Abstract
[Purpose] Diabetic peripheral neuropathy can often lead to balance impairment. The spinal reflex is a mechanism that is reportedly important for balance, but it has not been investigated in diabetic peripheral neuropathy patients. Moreover, inhibitory or facilitatory behavior of the spinal reflex—known as presynaptic inhibition—is essential for controlling postural sway. The purpose of this study was to compare the differences in as presynaptic inhibition and balance in subjects with and without diabetic peripheral neuropathy to determine the influence of presynaptic inhibition on balance in diabetic peripheral neuropathy patients. [Subjects and Methods] Presynaptic inhibition and postural sway were tested in eight patients (mean age, 58±6 years) and eight normal subjects (mean age, 59±7 years). The mean percent difference in conditioned reflex amplitude relative to the unconditioned reflex amplitude was assessed to calculate as presynaptic inhibition. The single-leg balance index was measured using a computerized balance-measuring device. [Results] The diabetic peripheral neuropathy group showed lower presynaptic inhibition (47±30% vs. 75±22%) and decreased balance (0.65±0.24 vs. 0.38±0.06) as compared with the normal group. No significant correlation was found between as presynaptic inhibition and balance score (R=0.37). [Conclusion] Although the decreased as presynaptic inhibition observed in diabetic peripheral neuropathy patients may suggest central nervous system involvement, further research is necessary to explore the role of presynaptic inhibition in decreased balance in diabetic peripheral neuropathy patients.
Key words: Presynaptic inhibition, Static postural sway, Diabetic peripheral neuropathy
INTRODUCTION
Nearly half of all diabetic patients also suffer from neuropathy, the most common form being diabetic peripheral neuropathy (DPN)1). DPN is characterized by a length-related distal distribution of sensory and motor symptoms, as well as autonomic involvement2, 3). Despite extensive research, the pathophysiology of DPN is still not well known2, 7, 8). Common symptoms of DPN include pain, tingling, loss of sensation, a feeling of heat in the lower limbs, and loss of balance4,5,6). DPN patients have been reported to have significantly decreased ankle movement perception and large changes in postural sway compared with normal individuals9). It has been proposed that the loss of sensation associated with DPN contributes to impaired balance and altered gait patterns that lead to the increased risk for falling seen in DPN patients10). However, the previously attempted therapeutic interventions targeting the peripheral nervous system in DPN patients11) suggests that other systems may be involved in the pathophysiology of this disease.
Although DPN has long been considered a disease of the peripheral nervous system, recent evidence has indicated central nervous system involvement12,13,14). Selvarajah et al.12) reported that a reduction in spinal cord area index was correlated with DPN, suggesting that even in the subclinical stages of DPN, extensive and possibly irreversible damage of the spinal cord may occur.
With this in mind, it is possible that the spinal reflex, which is critical for balance control, may be affected in DPN patients. The spinal reflex plays a critical role in group la monosynaptic projection and α-motorneuron activation15). Recent studies have reported that the inhibitory or excitatory influences of the spinal reflex are highly correlated with postural sway and stability16,17,18,19,20). It has been reported that balance tasks induce a decrease in Ia-motorneuron communication, leading to increased levels of presynaptic inhibition (PI)21, 22). PI is produced by primary afferent depolarization of axon terminals caused by increased Cl− permeability across the terminal membrane23). Furthermore, the frequency of spindle afferent feedback has been reported to increase during isometric contraction24), actively controlled standing balance25), and walking26).
To the authors’ knowledge, no studies have examined the levels of PI in DPN patients. It was hypothesized that since spinal cord activity has been reported to be affected in DPN patients, PI may be affected as well, indicating that it plays a role in the decreased balance observed in DPN patients. The purpose of this study was to compare the differences in PI and balance in subjects with and without DPN to determine the influence of PI on balance in DPN patients.
