Abstract
Endoscopic submucosal dissection (ESD) has been invented in Japan to provide resection for cure of early cancer in the gastrointestinal tract. Professional level of ESD requires excellent staging of early neoplasias with image enhanced endoscopy (IEE) to make correct indications for ESD, and high skills in endoscopic electrosurgical dissection. In Japan, endodiagnostic and endosurgical excellence spread through personal tutoring of skilled endoscopists by the inventors and experts in IEE and ESD. To translocate this expertise to other continents must overcome two fundamental obstacles: (1) inadequate expectations as to the complexity of IEE and ESD; and (2) lack of suitable lesions and master-mentors for ESD trainees. Leading endoscopic mucosal resection-proficient endoscopists must pioneer themselves through the long learning curve to proficient ESD experts. Major referral centers for ESD must arise in Western countries on comparable professional level as in Japan. In the second stage, the upcoming Western experts must commit themselves to teach skilled endoscopists from other referral centers, in order to spread ESD in Western countries. Respect for patients with early gastrointestinal cancer asks for best efforts to learn endoscopic categorization of early neoplasias and skills for ESD based on sustained cooperation with the masters in Japan. The strategy is discussed here.
Keywords: Endoscopic submucosal dissection, Early cancer, Endoscopic submucosal dissection clinical tutoring, Endoscopic submucosal dissection training, Gastrointestinal neoplasias, Endoscopic submucosal dissection learning curve, Endoscopic submucosal dissection techniques, Endoscopic submucosal dissection complications
Core tip: Endoscopic submucosal dissection (ESD) was developed in Japan for curative resection of early cancer. But Western countries take very long without tutoring to establish ESD on a professional level. A two-fold, sequential learning curve is necessary for endoscopic staging, and for endoluminal surgery of early neoplasias. This will need a sequential strategy: (1) education for diagnostic skills in routine endoscopy and in educational programs; and (2) endoscopists proficient in endoscopic snaring techniques must train for ESD and pass an untutored learning curve to become proficient. Then, Western ESD experts must instruct endoscopists from referral centers in their country.
INTRODUCTION
Endoscopic submucosal dissection (ESD) using electroknives was developed in Japan for curative en-bloc resection of early gastric cancer without risk of lymph node metastasis (LNM)[1,2]. Cancer of the gastrointestinal (GI) tract only is curable by resection. The obvious advantage of ESD over endoscopic mucosal resection (EMR) by electrosnaring is the ability to achieve en-bloc resection of even extended early neoplasias yielding accurate histological diagnosis and minimal recurrence rate[3]. EMR of larger lesions (> 2 cm) results in few or multiple pieces (piecemeal, PM), indeterminate histological resection status and high recurrence rate[3]. In contrast to surgery, ESD leaves the GI tract intact preserving the patient´s quality of life[4-6].
The decision for ESD or surgical full-wall resection with lymphadenectomy - is made by endoscopic staging of superficial gastrointestinal neoplasias using image-enhanced endoscopy (IEE, magnifying chromo- or NBI-endoscopy)[6,7]. Therefore, endoscopic findings describe the classical and expanded indication criteria for curative ESD (intention-to-treat)[4,8-16] (Table 1) that aim for curative resection by histologic outcome[17] (Table 2). Before resection, the neoplasia is usually confirmed by just a single targeted biopsy. Classical and Expanded Indications in Stomach and Esophagus, and Indications in Colorectum have been defined[1,8,10-14,16], evaluated by curative resection rates > 80%[18-23], and confirmed by excellent recurrence-free 3- to 5-years survival rates (> 96%) in large ESD studies performed by proficient operators in Japan18,23-30]. ESD has replaced EMR throughout Japan as state-of-the-art therapy for a wide spectrum of pre-/malignant early neoplasias in stomach, esophagus and colorectum[3].
Table 1.
Indications for endoscopic en-bloc resection of gastrointestinal neoplasias (modified from[6])
| Organ | Indications for … | Ref. |
| Stomach | ESD - classical indications1 | [1,4,5,13] |
| mucosal adenocarcinoma; intestinal type G1 or G2, size d ≤ 2 cm, no ulcer | ||
| ESD - expanded indications2 | ||
| adenocarcinoma, intestinal type, G1 or G2, any size without ulcer/adenocarcinoma, intestinal type, G1 or G2, sm-invasive < 500 μm/adenocarcinoma, intestinal type, G1 or G2, d ≤ 3 cm, with ulcer/adenocarcinoma diffuse type, G3 or G4, size d ≤ 2 cm, no ulcer | ||
| Esophagus | ESD - classical indications1 | [5,8,9,12,14,15] |
| SCC type 0-IIb (HGIN or G1, G2), intramucosal (m1, m2), any size | ||
| Barrett adenoca. type 0-II (G1, G2), intramucosal (m1, LPM), no ulcer | ||
| ESD - expanded indications2 | ||
| SCC type 0-II (HGIN, G1, G2) slightly invasive (m3, sm < 200 μm), any size3, clinical N 0 | ||
| Barrett adenocarcinoma type 0-II (HGIN or G1, G2), mucosal (≤ MM), clinical N 0 | ||
| Colorectum | ESD Indications | [5,10,11,16,64] |
| Any neoplasias > 20 mm in diameter without signs of deep submucosal invasion, indicative for en-bloc resection and unsuitable for EMR en-bloc: | ||
| LST-granular type d ≥ 4 cm (villous adenoma +/- HGIN)4 | ||
| LST-nongranular type d ≥ 2 cm | ||
| Mucosal carcinoma (HGIN, G1 or G2), or superficially sm-invasive5 | ||
| Depressed-type neoplasias (0-IIc) | ||
| Neoplasias type 0-I or 0-II with pit pattern type VI (irregular) | ||
| Sporadic localized neoplasias in chronic ulcerative colitis | ||
| Colorectal carcinoids of diameter < 20 mm (EMR, when diameter < 10 mm) |
Indications with risk of LNM < 1%;
Indications with risk of LNM or systemic M < 4%;
Increased risk for stricture formation, when ESD extends for ≥ 70% of circumference;
LST-granular type may also be resected in piecemeal fashion, the larger nodule resected first[10];
SM1 invasion of ≤ 1000 μm. LNM: Lymph node metastasis; ESD: Endoscopic submucosal dissection.
Table 2.
Criteria of curative endoscopic resection en-bloc in esophagus, stomach, and colorectum (modified from[17])
| Stomach |
| Guideline criteria1 |
| m-ca, diff. type, ly (-), v (-), and Ul (-) and ≤ 2 cm in size |
| Expanded criteria2 |
| m-ca, diff. type, ly (-), v (-), Ul (-) and any size > 2 cm |
| m-ca, diff. type, ly (-), v (-), Ul (+) and ≤ 3 cm in size |
| sm 1-ca (invasion depth < 500 μm3), diff. type, ly (-), v (-) |
| m-ca, undifferentiated type (G3), ly (-), v (-), Ul (-) and size < 2 cm |
| Esophagus (squamous lesions only) |
| Guideline criteria1 |
| pT1a-EP-ca/pT1a-LPM-ca |
| Expanded criteria2 |
| pT1a-MM-ca, ly (-), v (-), diff. type, expansive growth, ly (-), v (-) |
| cT1b-sm-ca (invasion < 200 μm3), ly (-), v (-), infiltrative growth pattern, expansive, diff. type, ly (-), v (-) |
| Colorectum |
| Guideline criteria1 |
| m-ca, diff. type, ly (-), v (-) |
| sm-ca (< 1000 μm3), diff. type, ly (-), v (-) |
Indications with risk of LNM < 1%;
Indications with risk of LNM or systemic M < 4%;
Measured as distance of maximum vertical invasion below MM. m: Mucosal; ca: Cancer; diff: Differentiated; ly: Lymphatic invasion; v: Vascular invasion; Ul: Ulceration; sm: Submucosal; EP: Epithelium; LPM: Lamina propria mucosae; MM: Muscularis mucosae.
