, and changed significantly over time (RM-ANOVA, F = 35.8, df = 2, P = 0.003). The mean concentrations of Cry2A detected in F. candida on days 14, 21, and 28 were 19.0 ± 5.0, 9.0 ± 1.0, and 7.0 ± 1.0 μg/g DW, respectively. The Cry2A concentration decreased over time but this was not significant (F = 8.7, df = 2, P = 0.064). No Cry protein was detected in F. candida fed on untreated control diet.
Stability and bioactivity of Cry proteins
Based on ELISA, the quantity of the two Cry proteins detected in the yeast powder diet supplemented with the proteins ranged from 66 to 88% of the nominal concentrations. The mean (±SE) concentration detected in the freshly prepared diet was 214.13 ± 10.32 μg/g FW for Cry1C (300 μg/g had been added to the diet) and 514.70 ± 9.66 μg/g FW for Cry2A (600 μg/g had been added to the diet). After 2 days of feeding exposure, the mean Cry protein concentration in the diet had declined by 10.9 to 17.0%. This decline was significant for Cry2A (442.9 ± 13.9 μg/g; Student’s t-test; t = 4.25, df = 4, P = 0.01) but not for Cry1C (186.9 ± 6.80 μg/g; t = 2.21, df = 4, P = 0.09).
Sensitive-insect bioassays were conducted to measure the bioactivity of Cry proteins in the yeast powder diet. The results showed that the mortality of Chilo suppressalis larvae was 0% when the larvae were fed a diet that contained the extract from the untreated yeast diet for 7 days. When C. suppressalis larvae were fed diets containing the extracts from freshly prepared Cry-containing yeast diets, the mortalities were 96.7% and 73.3% for Cry1C and Cry2A treatments, respectively; these values were not significantly different from those obtained with extracts from Cry-containing diets that had been exposed to F. candida for 2 days (Chi-square test; U = 2.965, df = 1, P = 0.195 for Cry1C; U = 0.32, df = 1, P = 0.779 for Cry2A).
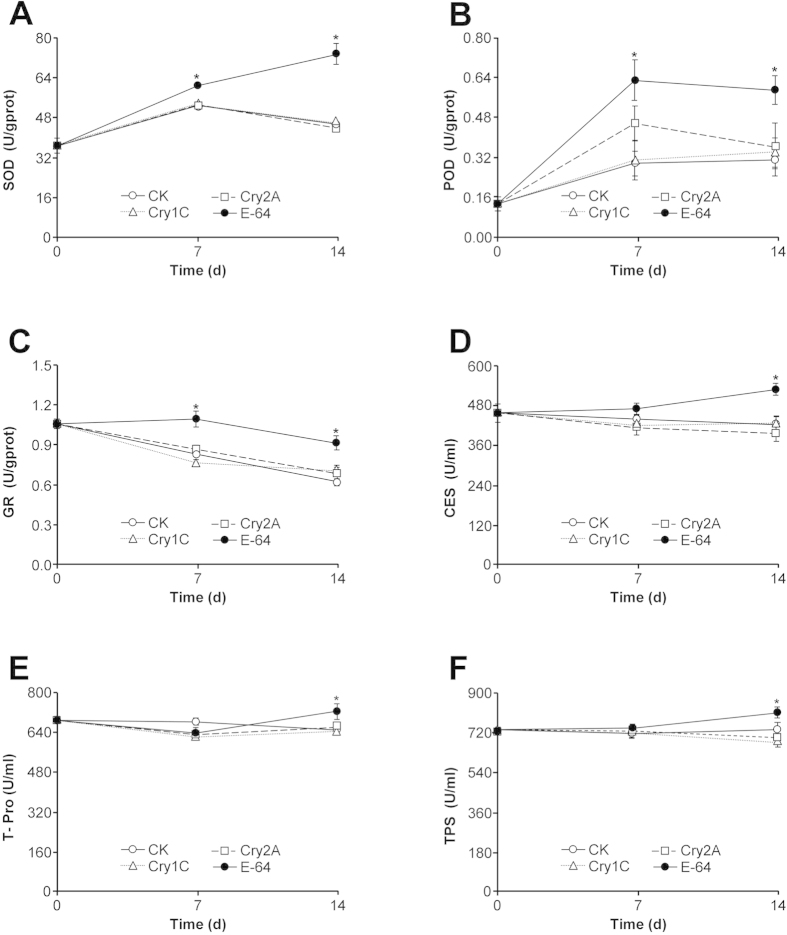
Enzyme activities in F. candida
We measured the activity of six enzymes in F. candida and none were shown to be significantly different in individuals fed diets containing Cry1C or Cry2A compared with individuals fed untreated diet (Dunett’s test; P > 0.05) (Fig. 4). In contrast, the activity of all six enzymes was significantly increased in F. candida when the diet contained E-64. For SOD, POD, and GR, the increase was significant on day 7 and day 14 (SOD: P < 0.001 on day 7 and day 14; POD: P = 0.006 on day 7 and P = 0.028 on day 14; GR: P < 0.001 on day 7 and day 14) (Fig. 4A–C). For CES, T-Pro or TPS, the activity was not significantly increased on day 7 (P > 0.05) but was significantly increased on day 14 (CES: P = 0.006; T-Pro: P = 0.029; TPS: P = 0.02) (Fig. 4D–F).
