Abstract
Inflammatory bowel diseases (IBD) comprise the two major entities Crohn’s disease and ulcerative colitis and endoscopic imaging of the gastrointestinal tract has always been an integral and central part in the management of IBD patients. Within the recent years, mucosal healing emerged as a key treatment goal in IBD that substantially decides about the clinical outcome of IBD patients, thereby demanding for a precise, timely and detailed endoscopic assessment of the mucosal inflammation associated with IBD. Further, molecular imaging has tremendously expanded the clinical utility and applications of modern endoscopy, now encompassing not only diagnosis, surveillance, and treatment but also the prediction of individual therapy response. Within this review we describe novel endoscopic approaches and advanced endoscopic imaging methods for the diagnosis, treatment and surveillance of IBD patients. We begin by providing an overview over novel and advanced imaging techniques such as magnification endoscopy and dye-based and dye-less chromoendoscopy, endomicroscopy and endocytoscopy. We then describe how these techniques can be utilized for the precise and ultrastructural assessment of mucosal inflammation and dysplasia development associated with IBD and outline how they have enabled the endoscopist to gain insight onto the cellular level in real-time. Finally, we provide an outlook on how molecular imaging has rapidly evolved in the recent past and can be used to make individual predictions about the therapeutic response towards biological treatment.
Keywords: Gastrointestinal endoscopy, Crohn’s disease, Ulcerative colitis, Inflammatory bowel diseases, Colon, Colorectal neoplasms
Core tip: Within this review we describe novel endoscopic techniques for the diagnosis, treatment and surveillance of inflammatory bowel diseases (IBD) patients. We begin by providing an overview over advanced imaging techniques such as magnification endoscopy, dye-based and dye-less chromoendoscopy, endomicroscopy and endocytoscopy. We then portray how these techniques provide insights on cellular level in real-time and how they can be utilized for the precise and ultrastructural assessment of mucosal inflammation and dysplasia development in IBD. Finally, we review how molecular imaging has rapidly evolved in the recent past and can now be used to make individual predictions about the therapeutic response towards biological treatment.
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) belong to the family of idiopathic inflammatory bowel diseases (IBD) in which an excessive mucosal immune response towards the complex enteric microbiota in a genetically predisposed host is believed to play a key role in disease pathophysiology[1-4]. It is well accepted that the chronic inflammatory stimulus within the gastrointestinal tract is associated with an increased risk for developing colitis associated cancer (CAC) in both, UC and CD[5] and the individual risk for colon cancer increases with the duration, severity and anatomic extent of colitis[6-10]. The close association between disease duration and the development of CAC represents the rationale for recommending regular surveillance endoscopy starting 6 to 8 years after first manifestation of the disease in current European and United States guidelines[11,12]. Despite the lack of randomized controlled trials directly assessing the reduction of CAC by surveillance colonoscopy, a large number of case series[13-16] and case-control studies[17-19] provided evidence of the clinical benefit of surveillance colonoscopy for IBD patients. However, dysplasia and intraepithelial neoplasia are frequently missed during routine white-light endoscopic examinations[20] and at the same time, random biopsies have a low yield for dysplasia detection[20,21].
The discovery that dye-based chromoendoscopy (e.g., with methylene blue) with targeted mucosal biopsies is superior for dysplasia detection in IBD patients[20,21] has led to the rapid evolvement of advanced endoscopic imaging techniques such as digital (i.e., FICE, i-scan, SPIES) or optical [i.e., narrow band imaging (NBI), Compound band imaging (CBI)] dye-less chromoendoscopy which offer the advantage of enhancing mucosal vascular and mucosal surface pattern morphology by just pushing a button on the handle of the endoscope thereby reducing time and costs associated with conventional dye-based chromoendoscopy[22,23] (Table 1).
Table 1.
Techniques and modes of advanced endoscopic imaging with advantages and indications
| Endoscopic technique | Modes | Advantages | Disadvantages | Indications |
| White light endoscopy (WLE) | Standard definition colonoscopy (SD) | Widely spread | No sufficient discrimination between inflammation and dysplasia | Routine assessment of mucosal inflammation |
| High definition colonoscopy(HD) | Can detect significantly more dysplastic lesion in IBD than SD[108] | Increased costs compared to SD | in combination with DBC: cancer surveillance of IBD patient[109,110] | |
| Dye based chromo-endoscopy (DBC) | Indigo-carmine (0.8%)[109,110] | Superior for the detection of dysplastic lesions in IBD[20,21,55,56,109,110] | Increase in time and effort, dye-pooling | Method of choice for cancer surveillance in IBD[12,34,35,109,110] |
| Methylene blue (1%)[109,110] | ||||
| Dye less chromo-endoscopy (DLC) | ||||
| Optical DLC | NBI CBI | Readily available (push-of-a-button technologies)[22,31,66] improved prediction of disease extent and disease activity compared to WLE[67-69,71] | NBI: results for dysplasia detection in IBD heterogenous | NBI: not recommended as a replacement for DBC for cancer surveillance in IBD[109,110] |
| Digital DLC | i-scan FICE SPIES | i-scan: no data for dysplasia detection in IBD yet | ||
| Confocal laser endomicroscopy (CLE) | pCLE iCLE | Real time histologic imaging with 1000-fold magnification, potentially improved delineation of degree and extent of mucosal inflammation[82] | Time- and cost-intensive procedure, expert skills required[22,31] | No routine indications |
| Endocytoscopy (EC) | Real time histologic imaging with up to 1390-fold magnification, potentially improved delineation of degree and extent of mucosal inflammation, can distinguish single inflammatory cells[88] | Time- and cost-intensive procedure, expert skills required[22,31,111] | No routine indications |
CBI: Compound band imaging; DBC: Dye-based chromoendoscopy; DLC: Dye-less chromoendoscopy; FICE: Fuji intelligent color enhancement; HD: High definition; IBD: Inflammatory bowel diseases; iCLE: Integrated confocal laser endomicroscop; NBI: Narrow band imaging; SD: Standard definition; SPIES: Storz professional image enhancement systems; pCLE: Probe based confocal endomicroscopy.
Apart from the detection of early colorectal cancer, endoscopic assessment of degree and severity of mucosal inflammation is another equally important aspect in the management of IBD patients. In this regard, mucosal healing has emerged as a key treatment goal in IBD in the recent past that predicts sustained clinical remission and resection-free survival of patients[24]. Hence, the precise assessment of intestinal inflammation is of pivotal importance for the management of IBD patients and advanced endoscopic imaging techniques including dye-less chromoendoscopy, endocytoscopy and confocal laser endomicroscopy have been shown to allow precise and ultrastructural characterization of the inflammation within the gut. Finally, by combining endoscopic imaging with the visualization of single molecular targets crucially involved in disease pathogenesis, in vivo endoscopic prediction of therapeutic response before the actual commencement of therapy is no longer a keen wish for distant future, but close to be ready for being integrated into daily practice. In this review we describe how novel and advanced endoscopic imaging techniques have been utilized for the diagnosis and surveillance of CAC and mucosal inflammation in IBD patients and follow a semantic structure “From the surface to the single cell”. Thus, we begin by reviewing imaging techniques that visualize the intestinal surface such as chromoendoscopy and subsequently discuss endoscopic approaches that go deeper within the intestinal layer and are capable of visualizing the submucosal architecture and single cells such as endocytoscopy and confocal endomicroscopy. Finally, we provide an outlook on how labelling molecular pathways and targets combined with endoscopy can be utilized to make predictions about therapeutic responses, thereby tremendously expanding the repertoire of modern endoscopy.
TECHNIQUES BEHIND ADVANCED ENDOSCOPIC IMAGING
Magnification endoscopy and chromoendoscopy
Magnification endoscopy utilizes a movable lens to vary the degree of magnification thereby allowing to magnify the mucosa of the gastrointestinal tract from 6-fold up to 150-fold[25]. In one of the earliest studies, magnification endoscopy has been shown to be able to differentiate true neoplasms from non-neoplastic colonic lesions, thereby providing an accurate instantaneous prediction of the histology of colorectal tumorous lesions[25]. This observation in colorectal polyps has now been dramatically extended to other neoplastic and non-neoplastic diseases in the upper and lower gastrointestinal tract, and especially in combination with chromoendoscopy, magnification endoscopy can be utilized for the precise diagnosis of a variety of diseases including dysplasia and early cancer in the esophagus, stomach and colorectum as well as intraepithelial neoplasia and disease extent in UC[26-32].