ESD technique has rapidly spread throughout Japan, because EMR-experienced endoscopists acquired the skills in clinical procedures under supervision by ESD experts and had a high case load of gastric neoplasias most suitable for learners[31]. The goal of the early learning curve is to achieve competence level, as defined by en-bloc resections in > 80% and complications in less than 10% of ESD procedures[31], qualifying to perform untutored ESD procedures. A skilled and well prepared endoscopist usually attains competence for gastric ESD after approximately 30 tutored procedures[31-34], and then proceeds with 30 to 40 tutored procedures for competence in colorectal ESD[35-38]. Even without experience in gastric ESD, about 40 tutored colorectal procedures are sufficient to attain competence level for colorectal ESD[35-37].
The ESD technique is quite slowly transferred to Western countries, because they must acquire double expertise - diagnostic and electrosurgical - and early gastric neoplasias most suitable for learning ESD are too rare[31,39]. A systematic strategy (Figure 1) to establish proficient ESD in Western countries needs to build on Western experience the main topics - Preparations for and Training in ESD, Clinical Learning Curve in ESD, and Continued Medical Education in IEE and ESD.
Figure 1.

Strategy how to learn and establish endoscopic submucosal dissection in the west. ESD: Endoscopic submucosal dissection.
WESTERN EXPERIENCE IN ESD
Over the past six years smaller prospective series were published from pioneering centers in Western countries. There were few preliminary reports without long-term outcome on heterogenous initial series from single centers[40-43] or cumulative multiinstitutional registries[44,45]. The prospective series with follow-up on gastric[46-51], esophageal[52-57], and colorectal ESD[38,58-61] all focussed on rate of en-bloc resection [median 92% (range 68%-100%)], complications [median 13% (7%-27%)] and speed of dissection. These series reported only moderately lower rates of en-bloc resection [median 92% (68%-100%)] and recurrence-free survival [median 96.7% (91%-100%)] at 1-2 years than in Japan. However, rates of curative resection were inferior, median 72%-75% per organ, but lowest rates per study were 64% for gastric cancer, 46% for esophageal squamous cell cancer, 39% for early Barrett adenocarcinoma, and 7% for rectosigmoidal cancer (Table 3). During tumor staging with IEE, lateral extension of early Barrett´s adenocarcinoma and invasiveness of esophageal squamous cell cancer and rectosigmoidal cancer had obviously been underdiagnosed. Accordingly, the rate of surgical resection for noncurative ESD was too high, median 8% and up to 28%. In general, the learning curve for ESD still is flat even in the pioneering Western centers and performance not yet on the professional level of leading centers in Japan. The strategy must be to establish Western reference centers on comparable professional level as in Japan (Figure 1).
Table 3.
Organ-specific outcome of endoscopic submucosal dissection (curative intention) for Western prospective studies
| Ref. | Malignant neo-plasia type6, n | ESD, n | Resection en-bloc, % | Resection curative6, % | Complications, % | Surgery, % | Mortal., % | Recurrence, % | Follow-up (med.) yr | DFS, %/yr |
| Gastric ESD | ||||||||||
| Cardoso et al[46], 2008 | GC 15 | 15 | 80 | 74 | 20 | 8 | 0 | 8 | 1 | 91/1 |
| Catalano et al[47], 2009 | GC 12 | 12 | 92 | 92 | 16 | 8 | 0 | 8 | 2.5 | 92/2 |
| Probst et al[49], 2010 | GC 66 | 91 | 87 | 72 | 10.6 | 11 | 0 | 5.6 | 2.3 | 96.7/2 |
| Schumacher et al[50], 2012 | GC 21 | 28 | 90 | 64 | 20 | 7 | 3.4 | 11 | 2 | 100/2 |
| Pimentel-Nunes et al[51], 2014 | GC 128 | 136 | 94 | 82 | 13 | 7 | 0 | 7 | 3.2 | 100/3 |
| median [range] | 90 [80-94] | 73 [64-92] | 15 [11-20] | 8 [7-11] | 0 [3.4] | 8 [5-11] | 2.3 [1-3] | 97 [91-100]/2 | ||
| Esophageal ESD | ||||||||||
| Repici et al[52], 2010 | SCC 20 | 20 | 100 | 90 | 15 | 10 | 0 | 0 | 1.5 | 100/1.5 |
| Neuhaus et al[53], 2012 | AC 26 | 29 | 90 | 39 | 17 | 0 | 0 | 4 | 1.5 | 96/1.5 |
| Arantes et al[54], 2012 | AC 25 | 25 | 92 | 80 | 12 | 4 | 0 | 8 | 1.5 | 96/1.5 |
| Höbel et al[56], 2014 | AC 22 | 22 | 96 | 77 | 27 | 23 | 0 | 6 | 1.6 | 94/1.6 |
| Chevaux et al[55], 2015 | AC and HG 66 | 73 | 90 | 64 | 7 (+603) | 10 | 0 (31) | (105) | 1.8 | 92/2 |
| Probst et al[57], 2015 | AC 87 | 87 | 95 | 72 (844) | 12.6 | 6 | 0 (21) | 5 | 2.0 | 98/2 |
| Probst et al[57], 2015 | SCC 24 | 24 | 100 | 46 (724) | 12.6 | 0 | 0 (41) | 4 | 3.2 | 96/3 |
| median [range] | 95 [90-100] | 72 [39-90] | 16 [12-66] | 6 [0-23] | 0 [0-4] | 4 [0-8] | 1.6 [1.5-3.2] | 96 [94-100]/2 | ||
| Colorectal ESD | ||||||||||
| Probst et al[59], 2012 | Rectosigm. LST | 76 | 82 | - | 9.2 | 15 | 0 | n.g. | 2.0 | 100/2 |
| 14 CRC | 86 | 7 | (792) | 0 | ||||||
| Iacopini et al[58], 2012 | Colorectal LST | 60 | 68 | - | 10 | 20 | 0 | n.g. | n.g. | n.g. |
| 29 CRC | 72 | n.g. | (282) | |||||||
| Repici et al[60], 2013 | Rectal LST | 40 | 90 | - | 7.5 | 5 | 0 | 2.5 | 0.5 | 100/0.5 |
| 8 RC | 75 | n.g. | (252) | |||||||
| Thorlacius et al[61], 2013 | Colorectal LST | 29 | 72 | 76 | 10 | 10 | 0 | n.g. | < 0.5 | n.g. |
| 10 HG and CRC | 80 | (202) | ||||||||
| Berr et al[38], 2014 | Colorectal LST | 39 | 76 | - | 17 | 3 | 0 | LG 9 | 1.5 | 100/1.5 |
| 12 HG | 83 | 83 | (02) | HG 0 | 100/1.5 | |||||
| median [range] | 83 [72-90] | 75 [7-83] | 10 [7.5-17] | 10 [3-20] | 0 | 8 [2.5-9] | 1.5 [0.5-2] | 100/1.5 |
Rate (%) due to cancer progression;
Surgery (%) for malignant lesion after ESD;
Plus stenoses (%);
Rate (%) of R0 resection;
Metachronous HGIN or cancer;
Curative resection does only apply for malignant neoplasias (cancer -/+ HGIN). AC: Adenocarcinoma; CRC: Colorectal carcinoma; GC: Gastric cancer; HG/LG: High/low-grade intraepithelial neoplasia; RC: Rectal carcinoma; SCC: Squamous cell cancer; DFS: Disease-free survival rate; n.g.: Not given; pmEMR: Piecemeal EMR.