Figure 4. Enzyme activities (U) in Folsomia candida when the collembolan was fed a pure artificial diet (negative control) or diets containing Cry1C, Cry2A, or E-64 (positive control) for 0, 7, or 14 days.
The following enzymes were analysed: (A) superoxide dismutase (SOD); (B) peroxidase (POD); (C) glutathione reductase (GR); (D)carboxylesterase (CES); (E) total proteases (T-Pro); (F) tryptase (TPS). Values are means + SE, n = 10. Asterisks indicate a significant difference between the treatment and the negative control (P < 0.05).
Discussion
Evaluation of the potential negative effects of an IRGE crop on non-target arthropods is an important part of the environmental risk assessment that is performed before a GE crop can be commercialized6. A typical hypothesis to be tested is that the plant-produced insecticidal compound does not harm the valued non-target species at concentrations present in the field, and a tiered framework has been recommended and widely accepted for such assessments6,23,24. The assessments typically begin with laboratory experiments, namely Tier-1 assays, whose main objective is to test the potential hazard of insecticidal proteins such as Bt proteins produced by IRGE plants on the surrogate species under controlled, worst-case exposure conditions20,21,24.
Ideally, a suitable artificial diet is available and can serve as a carrier for delivering the insecticidal proteins to the test organism20,21. In the current study, yeast powder was used as a diet for F. candida. When F. candida was fed with this diet, its survival was >85% during the 28-day feeding period, which fulfilled the criteria of such assays20. The test Cry proteins and the positive control compounds could be easily and uniformly mixed with the yeast powder, indicating that this diet is suitable for delivering test compounds to F. candida. The compounds E-64, GNA, and PA generated dose-dependent declines in F. candida survival and growth (measured as body length and head width) when compared to untreated control diet. This indicated that the experimental system was capable of detecting dietary effects caused by gut-active insecticidal compounds and that the compounds could serve as positive controls in subsequent bioassays. E-64 was selected as a positive control compound for the subsequent assays because it is less expensive than GNA and because PA may also cause some contact toxicity.
Once the new bioassay was found to be useful for detecting toxicity, the toxicity of purified Cry1C and Cry2A to F. candida was evaluated. The two proteins were selected because the transgenic rice line T1C-19 expresses cry1C and the transgenic rice line T2A-1 expresses cry2A; these lines were recently developed in China and exhibit high resistance to the target lepidopteran pests25. Our results indicate that the two Cry proteins have no adverse effects on the fitness of F. candida, even though the concentrations used were significantly higher than the highest concentrations of the Cry proteins contained in the tissues of the two Bt rice lines25. A diet containing E-64 significantly reduced survival, development, and reproduction of F. candida. This positive control suggests that the test proteins were actually ingested and again demonstrates that our experimental system was able to detect adverse effects caused by toxic compounds.
That the Cry proteins were ingested by F. candida in our assay was confirmed by ELISA. ELISA also indicated that >80% of the Cry proteins were still detectable in the diet after the 2-day feeding exposure. Furthermore, the bioactivity of the Cry proteins in the yeast diet was confirmed by a bioassay with larvae of the Bt protein-sensitive C. suppressalis. These results demonstrate that F. candida larvae were exposed to bioactive Cry1C and Cry2A protein in our feeding study. The results reinforce the conclusion that the species is not sensitive to the two Cry proteins. While Sims & Martin have already reported a lack of toxicity of Cry2A to F. candida26, we are not aware of any study that has assessed the impact of Cry1C. Similar dietary studies have been conducted for other beneficial arthropods, such as the green lacewing Chrysoperla sinica (Neuroptera: Chrysopidae), the ladybird beetle Propylea japonica (Coleoptera: Coccinellidae), and the honeybee Apis mellifera (Hymenoptera: Apidae); none of these studies reported any effect of feeding on a diet containing Cry1C or Cry2A on insects outside the order of Lepidoptera27,28,29,30.
