Abstract
The intestinal microbiome is a dynamic system of interactions between the host and its microbes. Under physiological conditions, a fine balance and mutually beneficial relationship is present. Disruption of this balance is a hallmark of inflammatory bowel disease (IBD). Whether an altered microbiome is the consequence or the cause of IBD is currently not fully understood. The pathogenesis of IBD is believed to be a complex interaction between genetic predisposition, the immune system and environmental factors. In the recent years, metagenomic studies of the human microbiome have provided useful data that are helping to assemble the IBD puzzle. In this review, we summarize and discuss current knowledge on the composition of the intestinal microbiota in IBD, host-microbe interactions and therapeutic possibilities using bacteria in IBD. Moreover, an outlook on the possible contribution of bacteriophages in the pathogenesis and therapy of IBD is provided.
Keywords: Microbiota, Inflammatory bowel disease, Gut, Bacteriophages, Bacterial therapy
Core tip: Inflammatory bowel diseases (IBD) are chronic disorders of the gastrointestinal tract, with multi-factorial pathogenesis, which affect millions of people worldwide and have a rising incidence. Dysbalanced intestinal microbiota is an important feature of IBD. The relationship between dysbalanced microbiota and IBD is not fully uncovered. We are only beginning to appreciate the role of microbiota in the pathogenesis, progression or prognosis of IBD. In this review, we deal with the composition of gut microbiota, microbe-host interactions, therapeutic potential of bacteria and discuss the possible roles of bacteriophages in IBD.
INTRODUCTION
Inflammatory bowel disease (IBD) is a term describing chronic inflammatory diseases of the gastrointestinal tract with a complex etiology caused by various genetic, immunological and environmental factors[1]. IBD refers to ulcerative colitis (UC) and Crohn’s disease (CD), which are diseases of the digestive tract with similar clinical, pathological and epidemiological features. They are characterized by recurrent episodes of disease exacerbations with associated abdominal pain, diarrhea, weight loss and rectal bleeding. It estimated that IBD currently affects more than 1 million people in the United States and 2 million people in Europe, with a rising incidence[2,3].
The healthy adult intestine contains about 1014 bacteria, a count that is 10x more than the total number of human cells. The total reported number of different bacterial strains in the human microbiota varies with regard to detection method used[4,5]. Recent data have suggested, that the intestinal microbiome comprises approximately 200 strains of bacteria, representing more than 100 bacterial species[6,7]. Advances in metagenomics have uncovered the complexity of this system. More than 90% of these bacterial species fall into three phyla - Firmicutes, Bacteroidetes and Proteobacteria[8].
The gastrointestinal (GI) tract and its microbiome represent a dynamic and mutually beneficial relationship that is thought to be a major determinant of health and disease. The intestinal immune system provides protection to prevent the penetration of an excessive amount of intraluminal bacteria into the systemic circulation. Commensal bacteria activate homeostatic processes based on molecular responses driven by epithelial cells, macrophages, dendritic cells, and T and B lymphocytes that mediate the coexistence with microbes and their products[6]. The gut provides nutrient rich environment for the microbiota, which in turn offers a huge diversity of metabolic functions that include digestion and absorption of non-digestible substrates, a barrier effect against pathogenic microbes and modulation of immune reactions. Disruption of this fine homeostasis on a certain level might lead to the chronic inflammation present in IBD, and also in other chronic inflammatory diseases.
MICROBIOTA AND IBD
Recently, microbial profiles at various stages of colitis have been described and characterized that depend on the time and location within the gastrointestinal tract[9]. It is not yet entirely clear whether changes in the composition of the microbiota are the cause or consequence of inflammatory processes in the intestinal tissue. The most consistent change observed among the vast majority of IBD patients is a decrease in intestinal microbiota diversity, with slightly different findings between CD and UC patients. In CD, a decrease in Firmicutes is often observed, including butyrate-producing bacteria such as Faecalibacterium prausnitzii. This leads to the overproduction of pro-inflammatory cytokines and downstream events[10]. In UC, several other groups of bacteria besides butyrate-producing Firmicutes are often reduced, including Bacteroides and Clostridium genera. On the other hand, Enterococcus and Gammaproteobacteria are found in higher amounts in fecal samples from UC patients[11]. However, the presence and abundance of specific bacterial species vary with disease activity and the site of sampling (fecal vs biopsy specimens).
Moreover, patterns of gut microbiome dysbiosis in IBD are inconsistent among published studies. A study by Gevers et al[12] defined a correlation between a specific microbial pattern and disease status. Samples were collected from multiple locations throughout the GI tract from treatment-naïve pediatric CD patients. The authors concluded that, in the early stages of disease, assessing the rectal-mucosal associated microbiome provides high-value information for a convenient and early diagnosis of CD. In addition, it is known from animal experiments that the presence of specifically altered (procolitic) intestinal microbiota has a direct correlation with the development of colon cancer associated with colitis (colitis-associated cancer - CAC)[13]. Such targeted change in the microbiota (dysbiosis), leading to an increased risk of both, colitis and CAC, is reversible and transmissible to another individual[14]. In this study, dysbiosis-associated disease risk was communicable via the gut microbiota to wild-type mice and reciprocal microbiota transplantation reduced disease risk in predisposed mice and led to long-term changes in the gut microbiota composition. Moreover, recent results suggest that intestinal tumorigenesis mediated by bacterial dysbiosis may be communicable through the microbiota among individuals with a genetic predisposition[15]. These studies highlight the potential of preventive and therapeutic manipulation of the intestinal microbiota.
