Abstract
The incidence of paediatric inflammatory bowel disease (PIBD) has dramatically increased in the last 20 years. Although first reported in mid 1970s’, diagnostic laparoscopy has started to be routinely adopted in paediatric surgical practice since late 1990s’. Minimally invasive surgery was first limited to diagnostic purposes. After 2002 it was also applied to the radical treatment of PIBD, either Crohn’s disease (CD) or Ulcerative colitis. During the last decade minimally invasive approaches to PIBD have gained popularity and have recently became the “gold standard” for the treatment of such invalidating and troublesome chronic diseases. The authors describe and track the historical evolution of minimally invasive surgery for PIBD and address all available opportunities, including most recent advancements such as robotic surgery, single port approaches and minimally invasive treatment of perianal fistulising CD. A systematic review of all series of PIBD treated with minimally invasive approaches published so far is provided in order to determine the incidence and type of patients’ complications reported up to present days. The authors also describe their experience with minimally invasive surgery for PIBD and will report the results of 104 laparoscopic procedures performed in a series of 61 patients between January 2006 and December 2014.
Keywords: Paediatric inflammatory bowel disease, Ulcerative colitis, Crohn’s disease, Laparoscopy, Minimally invasive approach, Complications
Core tip: This review aims at describing the historical evolution of minimally invasive surgery for paediatric inflammatory bowel diseases (PIBD). We will go through all recent technical advancements, provide an overview of our personal experience and perform an extensive systematic review of available data. The series of patients reported so far will be analysed and most relevant issues addressed in details. We do believe that this review will help physicians dealing with PIBDs by reporting and discussing the most advanced surgical opportunities. A special focus on complications and moreover, long-term outcome will help in implementing adequate education for parents.
INTRODUCTION
Inflammatory bowel disease (IBD) represents a group of chronic relapsing intestinal inflammatory conditions, namely Crohn’s disease (CD), ulcerative colitis (UC), and IBD unclassified (IBD-U, a form of colitis whose features made it impossible to discriminate between CD and UC)[1]. Although the exact aetiology of IBD remains unclear, these disorders are thought to result from the interactions of genetics, deranged host immunity and environmental factors[2].
Between 5% and 25% of IBDs occur in children[3]. Although the highest incidence of paediatric IBDs (PIBD) is during adolescence, with two-folds higher prevalence for CD over UC, these disorders can also occur in very young children (< 6 years of age, very early onset IBD). This latter age group is usually characterized by pancolonic inflammation (frequently IBD-U) with severe clinical course and high rate of resistance to immunosuppressive therapy. In these instances a primitive immunodeficiency should always be investigated. At present, the estimated incidence of PIBD ranges between 0.25 and 13.30 per 100000, with a dramatic increase over the last 20 years[2,4].
In children, clinical features of IBDs may be extremely diverse and somehow differ from those of adults, above all in CD. Bloody diarrhoea represents the most common symptoms at onset in UC. Abdominal pain, diarrhoea, weight loss, fever, fatigue, and growth retardation are typically reported in CD with a prevalence ranging between 95% and 25% of cases. As for adults, extraintestinal manifestations are not unlikely in patients with PIBD. Those are reported in 25% to 35% of cases. Interestingly, in children, these symptoms can precede the onset of gastrointestinal disease whereas in adults they tend to occur concurrently with the exacerbation of the disease[5].
Disease localization and severity in children with UC can vary. At onset UC involvement is extensive (pancolitis) in 60%-80% of all patients, while rectosigmoid and left-sided disease are less frequent. Disease extent is consistently associated with disease severity and children have more aggressive disease course with at least one acute severe colitis (ASC) before adulthood[6]. In case of CD, isolated involvement of terminal ileum (± limited to the caecum) is shown in 16% of cases. Isolated colonic disease is reported in 27% and ileocolonic in 53% of cases. Of note, although isolated upper gastrointestinal localization is reported in 4% of patients, 30% have esophagogastroduodenal involvement and 24% jejunal/proximal ileal disease[7]. Perianal disease accounts for 15% of patients. Of note, CD may have insidious onset that leads to delay in diagnosis[6,7].
Medical management of PIBD include nutritional therapy, aminosalicylates, steroids, antibiotics, immunomodulators (i.e., thiopurine, methotrexate), and biologic therapy (infliximab, adalimumab). All drugs can be administered in patients with mild to severe forms of PIBD in order to achieve or maintain remission.
In case of failure of medical treatment, surgical management is indicated to deal with complications. Indications to surgery include bleeding (UC and IBD-U), perforation/abscess (CD), obstruction (CD), stricture (CD), fistula (CD), toxic megacolon (all PIBD), failure of medical therapy (UC), severe growth retardation (UC), and dysplasia or malignancy (all PIBD). In CD a conservative surgical strategy is generally warranted. However, the specific surgical procedure adopted in each case (segmental resection partial colectomy, total colectomy with ileostomy or ileo-rectal anastomosis, and total proctocolectomy with end-ileostomy) depends upon the site of involvement and the type and severity of complications. In UC surgery is curative and includes total colectomy and J-pouch ileo-anal anastomosis with sphincter preservation either resorting to endorectal pull-through or subtotal proctectomy. Ultimately, more than 50% of patients with CD will require resections, whereas 15% to 40% of those with UC will require a colectomy[2,5].
In recent years, as for most of general surgery, surgeons have moved from conventional laparotomy to minimally invasive laparoscopic approach for PIBD. Below the Authors will provide a literature review of recent publications and reports on this regard and will provide details of a series of patients treated at Giannina Gaslini Institute during the last decade.
