Abstract
Background
Reliable implant-supported rehabilitation of an alveolar ridge needs sufficient volume of bone. In order to achieve a prosthetic-driven positioning, bone graft techniques may be required.
Purpose
This prospective cohort study aims to clinically evaluate the amount of resorption of corticocancellous fresh-frozen allografts bone blocks used in the reconstruction of the severe atrophic maxilla.
Materials and Methods
Twenty-two partial and totally edentulous patients underwent bone augmentation procedures with fresh-frozen allogenous blocks from the iliac crest under local anesthesia. Implants were inserted into the grafted sites after a healing period of 5 months. Final fixed prosthesis was delivered ± 4 months later. Ridge width analysis and measurements were performed with a caliper before and after grafting and at implant insertion. Bone biopsies were performed in 16 patients.
Results
A total of 98 onlay block allografts were used in 22 patients with an initial mean alveolar ridge width of 3.41 ± 1.36 mm. Early exposure of blocks was observed in four situations and one of these completely resorbed. Mean horizontal bone gain was 3.63 ± 1.28 mm (p < .01). Mean buccal bone resorption between allograph placement and the reopening stage was 0.49 ± 0.54 mm, meaning approximately 7.1% (95% confidence interval: [5.6%, 8.6%]) of total ridge width loss during the integration period. One hundred thirty dental implants were placed with good primary stability (≥ 30 Ncm). Four implants presented early failure before the prosthetic delivery (96.7% implant survival). All patients were successfully rehabilitated. Histomorphometric analysis revealed 20.9 ± 5.8% of vital bone in close contact to the remaining grafted bone. A positive strong correlation (adjusted R2 = 0.44, p = .003) was found between healing time and vital bone percentage.
Conclusions
Augmentation procedures performed using fresh-frozen allografts from the iliac crest are a suitable alternative in the reconstruction of the atrophic maxilla with low resorption rate at 5 months, allowing proper stability of dental implants followed by fixed prosthetic rehabilitation.
Keywords: allografts, alveolar ridge augmentation, corticocancellous block, fresh-frozen bone, iliac crest
Introduction
Bone augmentation techniques are widely used for the reconstruction of severely atrophic jaws prior to dental implants placement. The lack of appropriate volume of bone is caused by trauma, oncologic diseases, oral infections, congenitally missing teeth, or by the alveolar ridge tridimensional resorption process subsequent to dental extractions. The loss of teeth normally leads to progressive and irreversible bone atrophy resulting in bone volume diminution, more prominent in the first year. During this period, the horizontal dimension in the upper jaw can decrease, in many cases, 50% from the initial situation.1–3 The extent of undesirable changes in volume is also related to the elapsed time of premature loss of teeth and the severity of the mentioned etiology.4–6 Moreover, the remaining conditions with insufficient bone height and width of the alveolar ridge and an unfavorable maxillomandibular relation may prevent prosthetic-driven implant positioning and additional rehabilitations compromising both function and esthetics.7,8
In an attempt to correct the maxillary bone defects, many techniques for bone reconstruction and grafting materials have been described extensively.9–13 Although autografts remain the “gold standard,”14 there is an increased use of fresh-frozen bone allografts (FFBs) in orthopedics and in dentistry.15,16 The technique of onlay bone blocks is often indicated in the horizontal rehabilitation of large maxillary defects, where the biomechanical strength is required for the installation of implants and their prosthetic rehabilitation.9,17–19 Autogenous bone has always been the material of choice for cortical or corticocancellous onlay blocks.20,21 In contrast to particulate forms, which require additional materials to ensure space maintenance and graft containment, such as barrier membranes, tenting screws, and/or graft binders, onlay grafts are self-contained and provide an inherent ability to support the soft tissue. Both intra- and extra-oral donor sites22,23 have some drawbacks. Constraints in the size of autogenous block grafts from intraoral sites and the morbidity associated with graft harvesting often limit treatment recommendations and patient acceptance in practice. Complications associated with block grafts harvested from the symphysis or retromolar area, for example, can include nerve injury, soft tissue injury, wound dehiscence, and infection.24,25 In these instances, the most common extra-oral site is the iliac crest, and harvest-associated complications include pain, nerve damage, hematoma and wound complications, avulsion of the anterior superior iliac spine, herniation of the abdominal cavity contents, and cosmetic deformity.26 Additionally, published data on autografts rate resorption vary, showing reductions up to 30% at 1 year and a tendency to stagnate after that first year.27,28
Bone allografts allow the selection of blocks with a predefined configuration and a corticocancellous composition, and overcome disadvantages related to autografts such as availability and morbidity, allied to decrease blood loss.29–32 Also, its safety, biocompatibility, the less surgical time needed, the use of local anesthesia, decreasing the risks associated to general anesthesia and costs of an operating room, seem to be advantageous to the patients.33,34 Authorized tissue banks follow strict international guidelines for tissue harvesting and storing in order to ensure a more effective and safe application, hence making the risk of antigenicity and primary infections acceptably low.35–37 Various types of processing of allografts have been described and the FFBs have biomechanical advantages compared with freeze-dried and demineralized allografts.38,39 Therefore, FFB possesses necessary strength and rigidity to allow stable fixation in the recipient area.40 A systematic review41 included nine studies,42–50 but excluded the ones that examined fresh-frozen allografts. They were mainly case reports and case series describing the outcomes of freeze-dried allografts, and eight out of them used barrier membranes. Heterogeneity hampered generalization of results and turned interpretation somehow complex. Despite the reported successful outcome of ridge augmentation with onlay blocks, the review claimed that insufficient evidence is available to establish the treatment efficacy of allogeneic block grafts of that type relative to graft incorporation, alveolar ridge augmentation, and long-term dental implant survival.
Some case reports, case series, a nonrandomized clinical trial and a randomized controlled trial showed the feasibility of alveolar atrophy correction employing FFB blocks11,14,33,51–56; however, the data regarding the use of corticocancellous allografts from the iliac crest in the reconstruction of the severely atrophic maxilla remain limited.
The aim of our study is to evaluate horizontal bone resorption of corticocancellous fresh-frozen onlay bone blocks allografts from the iliac crest, under local anaesthesia, without the use of barrier membranes, at 5 months in the horizontal augmentation of atrophic jaws.
Materials and Methods
From August 2010 to January 2013, a total of 22 patients (2 men and 20 women, ranging from 35 to 62 years, mean age 49 ± 6 years), presenting severe bone deficiency in the maxilla and requiring bone augmentation procedures prior to implant-supported prosthesis, were recruited to this prospective cohort study. The protocol was approved by the Ethics Committee of the Faculty of Medicine of the University of Coimbra (Coimbra, Portugal) and all patients signed a written informed consent form.
Inclusion criteria were partial or totally edentulous patients with Cawood and Howell class IV maxillary atrophy3 requiring one or multiple implants, with absence of debilitating systemic diseases; patients smoking less than 10 cigarettes per day; and treatment protocol acceptance. General exclusion criteria were uncontrolled systemic diseases, use of medication interfering with bone metabolism, pregnancy or lactation, abuse of drugs or alcohol, use of tobacco equivalent to >10 cigarettes/day, and handicaps that would interfere with the ability to perform adequate oral hygiene or prevent completion of the study participation. Local exclusion criteria included untreated periodontitis, mucosal diseases, and local irradiation therapy.
All patients underwent clinical observation, which included the execution of panoramic radiographs and study models. Computerized tomography (CT) scans were obtained from bone volume analysis and measurements of the alveolar ridge after implant insertion (Figure 1).
Figure 1.
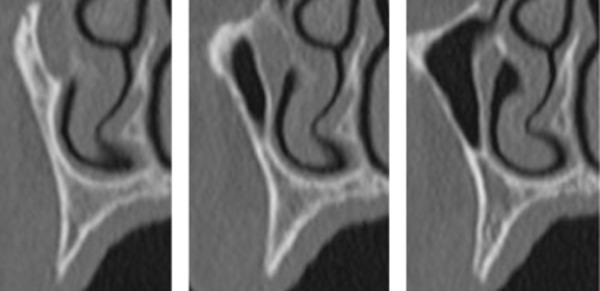
Preoperatory computerized tomography (CT) scan of the severely resorbed ridge.
The FFBs from the iliac crest (Figure 2) were processed according to the international guidelines in an authorized bone tissue bank (Bone and Tissue Bank of the Coimbra Hospital and Universitary Centre, Coimbra, Portugal). An experienced surgeon performed all the surgeries. During the first surgical phase, FFBs were thawed in a solution of sterile saline with vancomycin hydrochloride (Farma APS, Amadora, Portugal) 500 mL/500 mg for at least 40 minutes before the procedure, to hydrate and gradually get to room temperature.
Figure 2.