To determine whether the tested Cry proteins might have sublethal effects on F. candida, we measured the activities of antioxidant enzymes (SOD and POD), detoxification enzymes (GR and CES), and the proteases (T-Pro and TPS) after the collembolan had fed on a diet containing Cry1C, Cry2A, or E-64 for 7 and 14 days. SOD converts O2 to H2O2 through dismutation, and H2O2 is subsequently turned into H2O by POD; this series of reactions reduces or eliminates the damage that O2 can cause to membranes31. CES hydrolyzes ester, amide, and carbamate bonds and is important in pesticide and lipid metabolism32,33,34. GR is a flavoprotein that catalyzes the NADPH-dependent reduction of glutathione disulfide (GSSG) to glutathione (GSH); the reaction is essential for the maintenance of glutathione levels. Glutathione has a major role as a reductant in oxidation–reduction processes, serving in detoxification and several other important cellular functions35. T-Pro is widely used as an indicator of an insect’s adaptation to food; TPS has been suggested to be a key mediator in insects for resistance evolution to Bt insecticidal proteins36. The activities of such enzymes have been widely used as indicators for adverse effects caused by stomach poisons in arthropods17,37,38,39. The results from our study show that the activities of the test enzymes in F. candida were not affected by feeding on Cry1C or Cry2A. The results agree with those reported from previous studies. For example, Yuan et al. reported that the activity of antioxidant enzymes including SOD and POD in F. candida was not affected when the collembolan was fed yeast mixed with Cry1Ab and Cry1Ac proteins39. Bai et al. also found that the SOD activity was not significantly altered in F. candida after the collembolan had fed on Cry1Ab-containing rice tissue for 35 days17. To our knowledge, the current study is the first to measure the activities of the detoxification enzymes GR and CES and the proteases T-Pro and TPS in F. candida as a response to Cry protein uptake. Whereas the activities of the test enzymes in the current study were unaffected by the addition of Cry1C or Cry2A to the diet, the activities of all tested enzymes were significantly increased when F. candida was fed a diet containing E-64, which was toxic to the collembolan in the previous assays.
Because of their ecological importance as decomposers of plant litter in soil, collembolans in general and F. candida in particular have received much attention in the environmental risk assessment of IRGE crops. In most previous studies, F. candida was exposed to the Cry proteins by providing them with tissue from transgenic plants or with a mixture of plant tissue and soil. While most of these studies did not detect any adverse effects when F. candida fed on Bt plant tissue vs. non-Bt plant tissue17,18,39,40,41, negative effects were detected in a few studies. For example, F. candida produced significantly fewer fecal pellets when fed Bt (Cry1Ab) maize tissue rather than non-Bt maize tissue42,43. In such cases, however, it is not possible to determine whether the negative effects were caused by the Cry proteins or by other differences in the composition of the Bt vs. the non-Bt plants. In addition, plant material is less suitable than yeast as a food for F. candida44. By using the dietary exposure assay developed in the current study, we were able to measure the direct toxicity of Cry proteins to F. candida without confounding differences in plant tissue composition or other factors. In addition, the concentrations of the test compounds can be adjusted in the new assay, and the test species can be exposed to much higher concentrations of the test compounds than would be encountered in the field under realistic conditions which adds certainty to the conclusion of no effects6,19,20,21.
In summary, toxicological and biochemical techniques were used to assess the potential toxicity of Cry1C and Cry2A proteins to F. candida, and the results demonstrated that F. candida is insensitive to both Cry proteins. The results were consistent with previous reports that purified Bt proteins Cry1Ab, Cry1Ac, Cry2A, and Cry3A are not toxic to F. candida26,39,45. More importantly, the study describes a dietary exposure system that can be used to assess the direct toxic effects of orally active insecticidal compounds on F. candida.
Materials and Methods
Test organism
Folsomia candida was obtained from the Shanghai Institute for Biological Sciences, Chinese Academy of Sciences. Insects were cultured in Petri dishes (diameter 90 mm; height 10 mm) filled with a solidified mixture of plaster of Paris, activated charcoal, and distilled water at a ratio of 9 : 1 : 10 (w : w : w) and with a height of 3–5 mm (hereafter referred to as plaster-based Petri dishes). The plaster of Paris and activated charcoal base were kept moist by regularly adding distilled water, which resulted in a relative humidity close to 100% in the Petri dishes. Baker’s yeast (AB MAURI, Heben Mauri Foods Co., Ltd., Zhangjiakou, China) was provided as food for the insects and was renewed weekly to reduce fungal and bacterial contamination. The Petri dishes were kept in a dark growth chamber at 20 ± 1 °C. Insects used in the experiments were 10–12 days old.
Insecticidal compounds
Insecticidal compounds used in this study included lyophilized GNA, PA (KH2AsO4), protease inhibitor E-64 [(2S,3S)-trans-Epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester EST], and the Bt proteins Cry1C and Cry2A. GNA, PA, and E-64 were purchased from Sigma–Aldrich (St. Louis, MO), and the Bt proteins were purchased from Envirotest-China (agent for EnviroLogix Inc., Portland, Maine, USA; www.envirotest-china.com). The protoxins from Bt had been expressed as single-gene products in Escherichia coli (Cry1C) or in a cured Bt strain (Cry2A) at Case Western Reserve University (USA). The protoxin inclusion bodies were then dissolved and trypsinized, and then isolated and purified by ion exchange HPLC followed desalting and lyophilizing the pure fractions. The purity ranged from 94–96% (Marianne P. Carey, Case Western Reserve University, personal communication).