Patients with IBD are at increased risk of developing colorectal cancer and the risk increases with the duration and extent of colitis, positive family history and the degree of inflammation. The pathogenesis of CAC is multifactorial; the key factors are the mucosal inflammatory response, the presence of oxidative stress and the intestinal microbiota[16]. Some bacterial species of natural microbiota have a protective effect and conversely some contribute to the formation of CAC. The protective function is attributed to probiotic bacteria, which also have a stabilizing effect on the gut flora with the potential to reduce the pro-inflammatory response and thus the risk of development and progression of colitis and related cancer[13]. This effect is even transmissible to wild-type mice, which thus obtained reduced susceptibility to chemically induced colitis[17]. It only remains to be seen whether a dysbiotic state is enough to trigger IBD, which is a major risk factor for CAC. However, it seems that the licensing of dysbiotic microbiota is a key component of disease development. Therefore, manipulation of the gut microbiota certainly brings many opportunities for therapeutic intervention in IBD and CAC.
The whole situation is complicated by the fact that susceptibility to IBD mediated by specific bacterial microbiota also depends on a special diet[18]. This effect is not present in germ-free mice without a natural intestinal microbiota and in mice with a sterilized gut following antibiotic treatment. The absence of an intestinal microbiota thus has a protective effect on the formation and development of colitis and related cancer[13]. In our preliminary experiments, we found that sterilization of the bowel using antibiotics improves subsequent colitis and enhances the therapeutic effect of orally administered bacterial vectors[19]. Although the natural intestinal flora potentiates and promotes chronic inflammation and tumorigenesis, some authors suggest that it can also have the opposite effect - that it limits and reduces chemically induced damage and reduces inflammatory reactions that lead to the development of tumors in the colon[20]. Recolonization of germ-free mice with natural microbiota has been shown to decrease tumorigenesis. As with colitis, a significant change in the composition of the intestinal flora has been described in models of colorectal cancer[21].
The intestinal microbiota is a major component of several physiological processes, including the regulation of body weight and related metabolic balance, the immune system and epithelial cell responses. In recent years, a growing amount of knowledge has been published about the key role of the intestinal microbiota in the pathogenesis of a number of disease conditions, including obesity, cardiovascular diseases, multiple sclerosis, rheumatoid arthritis, diabetes, metabolic syndrome, chronic liver damage and growing evidence suggests chronic lung and kidney disease as well[6,22-25]. Furthermore, it is clear that the presence of a specific intestinal microbiota and interactions between the microbiota and the host organism (its immune system) are crucial for the successful treatment of certain diseases, including cancer, which is not even directly located in the gastrointestinal tract[26]; thus, the gut microbiota affects inflammation and immunity locally at the mucosal level as well as systemically. In this study, antibiotic-treated and germ-free mice showed significantly reduced tumor regression and survival after immunotherapy compared with control mice with natural gut microbiota. This effect was clearly based on decreased bacterial load leading to lower expression of TNF-α in tumors. Moreover, individual bacterial species have been identified that positively (Alistipes shahii) or negatively (Lactobacillus fermentum) correlate with TNF-α expression in tumors leading to an improved or worsened tumor response to immunotherapy, respectively. This study provided convincing evidence of the crucial influence of commensal bacteria on the therapeutic efficiency in systemic and distant-site diseases and identified individual members of the microbiota that can modulate this effect.
MECHANISMS OF HOST-MICROBE INTERACTIONS IN IBD
There is no question that interactions between microbes and the host play a central role in the development and severity of IBD. A growing body of experimental and clinical evidence has shown that IBD results from a dysregulated immune response to components of the normal gut flora in genetically susceptible individuals. Less is known about the mechanisms of such interactions. However, it is well-known that bacterial exposure is crucial for the development of colitis. In animal studies, genetically engineered mice developed spontaneous colitis when raised under standard conditions, but remained colitis-free when they were housed in germ-free conditions[27]. Moreover, it has been shown that antibiotic pretreatment seems to protect standard mice from the development of chemically induced colitis[19]. Similarly, antibiotic treatment has also been shown to be beneficial in a subset of IBD patients[28].
Lower temporal stability and reduced diversity of the microbiota along with a lower proportion of Gram-positive and a higher proportion of Gram-negative bacteria is frequently reported in IBD patients compared with healthy subjects. In a subset of IBD patients, certain bacterial strains with specific features promote the disease. However, the exact nature of host-microbe interactions that contribute to IBD development has not been assessed for the majority of IBD patients[29]. Apart from experimental studies on animal models of IBD, large genome-wide association studies (GWAS) may provide a relevant clue to explain this complex relationship between host genetic factors and the microbiome. A large meta-analysis was published that described an “IBD genome”, i.e., the possible causal genes that point to an essential role for host defense against infection in IBD. These genes are involved in defective processing of intracellular bacteria (NOD2, ATG16L1, IRGM), epithelial barrier function (HNF4A, CHD1, LAMB1), antigen presentation (HLA-DQA1) and inflammatory mediator production (TNFRSF14, TNFSF9, IL1R2, IL7R). These data confirm the key role of the interaction between the host mucosal immune system and microbes, both at the epithelial cell surface and within the gut lumen. Specifically, the study raises the question of what triggers components of the commensal microbiota to switch from a symbiotic to a pathogenic relationship with the host[30].