BACKGROUND AND PREPARATIONS FOR LEARNING ESD
Indications for ESD are pre/malignant superficial neoplasias without LNM. The risk of regional LNM rises from less than 5% to about 20% with increasing depth of vertical invasion into the submucosa (sm) layer, because lymphovascular supply increases deeper in the sm layer (sm2, sm3)[1,62,63]. Usual plane of dissection for ESD is the deeper third of the sm layer (sm3)[17]. Therefore, the probability of LNM has been determined in relation to the precise depth of sm invasion below the muscularis mucosae in very large series of differentiated early cancer (G1, G2) treated by surgical resection and lymphadenectomy. The maximum depth of superficial sm invasion consistent with minimum risk (< 4%) of lymph node metastasis is 500 μm in stomach, 1000 μm in colorectum, and 200 μm for esophageal squamous cell cancer with favourable prognostic indicators[1,8,13,64]. Evidence for massive sm invasion - deeper than this - is strict contraindication to endoscopic resection. Curative resection is reported when early cancer resected en- bloc reveals on serial sections differentiated carcinoma (G1/G2) without or with such extent of superficial sm invasion without discontinuous cancer cell nests at the invasion front, without lymphovascular invasion (L0, V0) and resected R0 with tumor-free margins[65] (Table 2). Therefore, curative ESD depends on accurate endoscopic categorization of early neoplasias (i.e., indication) as well as on dissection technique (i.e., operation).
Endoscopic categorization of of early neoplasias with IEE
Analysis with IEE can estimate the tumor category of superficial neoplasias. Optimized conditions require adequate preparation of the patient including sedation, cleaning of the mucosa from adherent mucus, use of a 60- to 100-fold magnifying endoscope with distal attachment to keep optimum distance for focussing on the microsurface (depth of field 3 mm) and mode for virtual chromoendoscopy (NBI, FICE, i-scan) with optimized processor settings[7]. Endoscopic analysis of the microsurface and capillary pattern of mucosal neoplasias in the GI tract is complex and requires special knowledge recently collected in an endoscopy atlas[66]. The staging diagnosis is substantiated by the type of atypias of the surface pattern on magnifying white light chromoendoscopy, and the type of alterations of sub-/mucosal capillary pattern using spectral light of 415 and 540 nm wavelength (virtual chromoendoscopy)[7]. IEE competence is indispensible to accurately analyze early carcinomas for grading (differentiated vs undifferentiated), lateral extension (margins) and extent of invasion (mucosal or slightly vs massively sm-invasive). The details are beyond the scope of this minireview (compare[67-73]). Most of this knowledge has been contributed over the past 15 years by endoscopic researchers from East Asia, but not widely introcuced to Western countries because comparable routine scopes for magnifying IEE were not marketed in the West until 2013. However, diagnostic proficiency to categorize early GI neoplasias with IEE at > 90% accuracy is fundamental to make correct indications for ESD vs surgery and achieve curative ESD. This skill must be well trained during routine endoscopy and at national continued medical education (CME) programs in the West.
Knowledge on ESD performed by experts and structural decisions
ESD is the new discipline of Endoluminal Surgery. N. Yahagi called ESD a “low tech, but highly skilled procedure” for the following reasons: (1) single handed resection procedure by endoscope movement (lack of countertraction); (2) complex high-frequency electrosurgery; (3) tissue recognition and diagnosis in intramural layers; and (4) skilled team approach required (operator and assistant).
At this stage of Western experience, EMR-experienced senior endoscopists in endoscopic and oncologic centers should approach to establish ESD up to professional level. The procedure should be learned from Japanese experts. The first step on the individual electrosurgical learning curve is to acquire background knowledge on ESD procedures and carefully observe at least 15 procedures performed in different locations of the GI tract by professional experts in Japan (Figure 1). The following decisions must be based on this practical experience and theoretical background highlighted in a recent textbook[74]: (a) The principal decision, whether to establish IEE and ESD competence in this hospital depending on existing experience with EMR and complication management, and on predicted case load (> 2 per month); (b) The subsequent decisions, how to assemble a team (operator, assistants, pathologist), select and provide best suitable equipment (special endoscopes with CO2-insufflation and electrosurgical unit), instruments and devices, organize top maintenance of endoscopes, and finally which type(s) of electroknife to use for start-up[75]. Beginners most easily control tip knives with flexible shaft (dual knife, hook knife) that use for lifting of the submucosa (sm) layer separate sm-injection of suitable solutions with a 25 gauge needle[74]. In the beginning this is less challenging than knives with integrated injection system (flush knife or hybrid knife), for the simple reason to control only three pedals for water jet, cutting and coagulation modes, but not an additional pedal for sm-injection via knife. On the other hand, knives with integrated injection system allow to maintain a safer, permament submucosal liquid cushion by frequent reinjection. At this point, accurate staging of neoplasias with IEE and thorough theoretical knowledge of ESD technique, equipment, complications and their management must be attained.
WESTERN DEMAND FOR TRAINING IN ESD
Ex-vivo training systems should first be used to acquire team coordination (operator and assistant) and basic dexterity for proper positioning of the scope in relation to the lesion, correct maneuvres of sm-injection, marginal incisions, submucosal access with the transparent distal attachment of the scope for sm-dissection, and electrothermal knife techniques - adequate current modes (“cut, coagulation, blended”), impulse duration, voltage and Watt settings, correct short duration of application by pedal tap[76-79]. Approach to the lifted lesion is preferably in knife position tangential to the proper muscle layer. Avoid to cut in perpendicular position to proper muscle layer or haustral folds, in order to prevent inadvertent muscle layer perforation. Train how to use the effect of gravity to keep the vision field clear and facilitate access to the submucosal space for further dissection. Basic dissection strategies such as initial complete circumferential incision (icci), partial circumferential incision method (pci, to longer maintain sm-lifting)[6,74], and hybrid-ESD-snaring[80] should be practiced, as well as clip closure techniques of the resection bed for complication management[81]. Thorough preparation of basic ESD techniques in ex-vivo systems - porcine stomach and esophagus and bovine colon[82-84] - contributes significantly to coordinated team work and training in individual ESD performance, and 20 to 30 procedures under preceptorship are sufficient to gain expertise[31,83,85,86].
Videotraining demonstrating typical high-risk maneuvers that have led to perforation or bleeding - compared to the correct strategy in that situation - could decrease complications during the early learning curve for untutored performance of clinical ESD, however is not yet available.
Experimental training in-vivo under expert supervision
In Western countries, training courses on in-vivo animal models generate additional progress after ex-vivo training[83,87-89]. Essential for optimal educational value is the expertise and teaching of the preceptors. For transition from ex-vivo training to clinical ESD, we recommend experimental training in-vivo - at least five gastric ESDs - under preceptorship of Japanese experts. This had been proposed in 2008 by T. OYAMA, because Japanese experts were not authorized to tutor trainees during ESD on patients in Western countries. After the first such expert training course in 2009, about two thirds of the participants increased their case load in gastric ESD (2.5-fold), colorectal ESD (3-fold), and esophageal ESD (8-fold) during the subsequent year at an acceptable rate of complications (9.7% perforations, 4.2% bleedings) without longterm morbidity[88]. Aims were propagation of technical skills, dissection maneuvers, specific electrocautery applications, strategy to keep a clear field of vision, and management of intentional complications (bleeding, perforation) in theory and practice. The experimental program and the personal tutoring by leading experts from Japan was ranked excellent by participating highly experienced endoscopists in their early learning curve, and therefore the course was repeated annually (Figure 2). Such a course is probably improving the outcome in the early untutored learning curve for ESD.