The bioactivity of the Cry proteins was confirmed by sensitive-insect, laboratory bioassays using neonate larvae of C. suppressalis that were fed an artificial diet containing a range of Cry protein concentrations for 7 days. The EC50 (toxin concentration resulting in 50% weight reduction compared to the control) was estimated to be 21.66 and 1302.60 ng/g for Cry1C and Cry2A, respectively (see Supplementary Information).
Artificial diet
Development of a robust and reliable dietary exposure assay requires an appropriate artificial diet to deliver the test compounds to the test organisms21. Baker’s yeast powder is an excellent food for F. candida44,46 and was used in the current study to deliver the test compounds. To incorporate Cry proteins, GNA, E-64, and PA into yeast powder, the compounds were first dissolved in distilled water at a defined concentration and then mixed with yeast powder at the ratio of 1 : 5 (w : w). The mixture was then lyophilized and ground into powder again. The diet was kept at −20°C until it was fed to F. candida.
Response of F. candida to PA, E-64, and GNA
Appropriate positive controls are necessary for a dietary exposure assay21. Bioassays were conducted to determine whether the yeast diet is appropriate for testing the toxicity of insecticidal compounds to F. candida and to clarify which of three compounds (GNA, PA, or E-64) is the most appropriate for use as the positive control in feeding assays with F. candida. Stock solutions of PA, E-64, and GNA were diluted with distilled water and incorporated into yeast powder as described in the previous section to obtain the following toxin concentrations: 4.5, 9, 18, and 36 μg/g fresh weight (FW) of diet for PA; 25, 75, and 225 μg/g FW of diet for E-64; and 100 and 1000 μg/g FW of diet for GNA. These concentrations were selected based on our preliminary experiments and the results from previous studies with other insect species47,48,49,50,51. Yeast powder treated with pure distilled water served as the negative control.
Adults of F. candida were placed in plaster-based Petri dishes and allowed to oviposit for 48 h before being removed. When the eggs hatched after approximately 7 days, the neonates were fed with untreated yeast powder for 11 days before they were used in the experiments. The age of the test organisms has been selected for two reasons: (i) According to the OECD test protocol52, it is recommended to use well fed F. candida at an age between 9 and 12 days; (ii) F. candida at this age have successfully been used to assess the impact of GM plant tissue or Cry proteins16,26. The insects were randomly selected and 10 individuals were kept in each plaster-based Petri dish, where they were fed with control diet or insecticide-treated diets as described earlier. The diets were replaced every 2 days. Each combination of insecticide and concentration was represented by three replicate, namely 3 Petri dishes with 10 insects per dish. Survival of the insects was recorded twice per day (9:00 am and 9:00 pm), and body length and head width were measured every 7 days. For measurement, pictures of the test organisms were taken by photomicroscope and subsequently body length and width were measured using a staff gauge. All living insects were measured. The experiment was terminated after 28 days. The bioassay was conducted in a climate chamber at 20 ± 1 °C with 70 ± 5% RH and a 12-h light/12-h dark cycle.
Toxicity of Cry proteins to F. candida
Effects on life-table parameters
The same experimental procedure that was used to assess the toxicity of PA, E-64, and GNA was used to assess the toxicity of Cry1C and Cry2A to F. candida. In contrast to the previous experiment, F. candida were individually kept in Petri dishes, and 40 insects were tested (40 replicates) in each treatment. The following four dietary treatments were tested: i) diet containing Cry1C protein; ii) diet containing Cry2A protein; iii) diet containing E-64 (positive control); and iv) diet containing no toxin (negative control). The nominal concentrations of Cry1C, Cry2A, and E-64 proteins in diets were 300, 600, and 75 μg/g FW diet, respectively. The Cry1C and Cry2A protein concentrations used are >10-times greater than the mean concentrations in different tissues of Bt rice lines recently developed in China (Cry1C: 0.90-3.65 μg/g FW leaves of T1C-19b rice; Cry2A: 0.76-87μg/g FW leave of T2A-1 rice)25. The concentration of E-64 was selected based on the results from the bioassay described earlier in this paper. Diets were prepared 3 days before initiation of the experiment and were stored at −20 °C until used. Diets were renewed every 2 days to prevent the degradation of the test compounds.
Survival of F. candida was assessed twice per day (9:00 am and 9:00 pm). The body length and head width were measured every 7 days as described above. The experiment was terminated after 28 days, at which time all F. candida in each Petri dish, including larvae and unhatched eggs, were counted.