There are a number of proposed mechanisms by which the intestinal microbiota interacts with the host cells[31], yet no definite explanation has been generally accepted. It has been found that the molecules secreted from bacteria can enter intestinal cells via transporters or endocytosis, and that they activate cell survival pathways. These findings indicate that the interactions between the gut microbiota and host cells are mediated, at least partly, by membrane transport systems[32]. In IBD, membrane function is frequently compromised, thus leading to an altered flow of information from beneficial as well as pathogenic members of gut microbiota. This, along with other processes of IBD pathogenesis, results in the complex clinical appearance of the disease. In general, four mechanisms have been proposed that drive pathogenic immunologic responses to luminal bacteria: (1) bacterial pathogens; (2) dysbiosis of commensal bacteria; (3) host genetic factors; and (4) defective host immunoregulation[33]. A scheme summarizing possible interactions between bacteria and components of the GI tract in health and IBD is depicted in Figure 1.
Figure 1.
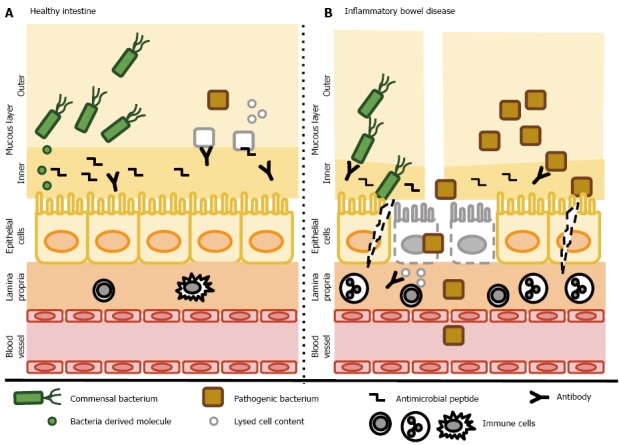
Host-bacteria interactions. A: In the healthy gut, both commensal and pathogenic bacteria reside in the outer layer of the intestinal mucous layer without coming into direct contact with epithelial cells. The inner layer contains abundant antibacterial peptides and secreted antibodies that prevent the invasion of bacteria. Pathogenic bacteria are eradicated by various mechanisms. Commensal bacteria secrete various molecules that help to maintain the intestinal barrier, activate cell survival pathways and suppress inflammatory responses. Epithelial cells form a continuous, selectively permeable layer connected by intercellular junctions. The lamina propria contains only a few resident immune cells; B: In inflammatory bowel disease, the mucous layer is reduced and contains fewer antimicrobial peptides and secretory antibodies. The abundance of commensal bacteria is reduced in favor of pathogenic bacteria and both types enter the inner mucosal barrier and interact directly with epithelial cells. Some epithelial cells undergo cell death and disruption of the epithelial barrier occurs. Cell components are released and trigger further inflammation. Disruption of the epithelial barrier enables bacteria to invade the submucosa and recruit inflammatory cells. Finally, chronic inflammation develops. A dysbalanced immune system leads to the production of antibodies recognizing both commensal bacteria (and further reduce their numbers) and cells of the host, leading to further tissue destruction and inflammation, creating the “circulus vitiosus” typical for inflammatory bowel diseases.
BACTERIAL THERAPY OF IBD
Although the interaction between the host and intestinal microbiota seems to play essential role in IBD pathogenesis, standard therapeutic approaches for treating IBD are typically based on suppression of the host immune response; these drugs mainly consist of 5-aminosalicylates, corticosteroids, thiopurines and biologicals[34]. Since a significant amount of IBD patients do not achieve clinical remission after conventional therapy, there is a legitimate need for new therapeutic approaches. Targeting IBD-related microbial dysbiosis can represent an attractive new alternative for IBD therapy. Therapies based on restoration of the intestinal microbiota that have been successfully used in IBD patients include fecal microbiota transplantation, probiotics, prebiotic antibiotics, helminth therapy and dietary polyphenols[8]. The use of antibiotics, probiotics, prebiotics and synbiotics in IBD patients has been extensively discussed in the literature[35]. Such selective manipulation of the intestinal microbiota has been evaluated as an attractive therapeutic option with few adverse effects.
The number of clinical trials that investigated the role of probiotics in IBD remains relatively low. The most extensively tested probiotic preparations include Escherichia coli (E. coli) Nissle 1917 and VSL#3 - a highly concentrated mixture of four strains of Lactobacillus (L. casei, L. plantarum, L. acidophilus and L. delbrueckii subsp. bulgaricus), three strains of Bifidobacterium (B. longum, B. breve and B. infantis) and one strain of Streptococcus (S. salivarius subsp. thermophilus). The published results clearly indicate that both the multispecies probiotic VSL#3 and E. coli Nissle 1917 can be as efficient as standard pharmacotherapy, but only in UC (both for active disease as well as to induce and maintain remission). On the other hand, results from CD trials are disappointing[33,36,37]. The differential effect of probiotic preparations in UC vs CD indicates that IBD is a multifactorial disease with considerable variety in terms of phenotypes and severity.