METHODOLOGY
Literature review
Two independent investigators performed the literature search, using PubMed, EMBASE, and Ovid database. The search terms were “laparoscopy”, “surgery”, “children”, and ”Inflammatory Bowel Disease”, or “Ulcerative colitis”, “Crohn Disease”, and “Indeterminate Colitis”. Inclusion criteria were: (1) paper fully written in English language; and (2) patients younger than 18 years of age. All prospective, observational, and retrospective studies were included. Case reports were excluded.
Data were extracted from articles that fulfilled inclusion and exclusion criteria and entered into tables. These data included first Author, country of origin, years of data collection, series size, PIBD types, and incidence of complications.
Personal series
The medical records of all patients affected by PIBD (CD, UC, IBD-U) from January 2006 to December 2014 who underwent minimally invasive surgery (laparoscopic or laparoscopic-assisted) at Giannina Gaslini Children Hospital were reviewed. We recorded demographic data, type of procedure performed (single or staged), operating time, morbidity and length of hospital stay from our centralized operating room database.
Data reporting and statistical analysis
Descriptive statistics were reported as absolute frequencies and percentages for qualitative data, mean ± SD or median and range (based on variability) were used to describe quantitative variables. Differences in the frequencies of each variable were evaluated by the χ2 test, or by Fisher’s exact test, when appropriate. All the statistical tests were two sided and a P value lower than 0.05 was considered as statistically significant.
LITERATURE REVIEW
Overall literature review
On the basis of the available literature data, we could address minimally invasive surgery for PIBDs focusing on diagnostic laparoscopy before 2000 and on laparoscopic treatment, afterwards. Furthermore, therapeutic laparoscopy can be divided basing on the adopted surgical procedure[8-26].
Diagnostic laparoscopy
The first report regarding diagnostic laparoscopy dates back to 1975 when Leape et al[27] reported the first series of children and infants undergoing diagnostic laparoscopy for various issues, including CD. Diagnostic laparoscopy was used to determine what surgical treatment was eventually required with a conventional laparotomy. Later on, in 1996, Miller and colleagues reported the use of minimally invasive surgery to detect the presence of abnormal mesenteric fat (“creeping fat”) in patients with suspected CD. The Authors described 6 children who underwent diagnostic laparoscopy. Three of them were suspected of having CD and underwent resection confirming the diagnosis[28]. Similarly, in 1998, Schier et al[29] reported a series of 11 children who underwent diagnostic laparoscopy without major complication. The Authors confirmed the usefulness of direct images in the early stages of CD for a better implementation of adequate medical treatments.
Laparoscopic treatment
Laparoscopy has been adopted in adults since early 90s’ either for the treatment of UC or CD with good results[30,31]. In children, therapeutic laparoscopy for IBDs has been introduced since early 2000s’. The first report of laparoscopic treatment of CD dates back to 2002, when Rothemberg reported his preliminary experience with his first 15 segmental bowel resections for CD[10].During the same year, Georgeson reported his preliminary experience with 18 patients with UC who underwent Laparoscopic assisted total colectomy with pouch reconstruction[9]. Both authors reported their preliminary experiences on relatively small pediatric series demonstrating the feasibility of minimally invasive surgery for the treatment of IBDs in children, either CD or UC. Similarly, Proctor in 2002 and Dutta in 2003 reinforced these considerations in two separate reports[8,12]. In particular, Proctor and colleagues reported a comparative retrospective analysis of the results of open vs laparoscopic subtotal colectomy for UC, treated in their institution between 1999 and 2001. The authors concluded that laparoscopy requires longer surgery but better cosmetic results with shorter return to normal activities and bowel function, being the incidence of major complications unaffected by the chosen approach. Furthermore, the authors underlined how length of surgery may be improved during the learning curve, as previously reported in adult literature[8].
Up to present years, a number of publications reported extensive use of laparoscopy for the treatment of PIBD and larger series have been reported. So far, the largest series published in international literature is that by Diamond and colleagues who reported a series of 136 patients who underwent 154 laparoscopic procedures for PIBD in the period between 1999 and 2007. The authors reported improved cosmetic results, a reduced hospitalisation but an incidence of major complications comparable to that of open surgery, particularly intestinal obstruction[18] (Table 1).
Table 1.
List of available publications concerning minimally invasive approach for inflammatory bowel diseases in pediatric population (PubMed, EMBASE, Ovid) n (%)
| Ref. | Country | Year | IBD | CD | UC | IBD-U | Complications | Years |
| Proctor et al[8] | Canada | 2002 | 8 | 1 | 5 | 2 | 4 (50) | 1999-2001 |
| Georgeson[9] | United States | 2002 | 18 | 0 | 18 | 0 | NS | NS |
| Rothenberg et al[10] | United States | 2002 | 15 | 15 | 0 | 0 | 1 (7) | NS |
| von Allmen et al[11] | United States | 2003 | 12 | 12 | 0 | 0 | 1 (8) | 1997-2002 |
| Dutta et al[12] | United States | 2003 | 15 | 15 | 0 | 0 | 2 (13) | 1998-2002 |
| Simon et al[13] | United States | 2003 | 29 | NS | NS | NS | 5 (17) | 1991-2002 |
| Bonnard et al[14] | France | 2006 | 11 | 11 | 0 | 0 | 2 (18) | 1999-2004 |
| Meier et al[15] | United States | 2007 | NS | NS | NS | NS | NS | NS |
| Fraser et al[16] | United States | 2010 | 27 | 0 | 27 | 0 | 18 (66) | 1998-2008 |
| Flores et al[17] | Argentina | 2010 | 13 | 0 | 13 | 0 | 4 (31) | 1991-2009 |
| Diamond et al[18] | Canada | 2010 | 136 | 83 | 50 | 3 | 50 (37) | 1999-2007 |
| Mattioli et al[19] | Italy | 2011 | 16 | 3 | 12 | 1 | 6 (24) | 2006-2010 |
| Laituri et al[20] | United States | 2011 | 30 | 30 | 0 | 0 | 3 (10) | 2000-2009 |
| Potter et al[21] | United States | 2012 | 9 | 2 | 6 | 1 | 5 (55) | 2010-2011 |
| Linden et al[22] | United States | 2013 | 68 | 0 | 68 | 0 | 13 (19) | 2003-2011 |
| Huang et al[23] | United States | 2013 | 44 | 25 | 16 | 3 | 10 (22) | 2002-2011 |
| Stephens[24] | Ireland | 2013 | 9 | 0 | 9 | 0 | 3 (33) | 2009-2011 |
| Sharp et al[25] | United States | 2014 | 28 | 28 | 0 | 0 | 8 (29) | 2009-2013 |
| Vrecenak et al[26] | United States | 2014 | 71 | 71 | 0 | 0 | 23 (32) | 2001-2010 |
| Total | 559 | 296 | 224 | 10 | 158 (29.2) | 1991-2013 |
Case reports or case series have been excluded. This table provides the overall number of IBDs treated in a selected time span. Whenever available, the series were differentiated into patients with UC, CD and IC. Overall, a relatively high prevalence of major complications requiring some sort of surgical intervention has been reported (higher than 29%), still similar or even lower when compared to what previously published for open surgery. Complications rate was calculated basing on series providing incidence (541 overall patients). IBD: Inflammatory bowel disease; CD: Crohn’s disease; UC: Ulcerative colitis; IBD-U: Inflammatory bowel disease - unclassified; NS: Not stated.