Chromoendoscopy encompasses dye-based chromoendoscopy (DBC) and dye-less chromoendoscopy (DLC) and enhances the mucosal architecture and/or submucosal microvasculature by the use of various dyes (DBC) or endoscopic optical and computer-based color programs (DLC). This contrast enhancement of the mucosal layer often results in the improved detection of lesions that are otherwise subtle or even invisible in conventional white-light endoscopy.
DBC uses different dye agents which are divided into absorptive agents (Lugol, methylene blue, toluidine blue, and cresyl violet), contrast agents (indigo carmine, acetic acid) and reactive staining agents (congo red, phenol red), all of which are mostly applied via standard spraying or plain biliary ERCP catheters[33]. As outlined below, DBC has been shown to improve detection of dysplasia in IBD, and chromoendoscopy is recommended as the preferred modality for surveillance in patients with colonic IBD by the British Society of Gastroenterology[34] and the European Crohn’s and Colitis organization[35]. However, DBC also requires increased effort, skill, time, and costs. These confinements associated with the use of traditional dye agents have finally led to the development of DLC techniques.
DLC is further subdivided into optical chromoendoscopy [Narrow band imaging (NBI), Olympus, Japan; Compound band imaging (CBI), Aohua, Shanghai, China] and digital chromoendoscopy [i-scan, Pentax, Tokyo, Japan; Fujinon Intelligent Color Enhancement (FICE), Fujifilm, Tokyo, Japan; Storz Professional Image Enhancement Systems (SPIES), Karl Storz, Tuttlingen, Germany]. Optical DLC such as NBI utilizes optical filters within the light source of the endoscope to narrow the bandwidth of spectral transmittance, thereby enhancing and facilitating the visualization of blood vessels. Digital DLC such as i-scan and FICE uses a digital postprocessing algorithm that reconstructs the endoscopic image from the video processor in real time resulting in an improved contrast of the capillary patterns and enhancement of the mucosal surface pattern morphology[22,33]. Representative images for the enhanced visualization and delineation of the mucosal surface pattern by dye-based chromoendoscopy, and the mucosal surface and vascular pattern by NBI and i-scan are shown in Figures 1 and 2, respectively. Importantly, both optical and digital DLC are simple “push-of-a-button” techniques that are readily available during the endoscopic examination. Thus, compared to dye-based chromoendoscopy, DLC offers the great advantage of dye-enhanced mucosal imaging without the efforts in time and costs of applying contrast agents during ongoing endoscopy. Further, data derived from the in vivo assessment of colorectal polyp histology impressively demonstrated that DLC can be readily learned even by “non-expert” endoscopists[36-38]. Hence, endoscopists with varying levels of experience can accurately use digital chromoendoscopy after a single training session[39,40] with comparable diagnostic accuracies between non-expert and expert endoscopists[41].
Figure 1.
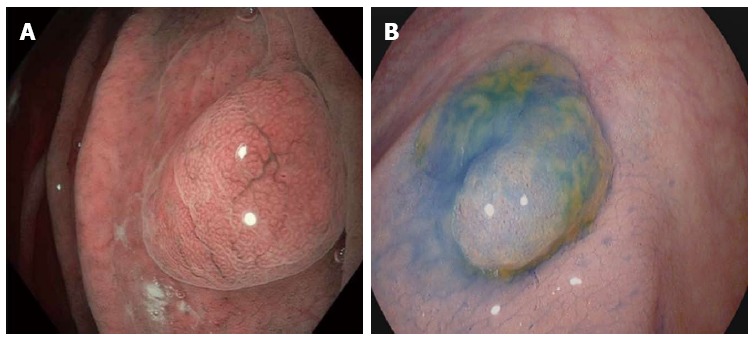
Dye-based chromoendoscopy and optical chromoendoscopy in the gastrointestinal tract. Left picture: Optical dye-less chromoendoscopy with narrow band imaging (NBI) is based on the utilization of optical filters within the light source of the endoscope to narrow the bandwidth of spectral transmittance, thereby enhancing and facilitating the visualization of blood vessels. As exemplified on a fundic gland polyp in the stomach, NBI allows a clear delineation of the mucosal surface pit pattern architecture. Right picture: Dye-based chromoendoscopy with indigo carmine. Indigo carmine is a blue contrast agent that is used primarily in the colon for enhancing the detection or differentiation of colorectal neoplasms. As shown for a small colon polyp here, application of indigo carmine via a spraying catheter enhances the contrast and allows to visualize the pit pattern and to delineate mucosal irregularities.
Figure 2.
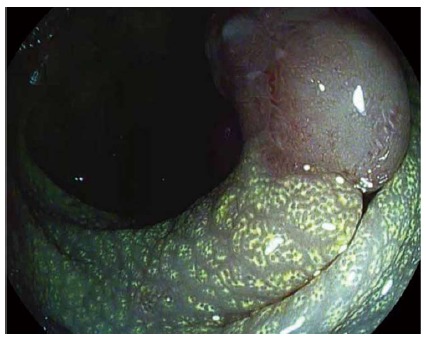
Digital dye-less chromoendocopy with i-scan in the lower gastrointestinal tract. i-scan uses a digital postprocessing algorithm that reconstructs the endoscopic image from the video processor in real time resulting in improved contrast of the capillary patterns and enhancement of the mucosal surface pattern morphology as exemplified on a adenomatous polyp in the colon. As a result of an accumulation of lipid-filled macrophages within the lamina propria, the mucosa adjacent to the polyp exhibits a chicken skin mucosa on digital chromoendoscopy.
Confocal laser endomicroscopy
Confocal laser endomicroscopy is a technique allowing to obtain images at the (sub)cellular level[42], and since its introduction in 2003 confocal laser endomicroscopy (CLE) has rapidly emerged as a powerful technique that enables precise histologic real time in vivo imaging of various diseases[43-47]. Technically, CLE is based on the emission of a low power blue laser into the tissue after topical (acriflavine hydrochloride, cresyl violet) or systemic (fluorescein sodium) administration of contrast agents. The emitted light is then reflected from the tissue and refocused on the detection system by the same lens, leading to microscopic imaging at 1000-fold magnification in real time. As shown in healthy mucosa in Figure 3, CLE allows a clear visualization of the colonic crypt architecture, single cells within the lamina propria and the microvasculature within the colon[45]. Currently, two FDA-approved and CE-certified CLE devices are available and used in clinical routine[48]: (1) a probe based CLE system that can be inserted into the accessory channel of any standard endoscope (pCLE, Cellvizio, Mauna Kea Technologies, Paris, France); and (2) an integrated system in which the CLE probe is integrated into the distal end of a high-resolution endoscope (“integrated”, iCLE; Pentax, Tokyo, Japan). Both system use a blue laser light source that delivers an excitation wavelength of 488 nm, and light emission from the tissue is detected at wavelengths between 205 and 585 nm. The iCLE-system collects images at a manually adjustable scan rate of 1.6 frames per second with a resolution of 1024 × 512 pixels, or at 0.8 frames per second with a resolution of 1024 × 1024 pixels. The depth of scanning can be dynamically adjusted ranging from 0 to 250 μm and the laser power can be manually adjusted between 0 and 1000 μW. The optical slice thickness is 7 μm, with lateral and axial resolution of 0.7 μm and a confocal image field of view of 475 μm × 475 μm. Since the laser probe is integrated into the endoscope, the accessory channel of the endoscope can still be used.
Figure 3.
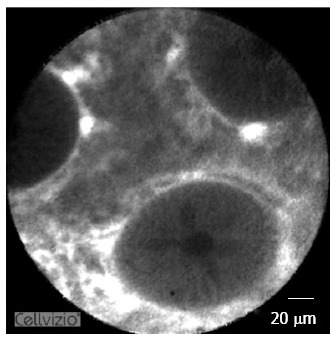
Confocal laser endomicroscopy in the lower gastrointestinal tract. Confocal laser endomicroscopy (CLE) is based on the emission of a low power blue laser into the tissue after topical or systemic administration of contrast agents. The emitted light is then reflected from the tissue and refocused on the detection system by the same lens, leading to microscopic imaging at 1000-fold magnification in real time. As shown in healthy colonic mucosa in this picture, CLE allows a clear visualization of the colonic crypt architecture, single cells within the lamina propria and the microvasculature within the colon.