Probiotic intestinal strains may provide their benefit in various pathological conditions and through different molecular mechanisms. One of these mechanisms which has been recently proposed could be reprogramming of cells in the intestinal wall into the state of pluripotency and subsequent differentiation into a phenotype resistant to pathological factors causing the disease[38]. In our experiments, we showed that dedifferentiation of intestinal cells during the development of colitis may result in resistance of these cells to adverse inflammatory events and ultimately give rise to new and fully functional healthy intestinal tissue. This hypothesis was first formulated theoretically and then supported by the results from a simple experiment[19,34,39].
Novel therapeutic modalities based on the restoration of intestinal homeostasis include fecal microbiota transplantation (FMT), an approach based on the transfer of a stool suspension obtained from a healthy person into the GI tract of diseased patient. FMT restores essential components of the microbiota which could reverse the inflammatory processes observed in IBD. FMT may possibly restore intestinal microbial homeostasis, and preliminary data have shown the clinical efficacy of FMT on refractory IBD or IBD combined with Clostridium difficile infection[35,40]. Although the evidence is still limited, the majority of the studies confirmed the efficiency of FMT in the therapy of IBD[37]. Recently, a meta-analysis of clinical studies was performed to evaluate the efficacy of FMT as a treatment for IBD[41]. Overall, 45% (54/119) of IBD patients achieved clinical remission during follow-up. Subgroup analyses demonstrated clinical remission of 22% (95%CI: 10.4%-40.8%) for UC (P = 0.37; I2 = 0%) and 60.5% (95%CI: 28.4%-85.6%) for CD (P = 0.05; I2 = 37%). However, more clinical studies have to be performed before FMT can become a part of standard medical care for IBD patients. Randomized controlled trials are currently ongoing that will shed more light into this topic, including an assessment of the long-term consequences of FMT such as infection, cancer, auto-immune and metabolic diseases.
Bacteria as vectors in gene therapy have been known for a long time and have a wide range of action and spectrum of use[42]. Partly justified concerns about the possible pathogenicity slowed their use in the clinic and in the experiment. This problem has been largely overcome by modern genetic engineering. Currently available strains are genetically modified to have reduced and strictly defined virulence, which allows them to enter cells in the target tissue while maintaining safe conditions. Bacterial vectors are especially appropriate for IBD therapy thanks to their natural ability to persist in the intestinal environment. Such bacterial therapy of IBD was first successfully applied more than a decade ago, when the bacterium Lactococcus lactis was administered in murine colitis found to secrete interleukin-10 (IL-10)[43]. Similar results were obtained in our experiment where we used recombinant probiotic strains of E. coli Nissle 1917 and L. lactis, which secreted IL-10 as a treatment for chemically induced colitis[44]. Numerous other studies have confirmed the validity of the bacterial approach in IBD using different combinations of vectors and therapeutic genes[45-50]. Moreover, various bacterial strains have been successfully used for the treatment of cancer[51]. Our results indicate that sterilization of the intestine using antibiotics (the absence of gut microbiota) improves colonization of the gut by administered bacterial vectors and thus enhances the transfer of genes into the intestine using these bacterial vectors[19]. New therapeutic strategies can be expected based on oral administration of genetically engineered live microorganisms producing or delivering anti-inflammatory or other novel agents into the target (intestinal) tissue.
BACTERIOPHAGES AND IBD
Recent studies have suggested that examination of the gut microbiome should not focus solely on the bacterial composition. Bacteriophages (or phages) are viruses that infect bacteria, but not eukaryotic cells. It is estimated that the human gut contains 1015 bacteriophages[52], which accounts for approximately 108-109 bacteriophages per gram of human feces[53]. The colonization of the gut by bacteriophages increases rapidly after birth, as infant feces contain 108 phage particles per gram of feces at the age of 1 wk[54]. Analysis of the human phage microbiome (phageome) from a fecal sample showed both phages and prophages (phage genomes incorporated in the bacterial genome) being present, while prophages contribute to approximately 28% of all phages[55]. Three dominant families from the order Caudovirales (Siphoviridae, Myoviridae and Podoviridae) have been shown to be the most abundant phages in the intestinal tissue, as confirmed by both electron microscopy in intestinal tissue[56] and by the metagenomic approach in intestinal tissue and gut wash[57]. These three families have been confirmed by other metagenomic analyses, with the addition of the family Microviridae in the feces[55]. On the other hand, the metagenomic approach showed that the majority of identified phage sequences are not yet identified (annotated sequences that do not exist in the databases), meaning that more exact information on individual phages in the gut is yet to be discovered[52]. This interest is further supported by the fact that, recently, phages have been shown to be a substantial player in mucosal immunity[58].
Although studies dealing with bacteriophages and IBD are rather scarce, several studies have already described differences in the phage population between CD patients and healthy individuals, both in children and adults[56,57,59]. The diversity of phage genomes was found to be lower in the feces of CD patients than in healthy individuals[59,60]. Interestingly, in the mucosa, CD patients were found to have more detectable bacteriophages than healthy individuals, but the ulcerated mucosa had a lower phage count than unaffected mucosa[56]. Whether an ulcerated mucosa has more or less bacteria than non-ulcerated parts of the gut is not entirely clear, as some studies suggest no differences between non-ulcerated and ulcerated mucosa[61] and some show less bacteria in ulcerated than in non-ulcerated parts of the mucosa in IBD patients[62]. Fewer bacteriophages could support the model with less bacteria, but this has yet to be determined. This is further complicated by the fact that phages directly interact with mucosal glycoproteins and thus their abundance in the gut is not entirely dependent on the bacteria present[58]. In feces, the relative amount and diversity of bacteria are decreased in IBD[60]. The study also showed an inverse correlation between Caudovirales and Microviridae in healthy individuals, as well as CD and UC patients. However, significantly higher amounts of Caudovirales compared to Microviridae were observed only in UC patients.