SUBJECTS AND METHODS
Two groups of age- and gender-matched participants were recruited for the study. Eight participants with diabetic peripheral neuropathy (4 males and 4 females) were assigned to one group, and eight healthy participants (4 males and 4 females) were assigned to the other group. The eight participants in the diabetic group had been diagnosed with diabetic peripheral neuropathy by their family practitioners and were recruited from local hospitals, pain clinics, rehabilitation centers, and diabetic support groups in the Salem and Portland, Oregon, areas. The exclusion criteria were participants who had sustained spinal or lower leg injuries (hip, knee and ankle) in the 12 months preceding the study. Written informed consent was obtained from each participant before the study. The Institutional Review Board at Willamette University reviewed and approved the study.
The double-leg static balance of the subjects was measured using the Biodex Balance System (Balance SystemTM SD). Prior to testing, a demonstration of the proper balance posture was given, followed by a 3-trial practice test. The proper posture consisted of an eyes-open double-leg static balance stance with the hands on the hips. Once the participant was familiarized with the testing protocol, the testing session, consisting of three trials for 20 seconds each, was started. Ten seconds of rest was allowed for the participants between each trial. The overall stability index was used to measure postural sway of the subjects. The overall stability index, presented as the deviation index (DI), shows the variance of foot platform displacement in degrees for all motions during the test. High scores indicate a mass amount of movement during the test, alluding to a less stable participant. Low scores denote the opposite: less movement indicates a more stable subject. The formulas used to calculate the overall stability index are shown below.
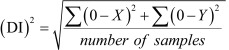 |
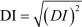 |
The “Center of Balance” (COB) is the reference point for a perfectly balanced state, i.e., COB x = 0; COB y = 027)
Prior to electrode placement, the skin of the lower leg of each subject was shaved and cleaned with alcohol to reduce signal impedance. Bipolar recording electrodes were then placed over the belly of the soleus. A self-adhesive ground electrode was secured over the lateral malleolus. The raw EMG signal was amplified and digitally converted at 2,000 Hz. The signal was band-pass filtered online (10–500 Hz) and collected for a 250 millisecond duration.
For paired reflex depression testing, the subjects were tested while lying prone on a padded table with the ankles positioned at 90°. Their faces were pointed down towards the relax pad on the table, and they were asked to relax their arms and put them at their sides and to keep their ankles dorsiflexed at the angle (90 degree) directed by the examiner. To elicit and record muscle responses and stimulation intensity, an EMG channel with surface electrodes (MP100, BIOPAC Systems Inc., Santa Barbara, California, USA) and a stimulating unit (STIM100C, BIOPAC Systems) and isolation adaptor unit (STIMSOC, BIOPAC Systems) was used to elicit the H-reflex. For the paired reflex depression (PRD) testing, the standardized H-reflex stimulation intensity was set up with an 80 ms delay between the first and second stimulation. The stimulus intensity was set to elicit H-reflexes at 25% of Mmax. Mmax was determined when the stimulus plateaued the motor response. A series of 10 paired reflex depression trials were completed in the dominant leg. The average change of the second H reflex relative to the first H-reflex amplitude was measured (unconditioned/conditioned * 100).
RESULTS
DPN patients showed less PI than the normal subjects (47±30% vs. 75±22%, p<0.05), as well as decreased balance (0.65 ±0.24 vs. 0.38±0.06, p<0.05) (Table 1). No significant correlation was found between PI and balance score (R=0.37, p=0.15) (Table 2).
Table 1. Differences in PRD and SI between the groups of DPN and healthy elderly participants.
| Groups | PRD (%) | SI |
|---|---|---|
| DPN | 46.6±29.7 | 0.65±0.24 |
| Normal | 74.7±22.3 | 0.38±0.06 |
PRD: paired reflex depression; SI: stability index; DPN: diabetic peripheral neuropathy
Table 2. Linear relationship between PRD and SI in the DPN and healthy elderly participants. The linear relationship was not statistically significant (p=0.15).