The pCLE system is based on stand-alone confocal probes, and specific probes available for different indications throughout the entire gastrointestinal tract are available. Probe-based CLE utilizes a fixed laser power and a fixed imaging plane depth for image acquisition. Lateral resolution ranges between 3.5 μm and 1 μm, resulting in a field of view of 600 μm-240 μm, depending on the confocal probe used. Images are acquired at 12 frames/s, leading to real-time videos of the intestinal mucosa and single video frames either in real time or post processed with an increased field of view (4 mm x 2 mm) can be reconstructed using a special computer algorithm (Mosaicing, Mauna Kea Technologies, Paris, France). Probe based CLE in IBD is mostly being performed by using the ColoFlex UHD probe which requires a 2.8 mm working channel. Hence, these probes can be fitted through the working channel of most endoscopes used in clinical practice.
Depending on the clinical question and the scenario in which they are used, both CLE-systems offer unique advantages and specifications. Advantages of the integrated system are its higher resolution and the possibility to alter the laser power and imaging plane depth, whereas the pCLE system allows ad hoc usage in existing endoscopes and enables real time video recording.
Endocytoscopy
Endocytoscopy (EC) allows in vivo microscopic imaging of the GI tract with an magnification ranging from 340-fold up to 1390-fold[49-51] and is based on the principle of contact light microscopy. EC utilizes a fixed-focus, high-power objective lens that projects highly magnified images from a sampling site onto a charge-coupled device[49-51]. The depth of field ranges from 0 to 50 μm and therefore only allows visualization of the very superficial mucosal layer. EC requires thorough mucolysis which can be performed with N-acetyl-cysteine. Further, prestaining of the mucosa with an absorptive agent such as methylene blue, toluidine blue, or cresyl violet is required. Optimal endocytoscopic mucosal imaging can be obtained with 1% methylene blue in the oesophagus and with 0.25% toluidine blue in the stomach and colon after 60 s of exposure to the dye[52].
In fact, a combination of different dye agents is often used to generate optimal tissue contrast and imaging modalities[53]. With the use of absorptive agents via spraying catheters, repeated staining of the mucosa may be needed when the clinical scenario requires extended visualization[50]. Endocytoscopy images are displayed on a monitor at 30 frames per second, which corresponds to the frame rate during routine high-resolution video endoscopy.
Currently, two systems of endocytoscopes are available[50]. Similar to the CLE devices, endocytoscopy devices can be integrated into the distal tip of a standard endoscope (iEC) or utilized as a probe that is advanced through the working channel of a standard endoscope (pEC). Endoscope-based instruments use two different lenses and integrate the EC component within upper (103 cm in length) and lower (133 cm in length) endoscopes and provide a 580x-fold image magnification on a 19-inch monitor, in addition to having conventional optical magnification and narrow band imaging capabilities. Recently, another endocytoscopy system (GIF-Y0002) was introduced consisting of only one lens that allows continuous increase of zooming power from the conventional endoscopy level up to 380-fold (tissue field of view, 700 mm x 600 mm) using a hand lever. Using digital magnification (x 1.6), the magnifying power can be increased to 600-fold, providing a tissue field of view measuring 440 mm x 380 mm[51,54]. For the first time, this new endoscope-generation enables continues magnification from standard overview to endocytoscopy therefore representing an “all-in-one” scope.
Currently, two different probe-based EC devices exist, providing either 450-fold (XEC 300F) or 1390-fold (XEC 120 U) magnification images on a 19-inch monitor[50,51]. The probes are 380 cm in length and 3.2 mm in diameter thus requiring an accessory channel of 3.7 mm. The horizontal observation field is given with 300 μm x 300 μm (0.09 mm2) for the 450-fold magnification probe and with 120 μm x 120 μm for the 1390-fold magnification probe. Similar to what is already discussed with different CLE devices, one of the major advantages of the probe-based EC system lies in its ad hoc usability in already existing endoscopes whereas the integrated EC devices allow to simultaneously take biopsies and thus to directly compare EC imaging with histopathological results.
ROLE AND APPLICTIONS OF ADVANCED ENDOSCOPIC IMAGING IN IBD
Surface level: Dye-based and dye-less chromoendoscopy
In one of the earliest prospective randomized trials on the relevance of dye-based chromoendoscopy (DBC) for the assessment of mucosal inflammation and dysplasia in UC, Kiesslich et al[21] directly compared DBC and conventional colonoscopy in a large cohort of UC patients. Importantly, DBC with methylene blue not only permitted a more accurate diagnosis of the extent and severity of the inflammatory activity in UC compared with conventional colonoscopy, but also significantly improved the early detection of intraepithelial neoplasia and CAC. Another “back-to-back” study evaluated pancolonic indigo carmine staining (0.1%) for the detection of UC-associated dysplasia[20]. As shown in this study, DBC with indigo carmine led to a higher dysplasia detection rate while at the same time reducing the total amount of biopsies[20]. Consistent with these results, another prospective trial also included patients with Crohn’s colitis (CC), and similarly, in both UC and CC, targeted biopsies with dye spray (methylene blue) detected significantly more dysplasia than random biopsies that were taken without the utilization of dye[55]. As shown in a recent meta-analysis of six randomized controlled trials, dye-based chromoendoscopy has a medium to high sensitivity and a high diagnostic accuracy for dysplastic lesions in UC[56] and the typical features of UC associated dysplasia on DBC (and conventional endoscopy) have been summarized by Matsumoto et al[57] from the Hyogo College of Medicine in Japan. Since white-light endoscopy exhibits only low interobserver agreement in differentiating dysplastic from non-dysplastic lesions during colitis surveillance, current guidelines recommend chromoendoscopy with targeted biopsies as the surveillance procedure of choice for appropriately trained endoscopists, whereas white-light endoscopy with random biopsies (quadrant biopsies every 10 cm) remains a reasonable alternative for cancer surveillance in IBD patients[11,12,58].
Since DBC is also associated with a potential increase in examination time, costs and overall effort, a recent study evaluated whether DBC is cost-effective for colorectal cancer surveillance in UC patients. Interestingly, DBC with targeted biopsies is not only more effective but also less costly compared to conventional white-light endoscopy with random biopsies[59]. In its totality, this profound evidence on the superiority of DBC for the detection of colitis-associated neoplasia, together with the knowledge of a cumulative CRC risk in UC patients of 18% after 30 years of disease[7], have led to the recommendation to perform chromoendoscopy with targeted biopsies as the surveillance procedure of choice in IBD patients in US and European guidelines[11,12,34,35].
The first case in which optical DLC was used to help in identifying colitis associated neoplasia was a 63 year old man with longstanding ulcerative colitis and a previous history of dysplasia associated lesions or masses (DALM). In this patient it was shown for the first time that visualization of the pit pattern and the vascular pattern intensity by NBI might help in DALM detection and to distinguish dysplastic from non-dysplastic mucosa in ulcerative colitis[60]. Especially the capillary vasculature in dysplastic lesions exhibited a higher vascular pattern and appeared darker on NBI compared to adjacent normal mucosa[60]. Since then, various trials have studied the potential of NBI to assess mucosal inflammation and colitis associated preneoplastic and neoplastic changes, with so far mixed results. In one of the earliest reports, the Amsterdam group compared the accuracy of NBI with standard colonoscopy for the detection of neoplasia in patients with longstanding ulcerative colitis[61]. Although more suspicious lesions were found during DLC with NBI, the sensitivity of NBI for neoplasia detection was similar to conventional white-light endoscopy[61]. Soon thereafter, the same group assessed the value of NBI for surveillance in UC in two other studies[62,63]. In these studies, pit pattern analysis of neoplastic lesions exhibited only a moderate accuracy for the prediction of histology[62] and also NBI did not improve the detection of UC associated neoplasia compared to high-definition endoscopy[63]. Nevertheless, NBI has been shown to be equally effective in detecting UC associated intraepithelial neoplasia compared to conventional dye-based endoscopy and exhibited a reduced false-positive biopsy rate and a similar true-positive rate[64]. However, the high miss rate with NBI, as pointed out by the authors themselves, makes NBI not advisable as the standard technique to detect dysplasia in patients with long-standing IBD[64] and clearly, higher powered studies are needed to address this question[65,66].