Given the abundance of phages in the gut, they likely substantially influence the abundance and diversity of bacteria in IBD. The mechanisms by which bacteriophages modulate the actual bacterial flora in the gut are likely multifactorial (Figure 2). Phages substantially contribute to the genetic variability of bacteria by horizontal gene transfer and increasing of the mutation rate[63]. Either way, they substantially influence bacterial fitness[64] and very likely modulate their behavior in IBD. For example, prophages carrying genes encoding antibiotic resistance may act either as procolitic factors, when incorporated into pathogenic bacteria, but may be beneficial when incorporated into probiotic bacteria. Also, stress-induced activation of a prophage dormant in commensal (or pathogenic) bacteria might lead to activation of its lytic cycle and subsequent reduction of the amount of the host bacteria. The vacated environmental niche might be then replaced by pathogenic (or commensal) bacteria with procolitic (or anti-colitic) effects. On the other side, an increase in a certain bacterial strain might lead to increased chance of infection by a specific phage. This is further supported by the fact that CD patients have higher amounts of bacteriophages with less diversity[56], suggesting a regulatory role for bacteriophages in a host-predator manner[55]. On the other hand, the number of bacteriophages may not necessarily correspond to the amount of bacteria[60,65].
Figure 2.
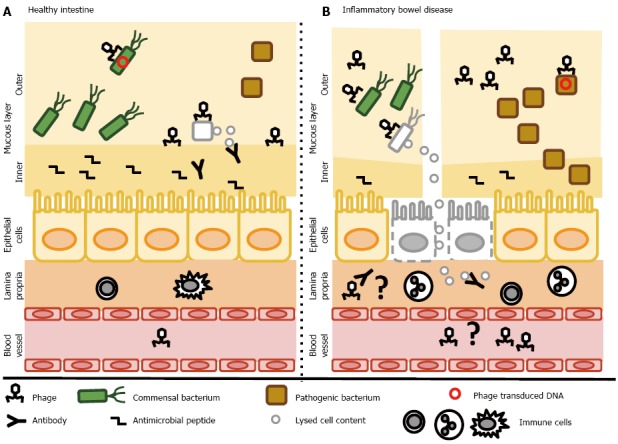
Putative contribution of bacteriophages to regulation of the intestinal bacteria - a simplified scheme. A: In the healthy gut, bacteriophages might increase the fitness of commensal bacteria by the delivery of genes with environmental benefit or contribute to reduction of the pathogenic bacteria. Moreover, phages directly interact with the glycoproteins of the mucous layer and provide protection against invading bacteria. In some healthy individuals, phages have been detected in the circulation, suggesting the possibility that they cross the intestinal epithelial barrier; B: In inflammatory bowel disease, more phages are found in the mucous layer. Higher numbers of phages may be involved in reducing the amount of commensal bacteria, and may drive the transfer of genes with environmental benefit to pathogenic bacteria. Due to the reduced mucosal layer, phage interactions with mucosal glycoproteins may be reduced. Moreover, disruptions in the epithelial barrier might lead to the migration of many phage particles into the lamina propria or even the circulation. In the lamina propria, phages may serve as a local trigger of the immune response. After translocation to the systemic circulation, a systemic immune response might occur.
Due to the coat proteins of bacteriophages, their effects on IBD could be also immunomodulatory. It has been shown that some phages are able to cross the mucosal barrier and stimulate immunity[66]. Although the available information is rather scarce, phages have been found to modulate both cellular and humoral immunity[66], but the mechanisms are largely unexplored[67]. In IBD, this effect might be further enhanced due to increased permeability of the intestinal mucosal barrier. Several studies have shown the presence of phages (“phagemia”) in the bloodstream of healthy individuals[66] and one study has shown phagemia (mycobacteriophages) in patients with CD[68]. This study did not show significant differences in the total amount of phages between healthy subjects and patients with IBD. However, it should be mentioned that the study was performed in the early 1970s and the only detection method available was based on phage titering. Metagenomic approach might provide more information in this subject. Finally, the lytic cycle of phages is followed by lysis of bacterial cells leading to release of macromolecules such as proteins, lipids and nucleic acids which may trigger the immune response and promote inflammation[60].
In recent years, phage therapy has re-gained attention as a therapeutic approach to combat bacterial infections[69]. For IBD patients, this approach could possibly reduce the number of specific disease-associated bacterial strains without a direct negative effect on commensal bacteria. Moreover, metagenomic studies unveiling the “phageome” of the gut of IBD patients might help to develop new strategies or screening methods for the prediction of disease progression, and/or serve as a prediction tool for choosing the optimal therapeutic strategy. On the other side, in light of recent findings focused on phages and their putative role in IBD, especially those that do not depend on bacteria, further studies on safety and efficacy are necessary.