Figure 2.
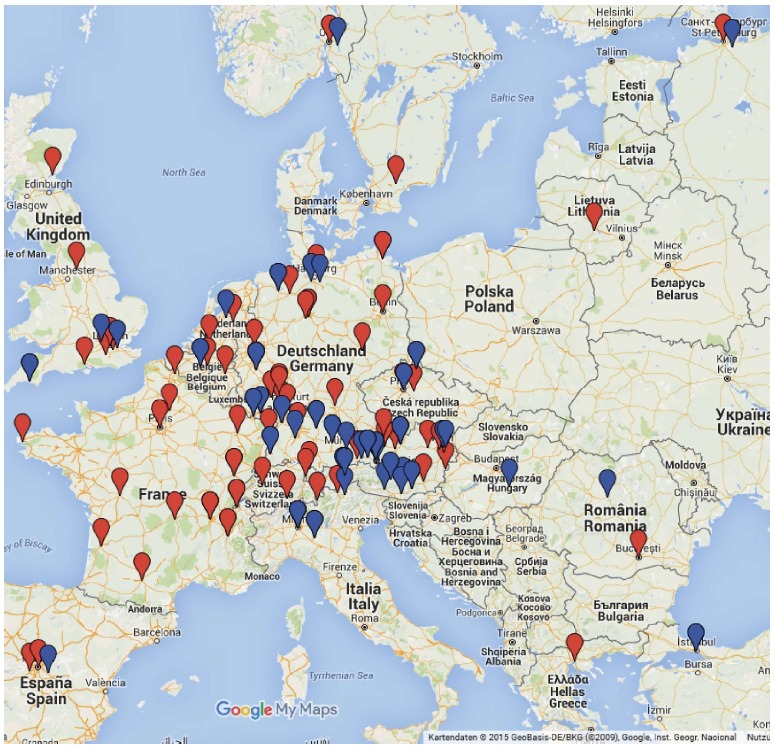
Distribution of reference centers with interventional endoscopists participating in the seven experimental endoscopic submucosal dissection workshops [red flags, n = 110, (30 two times)] and in the Clinical endoscopic submucosal dissection-Tutorings (blue flags, n = 51, repeatedly). Additional participants in workshops and clinical tutoring from Jerusalem, Amman and Cairo, respectively. Created with www.google.com/maps.
LEARNING CURVE IN ESD IN WESTERN COUNTRIES
Lesions suitable for initial learning of ESD must be strictly intramucosal, of moderate size (< 5 cm diameter) and in locations technically not very challenging[31,90]. Exclude any cancer with evidence for sm-invasive parts on IEE during the early learning curve, and avoid duodenal as well as very large (diameter > 6 cm) or very fibrotic lesions (e.g., recurrent neoplasias). However, the risk of inadequate oncological treatment due to an artefactual incomplete resection R1 is high during the initial learning curve[31], and may be more dangerous than minor perforation managed well by proficient clipping. Undertreatment (R1 resection) results in major resective surgery or high risk of recurrence and incurable disease[31,90]. Therefore, early cancer lesions in esophagus and upper half of stomach should be reserved for proficient endosurgeons, and not treated before the competence level for that organ has been accomplished[31,39]. This suggests that large part of untutored initial learning for ESD may better be passed on rather challenging adenomatous/dysplastic LST´s in the colorectum than on early cancer in stomach or esophagus[38].
Strategy for untutored learning of ESD
In 2008, a panel of experts had recommended a “step-up approach” in technical challenge for untutored learning of clinical ESD[90]. The first 20 ESD should be performed on neoplasias in the antrum and distal corpus of stomach, and in rectum, before more challenging locations are approached. This strategy has been very successful for tutored training in ESD in Japan, where however gastric cancer has 10 fold higher incidence and is more often detected as early cancer (in 70% vs 20%-30%) than in most Western countries[31,39,91]. Therefore, early gastric cancer is too rare in the West to achieve a useful case load of at least two ESD procedures per month. This recommendation would impede to establish ESD for decades - to the disadvantage of GI cancer patients. Alternatively, a “prevalence-based approach” allows for a reasonable case load, but requires learning ESD mainly in the colorectum and early on in difficult locations[38,91]. Such an approach has successfully been taken after the described basic preparations and experimental in-vivo training in gastric ESD supervised by Japanese experts[38,88].
Untutored learning of ESD
The risk of complications is highest during the early untutored learning curve[44]. We recommend that only the most skilled and EMR experienced endoscopist of the unit undertakes to establish ESD in the early untutored learning curve[31,38,39]. After rigorous theoretical and experimental preparation, a skilled interventional endoscopist can achieve competence level after 20 to 30 untutored ESD procedures, and needs twice that case load (e.g., 2 x 25 ESD) to prove outcome for competence level[38,59]. The outcome for untutored colorectal ESD without significant experience in gastric ESD[38] was quite similar as reported with the step-up approach by others[35,59,92]. However, this should not be endeavoured with little interventional expertise and low theoretical background. Meticulous preparation is important for any of those untutored ESD procedures (Table 4). In addition, close personal contact with the patient is essential before ESD for fully informed consent and after ESD to monitor/treat complications and in the long-term to detect and handle any local recurrence or delayed complication such as stenosis. The cooperating pathologist must receive IEE information about suspicious areas in the oriented specimens, and provide precise histologic work-up for tumor grading, sm invasiveness, lymphovascular infiltration and resection status - curative resection is critical for the patient[65]. Continuously register outcome quality to evaluate the level of performance and spur to improve ESD technique[38]. ESD on beginning proficiency level (en-bloc 90%, complications < 5%, curative resection > 80%, speed about 9 cm2/h) requires more than 100 self-completed procedures[31,35,85,93] and continued education by and feed-back with top experts.
Table 4.
Principles for establishing endoscopic submucosal dissection by an untutored learning curve (modified from[38])
| Evaluate the lesion during prior endoscopy for ESD indication and resection strategy |
| Avoid risk of any R2 resection of cancer (no signs for deep submucosal invasion!) |
| Avoid high risk lesions (> 5 cm diameter, or in fornix and cardia, duodenum, colonic flexures) |
| Safety comes first, procedure time of ESD is of minor importance in the beginning |
| Only cut tissue or fibers in submucosa that you clearly see and have identified |
| Keep the vision field clear, prevent and immediately stop bleeding |
| Close any perforation immediately by endoscopic clipping on expert level |
| Complete any started ESD procedure with intention for safe, curative resection |
| Guide personally the patient pre-ESD (informed consent) and post-ESD (for any complication) |
| Only a single endoscopist per unit should do untutored ESD until he is on competence level1 |
| Document all entire ESD procedures on DVD recordings (for evidence and error analysis) |
| Follow-up short-term and long-term (center Registry), trend in dozens |
Performance of 20 consecutive ESD procedures with < 10% complications. ESD: Endoscopic submucosal dissection.