The role of dye-less chromoendoscopy to assess mucosal inflammation associated with IBD has also been studied. In one of the earliest reports, Kudo et al[67] analyzed the mucosal vascular pattern (MVP) in patients with asymptomatic or mildly active UC using NBI and HD white-light endoscopy. The authors found that areas with obscure MVP on NBI exhibit increased numbers of acute inflammatory cell infiltrates, goblet cell depletion and basal plasmacytosis and that evaluation of the MVP with NBI yielded a more precise determination of acute microscopic inflammation in patients with quiescent UC[67]. The typical appearance of active UC and inactive, quiescent disease on NBI have been summarized by the same group of authors[68]. In addition to that, another pilot study on 14 IBD patients was able to demonstrate that areas that appear normal on WLE, but positive on NBI (as defined by a stronger capillary vascular pattern), exhibit an increased leukocyte infiltrate and a significantly increased microvessel density on immunohistology, thus providing first evidence that NBI might allow in vivo imaging of intestinal neoangiogenesis in IBD patients[69].
Data on the relevance of digital DLC for the assessment of mucosal inflammation in IBD patients are limited. To date, only one study evaluated FICE in IBD patients and showed that FICE is not helpful to improve the detection or delineation of ulcers and erosions in CD[70]. Just recently, a study on 78 IBD patients that were randomized to receive either HD white-light endoscopy or HD endoscopy with i-scan, was able to demonstrate that i-scan allows a considerably improved prediction of disease extent and disease activity compared to white-light endoscopy (i-scan: 92% and 90% vs WLE: 49% and 54%)[71]. Of note, examination time was not different between WLE and i-scan, consistent with the idea that dye-less chromoendoscopy is a push-of-a-button technology that can be readily incorporated into the existing examination[71]. Although no studies have directly assessed the relevance of digital chromoendoscopy for the detection of colitis-associated neoplasia and cancer, it has been shown that HD endoscopy with i-scan can detect significantly more neoplastic lesions and more flat adenomas than standard resolution endoscopy[72] and is as precise as dye-based chromoendoscopy for the characterization of small colorectal lesions[73]. Based on these results, data on the assessment of colitis associated dysplasia by digital DLC are eagerly awaited.
Cellular level: Confocal laser endomicroscopy
The technical application of confocal endomicroscopy and the interpretation of images for the utilization in IBD patients can be readily learned. In this regard, it has been shown that after an initial three examinations, performance of CLE significantly improves with a decreased confocal imaging time, successful CLE diagnosis and decline in overall procedural time[74]. In one of the first in vivo studies for dysplasia detection, it was shown that using chromoendoscopy (methylene blue) together with endomicroscopy can detect significantly more neoplasia compared to conventional white-light endoscopy while at the same time requiring 50% fewer biopsies[75]. Soon thereafter, CLE was proven to be accurate also for the differentiation between DALM and adenoma-like mass (ALM), thereby facilitating the clinical decision whether patients should receive endoluminal endoscopic resection or be rather referred for proctocolectomy[76]. Importantly, these studies utilized the integrated CLE system (iCLE) and subsequently, another pilot study utilizing probe-based CLE demonstrated that pCLE for dysplasia surveillance in UC is also feasible with reasonable diagnostic accuracy[77] and the typical appearance of DALM on CLE against inflammatory changes has been characterized as dark cells with crypt density attenuation, a ridged-lined irregular epithelial layer with loss of crypts and dilated and distorted vessels with elevated leakage and irregular vascular architecture[42,78]. A recent meta-analysis on the relevance of CLE for dysplasia detection in either patients with sporadic polyps or IBD patients calculated that CLE can distinguish neoplasms from non-neoplastic tissue in IBD patients with a sensitivity of 83% and specificity of 90%, thereby confirming that CLE can indeed differentiate between neoplastic and non-neoplastic tissue[79].
CLE also has been proven to be accurate and efficient for the real-time in vivo assessment of mucosal inflammation associated with IBD. One of the earliest pilot studies assessed the morphologic differences on CLE between active and inactive UC and it was shown that colonic crypts in non-active UC are small, round and slightly irregularly arranged with small and round crypt lumina, whereas colonic crypts in active UC appear large, variously shaped, irregularly arranged with numerous inflammatory cells and capillaries in the lamina propria[80]. Soon thereafter, Li et al[81] utilized a 4-grade classification of crypt architecture combined with an analysis of microvascular alterations and fluorescein leakage to establish a CLE based classification system for assessment of inflammatory activity in UC patients. All three parameters (crypt architecture, fluorescein leakage, microvasculature) did correlate well with histology, and more than 50% of the patients with normal appearing mucosa on conventional white-light endoscopy exhibited acute inflammation on histology whereas no patient with normal mucosa on CLE showed acute inflammation on histology[81]. Results from our own group indicate that CLE can also reliably assess Crohn’s disease activity: a significantly higher proportion of patients with active CD had increased colonic crypt tortuosity, enlarged crypt lumen, microerosions, augmented vascularization, and increased cellular infiltrates within the lamina propria. In quiescent CD, a significant increase in crypt and goblet cell number was detected compared with controls[82] and based on these findings, we proposed the Crohn’s Disease Endomicroscopic Activity Score (CDEAS) for the assessment of Crohn’s disease activity in vivo. The CDEAS does not only allow to differentiate between quiescent CD and controls but also between quiescent and active disease and shows strong correlation to serum levels of the C-reactive protein[82]. Hence, the CLE and the CDEAS are accurate tools for the accurate prediction of disease severity in CD patients[82].
Epithelial gaps, as originally described on CLE by Kiesslich et al[83] result from shedding of epithelial cells and are of particular relevance for the endomicroscopic evaluation of inflammatory activity in IBD patients. As shown by Liu et al[84], patients with CD exhibit a higher gap density than controls and increased epithelial gap density in the small intestine is a predictor for future hospitalization or surgery in IBD patients[85]. Further, increased cell shedding with fluorescein leakage is associated with subsequent relapse within 12 mo after confocal examination in IBD patients in remission and a CLE based grading system assessing cell shedding and local barrier dysfunction can predict disease flares with high specificity[86].
Taken together, these results demonstrate that CLE can be used to reliably assess the macro- and microscopic inflammatory activity in IBD patients and to obtain tissue histology in real-time. Since the precise determination of mucosal inflammation is of paramount importance to achieve mucosal healing as a key prognostic parameter and important treatment goal in IBD patients[24], it can be expected that CLE will experience a wider-spread utilization not only to facilitate and optimize the management and surveillance of IBD patients but also to prospectively identify patients that are under risk of experiencing a disease flare.
Cellular level: Endocytoscopy
Compared with CLE, less data on the role of EC for the evaluation of mucosal inflammation in IBD patients are available. In an initial report utilizing an EC system with 450-fold magnification, a newly introduced endocytoscopy score assessing the shape and distance between crypts as well as the visibility of superficial microvessels showed a strong correlation with Matts’ histopathological grading[87] and a high reproducibility between different investigators[87]. Recently, our own group tackled the issue whether EC can not only determine inflammatory activity in IBD, but also discriminate single inflammatory cells. For this purpose, we utilized a probe-based EC system with 1390-fold magnification on 19 patients with CD and 21 patients with UC[88]. In this report, we were able to demonstrate that EC is able to reliably distinguish single inflammatory cells, namely neutrophilic, basophilic and eosinophilic granulocytes, and lymphocytes[88]. Further, concordance between endocytoscopy and histopathologic grading of disease activity was 100% and EC exhibited a substantial interobserver and almost perfect intraobserver agreement[88]. The detection of colitis- associated neoplasia or cancer with EC has not been studied to date. However, first evidence suggests that EC can identify dysplasia in aberrant crypt foci as the earliest precursor lesions of colorectal cancer in the dysplasia-carcinoma sequence[89]. In colonic polyps, EC is capable to even detect and distinguish focal high-grade intraepithelial neoplasia[90]. Based on these results, EC is a promising imaging technique that might allow microscopic real-time identification of colitis-associated neoplasia.