Surgical procedures
Small bowel and/or ileocolic resection: Either laparoscopically-assisted or total intracorporeal ileo-colic resection and segmental ileal resection have been reported. Those procedures have been adopted in patients with CD and consist of laparoscopy with 3 to 4 ports. The whole bowel is inspected and the site of resection is determined by preoperative imaging matched to intraoperative evidences. The resection-anastomosis can be accomplished via a mini-laparotomy (either extending umbilical port incision or by means of a small modified Pfannenstiel incision) through which the bowel is exteriorized, resected and anatomised[13,18]. Alternatively, total intracorporeal resection and anastomosis can be performed as described by Rothemberg and Dutta in their previous reports[10,12].
Although some Authors suggested to resort to the “safer” extracorporeal anastomoses (laparoscopic-assisted approach) due to the inflamed and fragile bowel to be anastomized with staplers[13,14], both alternatives have proved to be safe and effective in experienced hands and are now used worldwide in CD.
Strictureplasty: The Heineke-Mikulicz strictureplasty can be performed with a minimally invasive approach, either totally intracorporeal or laparoscopic-assisted. Although mostly limited to upper gastrointestinal tract or to multisite CD involvement for intestinal preservation, in paediatric settings this procedure can be accomplished with good results. Of note, Romeo and colleagues demonstrated that the recurrence rate of strictureplasty overlaps that of resection-anastomosis, thus making this technique a valid option for selected CD patients at risk for short bowel syndrome following resection[32].
Total or subtotal colectomy: Either in elective or emergency setting, total or subtotal colectomy can be carried out with results that overlap and/or overcome those of conventional open surgery[18]. This procedure has been mostly used for the treatment of UC or IBD-U (occasionally for the treatment of pancolonic CD) and consists of a 4 to 5 ports laparoscopy. The left colon is approached first and divided close to peritoneal reflection with straight or angled stapling devices. Mesentery is divided using available laparoscopic sealing devices (Ligasure® in our experience) and colectomy is carried on in anticlockwise direction. Particular care must be taken when dividing midcolonic vessels in order to avoid lesions of first jejunal loop at Treitz ligament. Similarly, another key-point of colectomy is the right hepatic flexure when surgeons must spare and protect the duodenum and hepatic hilum. Once colonic isolation and resection reaches the caecum, the whole colon can be extracted through the right iliac fossa port and the same wound can be used to fashion the ileostomy. Alternatively, the colon can be extracted by everting the rectum through the anus and stapling the rectum from outside. This alternative approach can turn useful in the elective setting in case of small children, particularly those with IBD-U.
Straight or J-pouch reconstruction? Although a debate regarding the indication to fashion a pouch during reconstruction after colectomy in PIBD exists, most surgeons resort to this technique. A straight ileo-anal or ileo-rectal anastomosis is used only by a limited number of Authors. In 2006, Tilney and colleagues performed a meta-analysis demonstrating that, though basing on a very few good-quality studies, pouch procedures should be preferred in order to achieve better survival and functional outcome[33]. On the basis of these considerations, although pouch implies frequent endoscopic follow up and a relatively high incidence of pouchitis, (reported in up to 50% of patients[34-36]), pouch procedures represent at the moment the gold standard for reconstruction after colectomy in children with PIBD.
J-pouch ileo-rectal or ileo-anal anastomosis? This is one of the most controversial topics in the surgical treatment of PIBD and represents a key element in the radical treatment of UC/IBD-U and of pancolonic CD. Some surgeons opt for a mucosectomy, endorectal pull-through and J-pouch ileo-anal anastomosis[9]. Others do prefer subtotal proctocolectomy and J-pouch ileo-rectal anastomosis 2-3 cm above the dentate line. The most relevant issues leading surgeons’ choice are: (1) relatively high risk of leaving small islands of rectal diseased mucosa in the rectal cuff, in case of endorectal dissection; and (2) relatively high risk of dysplasia and cancer in the 2-3 residual centimetres of rectum left in place in case of J-pouch ileo-rectal anastomosis[9,37]. Regardless of the choice for reconstruction, both options are amenable of laparoscopic approach. The mesentery of the terminal ileum is widened and lengthened and a J-pouch is created through the site of the ileostomy. J-pouch length varies between short pouches[38] and longer ones[18] according to surgeons. The pouch is then returned into the abdomen and the rectal pouch is dissected with a sealing device (i.e., Ligasure®) down to the elevator ani and stapled. A circular stapling device is then inserted into the anus and the ileal-pouch is anastomized 2-3 cm above the dentate line. Alternatively, the endorectal dissection can be accomplished starting 1-2 mm above the dentate line in order to perform a classic endorectal pull-through, as described for Hirschsprung’s disease[39].