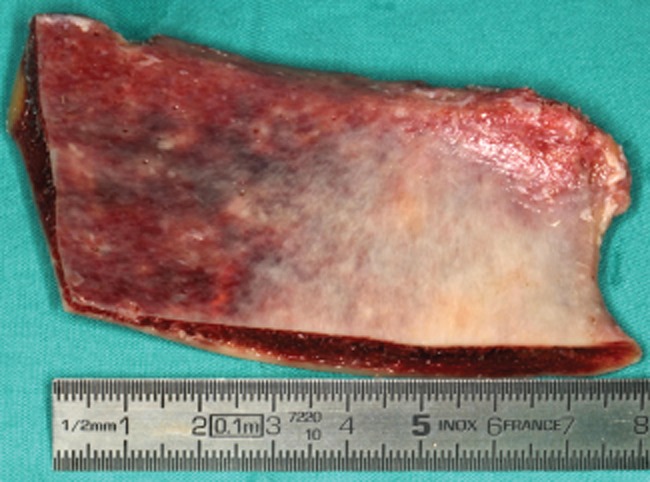
Allogenous fresh-frozen bone block from the iliac crest.
Prior to surgery, all patients rinse with chlorhexidine 0.12% (Pierre Fabre Portugal, Lisboa, Portugal). Under local anesthesia (4% articaine with 1:100,000 epinephrine), a full-thickness crestal incision with two vertical releasing incisions was attained to expose the three-dimensional aspect of the bone defects (Figure 3). After evaluation to determine the size and shape of the needed bone blocks, FFBs were cut and sculpted with rotary instruments and scissors. Meticulous removal of residual fibers adhered to the recipient bed was undertaken in order to promote optimal adaptation of bone blocks. Fifteen patients out of 22 needed simultaneous posterior vertical augmentation with sinus lift lateral window technique.
Figure 3.
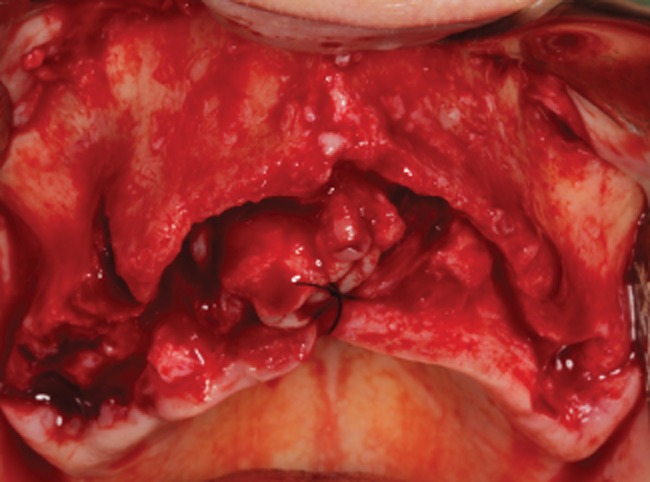
Occlusal view of the severely resorbed maxillary ridge.
Recipient site preparation included small perforations with spherical low-speed drill and adequate irrigation in order to promote the revascularization of the onlay grafts and the closest adaptation possible. The onlay bone blocks were positioned with the cancellous bone turning to the receptor site. All blocks were stabilized and then fixed to the residual crest at mid-height (5 mm) with 2-mm diameter round headed self-tapping micro-screws (Sistemas de Prótese, Conexão®, São Paulo, Brazil). In order to ensure reproducibility of the measurements upon initial flap reflection, after block placement, and at reentry, morphometric measurements of the width of the ridges were recorded with a dial caliper at the point immediately apical to the head of the fixation micro-screw, which corresponds to the stabilization of the caliper 6 mm apical to the crest.
The sharp angles and edges were gently reduced to avoid punctures of the overlying soft tissues and undesired exposure of the allografts. The gaps or voids at the periphery of the blocks were filled with FFB chips obtained by grinding the remaining allograft (Figure 4).
Figure 4.
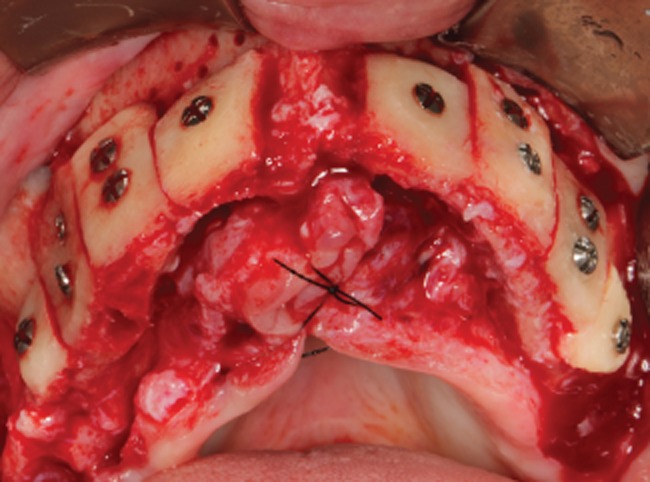
Immediate postoperatory. Occlusal view of the eight blocks fixed to the recipient site.
The flaps were repositioned without tension with nylon 4-0 (Lab. Aragó, S.L.Esp. ®, São Paulo, Brazil). None of the FFB onlay blocks was covered with membranes. After each surgical intervention, patients received antibiotics (amoxicillin + clavulanic acid 875 mg/125 mg [BIAL, Bial S.A., S. Mamede do Coronado, Portugal], twice a day for 7 days), nonsteroidal anti-inflammatory treatment (ibuprofen 600 mg [Abbott Laboratórios, Lda., Amadora, Portugal], twice a day for 5 days), and analgesics (paracetamol-codein phosphate 500 mg/30 mg [Bene Farmacêutica, Lisboa, Portugal], according to individual needs). Patients con tinued to rinse with chlorhexidine digluconate for the following 2 weeks.
Sutures were removed 12 days after the surgery and patients were observed weekly during the first 2 months and then monthly until the second stage. Some of the partial edentulous patients received adjacent tooth-supported provisional fixed restorations during the healing period, while total edentulous patients were instructed to remain without prosthesis.
Prior to the second stage, CT scans were taken in all patients, in order to evaluate good healing of the grafts (Figure 5). After a 5-month healing period, a two-stage approach for implant placement was performed, with the surgical exposure of the augmented sites (Figure 6). A third measurement of the ridge was taken. The micro-screws were removed and 130-standard diameter dental implants (Master Active, Conexão®, São Paulo, Brazil) were installed (Figure 7). Additional grafting procedures were not required for any of the patients.
Figure 5.
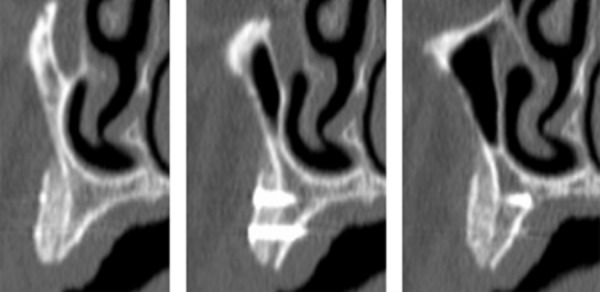
Postoperatory computerized tomography (CT) scan of the augmented area with the onlay blocks fixed to the recipient site.
Figure 6.
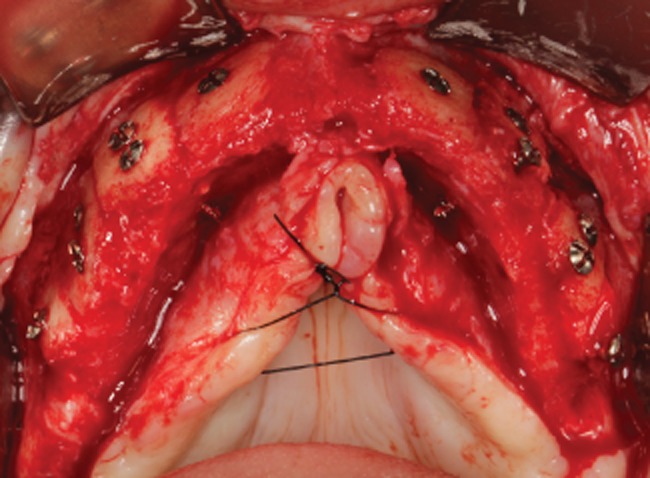
Occlusal view of the reconstructed ridge during reentry at 5 months with very good incorporation of the blocks.
Figure 7.