Subcellular level: Molecular targeting and molecular imaging
Molecular imaging is based on the utilization of fluorophores with specificity towards a defined molecular target, thereby allowing in vivo visualization on the sub-cellular molecular level. Molecular imaging is a rapidly evolving field and with the ongoing identification of crucial molecules involved in the immunopathogenesis of intestinal diseases in basic research, a steadily growing arsenal of targets that can be visualized with molecular imaging becomes available. The ideal probes utilized for molecular imaging in the gastrointestinal tract should exhibit the following characteristics: high diversity, high affinity binding, rapid binding kinetics within minutes, adequate tissue penetration, low immunogenicity, ability for large scale synthesis and florescent labelling[91]. Agents that have been utilized for molecular imaging include the following substance classes: antibodies, lectines, affinity peptides, activatable probes, nanoparticles and physiological substances[92-94]. So far, molecular imaging has been successfully evaluated in mucosal inflammation and cancer development in both, mice and humans. Just recently, Mitsunaga et al[95] utilized a topically applied enzymatically activatable probe (gGlu-HMRG) which fluoresces in the presence of γ-glutamyltranspeptidase (GGT), an enzyme associated with cancer, to study colitis-associated cancer detection in a murine model. Using fluorescence colonoscopy in mice, gGlu-HMRG fluorescent lesions were detected 5 min after topical administration, even in small lesions, and fluorescence persisted for at least 30 min. Importantly, at autopsy such lesions corresponded to tumour-containing lesions in all cases analyzed and microscopic inflammatory infiltration exhibited a much lower signal than cancer[95]. Consistent with these observations, others studies successfully detected intestinal dysplasia and polyps in murine and xenograft models via the utilization of protease-sensing probes such as a cathepsin reporter probes[96,97], MMP-activatable probes[98], substrates of the γ-glutamyltranspeptidase[95,99], or certain peptides[100,101]. Apart from that, other studies have chosen molecular targets that are known to be upregulated in colorectal cancer and are already established therapeutic targets such as epidermal growth factor receptor (EGFR) or vascular endothelial growth factor receptor (VEGFR) as fluorescent probes for the detection and precise discrimination of colorectal cancer[102,103]. When targeting VEGFR with fluorescently labeled antibodies, CLE visualized the distribution of VEGF in the malignantly transformed tissue in rodent and xenografted models of colon cancer, as well as in human specimens, and thus allowed identification of cancer cells on subcellular level[102]. For EGFR it was shown that EGFR expression levels of different tumors cell lines in xenograft models could be discriminated in vivo in mice with CLE and that topical administration, i.e., the incubation of human colon cancer specimens with the antibody, allowed discerning neoplastic tissue from healthy mucosa[103]. Similar observations were made in a murine model of gastric cancer[104]. In this study, anti-EGFR antibodies as molecular probes not only successfully identified tumor xenografts but also allowed to visualize the subcellular distribution of EGFR[104]. Based on these results, a first human study was conducted just recently in which the fluorescently-labeled anti-EGFR antibody cetuximab was topically applied in CRC patients[105]. Upon visualization with CLE, an EGFR-specific fluorescence signal was present in 18 out of 19 patients with CRC and 12 out of 18 patients with intestinal adenomas while normal mucosa exhibited no or only weak fluorescence[105].
These findings were directly translated into clinical applications and first pre-clinical trials have impressively demonstrated that the visualization of molecular targeted can be utilized for a risk-stratification of individual patients which allows to predict therapeutic response a priori to the initiation of treatment. One of the first studies that provided proof-of-concept utilized nude mice transplanted with colon cancer xenografts with either high or low EGFR expression[106]. CLE was performed 48 h after injection of a test dose of fluorescently labelled cetuximab and subsequently received cetuximab as a cancer treating agent. Importantly, the CLE-assessed fluorescence intensity before initiation of therapy predicted the response to subsequent cetuximab treatment as shown in a significantly slower tumor progression, better physical condition, and longer overall survival in mice that exhibited tumors with high anti-EGFR fluorescence at the initial evaluation[106].
Just recently it has been shown that molecular imaging with fluorescently labeled antibodies and CLE can successfully be used to stratify IBD patients prior to the initiation of treatment into responders and non-responders, thereby allowing a prediction on the therapeutic success.
In this seminal first phase 1 clinical trial, a fluorescently labeled anti-TNF antibody (FITC-adalimumab) was topically applied to the inflamed mucosa of IBD patients during endoscopy via a spraying catheter, and subsequently, the amount of intestinal mTNF+ cells was quantified via CLE[107]. Importantly, patients with high numbers of mTNF+ cells showed significantly higher short-term response rates (92%) at week 12 upon subsequent anti-TNF therapy as compared to patients with low amounts of mTNF(+) cells (15%), despite comparable severity of mucosal inflammation in both patient groups. This clinical response in patients with high amounts of intestinal mTNF+ cells was sustained over a follow-up period of 1 year and was associated with mucosal healing observed at follow-up endoscopy[107]. Hence, these data were the first to indicate that molecular imaging with fluorescent antibodies and CLE has the potential to predict therapeutic responses to biological treatment in CD and might be used for personalized medicine in IBD and potentially other autoimmune or inflammatory disorders. The establishment of this approach and its widespread integration into daily endoscopic routine and patient care would have a tremendous impact since it will not only allow to avoid unnecessary risk exposure associated with biological therapies but would also lead to a considerable economization of the treatment regimens.
In summary, as contoured by the studies described above, molecular imaging is a rapidly emerging field in advanced endoscopic imaging and will likely have paradigm-shifting consequences for daily practice in the foreseeable future. With this approach, endoscopy is in the center of attention and allows the endoscopist, apart from diagnosis and treatment, to acquire a third key competence in medicine: prediction on individual patient level.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 4, 2015
First decision: July 20, 2015
Article in press: September 15, 2015
P- Reviewer: Cheifetz AS, Zouiten-Mekki L S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 4.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 5.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 9.Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–105; quiz 1340-1. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, Tremaine WJ, Melton LJ, Munkholm P, Sandborn WJ. Incidence and prognosis of colorectal dysplasia in inflammatory bowel disease: a population-based study from Olmsted County, Minnesota. Inflamm Bowel Dis. 2006;12:669–676. doi: 10.1097/00054725-200608000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T, Lewis JD, Ullman TA, James T, McLeod R, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 12.Van Assche G, Dignass A, Bokemeyer B, Danese S, Gionchetti P, Moser G, Beaugerie L, Gomollón F, Häuser W, Herrlinger K, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1–33. doi: 10.1016/j.crohns.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Jonsson B, Ahsgren L, Andersson LO, Stenling R, Rutegård J. Colorectal cancer surveillance in patients with ulcerative colitis. Br J Surg. 1994;81:689–691. doi: 10.1002/bjs.1800810520. [DOI] [PubMed] [Google Scholar]