Single or staged procedures: Total colectomy and J-pouch reconstruction can be accomplished as a single stage (Total colectomy with J-pouch reconstruction and no protective ileostomy), two-stage (Total colectomy with ileostomy followed by J-pouch reconstruction without protective ileostomy) or three-stage procedure (Total colectomy with ileostomy followed by J-pouch reconstruction and protective ileostomy) depending on surgeons’ attitude and on patients’ general conditions. Protective ileostomy is chosen by most surgeons[8,9,18,38]. Nonetheless, as complications have been frequently related to the ileostomy (i.e., internal hernia, prolapse, adhesion, twisting)[18,19] most surgeons addressed this issue and questioned whether the routine use of protective ileostomy should be abandoned in favour of a strict selection of patients with the highest risk of anastomotic complications (fulminant colitis? very low body mass index? low albumin levels?). Anyway, a three-stage approach is recommended in all emergent situation, i.e., fulminant colitis, patients on high dose steroids, severe malnutrition and IBD-U.
Further technical improvements: Recently, SILSTM devices to perform single incision laparoscopic surgery have been adopted to further contain the trauma of abdominal wall and to improve the outcome of the patients both in terms of reduced pain, shorter postoperative stay, earlier recovery of normal bowel functions and improved cosmetic appearance[25,38]. Finally, robotic surgery is on its way to be applied to J-pouch ileo-rectal or ileo-anal anastomosis, given the possibility to apply this innovative technologies to rectal pouch dissection in order to further minimize the risk of damaging perirectal structures (personal unreported experience).
Complications and long-term functional outcome
Comparing open and laparoscopic surgery for PIBD it comes clear that the incidence of complications does not significantly differ[8,12,35]. Nonetheless, surgery for PIBD is somehow frustrating given the relatively high incidence of surgical problems that can occur in the postoperative period. In fact, complications requiring surgical intervention occurred with an average incidence of 29% (158 out of overall 541 pediatric patients in this review) (Table 1). This percentage is similar or even lower than that reported for open surgery. The most frequent issues are represented by intestinal obstruction, anastomotic leakage or stenosis, pouchitis and faecal incontinence[8,18,19,34,40-44].
In particular, the incidence of complications approaches 55% for UC, being that of intestinal obstruction of around 25% and that of pouchitis of nearly 50%[34]. Similarly, the incidence of complications following surgery for CD can be as high as 33% with both early and late complications reported in pediatric patients[40,41]. This should confirm a higher likelihood of complications for patients with UC.
Functional outcome is acceptable with a wide variability of outcomes in different literature reports. Stavlo et al[42] has reported normal continence in 100% of patients in 2003 and Wewer in less than 50% of patients in 2005[43]. This wide range of results is difficult to explain. Though, most surgeons report a relatively high incidence of soiling, urge incontinence and night-time faecal continence issues in the long term[18,19,42-44]. All these issues must be acknowledged to families approaching surgery for PIBD in order to achieve a better education and participation in the long term care of their relatives.
Perianal fistulising CD - minimally invasive surgical options
Since no effective and definitive therapeutic option have been identified for the treatment of perianal disease in children with CD but the need for reduced trauma and sphincters preservation[45], the issue of perianal abscesses and fistula remains difficult to deal with and extremely troublesome both for the patient and the care-giver. In 2013 the World Congress of Gastroenterology implemented shared guidelines and therapeutic options for the medical and surgical management of perianal fistulising CD. The most important aspect is to appropriately select patients for surgery. On this regards, it is widely accepted that surgery for fistulising CD should be used only following complete mucosal healing and no active disease[46]. Those patients with perianal fistulising CD and healthy rectal mucosa are amenable of various surgical options, namely fistulotomy, biological plugs, fibrin glue, advancement flaps, fistula resections (including the so-called “cone-like” resection), stem cells and gracilis muscle interposition[46]. Only some of them can be considered as truly minimally invasive, namely biological plugs, fibrin glue and stem cells based therapy. In particular, the promising fibrin glue treatment showed results similar to those obtained with other established surgical treatment[47]. Although stem cells based therapy proved to be similarly effective, its healing rate of roughly 80% showed a dramatic decrease to nearly 30% over time[46].
Recently, Meinero et al[45] described an innovative and minimally invasive technique for the treatment of anal fistula, named Video Assisted Anal Fistula Treatment. Though based on a small series of patients, Schwandner[48] showed the feasibility of this approach in fistulising CD and the possibility to treat transphincteric, suprashpincteric and rectovaginal fistulae with little to no complications and minimal discomfort. Although results are based on only 11 patients, over 80% success rate and absent continence deterioration are promising aspects for this innovative technique.
PERSONAL SERIES
Between January 2006 and December 2014 (9 years) a total of 104 laparoscopic procedures (98 primary laparoscopy, 6 reoperations for complication) were performed in 61 patients with PIBD. Diagnoses included 39 UC, 20 CD and 2 IBD-U.
Indications to surgery for patients with UC were mostly represented by haemorrhagic colitis, followed by failure of medical treatment and intestinal obstruction/stricture.