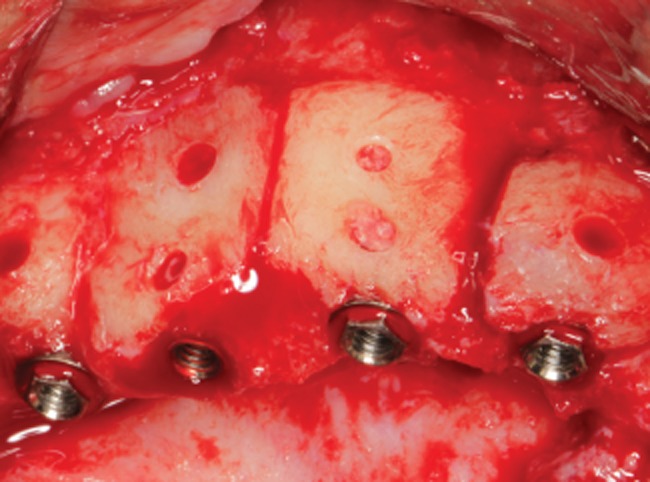
Detail view of four implants inserted into grafted bone.
Bone biopsies from the grafted areas were harvested with a 3-mm diameter trephine, provided that the procedure would not compromise implant placement, and routinely processed for serial decalcified sections. The retrieved biopsies were fixed by immersion in 10% neutral buffered formalin solution, decalcified with Morse solution, dehydrated in a graded series of ethanol, and finally embedded in Paraplast® Regular (Sigma-Aldrich Co., St. Louis, MO, USA). Longitudinal 7-μm thick sections were cut by microtome (Leica RM 2155, Leica Microsystems Nussloch GmbH, Nussloch, Germany) and stained with hematoxylin and eosin (HE). The histomorphometric analysis was carried out using a light stereomicroscope (Nikon® SMZ1500, Melville, NY, USA) connected to a high-resolution video camera (Optronics® DEI 750DCE, Goleta, CA, USA). The optical system was associated with the software package Bioquant Nova® (BIOQUANT Image Analysis Corporation, Nashville, TN, USA) with image-capturing capabilities. The measurements of vital bone (VB), residual graft or non-vital bone (NVB), and non-mineralized tissue (n-MT) were made as percentages of the area of a defined section. A VB/total bone ratio was calculated in percentage. Additionally, each section was further divided into four equal cross-sectional subsections from the allograft-native bone contact zone to the surface of the onlay to analyze the extent of new bone formation. Specimen classification was made from 1 (quartile of the allograft-native bone contact zone) to 4 (quartile of the allograft surface), taking into consideration the most superficial cross-sectional subsection where new bone was present.
After a 4-month healing period, the patients were rehabilitated with implant-supported fixed prosthesis (Figures 8– 10).
Figure 8.
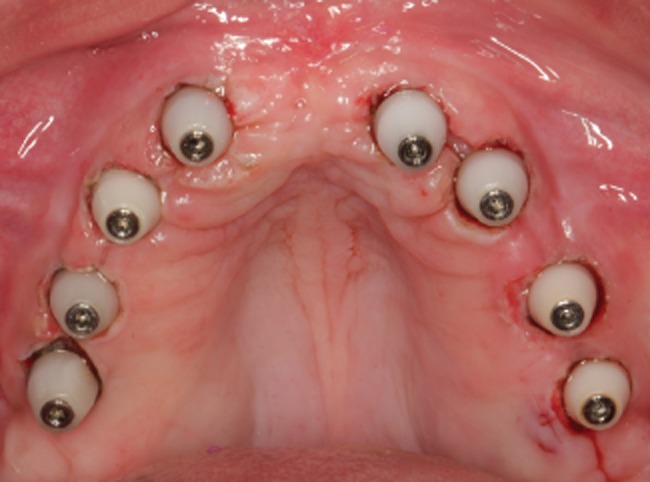
Occlusal view of the transmucosal exposition of the implants with healing caps.
Figure 10.
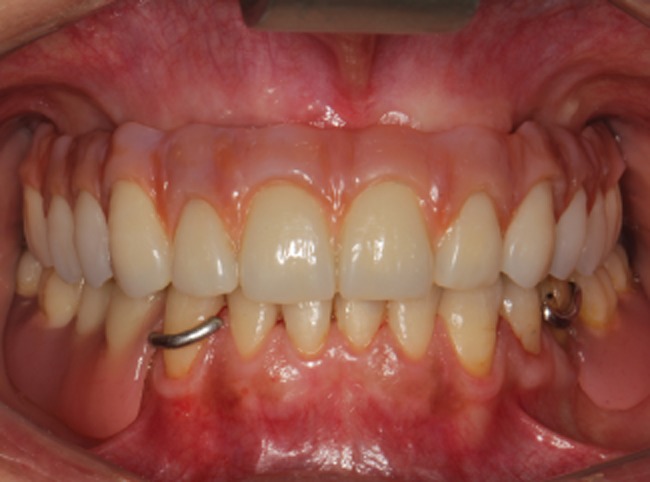
Frontal view of the final rehabilitation.
Figure 9.
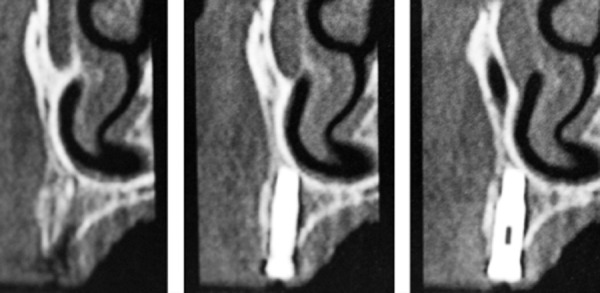
Computerized tomography (CT) slices of the grafted area with the implant placed. Note the presence of a good buccal plate from the allogenous block.
Results
A total of 22 patients with a mean age of 49 ± 6 years (ranging from 35 to 62) were selected to participate in the study. Fourteen patients were totally edentulous and the remaining 8 presented atrophic anterior maxillae. Ninety-eight fresh-frozen bone blocks were placed and each patient received from 1 to 8 corticocancellous allograft onlay bone blocks. The healing period was uneventful for all cases except for three onlay blocks that presented early exposure. Data for each patient are summarized in Table 1. At the crestal level, all patients met the inclusion criteria of ridge width less than 4 mm; however, caliper measurements were performed 6 mm apical to the crest to ensure the correct positioning after onlay installation.
Table 1.
Description of Patients Enrolled in the Study: Patient, Medical and Smoking Status, Number of Fresh-Frozen Onlay Blocks and Implants, Complications, and Mean Thickness Gain
| Patient | Sex | Age | Medical history | Smoking | Edentulism | FFB blocks | Sinus lift | Implants | Allograft complications | Preoperatory width (mm) | Postoperatory width (mm) | Stage II – reentry width (mm) | Resorption (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 46 | ASA I | No | Total | 4 | 2 | 8 | No | 5.37 | 8.25 | 7.75 | −0.5 |
| 2 | F | 62 | ASA I | No | Total | 4 | 2 | 6 | No | 5.00 | 7.00 | 7.00 | 0.00 |
| 3 | F | 52 | ASA I | No | Total | 6 | 2 | 6 | No | 3.18 | 6.35 | 5.62 | −0.73 |
| 4 | F | 56 | ASA I | No | Total | 5 | – | 6 | No | 3.26 | 7.38 | 6.34 | −1.04 |
| 5 | F | 47 | ASA I | No | Total | 4 | 2 | 6 | No | 5.37 | 8.62 | 8.25 | −0.37 |
| 6 | F | 50 | ASA I | Yes | Total | 6 | 2 | 8 | No | 3.41 | 5.58 | 5.33 | −0.25 |
| 7 | M | 39 | ASA I | No | Partial | 2 | – | 2 | No | 3.00 | 6.50 | 6.40 | −0.10 |
| 8 | F | 35 | ASA I | No | Partial | 2 | 1 | 3 | No | 2.50 | 5.20 | 4.85 | −0.35 |
| 9 | F | 41 | ASA I | No | Partial | 2 | 2 | 5 | No | 1.70 | 5.50 | 5.25 | −0.25 |
| 10 | F | 48 | ASA I | No | Total | 8 | 2 | 6 | Yes (1 exposure without infection and spontaneous resolution with clorhexidine gel and rinses) | 2.87 | 7.37 | 6.86 | −0.51 |
| 11 | F | 60 | ASA I | No | Partial | 1 | 2 | 5 | No | 5.20 | 9.20 | 8.50 | −0.70 |
| 12 | F | 36 | ASA I | No | Partial | 4 | – | 4 | No | 1.42 | 6.12 | 5.35 | −0.77 |
| 13 | M | 59 | ASA I | No | Total | 5 | 2 | 7 | No | 2.60 | 6.38 | 5.70 | −0.68 |
| 14 | F | 52 | ASA I | No | Total | 4 | 2 | 8 | No | 4.62 | 7.87 | 7.67 | −0.2 |
| 15 | F | 53 | ASA I | Yes | Total | 6 | 2 | 9 | Yes (1 exposure without infection and spontaneous resolution with clorhexidine gel and rinses) | 2.50 | 7.35 | 6.22 | −1.13 |
| 16 | F | 55 | ASA I | No | Partial | 2 | – | 2 | No | 1.00 | 5.90 | 5.80 | −0.10 |
| 17 | F | 55 | ASA I | No | Total | 6 | 2 | 8 | Yes (1 exposure without infection and spontaneous resolution with clorhexidine gel and rinses) | 2.20 | 5.95 | 5.33 | −0.62 |
| 18 | F | 45 | ASA I | No | Total | 6 | – | 6 | No | 4.48 | 8.00 | 7.12 | −0.88 |
| 19 | F | 45 | ASA I | No | Total | 6 | 2 | 8 | Yes (1 early exposure and total resorption) | 3.83 | 7.87 | 6.28* (7.54) | −1.58* (−0.40) |
| 20 | F | 47 | ASA I | No | Total | 6 | 2 | 8 | No | 4.62 | 7.20 | 7.07 | −0.13 |
| 21 | F | 48 | ASA I | No | Total | 8 | – | 8 | No | 3.20 | 7.25 | 7.12 | −0.13 |
| 22 | F | 44 | ASA I | No | Partial | 1 | – | 1 | No | 2.00 | 6.50 | 6.50 | 0.00 |
| Total | 98 | 29 | 130 | One block lost | 3.41 | 7.03 | 6.46* (6.53) | −0.56* (−0.49) |
*Considering the block that was completely resorbed by the second-stage surgery. Between brackets is presented the mean value without considering the allograft that had the postoperatory complication.