CONTINUED MEDICAL EDUCATION IN IEE AND ESD
Continued expert instruction for ESD
Nevertheless, immediate preceptorship of trainees by proficient ESD experts best guides through the early learning curve for ESD. This was the key for enormously rapid spreading of ESD throughout Japan. Intense observation of experts performing ESD (about 40 cases) can enhance performance of ESD during the clinical learning curve. At present this requires a sabbatical of four weeks in Japan. When combined with some ex-vivo technical training, performance and skills markedly increase during subsequent untutored ESD, as shown by doubling of dissection speed[39]. ESD clincal tutoring program was introduced by four top experts from Japan together with eight endoscopists in the ESD learning curve, former participants of the Experimental ESD Training Workshop[88], in order to enhance progression to professional level both in IEE categorization of mucosal neoplasias and ESD performance (Figure 2). The educational benefit is immediate preceptorship during the diagnostic and endosurgical procedures, assessment of differential indication and risk for ESD, tipps and tricks for strategy and technique of dissection for different lesions, preventive hemostasis and/or clipping of the resection bed, and documentation of the specimen for histopathology. The enrolled patients received excellent treatment, as shown by the outcome of a series of 116 ESD performed intention-to-treat in cooperation with expert tutors from Japan. The curative resection rate of all 49 malignant neoplasias and five symptomatic semimalignant submucosal tumors was an astonishing 100% at an acceptable rate of complications (14%) managed without surgery or long-term morbidity in these elderly, often comorbid patients[94]. We highly recommend such CME with Japanese experts for establishing ESD in Western countries.
ESD reference centers where ESD is performed on a professional level as high as in Japan must arise in Western countries to spread ESD to all referral centers (Figure 1). Expert instructors in ESD reference centers can better guide advanced trainees for ESD how to increase safety, dexterity (en-bloc resection, speed), specimen quality, and outcome (curative resection, low complication rate). These centers must take a leading role in national CME programs for IEE and ESD of early neoplasias.
Medical progress and patients rights
Diagnosis with IEE and performance of EMR/ESD/surgical resection with lymphadenectomy according to the criteria established in Japan is state-of-the-art for early neoplasias in the gastrointestinal tract (Table 1)[4,5,18], but requires proficient performance of diagnostic IEE and electrosurgical ESD with curative outcome. The benefit for patients with such early neoplasias is so enormous that major endoscopic referral hospitals have the duty to establish ESD. Typically, this should be a third level endoscopic referral center that manages a high volume of neoplastic lesions allowing a suitable case load of more than two ESD indications per month. Establishing ESD in such centers should be restricted to the endoscopist most experienced with EMR, emergency endoscopy and IEE staging of early neoplasias[39]. In many Western countries, performance of ESD by such a leading endoscopist under tutoring and, if necessary or better for the patient, with direct help by the Japanese expert, is permissible and compatible with legal and insurance rules. For untutored ESD in the learning phase, the indication must conform to criteria established in Japan and national guidelines, and in any doubt expert advice should be obtained via web-based image and video analysis. All lesions comprising early cancer must be presented to an interdisciplinary cancer board prior to ESD and again with the histopathological results. For advanced adenoma (e.g., in colorectum) the decision can be made by patient and treating physician after informed consent on ESD and alternative resection techniques, including the lower level of technical performance of ESD as compared with data from Japan. Since individual learning curves are involved, we recommend detailed documentation of all entire procedures with DVD recordings (for evidence and error analysis) and a continuously updated registry of the outcome data (procedure, complications, histopathology, follow-up) that also enhances the learning process.
CONCLUSION
To establish accurate endoscopic diagnosis and endosurgical treatment of early cancer in Western countries lasts longer than anticipated five years ago, but the strategy to achieve it is quite clear (Figure 1). IEE of early GI neoplasias must become daily practice and part of national CME programs. EMR-experienced senior endoscopists from major endoscopic referral centers ought to establish ESD on competence and subsequently proficiency level. They need to understand the present lack of competence and the long-lasting learning curve. When knowledgeable about IEE and ESD, they have to negotiate with the hospital for sustained funding of team and optimal equipment for IEE and ESD. Dedicated ex-vivo training must be combined with experimental ESD training in vivo supervised by experts from Japan before untutored performance of first clinical ESDs. Continued instruction by experts from Japan is highly recommended until proficiency in ESD of early GI cancer is confirmed by outcome data. Western experts in new reference centers must commit themselves to teach EMR-experienced senior endoscopists from other referral centers in order to spread ESD. ESD is high-end endoscopic patient care that will be largely confined to referral centers.
Footnotes
Supported by Leonie-Wild Charitable Foundation, Heidelberg-Eppelheim.
Conflict-of-interest statement: The authors have no conflict of interest to report.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 8, 2015
First decision: July 20, 2015
Article in press: September 30, 2015
P- Reviewer: Park KS S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 2.Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751–757. doi: 10.1055/s-0029-1215053. [DOI] [PubMed] [Google Scholar]
- 4.Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–4498. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 5.Fujishiro M. Perspective on the practical indications of endoscopic submucosal dissection of gastrointestinal neoplasms. World J Gastroenterol. 2008;14:4289–4295. doi: 10.3748/wjg.14.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oyama T, Yahagi N. Principles of Endoscopic Resection: Diagnostic and Curative Resection of Mucosal Neoplasias. In: Berr F, Oyama N, Ponchon T, Yahagi N, editors. Early Neoplasias of the Gastrointestinal Tract - Endoscopic Diagnosis and Therapeutic Decisions. New York: Springer; 2014. pp. 35–48. [Google Scholar]
- 7.Berr F, Uraoka T, Ponchon T, Yahagi N. Endoscopic Detection and Analysis of Mucosal Neoplastic Lesions: Enhanced Imaging and Tumor Morphology. In: Berr F, Oyama N, Ponchon T, Yahagi N, editors. Early Neoplasias of the Gastrointestinal Tract - Endoscopic Diagnosis and Therapeutic Decisions. New York: Springer; 2014. pp. 49–70. [Google Scholar]
