Abstract
Heroin addiction is a disease of chronic relapse affecting over half of its users. Therefore, modeling individual differences in addiction-like behavior is needed to better reflect the human condition. In a rodent model, avoidance of a cocaine-paired saccharin cue is associated with greater cocaine seeking and taking. Here, we tested whether rats would avoid a saccharin cue when paired with the opportunity to self-administer heroin and whether the rats that most greatly avoid the heroin-paired taste cue would exhibit the greatest drug escalation over time, the greatest willingness to work for drug, and the greatest heroin-induced relapse. Adult male Sprague-Dawley rats received 5 min access to a 0.15% saccharin solution followed by the opportunity to self-administer either saline or heroin for 3 h (short access) or 6 h (extended access). Following 16 – 18 pairings, terminal saccharin intake was used to categorize the rats into small (>200 licks/5min) or large (<200 licks/5min) suppressors and responding for drug was examined accordingly. Only 5% of the short access rats reached the criteria for large suppressors. This large suppressor did not differ from the small suppressors in drug taking behavior. Conversely, 50% of the extended access saccharin-heroin rats were large suppressors and showed the largest escalation of drug intake, drug-loading behavior, and the greatest relapse-like behaviors. Extended access small suppressors displayed drug-taking behaviors that were similar to rats in the short access heroin condition. Avoidance of a heroin-paired taste cue reliably identifies individual differences in addiction-like behavior for heroin using extended drug access.
Keywords: heroin, self-administration, reward, individual differences, motivation
Heroin addiction is a persistent disease that can lay dormant in an individual across years of abstinence only to resurface given the right circumstances. Recent reports have shown that heroin use is on the rise, partially due to a large number of individuals transitioning from prescription opiates to heroin, which is cheaper and easier to access (Lankenau et al., 2012; Peavy et al., 2012). This shift in heroin use also can be seen in the change in the demographics of users. Heroin use is becoming more widespread among white men and women in their late 20s rather than being concentrated among young men in urban environments as in years past (Cicero, Ellis, Surratt, & Kurtz, 2014). In addition, compared to other drugs, heroin has been shown to have a higher proportion of individuals switching from drug taking to substance use disorder. It has been reported that approximately 17–19% of individuals who engage in illicit drug use transition to develop drug addiction (Anthony, Warner, & Kessler, 1994; Grant & Dawson, 1998). With respect to heroin, however, it has been estimated that over half of first time users of heroin transition to drug dependence (SAMHSA, 2012). Therefore understanding how some individuals can be resilient to heroin addiction, while others fall victim to the disease should aid in combating this disease.
There are a number of ways to model drug addiction in rodents. The extended access procedure in which rats are given long periods of access to drug is of great interest given its ability to model many symptoms of human substance use disorder. Specifically, rats that receive prolonged periods of drug access show signs of drug escalation, increased motivation to work for drug, persistence to use even in the face of adversity, and greater relapse-like behavior after periods of enforced abstinence (Ahmed & Koob, 1998; Ahmed, Walker, & Koob, 2000). Despite this progress, few studies have examined whether there are individual differences in addiction-like behavior when tested using the extended access model. Given the degree of variability in the human population, there is a need for animal models that are sensitive to individual differences in vulnerability to, and resilience from, addiction-like behavior.
Recently a number of laboratories have begun to investigate individual differences in responding to drugs of abuse using other models. Deroche-Gamonet et al. (2004) used intermittent access to cocaine to stratify subjects by their addiction-like behavior, even when all of the rats consumed the same amount of drug during fixed ratio responding. Saunders and Robinson (2010) determined that subjects who attributed incentive salience to a drug cue showed greater motivation to work for drug and increased relapse-like behaviors than subjects who did not. Lenoir et al. (2013) utilized a discrete 2-choice procedure to determine individual differences in preference of a natural reward versus a drug of abuse. Our laboratory has shown that rodents that most greatly avoid a drug-paired taste cue exhibit the greatest responding for drug. Specifically, avoidance of an otherwise palatable saccharin cue following pairings with cocaine or morphine has been shown to be associated with blunted accumbal dopamine levels, an increased corticosterone response to presentation of the drug-paired cue, and increased withdrawal symptoms (Gomez, Leo, & Grigson, 2000; Grigson & Hajnal, 2007; Nyland & Grigson, 2013). Finally, when using self-administration, greater avoidance of a cocaine-paired saccharin cue was associated with greater seeking and taking of cocaine (Cason & Grigson, 2013; Grigson & Twining, 2002; Twining, Bolan, & Grigson, 2009).
The current study sought to determine if rats also will avoid intake of a saccharin cue when paired with the opportunity to self-administer heroin. In this case, however, heroin was provided in not only short (3h), but also extended (6h) daily access periods. Thus, the question under consideration was whether greater avoidance of the heroin-paired saccharin cue would be associated with not only a greater willingness to work for heroin and greater drug-induced reinstatement of heroin seeking behavior following a 6 h period of extinction, but also with greater escalation of heroin self-administration over time. Here we demonstrate that extended but not short access to heroin is sufficient to produce large individual differences in addiction-like behavior. Rats that most greatly avoid the drug-paired cue exhibit the greatest escalation, drug loading, and relapse.