Forty-five patients underwent laparoscopic subtotal colectomy (LSTC) and 38 laparoscopic J-Pouch Ileo-Rectal restorative Anastomoses (JPIRA) (Figures 1-3 illustrate our JPIRA technique according to what previously published[38]). In 41 patients LSTC was associated to a protective temporary ileostomy. Four patients underwent LSTC along with JPIRA in a single stage procedure. Definitive ileostomy closure was accomplished in 38 patients.
Figure 1.
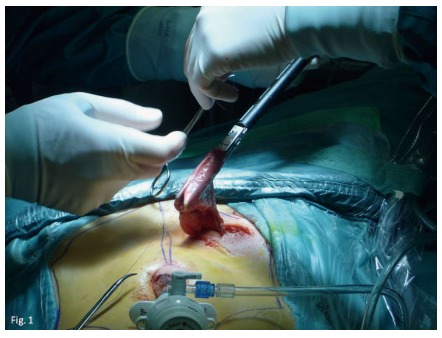
J-pouch is fashioned through the stoma site with a linear stapler.
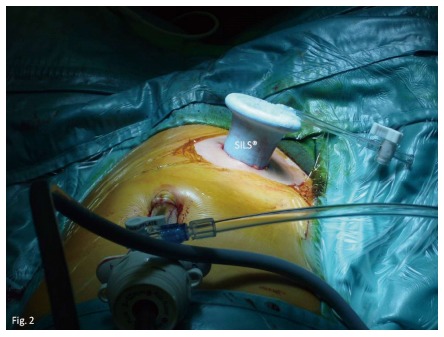
Figure 3.
Surgeon can end up with a four-ports procedure using a SILS® device and the ports sites used for the previous laparoscopic subtotal colectomy.
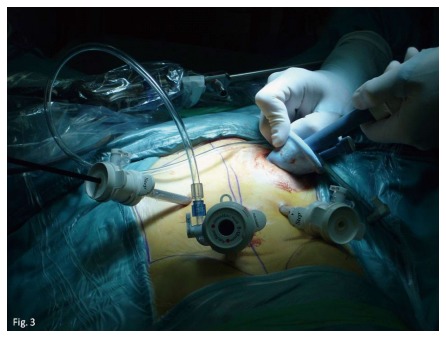
Figure 2.
SILS® device can be inserted in the stoma site to help with the perirectal dissection during the J-Pouch Ileo-Rectal restorative anastomoses procedure.
Fifteen laparoscopic segmental resections have been performed in patients with CD. Two patients required conversion to laparotomy due to the extremely difficult mobilization and manipulation of the inflamed and fragile small bowel. Twelve of these procedures were either ileo-colic resections or right hemicolectomies, all with extracorporeal anastomoses except one that was totally intracorporeal performed. Three were segmental small bowel resections with extracorporeal anastomoses. See Table 2 for details.
Table 2.
Personal series of paediatric inflammatory bowel disease treated laparoscopically
| UC | CD | IBD-U | Overall | ||
| Patients | 39 | 20 | 2 | 61 | |
| Males | 25 | M:F ratio 0.7:1 | |||
| Females | 36 | ||||
| Age at surgery (yr) | 9.5 (1.1-20.2) | ||||
| Indications | |||||
| Haemorrhagic colitis | 26 | 3 | 2 | 31 | |
| Failure of treatment | 13 | 3 | 0 | 16 | |
| Obstruction/strictures | 0 | 13 | 0 | 13 | |
| Other | 0 | 11 | 0 | 1 | |
| Procedures | |||||
| LSTC | 39 | 4 | 2 | 45 | Overall 98 procedures |
| Median operative time (min) (range) | 215 (80-350) | ||||
| Median length of hospitalization (d) (range) | 8.5 (3-11) | ||||
| JPIRA | 38 | 0 | 0 | 38 | |
| Median operative time (min) (range) | 195 (130-260) | ||||
| Median length of hospitalization (d) (range) | 3.5 (2-11) | ||||
| Segmental resection | 0 | 15 | 0 | 15 | |
| Median operative time (min) (range) | 145 (85-205) | ||||
| Median length of hospitalization (d) (range) | 7 (2-15) | ||||
| Complications (18 events), n (%) of procedures | 8 (13) | 4 (30) | 1 (50) | 18% of procedures | P = 0.08062 |
| Stoma-related issues (6 events), n (%) of procedures) | 4 (11) | 1 (33) | 1 (50) | 14% of procedures | P = 0.63223 |
Pelvic abscess due to fistulising CD;
Statistical analysis was performed to compare the prevalence of complications in CD and UC;
Statistical analysis was performed to compare the prevalence of complications related to the stoma (14.3%) and that of complications related to other aspect of surgery (12.2%). Overall, we performed 98 primary laparoscopic procedures and 6 laparoscopic management of surgical complications. Complications were experienced by 21% of our patients following 18% of procedures. Though without statistical significance, complications were more likely to occur in patients with CD when compared to those with UC (30% vs 13%). UC: Ulcerative colitis; CD: Crohn disease; IBD-U: Inflammatory bowel disease - unclassified; LSTC: Laparoscopic SubTotal colectomy; JPIRA: J-Pouch Ileo-Rectal restorative anastomosis.
A total of 18 complications requiring some sort of surgical intervention were experienced by 13 patients (21% of patients being 8 UC, 4 CD and 1 IBD-U). Patients with UC experienced 4 bowel obstruction (all dealt with laparoscopically), 2 anastomotic leaks, 1 endoperitoneal bleeding, 1 anastomotic stricture, 1 ileostomy prolapse, 1 J-pouch prolapse (treated by laparoscopic pouch-pexy). Complications occurred after an average of 2 years postoperatively (range 1 d - 4 years). Patients with CD (20% of patients, 30% of overall procedures) experienced 2 anastomotic leaks, 1 bowel obstruction due to anastomotic stricture, 1 anastomotic leak, 1 pelvic abscess, and 1 ileostomy prolapse. Complications occurred after an average of 52 d postoperatively (range 3-240 d). One patient with IBD-U experienced enterocutaneous fistula at the stoma site.