- 8.Oyama T, Miyata Y, Shimatani S, Tomori A, Hotta K, Yoshida M. Diagnosis and Long-term Results and Prognosis of m3 and sm1 Esophageal Cancer. Lymph Nodal Metastasis of m3, sm1 Esophageal Cancer. Stomach Intestine. 2002;37:71–74. [Google Scholar]
- 9.Paris Workshop on Columnar Metaplasia in the Esophagus and the Esophagogastric Junction, Paris, France, December 11-12 2004. Endoscopy. 2005;37:879–920. doi: 10.1055/s-2005-870305. [DOI] [PubMed] [Google Scholar]
- 10.Uraoka T, Saito Y, Matsuda T, Ikehara H, Gotoda T, Saito D, Fujii T. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592–1597. doi: 10.1136/gut.2005.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konishi T, Watanabe T, Kishimoto J, Kotake K, Muto T, Nagawa H. Prognosis and risk factors of metastasis in colorectal carcinoids: results of a nationwide registry over 15 years. Gut. 2007;56:863–868. doi: 10.1136/gut.2006.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwano H, Nishimura Y, Ohtsu A, Kato H, Kitagawa Y, Tamai S, Toh Y, Matsubara H. Guidelines for diagnosis and treatment of neoplasias of the esophagus. April 2007 edition: part I. Edited by the Japan Esophageal Society. Esophagus. 2008;5:61–73. [Google Scholar]
- 13.Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–152. doi: 10.1007/s10120-009-0515-x. [DOI] [PubMed] [Google Scholar]
- 14.Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: a systematic review. Am J Gastroenterol. 2012;107:850–62; quiz 863. doi: 10.1038/ajg.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oyama T. Diagnostic strategies of superficial Barrett’s esophageal cancer for endoscopic submucosal dissection. Dig Endosc. 2013;25 Suppl 1:7–12. doi: 10.1111/den.12036. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S, Saitoh Y, Matsuda T, Igarashi M, Matsumoto T, Iwao Y, Suzuki Y, Nishida H, Watanabe T, Sugai T, et al. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol. 2015;50:252–260. doi: 10.1007/s00535-014-1021-4. [DOI] [PubMed] [Google Scholar]
- 17.Toyonaga T, Nishino E, Man-I M, East JE, Azuma T. Principles of quality controlled endoscopic submucosal dissection with appropriate dissection level and high quality resected specimen. Clin Endosc. 2012;45:362–374. doi: 10.5946/ce.2012.45.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67–S70. doi: 10.1016/s1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 19.Nakamoto S, Sakai Y, Kasanuki J, Kondo F, Ooka Y, Kato K, Arai M, Suzuki T, Matsumura T, Bekku D, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy. 2009;41:746–750. doi: 10.1055/s-0029-1215010. [DOI] [PubMed] [Google Scholar]
- 20.Kuroki Y, Hoteya S, Mitani T, Yamashita S, Kikuchi D, Fujimoto A, Matsui A, Nakamura M, Nishida N, Iizuka T, et al. Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. J Gastroenterol Hepatol. 2010;25:1747–1753. doi: 10.1111/j.1440-1746.2010.06331.x. [DOI] [PubMed] [Google Scholar]
- 21.Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video) Gastrointest Endosc. 2010;72:1217–1225. doi: 10.1016/j.gie.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto Y, Fujisaki J, Hirasawa T, Ishiyama A, Yoshimoto K, Ueki N, Chino A, Tsuchida T, Hoshino E, Hiki N, et al. Therapeutic outcomes of endoscopic submucosal dissection of undifferentiated-type intramucosal gastric cancer without ulceration and preoperatively diagnosed as 20 millimetres or less in diameter. Dig Endosc. 2010;22:112–118. doi: 10.1111/j.1443-1661.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 23.Toyonaga T, Man-i M, East JE, Nishino E, Ono W, Hirooka T, Ueda C, Iwata Y, Sugiyama T, Dozaiku T, et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc. 2013;27:1000–1008. doi: 10.1007/s00464-012-2555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262–270. doi: 10.1007/s10120-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 25.Hirasawa K, Kokawa A, Oka H, Yahara S, Sasaki T, Nozawa A, Tanaka K. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:960–966. doi: 10.1016/j.gie.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Niimi K, Fujishiro M, Kodashima S, Goto O, Ono S, Hirano K, Minatsuki C, Yamamichi N, Koike K. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2010;42:723–729. doi: 10.1055/s-0030-1255675. [DOI] [PubMed] [Google Scholar]
- 27.Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, Omae M, Kubota M, Ishiyama A, Hirasawa T, et al. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122–127. doi: 10.1055/s-0031-1291486. [DOI] [PubMed] [Google Scholar]
- 28.Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703–707. doi: 10.1055/s-0033-1344396. [DOI] [PubMed] [Google Scholar]
- 29.Yoda Y, Ikematsu H, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, Fujii T, Oono Y, Sakamoto T, Nakajima T, et al. A large-scale multicenter study of long-term outcomes after endoscopic resection for submucosal invasive colorectal cancer. Endoscopy. 2013;45:718–724. doi: 10.1055/s-0033-1344234. [DOI] [PubMed] [Google Scholar]
- 30.Kosaka T, Endo M, Toya Y, Abiko Y, Kudara N, Inomata M, Chiba T, Takikawa Y, Suzuki K, Sugai T. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc. 2014;26:183–191. doi: 10.1111/den.12099. [DOI] [PubMed] [Google Scholar]
- 31.Gotoda T, Friedland S, Hamanaka H, Soetikno R. A learning curve for advanced endoscopic resection. Gastrointest Endosc. 2005;62:866–867. doi: 10.1016/j.gie.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 32.Choi IJ, Kim CG, Chang HJ, Kim SG, Kook MC, Bae JM. The learning curve for EMR with circumferential mucosal incision in treating intramucosal gastric neoplasm. Gastrointest Endosc. 2005;62:860–865. doi: 10.1016/j.gie.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Omata M. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy. 2006;38:991–995. doi: 10.1055/s-2006-944808. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto S, Uedo N, Ishihara R, Kajimoto N, Ogiyama H, Fukushima Y, Yamamoto S, Takeuchi Y, Higashino K, Iishi H, et al. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy. 2009;41:923–928. doi: 10.1055/s-0029-1215129. [DOI] [PubMed] [Google Scholar]
- 35.Hotta K, Oyama T, Shinohara T, Miyata Y, Takahashi A, Kitamura Y, Tomori A. Learning curve for endoscopic submucosal dissection of large colorectal tumors. Dig Endosc. 2010;22:302–306. doi: 10.1111/j.1443-1661.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- 36.Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum. 2011;54:1307–1312. doi: 10.1097/DCR.0b013e3182282ab0. [DOI] [PubMed] [Google Scholar]
- 37.Ohata K, Ito T, Chiba H, Tsuji Y, Matsuhashi N. Effective training system in colorectal endoscopic submucosal dissection. Dig Endosc. 2012;24 Suppl 1:84–89. doi: 10.1111/j.1443-1661.2012.01272.x. [DOI] [PubMed] [Google Scholar]
- 38.Berr F, Wagner A, Kiesslich T, Friesenbichler P, Neureiter D. Untutored learning curve to establish endoscopic submucosal dissection on competence level. Digestion. 2014;89:184–193. doi: 10.1159/000357805. [DOI] [PubMed] [Google Scholar]
- 39.Draganov PV, Coman RM, Gotoda T. Training for complex endoscopic procedures: how to incorporate endoscopic submucosal dissection skills in the West? Expert Rev Gastroenterol Hepatol. 2014;8:119–121. doi: 10.1586/17474124.2014.864552. [DOI] [PubMed] [Google Scholar]