Uptake of Cry protein by F. candida
More than 1000 F. candida (10–12 days old) were fed with diet containing no toxin, 300 μg/g Cry1C, or 600 μg/g Cry2A for 28 days as described earlier. Insects were collected after 14, 21, and 28 days. Fifty to 60 individuals were collected for each sample, and three samples were collected at each sampling date resulting in a total of 27 samples. The insect samples were frozen at −80 °C for ELISA analyses (see below).
Stability and bioactivity of Cry proteins
The temporal stability and bioactivity of the Cry proteins in the artificial diets were measured in three subsamples (2–3 mg FW of diet per subsample) that were collected from fresh diets taken from the freezer and from diets that had been exposed to F. candida for 2 days. The Cry protein concentrations and bioactivities were determined by ELISA and by a ‘‘sensitive-insect’’ bioassay as described in the following sections.
ELISA analyses
The Cry protein concentrations in F. candida samples and in artificial diet were measured by double-antibody sandwich enzyme-linked immunosorbent assays (DAS-ELISA) using Cry1C and Cry2A detection kits purchased from Enviro-Logix (Portland, Maine, USA). Before the analyses, all insects were washed in phosphate-buffered saline Tween (PBST) to remove any Bt toxin from their outer surface. For Cry protein extraction, samples of insects or artificial diets were weighed and mixed with PBST at a ratio of at least 1:10 to 1:100 (mg of sample : μl of buffer) in 1.5-ml centrifuge tubes. The samples were then fully ground by hand using an electric grinding rod. After centrifugation and appropriate dilution of the supernatants, ELISA was performed according to the manufacturer’s instructions. The optical density (OD) values were read with a microplate spectrophotometer (PowerWave XS2, BioTek, USA). The concentrations of Cry1C and Cry2A were calculated by calibrating the OD values to a range of concentrations of standard Cry1C and Cry2A samples.
Sensitive-insect bioassay
Bt-susceptible C. suppressalis larvae were used as sensitive insects to verify the bioactivity of the Cry proteins in the yeast diets before and after the 2-days feeding exposure. The Cry proteins were firstly extracted from the yeast diet samples as described in the ELISA analyses section, and the supernatants were appropriately diluted before being incorporated into the artificial diet for C. suppressalis53. The C. suppressalis diet must be heated during preparation. To avoid the degradation of the Cry proteins during heating, the supernatants were mixed into the diet when the temperature had decreased to less than 60 °C. Once the diet was solid, it was cut into slices and individually placed in Petri dishes (90 mm diameter, 15 mm height). Neonates of C. suppressalis were individually transferred to the Petri dishes, which were subsequently sealed with Parafilm. Thirty replicates were tested for each treatment. After 7 days, the mortality of C. suppressalis larvae in each treatment was recorded.
Determination of enzyme activity
F. candida larvae (10 to 12 days old) were exposed to 300 μg Cry1C/g diet or 600 Cry2A μg/g diet for 0, 7, and 14 days using the same procedure described earlier. At each sampling date, 200 to 300 F. candida were collected and stored at −20 °C before the activities of the following enzymes were quantified: digestion-related enzymes (total protease, T-Pro) and tryptase, TPS); the antioxidant-related enzymes (superoxide dismutase, SOD and peroxidase, POD); and the detoxification-related enzymes (carboxylesterase, CES and glutathione reductase, GR). SOD, POD, and GR activities were measured with ELISA kits from Nanjing Jiancheng Ltd. Co. (Nanjing, China), and TPS, CES, and T-Pro activities were measured with ELISA kits from Beijing Luyuan Byrd Biological Technology Ltd., Co. (Beijing, China).
Insect samples were homogenized at 4 °C in physiological saline solution at a ratio of 1 : 9 (w : v). The homogenates were then centrifuged at 2500–3000 × g for 10 min at 4 °C, and the resulting supernatants were used for analysis of the enzyme activities following the manufacturer’s instructions. The optical density (OD) values were read with a microplate spectrophotometer (PowerWave XS2, BioTek, USA). The activities of the enzymes were calculated by calibrating the OD values to a range of concentrations of standards provided with the kits.
Data analysis
In all bioassays with F. candida, statistical comparisons were made between each treatment and the control (pure diet). Data on body length, head width, and the number of offspring were compared by Dunnett’s tests. The offspring data were transformed by log (x) to satisfy the assumptions of parametric analysis (normal distribution of residues and homogeneity of error variances). In the bioassays with PA, E-64, and GNA, the survival rates were analyzed by Dunnett’s tests after the data were transformed by SQRT(x + 1). The effect of Cry protein dietary treatments on F. candida survival was analyzed with the Kaplan-Meier procedure and Logrank test.