Long term outcome is being assessed and exhaustive data are now available only for a minority of patients (10 out of 38 who underwent JPIRA, as previously published) with a minimum follow up of 15 mo with satisfactory results in terms of continence, perspectives and cosmetic results[19].
With regard to perianal fistulising CD we routinely apply the so called “cone like resection” with mucosal advancement flaps, which proved to be effective in solving fistulae with promising results. We recently adopted the VAAFT procedure to treat perianal fistula in patients without CD and this minimally invasive approach proved to be feasible and safe in the pediatric population (unpublished data). We now aim at applying this approach to a selected subpopulation of perianal fistulising CD that would benefit of this minimally invasive treatment.
All in all, our experience is in line to what previously published in international literature and confirms the feasibility, safety end effectiveness of minimally invasive surgery for PIBD. Though, complications can still occur and can involve roughly 20% of our patients. Of note, in contrast to what previously published, we observed a higher likelihood of complications in CD (Table 2) but this difference proved not to be statistically significant and deserves a larger series of patients to be confirmed. Anyway, families must be acknowledged on this regard.
CONCLUSION
Advances in surgical treatment of PIBD are striking and include the use of “conventional” laparoscopy, single-incision laparoscopy, robotic surgery and other minimally invasive approaches. Overall technical details and indications do not significantly differ between adults and children. In fact, minimally invasive surgery have been adopted either in the elective or emergency setting thanks to incidence of complications that proved not to significantly differ from that of conventional open surgery but shorter hospitalization and fewer long term sequelae[49]. According to literature review and personal experience, we can provide good results since indications are based on widely accepted international standards and surgery performed by highly experienced surgeons in third level hospitals. Minimally invasive surgery and fast-track concept of care have been confirmed to fit with PIBD management though a number of problems still occur. In fact, our extensive literature review showed an average incidence of complication of nearly 30% thus confirming the measure of risk for this surgery. On the grounds of these considerations, parents should be adequately acknowledged regarding the risk-benefit ratio of surgery in these high-risk cases. A strict cooperation between surgeons, gastroenterologists, anaesthetists and pathologists is thus required in a multidisciplinary approach to serve the best for our patients.
ACKNOWLEDGMENTS
We want to thank Dr. Francesca Fallabrino for her invaluable revision of English of the definitive version of the manuscript.
Footnotes
Supported by Italian Ministry of Health Ricerca Corrente.
Conflict-of-interest statement: All Authors declare to have no conflict of interest related to the manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 17, 2015
First decision: May 18, 2015
Article in press: August 31, 2015
P- Reviewer: Krawiec P, Woo SY, Yamamoto S S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Rabizadeh S, Dubinsky M. Update in pediatric inflammatory bowel disease. Rheum Dis Clin North Am. 2013;39:789–799. doi: 10.1016/j.rdc.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. doi: 10.1097/MPG.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 3.Ruel J, Ruane D, Mehandru S, Gower-Rousseau C, Colombel JF. IBD across the age spectrum: is it the same disease? Nat Rev Gastroenterol Hepatol. 2014;11:88–98. doi: 10.1038/nrgastro.2013.240. [DOI] [PubMed] [Google Scholar]
- 4.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Diefenbach KA, Breuer CK. Pediatric inflammatory bowel disease. World J Gastroenterol. 2006;12:3204–3212. doi: 10.3748/wjg.v12.i20.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner D, Levine A, Escher JC, Griffiths AM, Russell RK, Dignass A, Dias JA, Bronsky J, Braegger CP, Cucchiara S, et al. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012;55:340–361. doi: 10.1097/MPG.0b013e3182662233. [DOI] [PubMed] [Google Scholar]
- 7.de Bie CI, Paerregaard A, Kolacek S, Ruemmele FM, Koletzko S, Fell JM, Escher JC. Disease phenotype at diagnosis in pediatric Crohn’s disease: 5-year analyses of the EUROKIDS Registry. Inflamm Bowel Dis. 2013;19:378–385. doi: 10.1002/ibd.23008. [DOI] [PubMed] [Google Scholar]
- 8.Proctor ML, Langer JC, Gerstle JT, Kim PC. Is laparoscopic subtotal colectomy better than open subtotal colectomy in children? J Pediatr Surg. 2002;37:706–708. doi: 10.1053/jpsu.2002.32258. [DOI] [PubMed] [Google Scholar]
- 9.Georgeson KE. Laparoscopic-assisted total colectomy with pouch reconstruction. Semin Pediatr Surg. 2002;11:233–236. doi: 10.1053/spsu.2002.35361. [DOI] [PubMed] [Google Scholar]