- 40.Coda S, Trentino P, Antonellis F, Porowska B, Gossetti F, Ruberto F, Pugliese F, D’Amati G, Negro P, Gotoda T. A Western single-center experience with endoscopic submucosal dissection for early gastrointestinal cancers. Gastric Cancer. 2010;13:258–263. doi: 10.1007/s10120-010-0544-5. [DOI] [PubMed] [Google Scholar]
- 41.Sattianayagam PT, Desmond PV, Jayasekera C, Chen RY. Endoscopic submucosal dissection: experience in an Australian tertiary center. Ann Gastroenterol. 2014;27:212–218. [PMC free article] [PubMed] [Google Scholar]
- 42.Aslan F, Alper E, Cekıc C, Yurtlu DA, Ekıncı N, Arabul M, Unsal B, Mıura Y, Yamamoto H. Endoscopic submucosal dissection in gastric lesions: the 100 cases experience from a tertiary reference center in West. Scand J Gastroenterol. 2015;50:368–375. doi: 10.3109/00365521.2014.999253. [DOI] [PubMed] [Google Scholar]
- 43.Lang GD, Konda VJ, Siddiqui UD, Koons A, Waxman I. A single-center experience of endoscopic submucosal dissection performed in a Western setting. Dig Dis Sci. 2015;60:531–536. doi: 10.1007/s10620-014-3260-x. [DOI] [PubMed] [Google Scholar]
- 44.Farhat S, Chaussade S, Ponchon T, Coumaros D, Charachon A, Barrioz T, Koch S, Houcke P, Cellier C, Heresbach D, et al. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy. 2011;43:664–670. doi: 10.1055/s-0030-1256413. [DOI] [PubMed] [Google Scholar]
- 45.Chaves DM, Moura EG, Milhomem D, Arantes VN, Yamazaki K, Maluf F, Albuquerque W, Conrado AC, Araújo JC, Uejo PH, et al. Initial experience of endoscopic submucosal dissection in Brazil to treat early gastric and esophagheal cancer: a multi-institutional analysis. Arq Gastroenterol. 2013;50:148–152. doi: 10.1590/s0004-28032013000200025. [DOI] [PubMed] [Google Scholar]
- 46.Cardoso DM, Campoli PM, Yokoi C, Ejima FH, Barreto PA, de Brito AM, Mota ED, de Fraga Júnior AC, da Mota OM. Initial experience in Brazil with endoscopic submucosal dissection for early gastric cancer using insulation-tipped knife: a safety and feasibility study. Gastric Cancer. 2008;11:226–232. doi: 10.1007/s10120-008-0489-0. [DOI] [PubMed] [Google Scholar]
- 47.Catalano F, Trecca A, Rodella L, Lombardo F, Tomezzoli A, Battista S, Silano M, Gaj F, de Manzoni G. The modern treatment of early gastric cancer: our experience in an Italian cohort. Surg Endosc. 2009;23:1581–1586. doi: 10.1007/s00464-009-0350-5. [DOI] [PubMed] [Google Scholar]
- 48.Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa N, Lopes C, Moreira-Dias L. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69:350–355. doi: 10.1016/j.gie.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 49.Probst A, Pommer B, Golger D, Anthuber M, Arnholdt H, Messmann H. Endoscopic submucosal dissection in gastric neoplasia - experience from a European center. Endoscopy. 2010;42:1037–1044. doi: 10.1055/s-0030-1255668. [DOI] [PubMed] [Google Scholar]
- 50.Schumacher B, Charton JP, Nordmann T, Vieth M, Enderle M, Neuhaus H. Endoscopic submucosal dissection of early gastric neoplasia with a water jet-assisted knife: a Western, single-center experience. Gastrointest Endosc. 2012;75:1166–1174. doi: 10.1016/j.gie.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 51.Pimentel-Nunes P, Mourão F, Veloso N, Afonso LP, Jácome M, Moreira-Dias L, Dinis-Ribeiro M. Long-term follow-up after endoscopic resection of gastric superficial neoplastic lesions in Portugal. Endoscopy. 2014;46:933–940. doi: 10.1055/s-0034-1377348. [DOI] [PubMed] [Google Scholar]
- 52.Repici A, Hassan C, Carlino A, Pagano N, Zullo A, Rando G, Strangio G, Romeo F, Nicita R, Rosati R, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: results from a prospective Western series. Gastrointest Endosc. 2010;71:715–721. doi: 10.1016/j.gie.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 53.Neuhaus H, Terheggen G, Rutz EM, Vieth M, Schumacher B. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett’s esophagus. Endoscopy. 2012;44:1105–1113. doi: 10.1055/s-0032-1310155. [DOI] [PubMed] [Google Scholar]
- 54.Arantes V, Albuquerque W, Freitas Dias CA, Demas Alvares Cabral MM, Yamamoto H. Standardized endoscopic submucosal tunnel dissection for management of early esophageal tumors (with video) Gastrointest Endosc. 2013;78:946–952. doi: 10.1016/j.gie.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 55.Chevaux JB, Piessevaux H, Jouret-Mourin A, Yeung R, Danse E, Deprez PH. Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett’s neoplasia. Endoscopy. 2015;47:103–112. doi: 10.1055/s-0034-1390982. [DOI] [PubMed] [Google Scholar]
- 56.Höbel S, Dautel P, Baumbach R, Oldhafer KJ, Stang A, Feyerabend B, Yahagi N, Schrader C, Faiss S. Single center experience of endoscopic submucosal dissection (ESD) in early Barrett’s adenocarcinoma. Surg Endosc. 2015;29:1591–1597. doi: 10.1007/s00464-014-3847-5. [DOI] [PubMed] [Google Scholar]
- 57.Probst A, Aust D, Märkl B, Anthuber M, Messmann H. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy. 2015;47:113–121. doi: 10.1055/s-0034-1391086. [DOI] [PubMed] [Google Scholar]
- 58.Iacopini F, Bella A, Costamagna G, Gotoda T, Saito Y, Elisei W, Grossi C, Rigato P, Scozzarro A. Stepwise training in rectal and colonic endoscopic submucosal dissection with differentiated learning curves. Gastrointest Endosc. 2012;76:1188–1196. doi: 10.1016/j.gie.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 59.Probst A, Golger D, Anthuber M, Märkl B, Messmann H. Endoscopic submucosal dissection in large sessile lesions of the rectosigmoid: learning curve in a European center. Endoscopy. 2012;44:660–667. doi: 10.1055/s-0032-1309403. [DOI] [PubMed] [Google Scholar]
- 60.Repici A, Hassan C, Pagano N, Rando G, Romeo F, Spaggiari P, Roncalli M, Ferrara E, Malesci A. High efficacy of endoscopic submucosal dissection for rectal laterally spreading tumors larger than 3 cm. Gastrointest Endosc. 2013;77:96–101. doi: 10.1016/j.gie.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 61.Thorlacius H, Uedo N, Toth E. Implementation of endoscopic submucosal dissection for early colorectal neoplasms in Sweden. Gastroenterol Res Pract. 2013;2013:758202. doi: 10.1155/2013/758202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takubo K, Aida J, Sawabe M, Kurosumi M, Arima M, Fujishiro M, Arai T. Early squamous cell carcinoma of the oesophagus: the Japanese viewpoint. Histopathology. 2007;51:733–742. doi: 10.1111/j.1365-2559.2007.02766.x. [DOI] [PubMed] [Google Scholar]
- 63.Fujimori T, Fujii S, Saito N, Sugihara K. Pathological diagnosis of early colorectal carcinoma and its clinical implications. Digestion. 2009;79 Suppl 1:40–51. doi: 10.1159/000167865. [DOI] [PubMed] [Google Scholar]
- 64.Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534–543. doi: 10.1007/s00535-004-1339-4. [DOI] [PubMed] [Google Scholar]
- 65.Neureiter D, Kiesslich T. Histopathology of Early Mucosal Neoplasias: Morphologic Carcinogenesis in the GI Tract. In: Berr F, Oyama N, Ponchon T, Yahagi N, editors. Early Neoplasias of the Gastrointestinal Tract - Endoscopic Diagnosis and Therapeutic Decisions. New York: Springer; 2014. pp. 19–33. [Google Scholar]
- 66.Berr F, Oyama N, Ponchon T, Yahagi N. Early Neoplasias of the Gastrointestinal Tract - Endoscopic Diagnosis and Therapeutic Decisions. New York: Springer; 2014. [Google Scholar]