| No. | DPN | Normal | ||
|---|---|---|---|---|
| PRD (%) | SI | PRD (%) | SI | |
| 1 | 40.0 | 0.73 | 93.30 | 0.30 |
| 2 | 86.80 | 0.63 | 24.50 | 0.30 |
| 3 | 10.20 | 0.76 | 89.0 | 0.43 |
| 4 | 89.0 | 0.66 | 82.40 | 0.43 |
| 5 | 12.20 | 0.56 | 76.70 | 0.40 |
| 6 | 33.20 | 1.13 | 90.50 | 0.40 |
| 7 | 52.0 | 0.33 | 65.30 | 0.35 |
| 8 | 49.30 | 0.43 | 76.30 | 0.50 |
PRD: paired reflex depression; SI: stability index; DPN: diabetic peripheral neuropathy
DISCUSSION
DPN has been reported to cause significant impairments in diabetic patients, including loss of balance. Despite this serious clinical concern, the etiology of DPN’s role in loss of balance is still not well known. Recently, it has been suggested that in addition to the peripheral nervous system (PNS), the central nervous system (CNS) also plays a role in DPN. This was the first study to investigate the role of the spinal reflex in balance in DPN patients. The spinal mechanism was quantified through the level of PI of the spinal reflex. It was found that DPN patients possessed significantly lower PI and balance ability than normal subjects.
DPN patients possessed significantly lower balance ability than normal subjects (0.65 ±0.24 vs. 0.38±0.06, p<0.05). Although several previous studies have reported similar results10, 28,29,30,31), the etiology of this discrepancy is still not well known. Based on previous literature reporting CNS involvement in DPN12,13,14, 32) we hypothesized that the spinal cord may influence balance in DPN patients and therefore that PI may be affected as well.
DPN patients showed less PI than the normal subjects (47±30% vs. 75±22%, p<0.05). Although previous studies have conducted EMG tests to detect the H-reflex in diabetic patients33, 34), none have measured the level of PI using a paired-reflex depression protocol. These studies revealed that the H-reflex is often not even measurable in some patients. Trujillo-Hernández et al.33) reported that the H-reflex was absent in 22% of patients. Similarly, Marya et al.34) reported an H-reflex abnormality consisting of either prolonged latency or complete absence in 54% of diabetics.
Alternatively, many studies have investigated the influence of PI on balance in nondiabetic subjects16, 21, 35, 36). It has been reported that increased levels of PI lead to changes in postural sway21,22,23, 37). Kitano et al.21) measured PI through the H-reflex of the soleus muscle and found that PI increased by 50% after a complex balance test. PI is an indicator of the efficacy of the spinal pathway between group Ia afferent neurons and α neurons17). Furthermore, presynaptic modulation of the stretch reflex allows muscle stiffness to be controlled independent of the level of activation38). Therefore, PI is functionally significant in that it is an indicator of fine motor control39), and it is reasonable to assume that an individual with decreased levels of presynaptic modulation may experience decreased fine motor control and therefore balance.
There are several possible mechanisms that may contribute to the decreased level of PI seen in DPN patients in the present study. The decreased level of PI indicates that spinal cord involvement is evident. Although few studies have investigated the role of the spinal cord in DPN, several mechanisms have been suggested13). One mechanism proposed is damage to the peripheral nervous systems resulting in secondary spinal cord shrinkage, which causes the cord to die back11). In addition, Ziegler et al.40) suggested the subcortical lesions may be a contributing factor in the spinal cord damage in DPN patients. Furthermore, postmortem studies have suggested that axonal loss, gliosis, and demyelination within the spinal cord may contribute to the CNS involvement seen in DPN14, 41). However, these studies did not directly examine DPN patients specifically, and therefore they can only be used as the basis for speculation. This lack of research indicates that the role of the CNS and spinal cord involvement in DPN needs to be examined further.
When examining both DPN and normal subjects, we found a linear relationship between PI and balance (Table 2). Although this relationship was not significantly different between the groups, a notable trend was observed. A larger subject pool may have revealed significant results.