CONCLUSION
The pathogenesis of IBD involves several key processes, including disturbed activation of the mucosal immune system driven by abnormal intestinal microbiota in genetically predisposed individuals. Systematic shifts in the gut microbiome structure and function have been observed in patients with IBD, compared with healthy individuals. However, there are still no definitive microbial pathogens linked to the onset and development of IBD[70]. An overview of the literature has been provided that describes the causes of dysbiosis and the mechanisms evolved by the host to prevent these changes to community structure[71]. Nevertheless, results from previous studies indicate that the taxonomic composition of the microbiome can differ substantially between subjects with the same disease and, thus mere taxonomic characterization might not be sufficient to fully uncover the relationship between the microbiome and IBD. A more relevant and up-to-date method of studying the interactions between microbes and disease seems to be the analysis of microbiome biological properties (functional analysis). Altered intestinal tissue along with microbial dysbiosis both result into significant metabolic shifts within the intestinal microenvironments in IBD[72]. The Integrative Human Microbiome Project (iHMP) is an ongoing multi-omic longitudinal study focused on (1) explaining how the intestinal microbiome may trigger disease activity in IBD; (2) determining if the microbial composition predicts the risk of exacerbations; and (3) testing whether a successful therapeutic response can be predicted from the stool microbiota[73]. Such an approach should provide a complex view on the dynamics of host-microbiome interactions and link them to specific disease states, including IBD. One of the challenges that have to be addressed is the timing of the administration of bacterial therapeutics. Unlike animal models, most IBD patients are treated after the appearance of serious symptoms, i.e., after the diagnosis of IBD. Therefore, the therapeutic effect might be more variable, insufficient or prone to failure. Nevertheless, ever-growing knowledge about the transmission potential of the healthy gut microbiome supports the rationale of preventive manipulation of the gut microbiota even before the diagnosis and onset of symptoms.
In conclusion, we predict that rigorous gut microbiota profiling will soon become a part of complex phenotypic analysis in IBD patients. Moreover, interventions targeting the microbial composition (including FMT, bacterial gene therapy, synthetic and multimicrobial microbiota substitutes) and the correct timing of these procedures will define the future directions in the field of IBD.
Footnotes
Supported by Ministry of Education of the Slovak Republic VEGA 1/0206/2012.
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 1, 2015
First decision: June 23, 2015
Article in press: September 14, 2015
P- Reviewer: De Silva AP, Galvez J S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Mitsuoka T. Intestinal flora and aging. Nutr Rev. 1992;50:438–446. doi: 10.1111/j.1753-4887.1992.tb02499.x. [DOI] [PubMed] [Google Scholar]
- 5.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koboziev I, Reinoso Webb C, Furr KL, Grisham MB. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radic Biol Med. 2014;68:122–133. doi: 10.1016/j.freeradbiomed.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, et al. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen WX, Ren LH, Shi RH. Enteric microbiota leads to new therapeutic strategies for ulcerative colitis. World J Gastroenterol. 2014;20:15657–15663. doi: 10.3748/wjg.v20.i42.15657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belzer C, Gerber GK, Roeselers G, Delaney M, DuBois A, Liu Q, Belavusava V, Yeliseyev V, Houseman A, Onderdonk A, et al. Dynamics of the microbiota in response to host infection. PLoS One. 2014;9:e95534. doi: 10.1371/journal.pone.0095534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, Vandamme P, Vermeire S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut. 2011;60:631–637. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- 11.Nemoto H, Kataoka K, Ishikawa H, Ikata K, Arimochi H, Iwasaki T, Ohnishi Y, Kuwahara T, Yasutomo K. Reduced diversity and imbalance of fecal microbiota in patients with ulcerative colitis. Dig Dis Sci. 2012;57:2955–2964. doi: 10.1007/s10620-012-2236-y. [DOI] [PubMed] [Google Scholar]
- 12.Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klimesova K, Kverka M, Zakostelska Z, Hudcovic T, Hrncir T, Stepankova R, Rossmann P, Ridl J, Kostovcik M, Mrazek J, et al. Altered gut microbiota promotes colitis-associated cancer in IL-1 receptor-associated kinase M-deficient mice. Inflamm Bowel Dis. 2013;19:1266–1277. doi: 10.1097/MIB.0b013e318281330a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz MD, Atay C, Heringer J, Romrig FK, Schwitalla S, Aydin B, Ziegler PK, Varga J, Reindl W, Pommerenke C, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514:508–512. doi: 10.1038/nature13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;20:9872–9881. doi: 10.3748/wjg.v20.i29.9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bel S, Elkis Y, Elifantz H, Koren O, Ben-Hamo R, Lerer-Goldshtein T, Rahimi R, Ben Horin S, Nyska A, Shpungin S, et al. Reprogrammed and transmissible intestinal microbiota confer diminished susceptibility to induced colitis in TMF-/- mice. Proc Natl Acad Sci USA. 2014;111:4964–4969. doi: 10.1073/pnas.1319114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ooi JH, Waddell A, Lin YD, Albert I, Rust LT, Holden V, Cantorna MT. Dominant effects of the diet on the microbiome and the local and systemic immune response in mice. PLoS One. 2014;9:e86366. doi: 10.1371/journal.pone.0086366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardlik R, Wagnerova A, Celec P. Effects of bacteria-mediated reprogramming and antibiotic pretreatment on the course of colitis in mice. Mol Med Rep. 2014;10:983–988. doi: 10.3892/mmr.2014.2244. [DOI] [PubMed] [Google Scholar]