- 14.Löfberg R, Broström O, Karlén P, Tribukait B, Ost A. Colonoscopic surveillance in long-standing total ulcerative colitis--a 15-year follow-up study. Gastroenterology. 1990;99:1021–1031. doi: 10.1016/0016-5085(90)90622-8. [DOI] [PubMed] [Google Scholar]
- 15.Nugent FW, Haggitt RC, Gilpin PA. Cancer surveillance in ulcerative colitis. Gastroenterology. 1991;100:1241–1248. [PubMed] [Google Scholar]
- 16.Rosenstock E, Farmer RG, Petras R, Sivak MV, Rankin GB, Sullivan BH. Surveillance for colonic carcinoma in ulcerative colitis. Gastroenterology. 1985;89:1342–1346. doi: 10.1016/0016-5085(85)90653-5. [DOI] [PubMed] [Google Scholar]
- 17.Choi PM, Nugent FW, Schoetz DJ, Silverman ML, Haggitt RC. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993;105:418–424. doi: 10.1016/0016-5085(93)90715-o. [DOI] [PubMed] [Google Scholar]
- 18.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145–153. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 19.Karlén P, Kornfeld D, Broström O, Löfberg R, Persson PG, Ekbom A. Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis? A population based case control study. Gut. 1998;42:711–714. doi: 10.1136/gut.42.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutter MD, Saunders BP, Schofield G, Forbes A, Price AB, Talbot IC. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256–260. doi: 10.1136/gut.2003.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880–888. doi: 10.1053/gast.2003.50146. [DOI] [PubMed] [Google Scholar]
- 22.Neumann H, Neurath MF, Mudter J. New endoscopic approaches in IBD. World J Gastroenterol. 2011;17:63–68. doi: 10.3748/wjg.v17.i1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann H, Vieth M, Langner C, Neurath MF, Mudter J. Cancer risk in IBD: how to diagnose and how to manage DALM and ALM. World J Gastroenterol. 2011;17:3184–3191. doi: 10.3748/wjg.v17.i27.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619–1635. doi: 10.1136/gutjnl-2012-302830. [DOI] [PubMed] [Google Scholar]
- 25.Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. doi: 10.1016/s0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 26.Coda S, Thillainayagam AV. State of the art in advanced endoscopic imaging for the detection and evaluation of dysplasia and early cancer of the gastrointestinal tract. Clin Exp Gastroenterol. 2014;7:133–150. doi: 10.2147/CEG.S58157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayee B, Inoue H, Sato H, Santi EG, Yoshida A, Onimaru M, Ikeda H, Kudo SE. Magnification narrow-band imaging for the diagnosis of early gastric cancer: a review of the Japanese literature for the Western endoscopist. Gastrointest Endosc. 2013;78:452–461. doi: 10.1016/j.gie.2013.03.1333. [DOI] [PubMed] [Google Scholar]
- 28.Hurlstone DP, Sanders DS, Lobo AJ, McAlindon ME, Cross SS. Indigo carmine-assisted high-magnification chromoscopic colonoscopy for the detection and characterisation of intraepithelial neoplasia in ulcerative colitis: a prospective evaluation. Endoscopy. 2005;37:1186–1192. doi: 10.1055/s-2005-921032. [DOI] [PubMed] [Google Scholar]
- 29.Hurlstone DP, Sanders DS, McAlindon ME, Thomson M, Cross SS. High-magnification chromoscopic colonoscopy in ulcerative colitis: a valid tool for in vivo optical biopsy and assessment of disease extent. Endoscopy. 2006;38:1213–1217. doi: 10.1055/s-2006-944732. [DOI] [PubMed] [Google Scholar]
- 30.Inoue H, Kaga M, Ikeda H, Sato C, Sato H, Minami H, Santi EG, Hayee B, Eleftheriadis N. Magnification endoscopy in esophageal squamous cell carcinoma: a review of the intrapapillary capillary loop classification. Ann Gastroenterol. 2015;28:41–48. [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann H, Mönkemüller K, Günther C, Atreya R, Vieth M, Neurath MF. Advanced endoscopic imaging for diagnosis of Crohn’s disease. Gastroenterol Res Pract. 2012;2012:301541. doi: 10.1155/2012/301541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh R, Hussain A, Loong CK. Narrow band imaging with magnification for the diagnosis of lesions in the upper gastrointestinal tract. World J Gastrointest Endosc. 2013;5:584–589. doi: 10.4253/wjge.v5.i12.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mönkemüller K, Fry LC, Zimmermann L, Mania A, Zabielski M, Jovanovic I. Advanced endoscopic imaging methods for colon neoplasia. Dig Dis. 2010;28:629–640. doi: 10.1159/000320065. [DOI] [PubMed] [Google Scholar]
- 34.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 35.Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Ignjatovic A, Thomas-Gibson S, East JE, Haycock A, Bassett P, Bhandari P, Man R, Suzuki N, Saunders BP. Development and validation of a training module on the use of narrow-band imaging in differentiation of small adenomas from hyperplastic colorectal polyps. Gastrointest Endosc. 2011;73:128–133. doi: 10.1016/j.gie.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 37.Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc. 2010;72:572–576. doi: 10.1016/j.gie.2010.03.1124. [DOI] [PubMed] [Google Scholar]
- 38.Rastogi A, Pondugula K, Bansal A, Wani S, Keighley J, Sugar J, Callahan P, Sharma P. Recognition of surface mucosal and vascular patterns of colon polyps by using narrow-band imaging: interobserver and intraobserver agreement and prediction of polyp histology. Gastrointest Endosc. 2009;69:716–722. doi: 10.1016/j.gie.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 39.Bouwens MW, de Ridder R, Masclee AA, Driessen A, Riedl RG, Winkens B, Sanduleanu S. Optical diagnosis of colorectal polyps using high-definition i-scan: an educational experience. World J Gastroenterol. 2013;19:4334–4343. doi: 10.3748/wjg.v19.i27.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neumann H, Vieth M, Fry LC, Günther C, Atreya R, Neurath MF, Mönkemüller K. Learning curve of virtual chromoendoscopy for the prediction of hyperplastic and adenomatous colorectal lesions: a prospective 2-center study. Gastrointest Endosc. 2013;78:115–120. doi: 10.1016/j.gie.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Testoni PA, Notaristefano C, Di Leo M, Vailati C, Mazzoleni G, Viale E. High-definition with i-Scan gives comparable accuracy for detecting colonic lesions by non-expert and expert endoscopists. Dig Liver Dis. 2013;45:481–486. doi: 10.1016/j.dld.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 42.Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–713. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 43.Goetz M, Malek NP, Kiesslich R. Microscopic imaging in endoscopy: endomicroscopy and endocytoscopy. Nat Rev Gastroenterol Hepatol. 2014;11:11–18. doi: 10.1038/nrgastro.2013.134. [DOI] [PubMed] [Google Scholar]
- 44.Kiesslich R, Canto MI. Confocal laser endomicroscopy. Gastrointest Endosc Clin N Am. 2009;19:261–272. doi: 10.1016/j.giec.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Neumann H, Kiesslich R, Wallace MB, Neurath MF. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology. 2010;139:388–92, 392.e1-2. doi: 10.1053/j.gastro.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 46.Neumann H, Vieth M, Raithel M, Mudter J, Kiesslich R, Neurath MF. Confocal laser endomicroscopy for the in vivo detection of intraepithelial neoplasia in Peutz-Jeghers polyps. Endoscopy. 2010;42 Suppl 2:E139–E140. doi: 10.1055/s-0029-1244052. [DOI] [PubMed] [Google Scholar]
- 47.Tontini GE, Mudter J, Vieth M, Atreya R, Günther C, Zopf Y, Wildner D, Kiesslich R, Vecchi M, Neurath MF, et al. Confocal laser endomicroscopy for the differential diagnosis of ulcerative colitis and Crohn’s disease: a pilot study. Endoscopy. 2015;47:437–443. doi: 10.1055/s-0034-1391226. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Dlugosz A, Neumann H. Beyond white light endoscopy: the role of optical biopsy in inflammatory bowel disease. World J Gastroenterol. 2013;19:7544–7551. doi: 10.3748/wjg.v19.i43.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue H, Kudo SE, Shiokawa A. Technology insight: Laser-scanning confocal microscopy and endocytoscopy for cellular observation of the gastrointestinal tract. Nat Clin Pract Gastroenterol Hepatol. 2005;2:31–37. doi: 10.1038/ncpgasthep0072. [DOI] [PubMed] [Google Scholar]