- 10.Rothenberg SS. Laparoscopic segmental intestinal resection. Semin Pediatr Surg. 2002;11:211–216. doi: 10.1053/spsu.2002.35356. [DOI] [PubMed] [Google Scholar]
- 11.von Allmen D, Markowitz JE, York A, Mamula P, Shepanski M, Baldassano R. Laparoscopic-assisted bowel resection offers advantages over open surgery for treatment of segmental Crohn’s disease in children. J Pediatr Surg. 2003;38:963–965. doi: 10.1016/s0022-3468(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 12.Dutta S, Rothenberg SS, Chang J, Bealer J. Total intracorporeal laparoscopic resection of Crohn’s disease. J Pediatr Surg. 2003;38:717–719. doi: 10.1016/jpsu.2003.50191. [DOI] [PubMed] [Google Scholar]
- 13.Simon T, Orangio G, Ambroze W, Schertzer M, Armstrong D. Laparoscopic-assisted bowel resection in pediatric/adolescent inflammatory bowel disease: laparoscopic bowel resection in children. Dis Colon Rectum. 2003;46:1325–1331. doi: 10.1007/s10350-004-6742-7. [DOI] [PubMed] [Google Scholar]
- 14.Bonnard A, Fouquet V, Berrebi D, Hugot JP, Belarbi N, Bruneau B, Aigrain Y, de Lagausie P. Crohn’s disease in children. Preliminary experience with a laparoscopic approach. Eur J Pediatr Surg. 2006;16:90–93. doi: 10.1055/s-2006-924048. [DOI] [PubMed] [Google Scholar]
- 15.Meier AH, Roth L, Cilley RE, Dillon PW. Completely minimally invasive approach to restorative total proctocolectomy with j-pouch construction in children. Surg Laparosc Endosc Percutan Tech. 2007;17:418–421. doi: 10.1097/SLE.0b013e3180f61277. [DOI] [PubMed] [Google Scholar]
- 16.Fraser JD, Garey CL, Laituri CA, Sharp RJ, Ostlie DJ, St Peter SD. Outcomes of laparoscopic and open total colectomy in the pediatric population. J Laparoendosc Adv Surg Tech A. 2010;20:659–660. doi: 10.1089/lap.2010.0086. [DOI] [PubMed] [Google Scholar]
- 17.Flores P, Bailez MM, Cuenca E, Fraire C. Comparative analysis between laparoscopic (UCL) and open (UCO) technique for the treatment of ulcerative colitis in pediatric patients. Pediatr Surg Int. 2010;26:907–911. doi: 10.1007/s00383-010-2669-3. [DOI] [PubMed] [Google Scholar]
- 18.Diamond IR, Gerstle JT, Kim PC, Langer JC. Outcomes after laparoscopic surgery in children with inflammatory bowel disease. Surg Endosc. 2010;24:2796–2802. doi: 10.1007/s00464-010-1050-x. [DOI] [PubMed] [Google Scholar]
- 19.Mattioli G, Pini-Prato A, Barabino A, Gandullia P, Avanzini S, Guida E, Rossi V, Pio L, Disma N, Mameli L, et al. Laparoscopic approach for children with inflammatory bowel diseases. Pediatr Surg Int. 2011;27:839–846. doi: 10.1007/s00383-011-2885-5. [DOI] [PubMed] [Google Scholar]
- 20.Laituri CA, Fraser JD, Garey CL, Aguayo P, Sharp SW, Ostlie DJ, Holcomb GW, St Peter SD. Laparoscopic ileocecectomy in pediatric patients with Crohn’s disease. J Laparoendosc Adv Surg Tech A. 2011;21:193–195. doi: 10.1089/lap.2010.0169. [DOI] [PubMed] [Google Scholar]
- 21.Potter DD, Tung J, Faubion WA, Moir C. Single-incision laparoscopic colon and rectal surgery for pediatric inflammatory bowel disease and polyposis syndromes. J Laparoendosc Adv Surg Tech A. 2012;22:203–207. doi: 10.1089/lap.2011.0117. [DOI] [PubMed] [Google Scholar]
- 22.Linden BC, Bairdain S, Zurakowski D, Shamberger RC, Lillehei CW. Comparison of laparoscopic-assisted and open total proctocolectomy and ileal pouch anal anastomosis in children and adolescents. J Pediatr Surg. 2013;48:1546–1550. doi: 10.1016/j.jpedsurg.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Huang R, Koleilat I, Lee EC. Single surgeon experience with laparoscopic surgery in pediatric patients with inflammatory bowel disease. J Laparoendosc Adv Surg Tech A. 2013;23:61–64. doi: 10.1089/lap.2012.0062. [DOI] [PubMed] [Google Scholar]
- 24.Stephens L, Gillick J. Early experience in laparoscopic colectomy for refractory colitis in children. Ir Med J. 2013;106:20–21. [PubMed] [Google Scholar]
- 25.Sharp NE, Thomas P, St Peter SD. Single-incision laparoscopic ileocecectomy in children with Crohn’s disease. J Laparoendosc Adv Surg Tech A. 2014;24:589–592. doi: 10.1089/lap.2013.0517. [DOI] [PubMed] [Google Scholar]
- 26.Vrecenak JD, Mattei P. Fast-track management is safe and effective after bowel resection in children with Crohn’s disease. J Pediatr Surg. 2014;49:99–102; discussion 102-3. doi: 10.1016/j.jpedsurg.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 27.Leape LL, Ramenofsky ML. Laparoscopy in infants and children. J Pediatr Surg. 1977;12:929–938. doi: 10.1016/0022-3468(77)90603-0. [DOI] [PubMed] [Google Scholar]
- 28.Miller GG, Blair GK, Murphy JJ. Diagnostic laparoscopy in childhood Crohn’s disease. J Pediatr Surg. 1996;31:846–848. doi: 10.1016/s0022-3468(96)90150-5. [DOI] [PubMed] [Google Scholar]
- 29.Schier F, Kähler G, Kauff E. [Laparoscopy in suspected Crohn disease in childhood] Langenbecks Arch Chir Suppl Kongressbd. 1998;115:124–127. [PubMed] [Google Scholar]
- 30.Thibault C, Poulin EC. Total laparoscopic proctocolectomy and laparoscopy-assisted proctocolectomy for inflammatory bowel disease: operative technique and preliminary report. Surg Laparosc Endosc. 1995;5:472–476. [PubMed] [Google Scholar]