- 20.Zhan Y, Chen PJ, Sadler WD, Wang F, Poe S, Núñez G, Eaton KA, Chen GY. Gut microbiota protects against gastrointestinal tumorigenesis caused by epithelial injury. Cancer Res. 2013;73:7199–7210. doi: 10.1158/0008-5472.CAN-13-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Q, Jin Z, Wu W, Gao R, Guo B, Gao Z, Yang Y, Qin H. Analysis of the intestinal lumen microbiota in an animal model of colorectal cancer. PLoS One. 2014;9:e90849. doi: 10.1371/journal.pone.0090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 23.Henao-Mejia J, Elinav E, Thaiss CA, Licona-Limon P, Flavell RA. Role of the intestinal microbiome in liver disease. J Autoimmun. 2013;46:66–73. doi: 10.1016/j.jaut.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Marsland BJ, Gollwitzer ES. Host-microorganism interactions in lung diseases. Nat Rev Immunol. 2014;14:827–835. doi: 10.1038/nri3769. [DOI] [PubMed] [Google Scholar]
- 25.Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010–1016. doi: 10.1038/ki.2012.440. [DOI] [PubMed] [Google Scholar]
- 26.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK, Talley NJ, Moayyedi P. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:661–673. doi: 10.1038/ajg.2011.72. [DOI] [PubMed] [Google Scholar]
- 29.Loh G, Blaut M. Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes. 2012;3:544–555. doi: 10.4161/gmic.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D‘Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H; International IBD Genetics Consortium (IIBDGC), Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konishi H, Fujiya M, Kohgo Y. Host-microbe interactions via membrane transport systems. Environ Microbiol. 2015;17:931–937. doi: 10.1111/1462-2920.12632. [DOI] [PubMed] [Google Scholar]
- 33.Orel R, Kamhi Trop T. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J Gastroenterol. 2014;20:11505–11524. doi: 10.3748/wjg.v20.i33.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagnerova A, Gardlik R. In vivo reprogramming in inflammatory bowel disease. Gene Ther. 2013;20:1111–1118. doi: 10.1038/gt.2013.43. [DOI] [PubMed] [Google Scholar]
- 35.Bringiotti R, Ierardi E, Lovero R, Losurdo G, Di Leo A, Principi M. Intestinal microbiota: The explosive mixture at the origin of inflammatory bowel disease? World J Gastrointest Pathophysiol. 2014;5:550–559. doi: 10.4291/wjgp.v5.i4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Floch MH. Recommendations for probiotic use in humans-a 2014 update. Pharmaceuticals (Basel) 2014;7:999–1007. doi: 10.3390/ph7100999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verbeke KA, Boesmans L, Boets E. Modulating the microbiota in inflammatory bowel diseases: prebiotics, probiotics or faecal transplantation? Proc Nutr Soc. 2014;73:490–497. doi: 10.1017/S0029665114000639. [DOI] [PubMed] [Google Scholar]
- 38.Ohta K, Kawano R, Ito N. Lactic acid bacteria convert human fibroblasts to multipotent cells. PLoS One. 2012;7:e51866. doi: 10.1371/journal.pone.0051866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardlik R. Inducing pluripotency using in vivo gene therapy. Med Hypotheses. 2012;79:197–201. doi: 10.1016/j.mehy.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 40.Wang ZK, Yang YS, Chen Y, Yuan J, Sun G, Peng LH. Intestinal microbiota pathogenesis and fecal microbiota transplantation for inflammatory bowel disease. World J Gastroenterol. 2014;20:14805–14820. doi: 10.3748/wjg.v20.i40.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardlik R, Fruehauf JH. Bacterial vectors and delivery systems in cancer therapy. IDrugs. 2010;13:701–706. [PubMed] [Google Scholar]
- 43.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 44.Gardlik R, Palffy R, Celec P. Recombinant probiotic therapy in experimental colitis in mice. Folia Biol (Praha) 2012;58:238–245. [PubMed] [Google Scholar]
- 45.Pálffy R, Behuliak M, Gardlík R, Jáni P, Kádasi L, Turna J, Celec P. Oral in vivo bactofection in dextran sulfate sodium treated female Wistar rats. Folia Biol (Krakow) 2010;58:171–176. doi: 10.3409/fb58_3-4.171-176. [DOI] [PubMed] [Google Scholar]
- 46.Palffy R, Gardlik R, Behuliak M, Jani P, Balakova D, Kadasi L, Turna J, Celec P. Salmonella-mediated gene therapy in experimental colitis in mice. Exp Biol Med (Maywood) 2011;236:177–183. doi: 10.1258/ebm.2010.010277. [DOI] [PubMed] [Google Scholar]