ASAI = American Society of Anestheologists physical status classification system, status I - normal healthy patient; F = female; FFB = fresh-frozen bone allograft; M = male.
Exposure of the block allograft was observed in four patients, as described in Table 1. Three of these occurred in the first weeks of healing, prior to the second-stage surgery. Two were small soft tissue dehiscences that presented no signs of infection or necrosis, and patients were instructed to apply 1% chlorhexidine gel over the exposed areas and to perform regular rinses for 14 days. After this period, soft tissue had covered the dehiscence, and no further clinical signs of inflammation were observed. The third block exposed (patient 10) required a surgical intervention to smoothen the exposed areas with a round bur at low velocity and to cover them with a connective tissue graft from the palatal mucosa. The fourth block exposed after the second-stage surgery was close to the prosthetic delivery and was solved by the prescription of chlorhexidine as previously described. At the second-stage surgery, the allografts were analyzed for viability. Despite the apparent clinical resolution of all exposures, one of the blocks (from patient 19) had suffered complete resorption and was excluded from the analysis. The remaining blocks that had suffered exposure were viable. Excellent incorporation of the onlays was obtained in all other sites, thus providing adequate alveolar ridge augmentation for implant installation.
In this stage, a total of 130 implants were placed with a minimum torque of 30 Ncm, except for one implant that failed to achieve primary stability. Four of the implants were not osseointegrated at the time of implant exposure and were removed, denoting a survival rate of 96.7%. In one patient, the two implants that failed during the healing period were replaced. All patients successfully received fixed implant-supported prosthesis. No other events have been reported after prosthesis delivery and patients present a mean follow-up of 18 ± 9 months since loading, extending up to 32 months.
Morphometric measurements were performed during surgery with a dial caliper before graft placement, immediately after graft fixation and at the reentry surgery, before implant installation. Mean ridge thickness per patient at the three periods is summarized in Table 1.
Repeated measures analysis of variance accounting for individual responses was performed to detect differences between ridge thicknesses. The fixation of FFB blocks induced a statistically significant increase of ridge thickness, followed by slight yet significant decrease of ridge thickness during the incorporation period F(2, 194) = 490.035 | F(1, 96) = 0.061. No interaction was determined between subject and response to treatment (F(1) = 0.061, p = .805), meaning that all patients presented similar patterns of ridge augmentation and subsequent resorption.
This pattern is represented in Figure 1. No other factors such as age or type of edentulism revealed significant interactions to the model.
Figure 11.
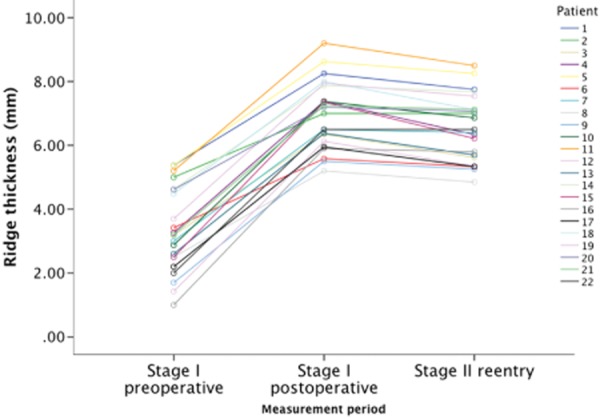
Graphic representation of the mean ridge thickness of each patient measured with a dial caliper during surgery before and after onlay fixation and at reentry, displaying a similar pattern for all cases. Values are in millimeters.
Paired comparisons revealed a significant mean increase in ridge thickness of 3.63 ± 1.28 mm from the preoperative measurement to the immediate postoperative measurement (p < .01). After this, during the incorporation period, there was a mean decrease in ridge thickness of 0.49 ± 0.54 mm (p < .01). Overall, there was a significant mean increase of 3.13 ± 1.12 mm in ridge thickness from the preoperative measurements to the second-stage reentry for implant placement (p < .01). The results are summarized in Table 2.
Table 2.
Ridge Width Variation between Surgical Stages and Paired Samples t-Test for the Difference
| Mean difference | SD | 95% CI for the difference | Paired samples t-test | |
|---|---|---|---|---|
| Stage I preoperative – Stage I postoperative | 3.63 | 1.28 | [3.37, 3.88] | t(96) = 27.87, p < .01 |
| Stage II – Stage I postoperative | −0.49 | 0.54 | [−0.60, −0.39] | t(96) = −9.05, p < .01 |
| Overall gain: Stage II – Stage I preoperative | 3.13 | 1.24 | [2.88, 3.39] | t(96) = 24.73, p < .01 |
α = 0.05.
Values expressed in millimeters.
CI = confidence interval; SD = standard deviation.
The bone resorption that occurred during the incorporation period corresponds to 7.1% (95% confidence interval [CI]: [5.6%, 8.6%]) of the measured postoperative ridge thickness. On the whole, there was a mean ridge thickness augmentation of 123.1% (95% CI: [102.8%, 143.4%]).
Histology could be performed in 16 samples, as in the other cases, either the harvested material proved inadequate for analysis or the biopsy could compromise implant placement. Histologic analysis of the sections stained with HE revealed signs of active remodeling in all specimens but with individual variability. VB and residual graft were in close contact, corroborating a creeping substitution process. In all specimens, residual graft was present with a typical lamellar arrangement around Haversian canals but with empty lacunae (NVB), whereas new bone was composed of both lamellar and woven VB-containing osteocytes in lacunae (Figure 2).
Figure 12.
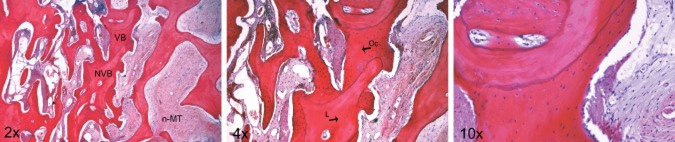
Photomicrograph of the bone biopsy stained with hematoxylin and eosin (HE), where vital bone (VB) surrounds the graft residual non-vital bone (NVB). The newly formed bone presents osteocytes (Oc) in the lacunae, whereas the graft residual is characterized by empty lacunae (L). Note the presence of osteoblast-like cells in the VB margin that contacts the non-mineralized tissue (n-MT). 20, 40 and 100x total magnification.
Histomorphometric analysis revealed that VB ranged from 12.2% to 36.0% with an overall average of 20.9 ± 5.8% of the total area of the section. Residual graft was present in 29.0 ± 11.8% of the area analyzed, varying from 12.9% (patient 7) to 50.9% (patient 1). n-MT extended from 26.7% to 67.7%, with a mean value of 49.1 ± 11.8%.
The mean of the VB/NVB ratio in the area of the section was 40.5 ± 16.0%, ranging from 22% to 67%.
A linear regression model was applied to the data in order to examine the correlation between healing time, corresponding to the elapsed time between onlay installation and biopsy harvesting (in weeks), and the VB percentage. The model obtained established that the healing time could statistically significantly predict the VB, F(1, 14) = 13.25, p = .003. The time elapsed between onlay installation and biopsy harvesting accounted for 44.9% of the explained variability in VB percentage.
Qualitative analysis of the slides showed that in 87.5% of the cases (14), VB was found across all subsections, from interface to the onlay surface. Only in 12.5% of the cases (2) the VB was spread up to the third subsection.