A paired-reflex depression protocol (PRD) was used to measure PI. This protocol has been used previously to measure PI20, 22, 42,43,44). Previous studies have reported that the PRD is a means of objectively and reliably measuring spinal mechanisms, with a reliability index of 0.93 to 0.9720). The PRD involves a pair of pulses separated by 80 ms. The 80 ms time interval negates influences of concurrent inhibition or Ib inhibition that would influence the first pulse45), and therefore the second reflex represents the true activation of the spinal reflex, or level of PI46). PI is an important measure of the spinal mechanism because it involves the depolarization of afferent neurons by inhibitory interneurons under descending control. Previous findings on the influence of descending pathways on primary afferent depolarization emphasize that the descending pathways can influence both the level of GABAa PI and effectiveness of peripheral afferent feedback of la afferents22, 47). Additional studies have indicated that the amplitude of the H-reflex varies directly with the afferent return arriving at the Ia afferent motor neuron synapse39).
Since this was the first study to measure PI in DPN patients, further research is needed to further examine the role of PI in DPN. MRI studies measuring the spinal cord area could provide further support for spinal cord and subsequent CNS damage in DPN patients. Furthermore, measuring the change in PI over time could help determine the progression of CNS damage throughout the course of the disease. Lastly, the influence of the metabolic effects of DPN on balance could also be investigated. It has been suggested that the metabolic changes seen in DPN (hyperglycemia, insulin resistance, dyslipidemia, hypertension, etc.) have a general effect on the nervous system48, 49) and thus may play a role in spinal cord atrophy12). Therefore, determining if there is a relationship between metabolic changes and balance may also provide insight in the pathophysiology of DPN.
References
- 1.Aring AM, Jones DE, Falko JM: Evaluation and prevention of diabetic neuropathy. Am Fam Physician, 2005, 71: 2123–2128. [PubMed] [Google Scholar]
- 2.Kang JH, Lee YS: Sensory nerve conduction studies in the diagnosis of diabetic sensorimotor polyneuropathy: electrophysiologicl features. J Phys Ther Sci, 2012, 24: 139–142. [Google Scholar]
- 3.Llewelyn JG: The diabetic neuropathies: types, diagnosis and management. J Neurol Neurosurg Psychiatry, 2003, 74: ii15–ii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesfaye S: Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig, 2011, 2: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han DW, Ha MS: The usefulness of current perception threshold test in both lower extremities with diabetic patient. J Phys Ther Sci, 2011, 23: 13–15. [Google Scholar]
- 6.Lee SW, Song CH: Virtual reality exercise improves balance of elderly persons with type 2diabetes: a randomized controlled trial. J Phys Ther Sci, 2012, 24: 261–265. [Google Scholar]
- 7.Cameron NE, Eaton SE, Cotter MA, et al. : Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia, 2001, 44: 1973–1988. [DOI] [PubMed] [Google Scholar]
- 8.Dyck PJ, Giannini C: Pathologic alterations in the diabetic neuropathies of humans: a review. J Neuropathol Exp Neurol, 1996, 55: 1181–1193. [DOI] [PubMed] [Google Scholar]
- 9.Horak FB, Hlavacka F: Somatosensory loss increases vestibulospinal sensitivity. J Neurophysiol, 2001, 86: 575–585. [DOI] [PubMed] [Google Scholar]