Cry protein concentrations in F. candida collected on different days during the feeding assay were analysed by repeated-measures ANOVA to test for changes in protein uptake over time. In addition, Student’s t-tests were used to compare Cry protein concentrations in the fresh diet vs. diet exposed to F. candida larvae for 2 days. Chi-square tests were used to compare the mortality of the C. suppressalis larvae that were fed with artificial diets containing the extracts from: untreated pure yeast diet; yeast diet containing fresh Cry; and yeast diet containing Cry that had been exposed to F. candida larvae for 2 days. The enzyme activities were compared between each toxin treatment and the pure diet control using Dunnett’s tests.
All statistical analyses were conducted using the software package SPSS (version 13 for windows, 2004).
Additional Information
How to cite this article: Yang, Y. et al. Toxicological and biochemical analyses demonstrate no toxic effect of Cry1C and Cry2A to Folsomia candida. Sci. Rep. 5, 15619; doi: 10.1038/srep15619 (2015).
Supplementary Material
Acknowledgments
We thank Yunxia Luan and Chengwang Huang (Shanghai Institute of Biological Science, Chinese Academy of Sciences) for providing the colony of Folsomia candida and the technique for rearing the insect. We acknowledge Bruce Jaffee for critical comments on an earlier version of the manuscript. The study was supported by the National GMO New Variety Breeding Program of PRC (2014ZX08011-02B) and (2014ZX08011-001).
Footnotes
Author Contributions Y.L. and Y.Y. designed the study. Y.Y. and X.C. performed all the experiments. Y.L., Y.Y., J.R. and X.C. analyzed data and wrote the manuscript. F.C., L.C. and Y.P. provided experimental materials. All authors have read and approved the manuscript for publication.
References
- Zwahlen C., Hilbeck A., Gugerli P. & Nentwig W. Degradation of the Cry1Ab protein within transgenic Bacillus thuringiensis corn tissue in the field. Mol. Ecol. 12, 765–775 (2003). [DOI] [PubMed] [Google Scholar]
- Li Y., Wu K., Zhang Y. & Yuan G. Degradation of Cry1Ac protein within transgenic Bacillus thuringiensis rice tissues under field and laboratory conditions. Environ. Entomol. 36, 1275–1282 (2007). [DOI] [PubMed] [Google Scholar]
- Zurbrügg C., Höhnemann L., Meissle M., Romeis J. & Nentwig W. Decomposition dynamics and structural plant components of genetically modified Bt maize leaves do not differ from conventional hybrids. Transgenic Res. 19, 257–267 (2010). [DOI] [PubMed] [Google Scholar]
- Saxena D., Florest S. & Stotzky G. Insecticidal toxin in root exudates from Bt corn. Nature 402, 480–481 (1999). [DOI] [PubMed] [Google Scholar]
- Tapp H. & Stotzky G. Insecticidal activity of the toxins from Bacillus thuringiensis subspecies kurstaki and tenebrionis adsorbed and bound on pure and soil clays. Appl. Environ. Microbiol. 61, 1786–1790 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis J. et al. Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nat. Biotechnol. 26, 203–208 (2008). [DOI] [PubMed] [Google Scholar]
- Romeis J. et al. Deriving criteria to select arthropod species for laboratory tests to assess the ecological risks from cultivating arthropod-resistant genetically engineered crops. Chemosphere 90, 901–909 (2013). [DOI] [PubMed] [Google Scholar]
- Rusek J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 7, 1207–1219 (1998). [Google Scholar]
- O'Callaghan M., Glare T. R., Burgess E. P. & Malone L. A. Effects of plants genetically modified for insect resistance on nontarget organisms. Annu. Rev. Entomol. 50, 271–292 (2005). [DOI] [PubMed] [Google Scholar]
- Kaneda S. & Kaneko N. Influence of soil quality on the growth of Folsomia candida (Willem) (Collembola). Pedobiologia 46, 428–439 (2002). [Google Scholar]
- Nelson K. L., Biiteau G., Lynch D. H., Peters R. D. & Fillmore S. Influence of agriculture soils on the growth and reproduction of the bio-indicator Folsomia candida. Pedobiologia 54, 79–86 (2011). [Google Scholar]
- de Boer M. E. et al. Reference genes for QRT-PCR tested under various stress conditions in Folsomia candida and Orchesella cincta (Insecta, Collembola). BMC Mol. Biol. 10, 54 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain M. T. & Hopkin S. P. Folsomia candida (Collembola): A “Standard” soil arthropod. Microbiol. Mol. Biol. Rev. 62, 775–806 (2004). [DOI] [PubMed] [Google Scholar]