Discussion
The severely atrophic maxillae require ridge augmentation procedures prior to implant placement to enable prosthetic-driven rehabilitations. Large horizontal defects of the maxilla have successfully received implants after lateral ridge augmentation either with autogenous or allogenous onlay blocks, split-crest procedures, and guided bone regeneration.57–59 For the reconstruction of “contour-forming” defects with only one supporting bony wall as presented in this study, some authors still consider autologous bone blocks as the most reliable and secure procedure,60,61 but no sufficient evidence is available to support the superiority of that option in comparison with other materials.58 In fact, two clinical trials have determined comparable clinical performances of autologous and allogenous bone grafts,55,62 and several other controlled studies15,29,46,48,51,56,63,64 have reported the clinical success of the last while disclosing some disadvantages for autografts due to the morbidity and discomfort of the patient plus the potential complications associated to the donor site.65 The autogenously harvested block grafts are also limited in volume and difficult to adapt to the receptor site as a consequence of their inherent shape of cortical bone graft and have been associated to uncertain quantitative and qualitative resorption despite the osteoconductive and osteoinductive properties, which has lead many authors to suggest the use of barrier membranes.66,67 However, there is no sufficient evidence that the use of barrier membranes prevents the resorption of autogenous onlay bone grafts, and the potential benefits of using either resorbable or nonresorbable barrier membranes do not overcome the risk of exposure during the healing period and subsequent complications. In fact, both the exposure of barrier membranes and the use of particulate autogenous bone collected with bone-trapping filters from intraoral locations have negative influence on bone gain after the augmentation procedures.58,68–70 On the contrary, allograft blocks also allow minor operative time and unlimited supply of bone volume for the conformation and shape to the desired height and width, exempting the procedure from the risks of additional coverage with bone substitute material and membrane barriers to protect from relevant resorption.71–74 Unfortunately, the lack of universally accepted ridge augmentation success criteria is a significant obstacle in comparing the different studies and surgical techniques using allogenous bone, and very often the success of grafting procedures has been measured in terms of implant survival in the areas subjected to bone augmentation. In our study, we have determined a survival rate of 96.7% for implants inserted into grafted bone prior to loading, which is slightly inferior to the results reported in the literature for two-stage procedures with allogenous bone blocks,41 but attributable to both smoking habits non-referred during the recruiting phase and the use of a removable prosthesis during the healing period, as no failures were reported after the prosthetic rehabilitation despite the short waiting period of 4 to 6 months. This is in accordance to the retrospective study of Carinci and colleagues15 that determined clinically safe a similar healing period after the evaluation of 88 implants inserted in fresh-frozen bone, as well as with the study by Barone and colleagues.51
Nevertheless, implant survival in grafted areas could be a function of residual bone supporting the dental implant rather than grafted bone75,76 and does not comply with the success criteria defined by Albrektsson and colleagues77. Accordingly, a recent systemic review concluded that survival rates of implants placed into augmented areas were comparable with those of implants placed into pristine bone irrespective of the graft resorption rate.67 In such cases, the primary outcome should be the creation of an alveolar ridge of adequate dimensions to facilitate the surgical placement of implants that eventually are able to osseointegrate into the host and regenerate bone and undergo functional load.59,69,75 Thus, both implant success and the extent of horizontal dimension gain should be clearly reported. In this study, the primary outcome was the mean gain in ridge width, and measurements were performed 6 mm apical to the crest, immediately above the head of the fixation screw, analogous to the method used by Heberer and colleagues.78 This modification of the traditional positioning prevents malposition of the caliper,46,48,79,80 ensures reproducibility during reentry, and provides a more accurate positioning for posterior radiographic measurements.79
The mean horizontal bone gain of 3.63 ± 1.28 mm presented in this study, ranging from 1 to 7 mm, is similar or superior to those presented in studies that use the same methodology for measurement.46,48,81,82 At reentry after 4 to 6 months of healing, little or no resorption was apparent, and the dimensions of the ridge plus the block were stable, as confirmed by the 7.1% variation in ridge width from onlay fixation to implant insertion. The single case of complete resorption after exposure was associated to the inadvisable use of the complete removable denture that caused mechanical trauma over the block during the healing phase. As a matter of fact, no studies on allogenous onlays report horizontal gain insufficient to promote subsequent implant placement. Nevertheless, the graft resorption rate seems to be dependent on the type of bone and processing method. Processing reduces the immunological potential of the graft but can significantly weaken the biologic and mechanical properties initially present in the bone tissue and delay the incorporation period.55,62,83 For instance, freeze-dried grafts have been associated to fast resorption of the particles used to fill bone defects by a process not mediated by creeping substitution.84 These grafts work mostly by osteoconduction despite expression of the osteoinductive bone morphogenetic protein, which is dependent on donor age and induces high variations in the ability to induce new bone formation.85,86 This variability and the reported fast resorption together with the modifications in the properties of the bone caused by the lyophilization process might have an effect on the long-term strength and mechanical properties of the graft.87 On the contrary, the fresh-frozen method of allograft preservation used in this study consists of sterile procurement followed by sterile wrapping and deep-freezing to −70°C, and can be used up immediately on thawing retaining texture and strength characteristics similar to those of autogenous bone.88
Some studies using fresh-frozen bone blocks for horizontal ridge augmentation have been reported but are highly heterogeneous regarding the type and origin of the blocks, size of the defects, number of blocks per patient, and type of rehabilitation, which constrains the interpretation of results.
Some authors report the use of cortical blocks of different origins (tibia, femur) with good clinical results and viable histological results with delayed remodeling,33,52,55,81,82,89 while others reveal greater resorption but higher histological incorporation of cancellous bone blocks on a short evaluation period.56,90 The histological analysis of the samples collected in the present study demonstrated the apposition of new VB surrounding or completely engulfing the non-vital trabeculae of the cancellous portion of the allograft, showing the fresh-frozen corticocancellous grafts from the iliac crest present good regenerative capacities. In fact, after 4 months, the mean VB present was 20.9 ± 5.8%, which represents a remodeling similar to autogenous grafts presented in other studies82,89 and even superior to the results of other studies on fresh-frozen bone.81,90 Chiapasco and colleagues62 found no differences on the histomorphometric measurements of new VB and NVB over a period ranging from 4 to 9 months between autogenous and fresh-frozen bone grafts from the same origin, which contradicts the results of Spin-Neto and colleagues.55 Nevertheless, one must consider that both studies have a limited number of samples and that the latter does not present quantitative analysis of the sections. When comparing fresh-frozen allografts from different origins and formulations, the corticocancellous blocks from the iliac crest appear to have slower remodeling than particulate forms or purely cancellous blocks because of the presence of compact cortical bone with limited vascularization.88,90
It is well known that the important factors affecting bone graft survival and hence the bone quality of the implant site are graft stability, the vascularity of the recipient bed, and the osteogenic potential of the grafted bone. Allogenous cancellous block grafts seem to be an adequate scaffold for good vascularization with osteoinductive and osteoconductive properties, but do not provide sufficient rigidity to withstand tension from the overlying soft tissues or from the compression by provisional restorations and may compromise the stability, determining greater and fast bone resorption.46,76 On the contrary, blocks with cortical bone will provide rigidity for fixation and also prevent resorption during the healing phase,33,43,45 but impair the remodeling process and integration of the graft because of the poor vascularization of the onlay. Acocella and colleagues determined that, at 4 months of healing, cortical fresh-frozen blocks from the tibia present around 70% NVB, which is slowly replaced overtime by new VB and expected to be completely revascularized only 16 months after surgery.82 Similarly, we were able to find a correlation between time elapsed between surgery and biopsy retrieval (in weeks) and the VB present in the sample. The healing time explained 44.9% of the variability of the VB present in the sample and the regression equation was VB = −2.07 + 0.965× (healing weeks). This means that the VB increases approximately 1% per healing week, which is compatible with the decrease in NVB of 4.7% to 6.9% per month, estimated by Acocella and colleagues.81
Thus, the corticocancellous blocks used in the present study conceal the mechanical advantages of the cortical and the biological advantages of the cancellous bone. The compact lamellar bone offers a good surface for the insertion of the osteosynthesis screws, while the cancellous part of the graft provides a wider interface between donor and recipient bone, allowing early revascularization.76 More, close adaptation of the graft to the recipient site avoids the use of particulate bone and membrane barriers, facilitating rapid integration of the graft.64 The better clinical performance of fresh-frozen corticocancellous blocks over cancellous blocks is well patent in the clinical trial by Buffoli and colleagues90 where femoral head allografts were compared with iliac crest allografts and revealed greater bone resorption and bleeding.