- 10.Menz HB, Lord SR, St George R, et al. : Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil, 2004, 85: 245–252. [DOI] [PubMed] [Google Scholar]
- 11.Pfeifer MA, Schumer MP: Clinical trials of diabetic neuropathy: past, present, and future. Diabetes, 1995, 44: 1355–1361. [DOI] [PubMed] [Google Scholar]
- 12.Selvarajah D, Wilkinson ID, Emery CJ, et al. : Early involvement of the spinal cord in diabetic peripheral neuropathy. Diabetes Care, 2006, 29: 2664–2669. [DOI] [PubMed] [Google Scholar]
- 13.Selvarajah D, Wilkinson ID, Davies J, et al. : Central nervous system involvement in diabetic neuropathy. Curr Diab Rep, 2011, 11: 310–322. [DOI] [PubMed] [Google Scholar]
- 14.Eaton SE, Harris ND, Rajbhandari SM, et al. : Spinal-cord involvement in diabetic peripheral neuropathy. Lancet, 2001, 358: 35–36. [DOI] [PubMed] [Google Scholar]
- 15.Magladery JW, Porter WE, Park AM, et al. : Electrophysiological studies of nerve and reflex activity in normal man. IV. The two-neurone reflex and identification of certain action potentials from spinal roots and cord. Bull Johns Hopkins Hosp, 1951, 88: 499–519. [PubMed] [Google Scholar]
- 16.Taube W, Leukel C, Gollhofer A: Influence of enhanced visual feedback on postural control and spinal reflex modulation during stance. Exp Brain Res, 2008, 188: 353–361. [DOI] [PubMed] [Google Scholar]
- 17.Tokuno CD, Carpenter MG, Thorstensson A, et al. : Control of the triceps surae during the postural sway of quiet standing. Acta Physiol (Oxf), 2007, 191: 229–236. [DOI] [PubMed] [Google Scholar]
- 18.Earles DR, Morris HH, Peng CY, et al. : Assessment of motoneuron excitability using recurrent inhibition and paired reflex depression protocols: a test of reliability. Electromyogr Clin Neurophysiol, 2002, 42: 159–166. [PubMed] [Google Scholar]
- 19.Shin SS, Lee YW, Song CH: Effects of lumber stabilization exercise on postural sway of patients with adolescent idiopathic scoliosis during quiet sitting. J Phys Ther Sci, 2012, 24: 211–215. [Google Scholar]
- 20.Ko DS, Jung DI, Bae SY: Effect of lumbar stabilization exercises on the balance ability of patients with stroke: a systematic review. J Phys Ther Sci, 2014, 26: 1993–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitano K, Tsuruike M, Robertson CT, et al. : Effects of a complex balance task on soleus H-reflex and presynaptic inhibition in humans. Electromyogr Clin Neurophysiol, 2009, 49: 235–243. [PubMed] [Google Scholar]
- 22.Jeon HS, Kukulka CG, Brunt D, et al. : Soleus H-reflex modulation and paired reflex depression from prone to standing and from standing to walking. Int J Neurosci, 2007, 117: 1661–1675. [DOI] [PubMed] [Google Scholar]
- 23.Rudomin P, Schmidt RF: Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res, 1999, 129: 1–37. [DOI] [PubMed] [Google Scholar]
- 24.Vallbo AB, Hulliger M: The dependence of discharge rate of spindle afferent units on the size of the load during isotonic position holding in man. Brain Res, 1982, 237: 297–307. [DOI] [PubMed] [Google Scholar]
- 25.Aniss AM, Diener HC, Hore J, et al. : Behavior of human muscle receptors when reliant on proprioceptive feedback during standing. J Neurophysiol, 1990, 64: 661–670. [DOI] [PubMed] [Google Scholar]
- 26.Hultborn H, Illert M, Nielsen J, et al. : On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res, 1996, 108: 450–462. [DOI] [PubMed] [Google Scholar]
- 27.Balance SystemTMSD-Balance, Physical Medicine/Biodex. http://www.biodex.com/sites/defult/files/950440 Man_10205revc.pdf. Balance System SD/Operation/Service Manual, P9-1-P9-2.