- Hopkin S. P. Biology of the springtails (Insecta: Collembola) Oxford University Press, Oxford (1997). [Google Scholar]
- Yu L., Berry R. E. & Croft B. A. Effects of Bacillus thuringiensis toxins in transgenic cotton and potato on Folsomia candida (Collembola: Isotomidae) and Oppia nitens (Acari: Orbatidae). J. Econ. Entomol. 90, 113–118 (1997). [Google Scholar]
- Romeis J., Battini M. & Bigler F. Transgenic wheat with enhanced fungal resistance causes no effects on Folsomia candida (Collembola: Isotomidae). Pedobiologia 47, 141–147 (2003). [Google Scholar]
- Bai Y. et al. Effects of transgenic Bt rice on growth, reproduction, and superoxide dismutase activity of Folsomia candida (Collembola: Isotomidae) in laboratory studies. J. Econ. Entomol. 104, 1892–1899 (2011). [DOI] [PubMed] [Google Scholar]
- Yuan Y., Xiao N., Krogh P. H., Chen F. & Ge F. Laboratory assessment of the impacts of transgenic Bt rice on the ecological fitness of the soil non-target arthropod, Folsomia candida (Collembola: Isotomidae). Transgenic Res. 22, 791–803 (2013). [DOI] [PubMed] [Google Scholar]
- Duan J. J., Lundgren J. G., Naranjo S. & Marvier M. Extrapolating non-target risk of Bt crops from laboratory to field. Biol. Lett. 6, 74–77 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis J. et al. Recommendations for the design of laboratory studies on non-target arthropods for risk assessment of genetically engineered plants. Transgenic Res. 20, 1–22 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. H., Romeis J., Wu K. M. & Peng Y. F. Tier-1 assays for assessing the toxicity of insecticidal proteins produced by genetically engineered plants to non-target arthropods. Insect Sci. 21, 125–134 (2014). [DOI] [PubMed] [Google Scholar]
- van Frankenhuyzen K. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 101, 1–16 (2009). [DOI] [PubMed] [Google Scholar]
- Garcia-Alonso M., Jacobs E., Raybould A., Nickson T. E. & Sowig P. A tiered system for assessing the risk of genetically modified plants to non-target organisms. Environ. Biosafe. Res. 5, 57–65 (2006). [DOI] [PubMed] [Google Scholar]
- Rose R. I. White paper on tier-based testing for the effects of proteinaceous insecticidal plant-incorporated protectants on non-target invertebrates for regulatory risk assessment (USDA-APHIS and US Environmental Protection Agency, Washington, DC, USA, 2007) http://www.epa.gov/pesticides/biopesticides/pips/non-target-arthropods.pdf (Date of access: 10/03/2015).
- Wang Y. N. et al. Comparison of three transgenic Bt rice lines for insecticidal protein expression and resistance against a target pest, Chilo suppressalis (Walker) (Lepidoptera: Crambidae). Insect Sci. Published on line, 10.1111/1744-7917.12178 (2014). [DOI] [PubMed]
- Sims S. R. & Martin J. W. Effect of the Bacillus thuringiensis insecticidal proteins CryIA(b), CryIA(c), CryIIA, and CryIIIA on Folsomia candida and Xenylla grisea (Insecta: Collembola). Pedobiologia 41, 412–416 (1997). [Google Scholar]
- Wang Y. Y. et al. Consumption of Bt rice pollen expressing Cry2Aa does not cause adverse effects on adult Chrysoperla sinica Tjeder (Neuroptera: Chrysopidae). Biol Control 61, 246–251 (2012). [Google Scholar]
- Li Y. H. et al. Bt rice expressing Cry2Aa does not cause direct detrimental effects on larvae of Chrysoperla sinica. Ecotoxiology 22, 1413–1421 (2013). [DOI] [PubMed] [Google Scholar]
- Li Y. H., Chen X. P., Hu L., Romeis J. & Peng Y. F. Bt rice producing Cry1C protein does not have direct detrimental effects on the green lacewing Chrysoperla sinica (Tjeder). Environ. Toxicol. Chem. 33, 1391–1397 (2014). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Consumption of Bt rice pollen containing Cry1C or Cry2A does not pose a risk to Propylea japonica Thunberg (Coleoptera: Coccinellidae). Sci. Rep. 5, 7679 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S. Y., Li H. Y., Li X. F. & Zhang Z. Y. Effects of two kinds of transgenic poplar on protective enzymes system in the midgut of larvae of American white moth. J. Forest. Res. 12, 119–122 (2001). [Google Scholar]
- Gao X., Zhao Y., Wang X., Dong X. & Zheng B. Induction of carboxylesterase in Helicoverpa armigera by insecticides and plant allelochemicals. Acta Entomol. Sin. 41, S7–13 (1998). [Google Scholar]
- Mastumura F. Metabolism of insecticides by animals and plants in Toxicology of Insecticides (ed. Mastumura F.) 203–298 (Plenum Press, New York, 1985). [Google Scholar]