Actually, only two studies have been published reporting the use of corticocancellous blocks from the iliac crest as in the present work,15,90 but none presented measurements of the ridge width after the grafting procedure or at the reentry surgery. Otherwise, this work reported the resorption rate of fresh-frozen corticocancellous bone blocks from the iliac crest while presenting a considerable sample of 98 blocks analyzed.
Conclusion
Augmentation procedures performed using corticocancellous fresh-frozen bone blocks from the iliac crest, under local anesthesia and without the use of barrier membranes, are a suitable alternative for the reconstruction of the atrophic maxillae at a short-term observation. The low resorption rate of the graft, at 5 months, allowed proper stability for placement of standard diameter dental implants followed by conventional fixed prosthetic rehabilitation. Further measurements in follow-up periods and histologic data at the interface of this type of grafted bone are needed.
References
- Tallgren A. The continuing reduction of the residual alveolar ridges in complete denture wearers: A mixed-longitudinal study covering 25 years. J Prosthet Dent. 2003;89(5):427–435. doi: 10.1016/s0022-3913(03)00158-6. [DOI] [PubMed] [Google Scholar]
- Cardaropoli G, Araújo M, Hayacibara R, Sukekava F, Lindhe J. Healing of extraction sockets and surgically produced - augmented and non-augmented - defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol. 2005;32:435–440. doi: 10.1111/j.1600-051X.2005.00692.x. [DOI] [PubMed] [Google Scholar]
- Cawood JI, Howell RA. A classification of the edentulous jaws. Int J Oral Maxillofac Surg. 1988;17(4):232–236. doi: 10.1016/s0901-5027(88)80047-x. [DOI] [PubMed] [Google Scholar]
- Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313–323. [PubMed] [Google Scholar]
- Nevins M, Camelo M, De Paoli S, Friedland B, Schenk RK, Parma-Benfenati S, et al. A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Int J Periodontics Restorative Dent. 2006;26(1):19–29. [PubMed] [Google Scholar]
- Iasella JM, Greenwell H, Miller RL, Hill M, Drisko C, Bohra AA, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74:990–999. doi: 10.1902/jop.2003.74.7.990. [DOI] [PubMed] [Google Scholar]
- Nevins M, Mellonig JT. The advantages of localized ridge augmentation prior to implant placement: a staged event. Int J Periodontics Restorative Dent. 1994;14(2):96–111. [PubMed] [Google Scholar]
- Oikarinen KS, Sàndor GKB, Kainulainen VT, Salonen-Kemppi M. Augmentation of the narrow traumatized anterior alveolar ridge to facilitate dental implant placement. Dent Traumatol. 2003;19(1):19–29. doi: 10.1034/j.1600-9657.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- Lundgren S, Sjöström M, Nyström E, Sennerby L. Strategies in reconstruction of the atrophic maxilla with autogenous bone grafts and endosseous implants. Periodontol 2000. 2008;47(1):143–161. doi: 10.1111/j.1600-0757.2008.00265.x. [DOI] [PubMed] [Google Scholar]
- Cordaro L, Amadé DS, Cordaro M. Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin Oral Implants Res. 2002;13(1):103–111. doi: 10.1034/j.1600-0501.2002.130113.x. [DOI] [PubMed] [Google Scholar]
- Carinci F, Brunelli G, Franco M, Viscioni A, Rigo L, Guidi R, et al. A retrospective study on 287 implants installed in resorbed maxillae grafted with fresh frozen allogenous bone. Clin Implant Dent Relat Res. 2010;12(2):91–98. doi: 10.1111/j.1708-8208.2008.00133.x. [DOI] [PubMed] [Google Scholar]
- Novell J, Novell-Costa F, Ivorra C, Fariñas O, Munilla A, Martinez C. Five-year results of implants inserted into freeze-dried block allografts. Implant Dent. 2012;21(2):129–135. doi: 10.1097/ID.0b013e31824bf99f. [DOI] [PubMed] [Google Scholar]
- Chiapasco M, Zaniboni M, Rimondini L. Autogenous onlay bone grafts vs. alveolar distraction osteogenesis for the correction of vertically deficient edentulous ridges: a 2–4-year prospective study on humans. Clin Oral Implants Res. 2007;18:432–440. doi: 10.1111/j.1600-0501.2007.01351.x. [DOI] [PubMed] [Google Scholar]
- Khan SN, Cammisa FP, Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The Biology of Bone Grafting. J Am Acad Orthop Surg. 2005;13(1):77–86. [PubMed] [Google Scholar]
- Carinci F, Guidi R, Franco M, Viscioni A, Rigo L, De Santis B, et al. Implants inserted in fresh-frozen bone: a retrospective analysis of 88 implants loaded 4 months after insertion. Quintessence Int. 2009;40(5):413–419. [PubMed] [Google Scholar]
- Tomford WW, Mankin HJ. Bone banking: Update on methods and materials. Orthop Clin North Am. 1999;30(4):565–570. doi: 10.1016/s0030-5898(05)70109-7. [DOI] [PubMed] [Google Scholar]
- Sjöström M, Lundgren S, Nilson H, Sennerby L. Monitoring of implant stability in grafted bone using resonance frequency analysis A clinical study from implant placement to 6 months of loading. Int J Oral Maxillofac Surg. 2005;34(1):45–51. doi: 10.1016/j.ijom.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Nyström E, Ahlqvist J, Gunne J, Kahnberg KE. 10-year follow-up of onlay bone grafts and implants in severely resorbed maxillae. Int J Oral Maxillofac Surg. 2004;33(3):258–262. doi: 10.1006/ijom.2003.0512. [DOI] [PubMed] [Google Scholar]
- Levin L, Nitzan D, Schwartz-Arad D. Success of dental implants placed in intraoral block bone grafts. J Periodontol. 2007;78:18–21. doi: 10.1902/jop.2007.060238. [DOI] [PubMed] [Google Scholar]
- Nyström E, Ahlqvist J, Legrell PE, Kahnberg KE. Bone graft remodelling and implant success rate in the treatment of the severely resorbed maxilla: a 5-year longitudinal study. Int J Oral Maxillofac Surg. 2002;31(2):158–164. doi: 10.1054/ijom.2001.0197. [DOI] [PubMed] [Google Scholar]
- Sjöström M, Sennerby L, Nilson H, Lundgren S. Reconstruction of the atrophic edentulous maxilla with free iliac crest grafts and implants: a 3-year report of a prospective clinical study. Clin Implant Dent Relat Res. 2007;9(1):46–59. doi: 10.1111/j.1708-8208.2007.00034.x. [DOI] [PubMed] [Google Scholar]
- Jensen J, Sindet-Pedersen S. Autogenous mandibular bone grafts and osseointegrated implants for reconstruction of the severely atrophied maxilla: a preliminary report. J Oral Maxillofac Surg. 1991;49(12):1277–1287. doi: 10.1016/0278-2391(91)90303-4. [DOI] [PubMed] [Google Scholar]
- Hunt DR, Jovanovic SA. Autogenous bone harvesting: a chin graft technique for particulate and monocortical bone blocks. Int J Periodontics Restorative Dent. 1999;19(2):165–173. [PubMed] [Google Scholar]
- Misch CM. Comparison of intraoral donor sites for onlay grafting prior to implant placement. Int J Oral Maxillofac Implants. 1997;12(6):767–776. [PubMed] [Google Scholar]
- Pikos MA. Mandibular block autografts for alveolar ridge augmentation. Atlas Oral Maxillofac Surg Clin North Am. 2005;13(2):91–107. doi: 10.1016/j.cxom.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Cooper MT, Coughlin MJ. Surgical technique: iliac crest corticocancellous bone graft harvest using a trap-door technique. Médecine Chir du Pied. 2009;25(4):127–132. [Google Scholar]
- Van der Meij AJ, Baart JA, Prahl-Andersen B, Valk J, Kostense PJ, Tuinzing DB. Computed tomography in evaluation of early secondary bone grafting. Int J Oral Maxillofac Surg. 1994;23(3):132–136. doi: 10.1016/s0901-5027(05)80286-3. [DOI] [PubMed] [Google Scholar]
- Reinert S, König S, Bremerich A, Eufinger H, Krimmel M. Stability of bone grafting and placement of implants in the severely atrophic maxilla. Br J Oral Maxillofac Surg. 2003;41(4):249–255. doi: 10.1016/s0266-4356(03)00078-0. [DOI] [PubMed] [Google Scholar]