- 50.Kwon RS, Wong Kee Song LM, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kethu SR, Mamula P, et al. Endocytoscopy. Gastrointest Endosc. 2009;70:610–613. doi: 10.1016/j.gie.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 51.Neumann H, Fuchs FS, Vieth M, Atreya R, Siebler J, Kiesslich R, Neurath MF. Review article: in vivo imaging by endocytoscopy. Aliment Pharmacol Ther. 2011;33:1183–1193. doi: 10.1111/j.1365-2036.2011.04647.x. [DOI] [PubMed] [Google Scholar]
- 52.Kodashima S, Fujishiro M, Takubo K, Kammori M, Nomura S, Kakushima N, Muraki Y, Tateishi A, Kaminishi M, Omata M. Ex-vivo study of high-magnification chromoendoscopy in the gastrointestinal tract to determine the optimal staining conditions for endocytoscopy. Endoscopy. 2006;38:1115–1121. doi: 10.1055/s-2006-944915. [DOI] [PubMed] [Google Scholar]
- 53.Minami H, Inoue H, Yokoyama A, Ikeda H, Satodate H, Hamatani S, Haji A, Kudo S. Recent advancement of observing living cells in the esophagus using CM double staining: endocytoscopic atypia classification. Dis Esophagus. 2012;25:235–241. doi: 10.1111/j.1442-2050.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- 54.Kumagai Y, Kawada K, Yamazaki S, Iida M, Odajima H, Ochiai T, Kawano T, Takubo K. Current status and limitations of the newly developed endocytoscope GIF-Y0002 with reference to its diagnostic performance for common esophageal lesions. J Dig Dis. 2012;13:393–400. doi: 10.1111/j.1751-2980.2012.00612.x. [DOI] [PubMed] [Google Scholar]
- 55.Marion JF, Waye JD, Present DH, Israel Y, Bodian C, Harpaz N, Chapman M, Itzkowitz S, Steinlauf AF, Abreu MT, et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342–2349. doi: 10.1111/j.1572-0241.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 56.Wu L, Li P, Wu J, Cao Y, Gao F. The diagnostic accuracy of chromoendoscopy for dysplasia in ulcerative colitis: meta-analysis of six randomized controlled trials. Colorectal Dis. 2012;14:416–420. doi: 10.1111/j.1463-1318.2010.02505.x. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto T, Iwao Y, Igarashi M, Watanabe K, Otsuka K, Watanabe T, Iizuka B, Hida N, Sada M, Chiba T, et al. Endoscopic and chromoendoscopic atlas featuring dysplastic lesions in surveillance colonoscopy for patients with long-standing ulcerative colitis. Inflamm Bowel Dis. 2008;14:259–264. doi: 10.1002/ibd.20267. [DOI] [PubMed] [Google Scholar]
- 58.Wanders LK, Mooiweer E, Wang J, Bisschops R, Offerhaus GJ, Siersema PD, D’Haens GR, Oldenburg B, Dekker E. Low interobserver agreement among endoscopists in differentiating dysplastic from non-dysplastic lesions during inflammatory bowel disease colitis surveillance. Scand J Gastroenterol. 2015;50:1011–1017. doi: 10.3109/00365521.2015.1016449. [DOI] [PubMed] [Google Scholar]
- 59.Konijeti GG, Shrime MG, Ananthakrishnan AN, Chan AT. Cost-effectiveness analysis of chromoendoscopy for colorectal cancer surveillance in patients with ulcerative colitis. Gastrointest Endosc. 2014;79:455–465. doi: 10.1016/j.gie.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.East JE, Suzuki N, von Herbay A, Saunders BP. Narrow band imaging with magnification for dysplasia detection and pit pattern assessment in ulcerative colitis surveillance: a case with multiple dysplasia associated lesions or masses. Gut. 2006;55:1432–1435. doi: 10.1136/gut.2005.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dekker E, van den Broek FJ, Reitsma JB, Hardwick JC, Offerhaus GJ, van Deventer SJ, Hommes DW, Fockens P. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39:216–221. doi: 10.1055/s-2007-966214. [DOI] [PubMed] [Google Scholar]
- 62.van den Broek FJ, Fockens P, van Eeden S, Reitsma JB, Hardwick JC, Stokkers PC, Dekker E. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut. 2008;57:1083–1089. doi: 10.1136/gut.2007.144097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Broek FJ, Fockens P, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Dekker E. Narrow-band imaging versus high-definition endoscopy for the diagnosis of neoplasia in ulcerative colitis. Endoscopy. 2011;43:108–115. doi: 10.1055/s-0030-1255956. [DOI] [PubMed] [Google Scholar]
- 64.Pellisé M, López-Cerón M, Rodríguez de Miguel C, Jimeno M, Zabalza M, Ricart E, Aceituno M, Fernández-Esparrach G, Ginès A, Sendino O, et al. Narrow-band imaging as an alternative to chromoendoscopy for the detection of dysplasia in long-standing inflammatory bowel disease: a prospective, randomized, crossover study. Gastrointest Endosc. 2011;74:840–848. doi: 10.1016/j.gie.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 65.Pai CG. Dysplasia detection in inflammatory bowel diseases: is narrow-band imaging in the race at all? Gastrointest Endosc. 2012;75:927–98; author rreply 928. doi: 10.1016/j.gie.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Sinha SR, Shah SB. Enhanced imaging technologies in detecting dysplasia in IBD: narrowing or widening our options? Gastroenterology. 2012;143:1108–1110. doi: 10.1053/j.gastro.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 67.Kudo T, Matsumoto T, Esaki M, Yao T, Iida M. Mucosal vascular pattern in ulcerative colitis: observations using narrow band imaging colonoscopy with special reference to histologic inflammation. Int J Colorectal Dis. 2009;24:495–501. doi: 10.1007/s00384-008-0631-9. [DOI] [PubMed] [Google Scholar]
- 68.Esaki M, Kubokura N, Kudo T, Matsumoto T. Endoscopic findings under narrow band imaging colonoscopy in ulcerative colitis. Dig Endosc. 2011;23 Suppl 1:140–142. doi: 10.1111/j.1443-1661.2011.01110.x. [DOI] [PubMed] [Google Scholar]
- 69.Danese S, Fiorino G, Angelucci E, Vetrano S, Pagano N, Rando G, Spinelli A, Malesci A, Repici A. Narrow-band imaging endoscopy to assess mucosal angiogenesis in inflammatory bowel disease: a pilot study. World J Gastroenterol. 2010;16:2396–2400. doi: 10.3748/wjg.v16.i19.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neumann H, Fry LC, Bellutti M, Malfertheiner P, Mönkemüller K. Double-balloon enteroscopy-assisted virtual chromoendoscopy for small-bowel disorders: a case series. Endoscopy. 2009;41:468–471. doi: 10.1055/s-0029-1214603. [DOI] [PubMed] [Google Scholar]
- 71.Neumann H, Vieth M, Günther C, Neufert C, Kiesslich R, Grauer M, Atreya R, Neurath MF. Virtual chromoendoscopy for prediction of severity and disease extent in patients with inflammatory bowel disease: a randomized controlled study. Inflamm Bowel Dis. 2013;19:1935–1942. doi: 10.1097/MIB.0b013e318290550e. [DOI] [PubMed] [Google Scholar]
- 72.Hoffman A, Sar F, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy. 2010;42:827–833. doi: 10.1055/s-0030-1255713. [DOI] [PubMed] [Google Scholar]
- 73.Hoffman A, Kagel C, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-Scan is as precise as chromoendoscopy. Dig Liver Dis. 2010;42:45–50. doi: 10.1016/j.dld.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 74.Neumann H, Vieth M, Atreya R, Neurath MF, Mudter J. Prospective evaluation of the learning curve of confocal laser endomicroscopy in patients with IBD. Histol Histopathol. 2011;26:867–872. doi: 10.14670/HH-26.867. [DOI] [PubMed] [Google Scholar]
- 75.Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR, Neurath MF. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874–882. doi: 10.1053/j.gastro.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 76.Hurlstone DP, Thomson M, Brown S, Tiffin N, Cross SS, Hunter MD. Confocal endomicroscopy in ulcerative colitis: differentiating dysplasia-associated lesional mass and adenoma-like mass. Clin Gastroenterol Hepatol. 2007;5:1235–1241. doi: 10.1016/j.cgh.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 77.van den Broek FJ, van Es JA, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Fockens P, Dekker E. Pilot study of probe-based confocal laser endomicroscopy during colonoscopic surveillance of patients with longstanding ulcerative colitis. Endoscopy. 2011;43:116–122. doi: 10.1055/s-0030-1255954. [DOI] [PubMed] [Google Scholar]
- 78.De Palma GD, Staibano S, Siciliano S, Maione F, Siano M, Esposito D, Persico G. In-vivo characterization of DALM in ulcerative colitis with high-resolution probe-based confocal laser endomicroscopy. World J Gastroenterol. 2011;17:677–680. doi: 10.3748/wjg.v17.i5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su P, Liu Y, Lin S, Xiao K, Chen P, An S, He J, Bai Y. Efficacy of confocal laser endomicroscopy for discriminating colorectal neoplasms from non-neoplasms: a systematic review and meta-analysis. Colorectal Dis. 2013;15:e1–12. doi: 10.1111/codi.12033. [DOI] [PubMed] [Google Scholar]