- 67.Berr F, Uraoka T, Yahagi N. Colorectum: Mucosal Neoplasias. In: Berr F, Oyama N, Ponchon T, Yahagi N, editors. Early Neoplasias of the Gastrointestinal Tract - Endoscopic Diagnosis and Therapeutic Decisions. New York: Springer; 2014. pp. 193–239. [Google Scholar]
- 68.Kiesslich R. Columnar Epithelium-Lined (Barrett’s) Esophagus: Mucosal Neoplasias. In: Berr F, Oyama N, Ponchon T, Yahagi N, editors. Early Neoplasias of the Gastrointestinal Tract - Endoscopic Diagnosis and Therapeutic Decisions. New York: Springer; 2014. pp. 115–127. [Google Scholar]
- 69.Kiesslich R. Chronic Inflammatory Bowel Disease in Remission: Mucosal Neoplasias. In: Berr F, Oyama N, Ponchon T, Yahagi N, editors. Early Neoplasias of the Gastrointestinal Tract - Endoscopic Diagnosis and Therapeutic Decisions. New York: Springer; 2014. pp. 241–259. [Google Scholar]
- 70.Kuroki Y, Uraoka T, Wolkersdörfer GW. High-Resolution Endoscopic Ultrasound: Clinical T-Staging of Mucosal Neoplasms. In: Berr F, Oyama N, Ponchon T, Yahagi N, editors. Early Neoplasias of the Gastrointestinal Tract - Endoscopic Diagnosis and Therapeutic Decisions. New York: Springer; 2014. pp. 71–82. [Google Scholar]
- 71.Oyama T. Squamous Cell-Lined Esophagus and Hypopharynx: Mucosal Neoplasias. In: Berr F, Oyama N, Ponchon T, Yahagi N, editors. Early Neoplasias of the Gastrointestinal Tract - Endoscopic Diagnosis and Therapeutic Decisions. New York: Springer; 2014. pp. 85–113. [Google Scholar]
- 72.Oyama T. Stomach: Mucosal Neoplasias. In: Berr F, Oyama N, Ponchon T, Yahagi N, editors. Early Neoplasias of the Gastrointestinal Tract - Endoscopic Diagnosis and Therapeutic Decisions. New York: Springer; 2014. pp. 129–171. [Google Scholar]
- 73.Ponchon T. Duodenum and Small Bowel: Mucosal Neoplasias. In: Berr F, Oyama N, Ponchon T, Yahagi N, editors. Early Neoplasias of the Gastrointestinal Tract - Endoscopic Diagnosis and Therapeutic Decisions. New York: Springer; 2014. pp. 173–192. [Google Scholar]
- 74.Fukami N. Endoscopic Submucosal Dissection - Principles and Practice. New York: Springer; 2015. [Google Scholar]
- 75.Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014;6:112–120. doi: 10.4253/wjge.v6.i4.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fukami N, Bults A. Electrocautery for ESD. In: Fukami N, editor. Endoscopic Submucosal Dissection - Principles and Practice. New York: Springer; 2015. pp. 75–83. [Google Scholar]
- 77.Ono T. ESD Technique: Stomach. In: Fukami N, editor. Endoscopic Submucosal Dissection - Principles and Practice. New York: Springer; 2015. pp. 95–101. [Google Scholar]
- 78.Oyama T. Endoscopic Submucosal Dissection for Superficial Esophageal Cancer. In: Fukami N, editor. Endoscopic Submucosal Dissection - Principles and Practice. New York: Springer; 2015. pp. 85–94. [Google Scholar]
- 79.Yahagi N. ESD for Colorectal Lesions. In: Fukami N, editor. Endoscopic Submucosal Dissection - Principles and Practice. New York: Springer; 2015. pp. 103–113. [Google Scholar]
- 80.Toyonaga T, Man-I M, Morita Y, Sanuki T, Yoshida M, Kutsumi H, Inokuchi H, Azuma T. The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc. 2009;21 Suppl 1:S31–S37. doi: 10.1111/j.1443-1661.2009.00872.x. [DOI] [PubMed] [Google Scholar]
- 81.Thirumurthi S, Gottumukkala SR. Management of Gastrointestinal EMR and ESD Perforation: From Lab to Practice. In: Fukami N, editor. Endoscopic Submucosal Dissection - Principles and Practice. New York: Springer; 2015. pp. 161–176. [Google Scholar]
- 82.Tanaka S, Morita Y, Fujita T, Wakahara C, Ikeda A, Toyonaga T, Azuma T. Ex vivo pig training model for esophageal endoscopic submucosal dissection (ESD) for endoscopists with experience in gastric ESD. Surg Endosc. 2012;26:1579–1586. doi: 10.1007/s00464-011-2074-6. [DOI] [PubMed] [Google Scholar]
- 83.Kato M, Gromski M, Jung Y, Chuttani R, Matthes K. The learning curve for endoscopic submucosal dissection in an established experimental setting. Surg Endosc. 2013;27:154–161. doi: 10.1007/s00464-012-2402-5. [DOI] [PubMed] [Google Scholar]
- 84.Pioche M, Rivory J, Aguero-Garcete G, Guillaud O, O’Brien M, Lafon C, Reversat N, Uraoka T, Yahagi N, Ponchon T. New isolated bovine colon model dedicated to colonic ESD hands-on training: development and first evaluation. Surg Endosc. 2015:Epub ahead of print. doi: 10.1007/s00464-014-4062-0. [DOI] [PubMed] [Google Scholar]
- 85.Coman RM, Gotoda T, Draganov PV. Training in endoscopic submucosal dissection. World J Gastrointest Endosc. 2013;5:369–378. doi: 10.4253/wjge.v5.i8.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parra-Blanco A, Arantes V, Gonzalez N, Herreros de Tejada A, Donoso A. Endoscopic Submucosal Dissection Training in Western Countries. In: Fukami N, editor. Endoscopic Submucosal Dissection - Principles and Practice. New York: Springer; 2015. pp. 237–256. [Google Scholar]
- 87.Tanimoto MA, Torres-Villalobos G, Fujita R, Santillan-Doherty P, Albores-Saavedra J, Gutierrez G, Martin-del-Campo LA, Bravo-Reyna C, Villanueva O, Villalobos JJ, et al. Endoscopic submucosal dissection in dogs in a World Gastroenterology Organisation training center. World J Gastroenterol. 2010;16:1759–1764. doi: 10.3748/wjg.v16.i14.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berr F, Ponchon T, Neureiter D, Kiesslich T, Haringsma J, Kaehler GF, Schmoll F, Messmann H, Yahagi N, Oyama T. Experimental endoscopic submucosal dissection training in a porcine model: learning experience of skilled Western endoscopists. Dig Endosc. 2011;23:281–289. doi: 10.1111/j.1443-1661.2011.01129.x. [DOI] [PubMed] [Google Scholar]
- 89.González N, Parra-Blanco A, Villa-Gómez M, Gamba A, Taullard A, Silveira A, Sanguinetti A, Olano C, Cohen H. Gastric endoscopic submucosal dissection: from animal model to patient. World J Gastroenterol. 2013;19:8326–8334. doi: 10.3748/wjg.v19.i45.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deprez PH, Bergman JJ, Meisner S, Ponchon T, Repici A, Dinis-Ribeiro M, Haringsma J. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy. 2010;42:853–858. doi: 10.1055/s-0030-1255563. [DOI] [PubMed] [Google Scholar]
- 91.Uraoka T, Parra-Blanco A, Yahagi N. Colorectal endoscopic submucosal dissection: is it suitable in western countries? J Gastroenterol Hepatol. 2013;28:406–414. doi: 10.1111/jgh.12099. [DOI] [PubMed] [Google Scholar]
- 92.Niimi K, Fujishiro M, Goto O, Kodashima S, Koike K. Safety and efficacy of colorectal endoscopic submucosal dissection by the trainee endoscopists. Dig Endosc. 2012;24 Suppl 1:154–158. doi: 10.1111/j.1443-1661.2012.01251.x. [DOI] [PubMed] [Google Scholar]
- 93.Yamamoto Y, Fujisaki J, Ishiyama A, Hirasawa T, Igarashi M. Current status of training for endoscopic submucosal dissection for gastric epithelial neoplasm at Cancer Institute Hospital, Japanese Foundation for Cancer Research, a famous Japanese hospital. Dig Endosc. 2012;24 Suppl 1:148–153. doi: 10.1111/j.1443-1661.2012.01278.x. [DOI] [PubMed] [Google Scholar]
- 94.Wagner A, Neureiter D, Kiesslich T, Allgaier H, Kleber G, Ziachehabi A, Heiler K, Plamenig D, Friesenbichler P, Wolkersdorfer G, et al. Endoscopic Submucosal Dissection (ESD) unter Tutoring durch Experten. Z Gastroenterol. 2015;53:P17. [Google Scholar]