- Contar CMM, Sarot JR, Bordini J, Galvão GH, Nicolau GV, Machado MAN. Maxillary ridge augmentation with fresh-frozen bone allografts. J Oral Maxillofac Surg. American Association of Oral and Maxillofacial Surgeons. 2009;67(6):1280–1285. doi: 10.1016/j.joms.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Jt Surg. 2001;83-A(Pt 2):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- Schaaf H, Lendeckel S, Howaldt H-P, Streckbein P. Donor site morbidity after bone harvesting from the anterior iliac crest. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(1):52–58. doi: 10.1016/j.tripleo.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Bauer TW. An overview of the histology of skeletal substitute materials. Arch Pathol Lab Med. 2007;131(2):217–224. doi: 10.5858/2007-131-217-AOOTHO. [DOI] [PubMed] [Google Scholar]
- Gomes KU, Carlini JL, Biron C, Rapoport A, Dedivitis RA. Use of allogeneic bone graft in maxillary reconstruction for installation of dental implants. J Oral Maxillofac Surg. 2008;66(11):2335–2338. doi: 10.1016/j.joms.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Köndell PA, Mattsson T, Astrand P. Immunological responses to maxillary on-lay allogeneic bone grafts. Clin Oral Implants Res. 1996;7(4):373–377. doi: 10.1034/j.1600-0501.1996.070411.x. [DOI] [PubMed] [Google Scholar]
- Joyce MJ. American association of tissue banks: a historical reflection upon entering the 21st century. Cell Tissue Bank. 2000;1(1):5–8. doi: 10.1023/A:1010136408283. [DOI] [PubMed] [Google Scholar]
- Costain DJ, Crawford RW. Fresh-frozen vs. irradiated allograft bone in orthopaedic reconstructive surgery. Injury. 2009;40(12):1260–1264. doi: 10.1016/j.injury.2009.01.116. [DOI] [PubMed] [Google Scholar]
- Simonds RJ, Holmberg SD, Hurwitz RL, Coleman TR, Bottenfield S, Conley LJ, et al. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med. 1992;326(11):726–732. doi: 10.1056/NEJM199203123261102. [DOI] [PubMed] [Google Scholar]
- Ryu SI, Lim JT, Kim S-M, Paterno J, Willenberg R, Kim DH. Comparison of the biomechanical stability of dense cancellous allograft with tricortical iliac autograft and fibular allograft for cervical interbody fusion. Eur Spine J. 2006;15(9):1339–1345. doi: 10.1007/s00586-005-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board TN, Brunskill S, Doree C, Hyde C, Kay PR, Meek RD, et al. Processed versus fresh frozen bone for impaction bone grafting in revision hip arthroplasty. Cochrane Database Syst Rev. 2009;(4) doi: 10.1002/14651858.CD006351.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocini PF, Bertossi D, Albanese M, D'Agostino A, Chilosi M, Procacci P. Severe maxillary atrophy treatment with Le Fort I, allografts, and implant-supported prosthetic rehabilitation. J Craniofac Surg. 2011;22(6):2247–2254. doi: 10.1097/SCS.0b013e3182327817. [DOI] [PubMed] [Google Scholar]
- Waasdorp J, Reynolds MA. Allogeneic bone onlay grafts for alveolar ridge augmentation: a systematic review. Int J Oral Maxillofac Implants. 2008;25(3):525–531. [PubMed] [Google Scholar]
- Keith JD, Petrungaro P, Leonetti JA, Elwell CW, Zeren KJ, Caputo C, et al. Clinical and histologic evaluation of a mineralized block allograft: results from the developmental period (2001–2004) Int J Periodontics Restorative Dent. 2006;26(4):321–327. [PubMed] [Google Scholar]
- Keith JD. Localized ridge augmentation with a block allograft followed by secondary implant placement: a case report. Int J Periodontics Restorative Dent. 2004;24(1):11–17. [PubMed] [Google Scholar]
- Wallace S, Gellin R. Clinical evaluation of a cancellous block allograft for ridge augmentation and implant placement: a case report. Implant Dent. 2008;17(2):151–158. doi: 10.1097/ID.0b013e318177784a. [DOI] [PubMed] [Google Scholar]
- Leonetti JA, Koup R. Localized maxillary ridge augmentation with a block allograft for dental implant placement: case reports. Implant Dent. 2003;12(3):217–226. doi: 10.1097/01.id.0000078233.89631.f8. [DOI] [PubMed] [Google Scholar]
- Lyford RH, Mills MP, Knapp CI, Scheyer ET, Mellonig JT. Clinical evaluation of freeze-dried block allografts for alveolar ridge augmentation: a case series. Int J Periodontics Restorative Dent [Internet] 2003;23(5):417–425. Oct; [PubMed] [Google Scholar]
- Nissan J, Romanos GE, Mardinger O, Chaushu G. Immediate nonfunctional loading of single-tooth implants in the anterior maxilla following augmentation with freeze-dried cancellous block allograft: a case series. Int J Oral Maxillofac Implants. 2008;23(4):709–716. [PubMed] [Google Scholar]
- Pendarvis WT, Sandifer JB. Localized ridge augmentation using a block allograft with subsequent implant placement: a case series. Int J Periodontics Restorative Dent. 2008;28(5):509–515. Oct; [PubMed] [Google Scholar]
- Petrungaro PS, Amar S. Localized ridge augmentation with allogenic block grafts prior to implant placement: case reports and histologic evaluations. Implant Dent. 2005;14(2):139–148. doi: 10.1097/01.id.0000163805.98577.ab. [DOI] [PubMed] [Google Scholar]
- Soltan M, Smiler D, Prasad HS, Rohrer MD. Bone block allograft impregnated with bone marrow aspirate. Implant Dent. 2007;16(4):329–339. doi: 10.1097/ID.0b013e31815c8ef4. [DOI] [PubMed] [Google Scholar]
- Barone A, Varanini P, Orlando B, Tonelli P, Covani U. Deep-frozen allogeneic onlay bone grafts for reconstruction of atrophic maxillary alveolar ridges: a preliminary study. J Oral Maxillofac Surg [Internet] American Association of Oral and Maxillofacial Surgeons. 2009;67(6):1300–1306. doi: 10.1016/j.joms.2008.12.043. Jun [cited 2014 Jan 23]; [DOI] [PubMed] [Google Scholar]
- Contar CMM, Sarot JR, da Costa MB, Bordini J, de Lima AAS, Alanis LRA, et al. Fresh-frozen bone allografts in maxillary ridge augmentation: histologic analysis. J Oral Implantol. 2011;37(2):223–231. doi: 10.1563/AAID-JOI-D-09-00108. [DOI] [PubMed] [Google Scholar]
- D'Aloja E, Santi E, Aprili G, Franchini M. Fresh frozen homologous bone in oral surgery: case reports. Cell Tissue Bank. 2008;9(1):41–46. doi: 10.1007/s10561-007-9053-0. [DOI] [PubMed] [Google Scholar]
- Franco M, Viscioni A, Rigo L, Guidi R, Brunelli G, Carinci F. Iliac crest fresh frozen homografts used in pre-prosthetic surgery: a retrospective study. Cell Tissue Bank. 2009;10(3):227–233. doi: 10.1007/s10561-008-9118-8. [DOI] [PubMed] [Google Scholar]
- Spin-Neto R, Landazuri Del Barrio RA, Pereira LAVD, Marcantonio RAC, Marcantonio E. Clinical similarities and histological diversity comparing fresh frozen onlay bone blocks allografts and autografts in human maxillary reconstruction. Clin Implant Dent Relat Res. 2013;15(4):490–497. doi: 10.1111/j.1708-8208.2011.00382.x. [DOI] [PubMed] [Google Scholar]
- Lumetti S, Consolo U, Galli C, Multinu A, Piersanti L, Bellini P, et al. Fresh-frozen bone blocks for horizontal ridge augmentation in the upper maxilla: 6-month outcomes of a randomized controlled trial. Clin Implant Dent Relat Res. 2014;16(1):116–123. doi: 10.1111/j.1708-8208.2012.00458.x. [DOI] [PubMed] [Google Scholar]
- Garcez-Filho J, Tolentino L, Sukekava F, Seabra M, Cesar-Neto JB, Araújo MG. Long-term outcomes from implants installed by using split-crest technique in posterior maxillae: 10 years of follow-up. Clin Oral Implants Res. 2014;1–6 doi: 10.1111/clr.12330. . doi: 10.1111/clr.12330. [DOI] [PubMed] [Google Scholar]
- Esposito M, Grusovin MG, Coulthard P, Worthington HV. The efficacy of various bone augmentation procedures for dental implants: a Cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants. 2006;21(5):696–710. [PubMed] [Google Scholar]
- Donos N, Mardas N, Chadha V. Clinical outcomes of implants following lateral bone augmentation: systematic assessment of available options (barrier membranes, bone grafts, split osteotomy) J Clin Periodontol. 2008;35(8 Suppl):173–202. doi: 10.1111/j.1600-051X.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78(3):377–396. doi: 10.1902/jop.2007.060048. [DOI] [PubMed] [Google Scholar]
- Jensen SS, Terheyden H. Bone augmentation procedures in localized defects in the alveolar ridge: clinical results with different bone grafts and bone-substitute materials. Int J Oral Maxillofac Implants. 2009;24(Suppl):218–236. Jan; [PubMed] [Google Scholar]