- 31.Bauer JJ, Harris MT, Grumbach NM, Gorfine SR. Laparoscopic-assisted intestinal resection for Crohn‘s disease. Dis Colon Rectum. 1995;38:712–715. doi: 10.1007/BF02048027. [DOI] [PubMed] [Google Scholar]
- 32.Romeo E, Jasonni V, Caldaro T, Barabino A, Mattioli G, Vignola S, di Abriola GF, De Angelis P, Pane A, Torroni F, et al. Strictureplasty and intestinal resection: different options in complicated pediatric-onset Crohn disease. J Pediatr Surg. 2012;47:944–948. doi: 10.1016/j.jpedsurg.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 33.Tilney HS, Constantinides V, Ioannides AS, Tekkis PP, Darzi AW, Haddad MJ. Pouch-anal anastomosis vs straight ileoanal anastomosis in pediatric patients: a meta-analysis. J Pediatr Surg. 2006;41:1799–1808. doi: 10.1016/j.jpedsurg.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Koivusalo A, Pakarinen MP, Rintala RJ. Surgical complications in relation to functional outcomes after ileoanal anastomosis in pediatric patients with ulcerative colitis. J Pediatr Surg. 2007;42:290–295. doi: 10.1016/j.jpedsurg.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Seetharamaiah R, West BT, Ignash SJ, Pakarinen MP, Koivusalo A, Rintala RJ, Liu DC, Spencer AU, Skipton K, Geiger JD, et al. Outcomes in pediatric patients undergoing straight vs J pouch ileoanal anastomosis: a multicenter analysis. J Pediatr Surg. 2009;44:1410–1417. doi: 10.1016/j.jpedsurg.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Wu XR, Kirat HT, Xhaja X, Hammel JP, Kiran RP, Church JM. The impact of mesenteric tension on pouch outcome and quality of life in patients undergoing restorative proctocolectomy. Colorectal Dis. 2014;16:986–994. doi: 10.1111/codi.12748. [DOI] [PubMed] [Google Scholar]
- 37.Griffen FD, Knight CD, Whitaker JM, Knight CD. The double stapling technique for low anterior resection. Results, modifications, and observations. Ann Surg. 1990;211:745–751; discussion 751-752. doi: 10.1097/00000658-199006000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattioli G, Guida E, Pini-Prato A, Avanzini S, Rossi V, Barabino A, Coran AG, Jasonni V. Technical considerations in children undergoing laparoscopic ileal-J-pouch anorectal anastomosis for ulcerative colitis. Pediatr Surg Int. 2012;28:351–356. doi: 10.1007/s00383-011-3030-1. [DOI] [PubMed] [Google Scholar]
- 39.Georgeson KE. Laparoscopic-assisted pull-through for Hirschsprung’s disease. Semin Pediatr Surg. 2002;11:205–210. doi: 10.1053/spsu.2002.35350. [DOI] [PubMed] [Google Scholar]
- 40.Frolkis A, Kaplan GG, Patel AB, Faris P, Quan H, Jette N, deBruyn J. Postoperative complications and emergent readmission in children and adults with inflammatory bowel disease who undergo intestinal resection: a population-based study. Inflamm Bowel Dis. 2014;20:1316–1323. doi: 10.1097/MIB.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 41.Blackburn SC, Wiskin AE, Barnes C, Dick K, Afzal NA, Griffiths DM, Beattie RM, Stanton MP. Surgery for children with Crohn’s disease: indications, complications and outcome. Arch Dis Child. 2014;99:420–426. doi: 10.1136/archdischild-2013-305214. [DOI] [PubMed] [Google Scholar]
- 42.Stavlo PL, Libsch KD, Rodeberg DA, Moir CR. Pediatric ileal pouch-anal anastomosis: functional outcomes and quality of life. J Pediatr Surg. 2003;38:935–939. doi: 10.1016/s0022-3468(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 43.Wewer V, Hesselfeldt P, Qvist N, Husby S, Paerregaard A. J-pouch ileoanal anastomosis in children and adolescents with ulcerative colitis: functional outcome, satisfaction and impact on social life. J Pediatr Gastroenterol Nutr. 2005;40:189–193. doi: 10.1097/00005176-200502000-00020. [DOI] [PubMed] [Google Scholar]
- 44.Robb BW, Gang GI, Hershko DD, Stoops MM, Seeskin CS, Warner BW. Restorative proctocolectomy with ileal pouch-anal anastomosis in very young patients with refractory ulcerative colitis. J Pediatr Surg. 2003;38:863–867. doi: 10.1016/s0022-3468(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 45.Meinero P, Mori L. Video-assisted anal fistula treatment (VAAFT): a novel sphincter-saving procedure for treating complex anal fistulas. Tech Coloproctol. 2011;15:417–422. doi: 10.1007/s10151-011-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gecse KB, Bemelman W, Kamm MA, Stoker J, Khanna R, Ng SC, Panés J, van Assche G, Liu Z, Hart A, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381–1392. doi: 10.1136/gutjnl-2013-306709. [DOI] [PubMed] [Google Scholar]
- 47.Cirocchi R, Santoro A, Trastulli S, Farinella E, Di Rocco G, Vendettuali D, Giannotti D, Redler A, Coccetta M, Gullà N, et al. Meta-analysis of fibrin glue versus surgery for treatment of fistula-in-ano. Ann Ital Chir. 2010;81:349–356. [PubMed] [Google Scholar]
- 48.Schwandner O. Video-assisted anal fistula treatment (VAAFT) combined with advancement flap repair in Crohn’s disease. Tech Coloproctol. 2013;17:221–225. doi: 10.1007/s10151-012-0921-7. [DOI] [PubMed] [Google Scholar]
- 49.Zoccali M, Fichera A. Minimally invasive approaches for the treatment of inflammatory bowel disease. World J Gastroenterol. 2012;18:6756–6763. doi: 10.3748/wjg.v18.i46.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]