- Ross M. K. & Edelmann M. J. Carboxylesterases: a multifunctional enzyme involved in pesticide and lipid metabolism in Parameters for pesticide QSAR and PBPK/PD models for human risk assessment. Vol. 1099 (eds Knaak J.B., Timchalk C. & Tornero-Velez R.) Ch 10, 149–164 (Oxford University Press, Oxford, 2012). [Google Scholar]
- Carlberg I. & Mannervik B. Glutathione reductase. Method. Enzymol. 113, 484–490 (1985). [DOI] [PubMed] [Google Scholar]
- Shi Y. X. et al. Cloning and relative quantitative determination of trypsin-like enzyme and chymotrypsin-like enzyme genes in larval midgut of Bt susceptible and resistant Helicoverpa armigera. Chin. J. Appl. Environ. Biol. 15, 630–633 (2009). [Google Scholar]
- Li L., Liu X., Guo Y. & Ma E. Activity of the enzymes of the antioxidative system in cadmium-treated Oxya chinensis (Orthoptera Acridoidae). Environ. Toxicol. Pharmacol. 20, 412–416 (2005). [DOI] [PubMed] [Google Scholar]
- Passay C. et al. The effect of insecticide synergists on the response of scabies mites to pyrethroid acaricides. PLoS Negl. Trop. Dis. 3, e354 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Ke X., Chen F., Krogh P. H. & Ge F. Decrease in catalase activity of Folsomia candida fed a Bt rice diet. Environ. Pollut. 159, 3714–3720 (2011). [DOI] [PubMed] [Google Scholar]
- Yu L., Berry R. E. & Croft B. A. Effects of Bacillus thuringiensis toxins in transgenic cotton and potato on Folsomia candida (Collembola: Isotomidae) and Oppia nitens (Acari: Orbatidae). J. Econ. Entomol. 90, 113–118 (1997). [Google Scholar]
- Clark B. W. & Coats J. R. Subacute effects of Cry1Ab Bt corn litter on the earthworm Eisenia fetida and the springtail Folsomia candida. Environ. Entomol. 35, 1121–1129 (2006). [Google Scholar]
- Bakonyi G. et al. Preference tests with Collembolas on isogenic and Bt-maize. Eur. J. Soil Biol. 42, S132–135 (2006). [Google Scholar]
- Bakonyi G., Dolezsai A., Mátrai N. & Székács A. Effects of consumption of Bt-maize (MON 810) on the Collembolan Folsomia candida, over multiple generations: A laboratory study. Insects 2, 243–252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Amelsvoort P. A. M. & Usher M. B. A method for assessing the palatability of senesced leaf litter using Folsomia candida (Collembola: Isotomidae). Pedobiologia 33, 193–198 (1989). [Google Scholar]
- Yuan Y. et al. Microarray detection and qPCR screening of potential biomarkers of Folsomia candida (Collembola: Isotomidae) exposed to Bt proteins (Cry1Ab and Cry1Ac). Environ. Pollut. 184, 170–178 (2014). [DOI] [PubMed] [Google Scholar]
- Snider R. Laboratory observations on the biology of Folsomia candida (Willem) (Collembola: Isotomidae). Rev. Ecol. Biol. Sol. 10, 103–124 (1973). [Google Scholar]
- Duan J. J., Huesing J. & Teixeira D. Development of tier-1 toxicity assays for Orius insidiosus (Heteroptera: Anthocoridae) for assessing the risk of plant-incorporated protectants to nontarget heteropterans. Environ. Entomol. 36, 982–988 (2007). [DOI] [PubMed] [Google Scholar]
- Duan J. J., Teixeira D., Huesing J. E. & Jiang C. Assessing the risk to nontarget organisms from Bt corn resistant to corn rootworm (Coleoptera: Chrysomelidae): Tier-1 testing with Orius insidiosus (Heteroptera: Anthocoridae). Environ. Entomol. 37, 838–844 (2008). [DOI] [PubMed] [Google Scholar]
- Li Y. H. et al. Development of a tier-1 assay for assessing the toxicity of insecticidal substances against Coleomegilla maculata. Environ. Entomol. 40, 496–502 (2011). [Google Scholar]
- Li Y. H. et al. Use of an artificial diet system to study the toxicity of gut-active insecticidal compounds on larvae of the green lacewing Chrysoperla sinica. Biol. Control 69, 45–51 (2014). [Google Scholar]
- Zhang X. et al. Use of a pollen-based diet to expose the ladybird beetle Propylea japonica to insecticidal proteins. PLoS ONE 9, e85395 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisation for Economic Cooperation and Development (OECD). Guidelines for testing chemicals: collembolan reproduction test in soil. (OECD, Paris, 2009) http://www.oecd-ilibrary.org/environment/test-no-232-collembolan-reproduction-test-in-soil_9789264076273-en (Date of access: 1/6/2015).
- Han L. Z., Li S. B., Liu P. L., Peng Y. F. & Hou M. L. New artificial diet for continuous rearing of Chilo suppressalis (Lepidoptera: Crambidae). Ann. Entomol. Soc. Am. 105, 253–258 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.