- 80.Watanabe O, Ando T, Maeda O, Hasegawa M, Ishikawa D, Ishiguro K, Ohmiya N, Niwa Y, Goto H. Confocal endomicroscopy in patients with ulcerative colitis. J Gastroenterol Hepatol. 2008;23 Suppl 2:S286–S290. doi: 10.1111/j.1440-1746.2008.05559.x. [DOI] [PubMed] [Google Scholar]
- 81.Li CQ, Xie XJ, Yu T, Gu XM, Zuo XL, Zhou CJ, Huang WQ, Chen H, Li YQ. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am J Gastroenterol. 2010;105:1391–1396. doi: 10.1038/ajg.2009.664. [DOI] [PubMed] [Google Scholar]
- 82.Neumann H, Vieth M, Atreya R, Grauer M, Siebler J, Bernatik T, Neurath MF, Mudter J. Assessment of Crohn’s disease activity by confocal laser endomicroscopy. Inflamm Bowel Dis. 2012;18:2261–2269. doi: 10.1002/ibd.22907. [DOI] [PubMed] [Google Scholar]
- 83.Kiesslich R, Goetz M, Angus EM, Hu Q, Guan Y, Potten C, Allen T, Neurath MF, Shroyer NF, Montrose MH, et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology. 2007;133:1769–1778. doi: 10.1053/j.gastro.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 84.Liu JJ, Madsen KL, Boulanger P, Dieleman LA, Meddings J, Fedorak RN. Mind the gaps: confocal endomicroscopy showed increased density of small bowel epithelial gaps in inflammatory bowel disease. J Clin Gastroenterol. 2011;45:240–245. doi: 10.1097/MCG.0b013e3181fbdb8a. [DOI] [PubMed] [Google Scholar]
- 85.Turcotte JF, Wong K, Mah SJ, Dieleman LA, Kao D, Kroeker K, Claggett B, Saltzman JR, Wine E, Fedorak RN, et al. Increased epithelial gaps in the small intestine are predictive of hospitalization and surgery in patients with inflammatory bowel disease. Clin Transl Gastroenterol. 2012;3:e19. doi: 10.1038/ctg.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, Pritchard DM, Galle PR, Neurath MF, Watson AJ. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012;61:1146–1153. doi: 10.1136/gutjnl-2011-300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bessho R, Kanai T, Hosoe N, Kobayashi T, Takayama T, Inoue N, Mukai M, Ogata H, Hibi T. Correlation between endocytoscopy and conventional histopathology in microstructural features of ulcerative colitis. J Gastroenterol. 2011;46:1197–1202. doi: 10.1007/s00535-011-0439-1. [DOI] [PubMed] [Google Scholar]
- 88.Neumann H, Vieth M, Neurath MF, Atreya R. Endocytoscopy allows accurate in vivo differentiation of mucosal inflammatory cells in IBD: a pilot study. Inflamm Bowel Dis. 2013;19:356–362. doi: 10.1002/ibd.23025. [DOI] [PubMed] [Google Scholar]
- 89.Cipolletta L, Bianco MA, Rotondano G, Piscopo R, Meucci C, Prisco A, Cipolletta F, de Gregorio A, Salvati A. Endocytoscopy can identify dysplasia in aberrant crypt foci of the colorectum: a prospective in vivo study. Endoscopy. 2009;41:129–132. doi: 10.1055/s-0028-1103452. [DOI] [PubMed] [Google Scholar]
- 90.Neumann H, Vieth M, Neurath MF. Image of the month. Endocytoscopy-based detection of focal high-grade intraepithelial neoplasia in colonic polyps. Clin Gastroenterol Hepatol. 2011;9:e13. doi: 10.1016/j.cgh.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 91.Li M, Wang TD. Targeted endoscopic imaging. Gastrointest Endosc Clin N Am. 2009;19:283–298. doi: 10.1016/j.giec.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Atreya R, Goetz M. Molecular imaging in gastroenterology. Nat Rev Gastroenterol Hepatol. 2013;10:704–712. doi: 10.1038/nrgastro.2013.125. [DOI] [PubMed] [Google Scholar]
- 93.Atreya R, Neurath MF. Novel imaging modalities for immune cell monitoring in the intestine. Curr Opin Gastroenterol. 2014;30:553–558. doi: 10.1097/MOG.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 94.Hoetker MS, Goetz M. Molecular imaging in endoscopy. United European Gastroenterol J. 2013;1:84–92. doi: 10.1177/2050640613483291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mitsunaga M, Kosaka N, Choyke PL, Young MR, Dextras CR, Saud SM, Colburn NH, Sakabe M, Nagano T, Asanuma D, et al. Fluorescence endoscopic detection of murine colitis-associated colon cancer by topically applied enzymatically rapid-activatable probe. Gut. 2013;62:1179–1186. doi: 10.1136/gutjnl-2011-301795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alencar H, Funovics MA, Figueiredo J, Sawaya H, Weissleder R, Mahmood U. Colonic adenocarcinomas: near-infrared microcatheter imaging of smart probes for early detection--study in mice. Radiology. 2007;244:232–238. doi: 10.1148/radiol.2441052114. [DOI] [PubMed] [Google Scholar]
- 97.Marten K, Bremer C, Khazaie K, Sameni M, Sloane B, Tung CH, Weissleder R. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology. 2002;122:406–414. doi: 10.1053/gast.2002.30990. [DOI] [PubMed] [Google Scholar]
- 98.Yoon SM, Myung SJ, Kim IW, Do EJ, Ye BD, Ryu JH, Park K, Kim K, Kwon IC, Kim MJ, et al. Application of near-infrared fluorescence imaging using a polymeric nanoparticle-based probe for the diagnosis and therapeutic monitoring of colon cancer. Dig Dis Sci. 2011;56:3005–3013. doi: 10.1007/s10620-011-1685-z. [DOI] [PubMed] [Google Scholar]
- 99.Urano Y, Sakabe M, Kosaka N, Ogawa M, Mitsunaga M, Asanuma D, Kamiya M, Young MR, Nagano T, Choyke PL, et al. Rapid cancer detection by topically spraying a γ-glutamyltranspeptidase-activated fluorescent probe. Sci Transl Med. 2011;3:110ra119. doi: 10.1126/scitranslmed.3002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joshi BP, Liu Z, Elahi SF, Appelman HD, Wang TD. Near-infrared-labeled peptide multimer functions as phage mimic for high affinity, specific targeting of colonic adenomas in vivo (with videos) Gastrointest Endosc. 2012;76:1197–206.e1-5. doi: 10.1016/j.gie.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Z, Miller SJ, Joshi BP, Wang TD. In vivo targeting of colonic dysplasia on fluorescence endoscopy with near-infrared octapeptide. Gut. 2013;62:395–403. doi: 10.1136/gutjnl-2011-301913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foersch S, Kiesslich R, Waldner MJ, Delaney P, Galle PR, Neurath MF, Goetz M. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut. 2010;59:1046–1055. doi: 10.1136/gut.2009.202986. [DOI] [PubMed] [Google Scholar]
- 103.Goetz M, Ziebart A, Foersch S, Vieth M, Waldner MJ, Delaney P, Galle PR, Neurath MF, Kiesslich R. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology. 2010;138:435–446. doi: 10.1053/j.gastro.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 104.Hoetker MS, Kiesslich R, Diken M, Moehler M, Galle PR, Li Y, Goetz M. Molecular in vivo imaging of gastric cancer in a human-murine xenograft model: targeting epidermal growth factor receptor. Gastrointest Endosc. 2012;76:612–620. doi: 10.1016/j.gie.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 105.Liu J, Zuo X, Li C, Yu T, Gu X, Zhou C, Li Z, Goetz M, Kiesslich R, Li Y. In vivo molecular imaging of epidermal growth factor receptor in patients with colorectal neoplasia using confocal laser endomicroscopy. Cancer Lett. 2013;330:200–207. doi: 10.1016/j.canlet.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 106.Goetz M, Hoetker MS, Diken M, Galle PR, Kiesslich R. In vivo molecular imaging with cetuximab, an anti-EGFR antibody, for prediction of response in xenograft models of human colorectal cancer. Endoscopy. 2013;45:469–477. doi: 10.1055/s-0032-1326361. [DOI] [PubMed] [Google Scholar]
- 107.Atreya R, Neumann H, Neufert C, Waldner MJ, Billmeier U, Zopf Y, Willma M, App C, Münster T, Kessler H, et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med. 2014;20:313–318. doi: 10.1038/nm.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Subramanian V, Ramappa V, Telakis E, Mannath J, Jawhari AU, Hawkey CJ, Ragunath K. Comparison of high definition with standard white light endoscopy for detection of dysplastic lesions during surveillance colonoscopy in patients with colonic inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:350–355. doi: 10.1002/ibd.23002. [DOI] [PubMed] [Google Scholar]
- 109.Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489–501.e26. doi: 10.1016/j.gie.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 110.Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639–651.e28. doi: 10.1053/j.gastro.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 111.Neumann H, Kudo SE, Kiesslich R, Neurath MF. Advanced colonoscopic imaging using endocytoscopy. Dig Endosc. 2015;27:232–238. doi: 10.1111/den.12395. [DOI] [PubMed] [Google Scholar]