- 47.Foligne B, Dessein R, Marceau M, Poiret S, Chamaillard M, Pot B, Simonet M, Daniel C. Prevention and treatment of colitis with Lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein. Gastroenterology. 2007;133:862–874. doi: 10.1053/j.gastro.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Vandenbroucke K, de Haard H, Beirnaert E, Dreier T, Lauwereys M, Huyck L, Van Huysse J, Demetter P, Steidler L, Remaut E, et al. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010;3:49–56. doi: 10.1038/mi.2009.116. [DOI] [PubMed] [Google Scholar]
- 49.Gardlik R, Bartonova A, Celec P. Therapeutic DNA vaccination and RNA interference in inflammatory bowel disease. Int J Mol Med. 2013;32:492–496. doi: 10.3892/ijmm.2013.1388. [DOI] [PubMed] [Google Scholar]
- 50.Hamady ZZ, Scott N, Farrar MD, Lodge JP, Holland KT, Whitehead T, Carding SR. Xylan-regulated delivery of human keratinocyte growth factor-2 to the inflamed colon by the human anaerobic commensal bacterium Bacteroides ovatus. Gut. 2010;59:461–469. doi: 10.1136/gut.2008.176131. [DOI] [PubMed] [Google Scholar]
- 51.Gardlik R, Behuliak M, Palffy R, Celec P, Li CJ. Gene therapy for cancer: bacteria-mediated anti-angiogenesis therapy. Gene Ther. 2011;18:425–431. doi: 10.1038/gt.2010.176. [DOI] [PubMed] [Google Scholar]
- 52.Dalmasso M, Hill C, Ross RP. Exploiting gut bacteriophages for human health. Trends Microbiol. 2014;22:399–405. doi: 10.1016/j.tim.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Kim MS, Park EJ, Roh SW, Bae JW. Diversity and abundance of single-stranded DNA viruses in human feces. Appl Environ Microbiol. 2011;77:8062–8070. doi: 10.1128/AEM.06331-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Breitbart M, Haynes M, Kelley S, Angly F, Edwards RA, Felts B, Mahaffy JM, Mueller J, Nulton J, Rayhawk S, et al. Viral diversity and dynamics in an infant gut. Res Microbiol. 2008;159:367–373. doi: 10.1016/j.resmic.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Breitbart M, Hewson I, Felts B, Mahaffy JM, Nulton J, Salamon P, Rohwer F. Metagenomic analyses of an uncultured viral community from human feces. J Bacteriol. 2003;185:6220–6223. doi: 10.1128/JB.185.20.6220-6223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lepage P, Colombet J, Marteau P, Sime-Ngando T, Doré J, Leclerc M. Dysbiosis in inflammatory bowel disease: a role for bacteriophages? Gut. 2008;57:424–425. doi: 10.1136/gut.2007.134668. [DOI] [PubMed] [Google Scholar]
- 57.Wagner J, Maksimovic J, Farries G, Sim WH, Bishop RF, Cameron DJ, Catto-Smith AG, Kirkwood CD. Bacteriophages in gut samples from pediatric Crohn’s disease patients: metagenomic analysis using 454 pyrosequencing. Inflamm Bowel Dis. 2013;19:1598–1608. doi: 10.1097/MIB.0b013e318292477c. [DOI] [PubMed] [Google Scholar]
- 58.Barr JJ, Auro R, Furlan M, Whiteson KL, Erb ML, Pogliano J, Stotland A, Wolkowicz R, Cutting AS, Doran KS, et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Natl Acad Sci USA. 2013;110:10771–10776. doi: 10.1073/pnas.1305923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pérez-Brocal V, García-López R, Vázquez-Castellanos JF, Nos P, Beltrán B, Latorre A, Moya A. Study of the viral and microbial communities associated with Crohn’s disease: a metagenomic approach. Clin Transl Gastroenterol. 2013;4:e36. doi: 10.1038/ctg.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norman JM, Handley SA, Baldridge MT, Droit L, Liu CY, Keller BC, Kambal A, Monaco CL, Zhao G, Fleshner P, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–460. doi: 10.1016/j.cell.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 62.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abeles SR, Pride DT. Molecular bases and role of viruses in the human microbiome. J Mol Biol. 2014;426:3892–3906. doi: 10.1016/j.jmb.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penadés JR, Chen J, Quiles-Puchalt N, Carpena N, Novick RP. Bacteriophage-mediated spread of bacterial virulence genes. Curr Opin Microbiol. 2015;23:171–178. doi: 10.1016/j.mib.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 65.Pride DT, Salzman J, Haynes M, Rohwer F, Davis-Long C, White RA, Loomer P, Armitage GC, Relman DA. Evidence of a robust resident bacteriophage population revealed through analysis of the human salivary virome. ISME J. 2012;6:915–926. doi: 10.1038/ismej.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Górski A, Wazna E, Dabrowska BW, Dabrowska K, Switała-Jeleń K, Miedzybrodzki R. Bacteriophage translocation. FEMS Immunol Med Microbiol. 2006;46:313–319. doi: 10.1111/j.1574-695X.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- 67.Duerkop BA, Hooper LV. Resident viruses and their interactions with the immune system. Nat Immunol. 2013;14:654–659. doi: 10.1038/ni.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parent K, Wilson ID. Mycobacteriophage in Crohn’s disease. Gut. 1971;12:1019–1020. doi: 10.1136/gut.12.12.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014;5:226–235. doi: 10.4161/viru.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588:4223–4233. doi: 10.1016/j.febslet.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16:1024–1033. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colgan SP, Curtis VF, Campbell EL. The inflammatory tissue microenvironment in IBD. Inflamm Bowel Dis. 2013;19:2238–2244. doi: 10.1097/MIB.0b013e31828dcaaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project: dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe. 2014;16:276–289. doi: 10.1016/j.chom.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