- Chiapasco M, Giammattei M, Carmagnola D, Autelitano L, Rabbiosi D, Dellavia C. Iliac crest fresh-frozen allografts and autografts in maxillary and mandibular reconstruction: a histologic and histomorphometric evaluation. Minerva Stomatol. 2013;62(1–2):3–16. [PubMed] [Google Scholar]
- Viscioni A, Franco M, Rigo L, Guidi R, Spinelli G, Carinci F. Retrospective study of standard-diameter implants inserted into allografts. J Oral Maxillofac Surg. 2009;67(2):387–393. doi: 10.1016/j.joms.2008.06.099. [DOI] [PubMed] [Google Scholar]
- Orsini G, Stacchi C, Visintini E, Di Iorio D, Putignano A, Breschi L, et al. Clinical and histologic evaluation of fresh frozen human bone grafts for horizontal reconstruction of maxillary alveolar ridges. Int J Periodontics Restorative Dent. 2011;31(5):535–544. [PubMed] [Google Scholar]
- Chiapasco M, Zaniboni M. Failures in Jaw Reconstructive Surgery with Autogenous Onlay Bone Grafts for Pre-implant Purposes: Incidence, Prevention and Management of Complications. Oral Maxillofac Surg Clin North Am. 2011;23(1):1–15. doi: 10.1016/j.coms.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res. 2006;17(Suppl 2):136–159. doi: 10.1111/j.1600-0501.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- Klein MO, Al-Nawas B. For which clinical indications in dental implantology is the use of bone substitute materials scientifically substantiated? Eur J Oral Implantol. 2011;4(Suppl):11–29. [Google Scholar]
- Beitlitum I, Artzi Z, Nemcovsky CE. Clinical evaluation of particulate allogeneic with and without autogenous bone grafts and resorbable collagen membranes for bone augmentation of atrophic alveolar ridges. Clin Oral Implants Res. 2010;21(11):1242–1250. doi: 10.1111/j.1600-0501.2010.01936.x. [DOI] [PubMed] [Google Scholar]
- Gielkens PFM, Bos RRM, Raghoebar GM, Stegenga B. Is there evidence that barrier membranes prevent bone resorption in autologous bone grafts during the healing period? A systematic review. Int J Oral Maxillofac Implants. 2007;22(3):390–398. [PubMed] [Google Scholar]
- Chiapasco M, Abati S, Romeo E, Vogel G. Clinical outcome of autogenous bone blocks or guided bone regeneration with e-PTFE membranes for the reconstruction of narrow edentulous ridges. Clin Oral Implants Res. 1999;10(4):278–288. doi: 10.1034/j.1600-0501.1999.100404.x. [DOI] [PubMed] [Google Scholar]
- Maiorana C, Beretta M, Salina S, Santoro F. Reduction of autogenous bone graft resorption by means of bio-oss coverage: a prospective study. Int J Periodontics Restorative Dent. 2005;25:19–25. [PubMed] [Google Scholar]
- Adeyemo WL, Reuther T, Bloch W, Korkmaz Y, Fischer JH, Zöller JE, et al. Healing of onlay mandibular bone grafts covered with collagen membrane or bovine bone substitutes: A microscopical and immunohistochemical study in the sheep. Int J Oral Maxillofac Surg. 2008;37:651–659. doi: 10.1016/j.ijom.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Cordaro L, Torsello F, Morcavallo S, di Torresanto VM. Effect of bovine bone and collagen membranes on healing of mandibular bone blocks: a prospective randomized controlled study. Clin Oral Implants Res. 2011;22:1145–1150. doi: 10.1111/j.1600-0501.2010.02093.x. [DOI] [PubMed] [Google Scholar]
- Von Arx T, Buser D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: a clinical study with 42 patients. Clin Oral Implants Res. 2006;17:359–366. doi: 10.1111/j.1600-0501.2005.01234.x. [DOI] [PubMed] [Google Scholar]
- Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007;22(Suppl):49–70. [PubMed] [Google Scholar]
- Laverick S, Summerwill A, Cawood JI. Ten years of experience with the anterior maxillary and mandibular osteoplasty (class IV ridges): a retrospective analysis of implant survival rates. Int J Oral Maxillofac Surg. 2008;37(5):415–418. doi: 10.1016/j.ijom.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11–25. [PubMed] [Google Scholar]
- Heberer S, Rühe B, Krekeler L, Schink T, Nelson JJ, Nelson K. A prospective randomized split-mouth study comparing iliac onlay grafts in atrophied edentulous patients: covered with periosteum or a bioresorbable membrane. Clin Oral Implants Res. 2009;20(3):319–326. doi: 10.1111/j.1600-0501.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- Sterio T, Katancik J. A Prospective, Multicenter Study of Bovine Pericardium Membrane with Cancellous Particulate Allograft for Localized Alveolar Ridge Augmentation. Int J Periodontics Restorative Dent. 2013;33(4):499–507. doi: 10.11607/prd.1704. [DOI] [PubMed] [Google Scholar]
- Feuille F, Knapp CI, Brunsvold MA, Mellonig JT. Clinical and histologic evaluation of bone-replacement grafts in the treatment of localized alveolar ridge defects. Part 1: Mineralized freeze-dried bone allograft. Int J Periodontics Restorative Dent. 2003;23(1):29–35. Feb; [PubMed] [Google Scholar]
- Acocella A, Bertolai R, Ellis E, Nissan J, Sacco R. Maxillary alveolar ridge reconstruction with monocortical fresh-frozen bone blocks: A clinical, histological and histomorphometric study. J Cranio-Maxillofacial Surg. 2012;40(6):525–533. doi: 10.1016/j.jcms.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Acocella A, Bertolai R, Colafranceschi M, Sacco R. Clinical, histological and histomorphometric evaluation of the healing of mandibular ramus bone block grafts for alveolar ridge augmentation before implant placement. J Cranio-Maxillofacial Surg. 2010;38:222–230. doi: 10.1016/j.jcms.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Deluiz D, Oliveira LS, Pires FR, Tinoco EMB. Time-Dependent Changes in Fresh-Frozen Bone Block Grafts: Tomographic, Histologic, and Histomorphometric Findings. Clin Implant Dent Relat Res. 2013 doi: 10.1111/cid.12108. . doi: 10.1111/cid.12108. [DOI] [PubMed] [Google Scholar]
- Yang S, Lan L, Miron RJ, Wei L, Zhang M, Zhang Y. Variability in Particle Degradation of Four Commonly Employed Dental Bone Grafts. Clin Implant Dent Relat Res. 2014 doi: 10.1111/cid.12196. . doi: 10.1111/cid.12196. [DOI] [PubMed] [Google Scholar]
- Bormann N, Pruss A, Schmidmaier G, Wildemann B. In vitro testing of the osteoinductive potential of different bony allograft preparations. Arch Orthop Trauma Surg. 2010;130(1):143–149. doi: 10.1007/s00402-009-0908-7. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Somers A, Mellonig JT, Carnes DL, Dean DD, Cochran DL, et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but not gender. J Periodontol. 1998;69:470–478. doi: 10.1902/jop.1998.69.4.470. [DOI] [PubMed] [Google Scholar]
- Steinberg EL, Luger E, Zwas T, Katznelson A. Very long-term radiographic and bone scan results of frozen autograft and allograft bone grafting in 17 patients (20 grafts) a 30- to 35-year follow-up. Cell Tissue Bank. 2004;5(2):97–104. doi: 10.1023/B:CATB.0000034084.27772.66. [DOI] [PubMed] [Google Scholar]
- Acocella A, Bertolai R, Nissan J, Sacco R. Clinical, histological and histomorphometrical study of maxillary sinus augmentation using cortico-cancellous fresh frozen bone chips. J Cranio-Maxillo-Facial Surg. 2011;39(3):192–199. doi: 10.1016/j.jcms.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Spin-Neto R, Stavropoulos A, Coletti FL, Faeda RS, Pereira LAVD, Marcantonio E. Graft incorporation and implant osseointegration following the use of autologous and fresh-frozen allogeneic block bone grafts for lateral ridge augmentation. Clin Oral Implants Res. 2014;25(2):226–233. doi: 10.1111/clr.12107. [DOI] [PubMed] [Google Scholar]
- Buffoli B, Boninsegna R, Rezzani R, Poli PP, Santoro F, Rodella LF. Histomorphometrical evaluation of fresh frozen bone allografts for alveolar bone reconstruction: preliminary cases comparing femoral head with iliac crest grafts. Clin Implant Dent Relat Res. 2013;15(6):791–798. doi: 10.1111/cid.12028. [DOI] [PubMed] [Google Scholar]