- 28.Conner-Kerr T, Templeton MS: Chronic fall risk among aged individuals with type 2 diabetes. Ostomy Wound Manage, 2002, 48: 28–34, 35. [PubMed] [Google Scholar]
- 29.Richardson JK, Hurvitz EA: Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sci, 1995, 50: M211–M215. [DOI] [PubMed] [Google Scholar]
- 30.Richardson JK, Ashton-Miller JA, Lee SG, et al. : Moderate peripheral neuropathy impairs weight transfer and unipedal balance in the elderly. Arch Phys Med Rehabil, 1996, 77: 1152–1156. [DOI] [PubMed] [Google Scholar]
- 31.Sorock GS, Labiner DM: Peripheral neuromuscular dysfunction and falls in an elderly cohort. Am J Epidemiol, 1992, 136: 584–591. [DOI] [PubMed] [Google Scholar]
- 32.Verma A, Bisht MS, Ahuja GK: Involvement of central nervous system in diabetes mellitus. J Neurol Neurosurg Psychiatry, 1984, 47: 414–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trujillo-Hernández B, Huerta M, Trujillo X, et al. : F-wave and H-reflex alterations in recently diagnosed diabetic patients. J Clin Neurosci, 2005, 12: 763–766. [DOI] [PubMed] [Google Scholar]
- 34.Marya RK, Chandran AP, Maini BK, et al. : Role of H-reflex latency studies in the diagnosis of subclinical diabetic neuropathy. Indian J Physiol Pharmacol, 1986, 30: 133–138. [PubMed] [Google Scholar]
- 35.Chen YS, Zhou S: Soleus H-reflex and its relation to static postural control. Gait Posture, 2011, 33: 169–178. [DOI] [PubMed] [Google Scholar]
- 36.Tokuno CD, Garland SJ, Carpenter MG, et al. : Sway-dependent modulation of the triceps surae H-reflex during standing. J Appl Physiol 1985, 2008, 104: 1359–1365. [DOI] [PubMed] [Google Scholar]
- 37.Trimble MH, Koceja DM: Modulation of the triceps surae H-reflex with training. Int J Neurosci, 1994, 76: 293–303. [DOI] [PubMed] [Google Scholar]
- 38.Trimble MH: Postural modulation of the segmental reflex: effect of body tilt and postural sway. Int J Neurosci, 1998, 95: 85–100. [DOI] [PubMed] [Google Scholar]
- 39.Zehr EP: Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol, 2002, 86: 455–468. [DOI] [PubMed] [Google Scholar]
- 40.Ziegler D, Mühlen H, Dannehl K, et al. : Tibial nerve somatosensory evoked potentials at various stages of peripheral neuropathy in insulin dependent diabetic patients. J Neurol Neurosurg Psychiatry, 1993, 56: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reske-Nielsen E, Lundbaek K, Gregersen G, et al. : Pathological changes in the central and peripheral nervous system of young long-term diabetics. The terminal neuro-muscular apparatus. Diabetologia, 1970, 6: 98–103. [DOI] [PubMed] [Google Scholar]
- 42.Sefton JM, Hicks-Little CA, Koceja DM, et al. : Modulation of soleus H-reflex by presynaptic spinal mechanisms during varying surface and ankle brace conditions. Neurophysiol Clin, 2007, 37: 15–21. [DOI] [PubMed] [Google Scholar]
- 43.Sefton JM, Hicks-Little CA, Hubbard TJ, et al. : Segmental spinal reflex adaptations associated with chronic ankle instability. Arch Phys Med Rehabil, 2008, 89: 1991–1995. [DOI] [PubMed] [Google Scholar]
- 44.Sefton JM, Yarar C, Hicks-Little CA, et al. : Six weeks of balance training improves sensorimotor function in individuals with chronic ankle instability. J Orthop Sports Phys Ther, 2011, 41: 81–89. [DOI] [PubMed] [Google Scholar]
- 45.Trimble MH, Du P, Brunt D, et al. : Modulation of triceps surae H-reflexes as a function of the reflex activation history during standing and stepping. Brain Res, 2000, 858: 274–283. [DOI] [PubMed] [Google Scholar]
- 46.Palmieri RM, Ingersoll CD, Hoffman MA, et al. : Arthrogenic muscle response to a simulated ankle joint effusion. Br J Sports Med, 2004, 38: 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudomin P: Selectivity of presynaptic inhibition: a mechanism for independent control of information flow through individual collaterals of single muscle spindle afferents. Prog Brain Res, 1999, 123: 109–117. [DOI] [PubMed] [Google Scholar]
- 48.Tesfaye S, Stevens LK, Stephenson JM, et al. : Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia, 1996, 39: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 49.DeFronzo RA, Hendler R, Simonson D: Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes, 1982, 31: 795–801. [DOI] [PubMed] [Google Scholar]