Method
Experiment 1: Short Access Procedure
Subjects and surgeries
The subjects were 19 naive, male Sprague-Dawley rats obtained from Charles River at approximately 90 days of age. The rats were housed singly in suspended, stainless steel cages in a humidity-controlled environment under a 12/12 h light/dark cycle. Food and water were available ad libitum, except where noted. The rats were implanted with intravenous jugular catheters as described previously by (Grigson & Twining, 2002). After surgeries, the rats were given 1 week to recover prior to the start of testing. General maintenance of catheter patency included daily examination and flushing of catheters using heparinized saline (0.2 ml of 30 IU/ml heparin). Catheter patency was verified when necessary using 0.2 ml of propofol (Diprivan 1%) administered intravenously. All studies were approved by the Pennsylvania State University College of Medicine, Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health specifications outlined in their Guide for the Care and Use of Laboratory Animals.
Apparatus
Testing was conducted in 12 self-administration chambers as previously described by Puhl et al. (2013). Each chamber measured 30.5 cm in length, 24.0 cm in width, 29.0 cm in height, and was equipped with three retractable sipper tubes that entered the chamber through three holes. A stimulus light was located above each tube. A lickometer circuit was used to monitor licking on the leftmost saccharin spout, the middle inactive spout (i.e., the spout upon which responding elicited no consequence), and the rightmost active spout (the spout upon which a set of fixed ratio (FR) responses led to an iv infusion of drug). Each chamber also was equipped with a house light (25 W), a tone generator, and a speaker for white noise. Events in the chamber and collection of the data were controlled on-line with a Pentium computer that used programs written in the Medstate notation language (MED Associates, Inc., St. Albans, VT).
Acquisition
Rats were habituated in the self-administration chambers 5 min per day for 3 days prior to the start of acquisition. During habituation, the rats were on a water restriction regimen in which they received 5 min access to water through 1 of the 3 spouts in the chambers and 25 ml of water in the home cage overnight. This habituation occurred over 3 days until each animal experienced each of the 3 spouts. Thereafter, water was returned to the rats to allow for an examination of responding in the absence of need (While it is the case that avoidance of a drug-paired saccharin cue can be quite pronounced when examined in a need-free state (Grigson et al., 2000), individual differences in suppression of CS intake have been obtained in both water deprived (Grigson & Twining, 2002; Twining et al., 2009) and water replete rats (Puhl et al., 2012)). During acquisition, rats were given 5 min access to 0.15% saccharin via the leftmost spout, after which the saccharin spout retracted, and the 2 empty spouts advanced. Rats were placed on a fixed ratio (FR) 10 schedule of reinforcement where 10 licks on the rightmost empty active spout resulted in a 6 sec iv infusion of either saline (n=4) or a 0.06 mg/0.2ml of heroin (n=15) as previously described by Kuntz et al. (2008). Drug or saline delivery was signaled by offset of the stimulus light and onset of the tone and house light, which remained on for a total of 20 sec. Further responding during this time was not reinforced. The access period for heroin was 3 h. There was one such taste-drug pairing/day for 18 days in succession.
Experiment 2: Extended Access Procedure
Subjects and surgeries
The subjects were 48 naïve, male Sprague-Dawley rats obtained and maintained as described in Experiment 1. This experiment was conducted over two replications, each with 24 rats. The subjects were implanted with jugular catheters and were again given 1 week to recover before testing began.
Apparatus
The apparatus was the same as that described above.
Drug acquisition
The extended access procedure followed primarily those described in Experiment 1 with a few exceptions. During acquisition, rats were given 5 min access to 0.15% saccharin. Thereafter, the saccharin spout retracted and the 2 empty spouts advanced. Completion of 10 licks on the active spout resulted in a 6 sec iv infusion of either saline (n=16) or a 0.06 mg/0.2ml of heroin (n=32). The access period for heroin was 6 h. There was one such taste-drug pairing/day, 5 days a week, for a total of 16 taste-drug trials.
Progressive ratio (PR)
At the completion of trial 16 of the drug acquisition phase, a single PR test was conducted on the following day to assess motivation. On the PR test, the rat received 5 min access to 0.15% saccharin via the leftmost spout. After 5 min, the saccharin spout retracted and the 2 empty spouts advanced. Each subject began on an FR 10 on the active spout for the 1st infusion, with subsequent infusions requiring the completion of the following progression of licks to obtain the next infusion: 10, 12, 16, 22, 30, 40, 52, 66, 82, 100, 120, 142, 166. Breakpoint was defined as the last ratio completed. The trial ended when 30 min elapsed without having earned an infusion. This PR regimen was based on that used by Puhl et al. (2009).
Maintenance phase
After the PR test, all rats were given 3 additional saccharin-saline or saccharin-heroin pairings on the FR10 schedule of reinforcement to reestablish drug-taking behavior prior to the extinction and reinstatement test.
Extinction and reinstatement test
Twenty-four hours later, all rats participated in a one-day extinction and reinstatement test. The rats were given 5 min access to the saccharin cue. The saccharin spout retracted and the 2 empty spouts advanced for a 6 h extinction session during which responding on the active spout was not reinforced. Otherwise, the session was conducted as described in acquisition. Immediately thereafter, rats received a single non-contingent 6 sec iv infusion of 0.06 mg/0.2 ml heroin and responding on the active and inactive empty spouts was recorded for an additional hour.
Behavioral stratification
Rats trained in the short access and extended access condition were behaviorally stratified using criteria from Grigson and Twining (2002). Specifically, terminal saccharin intake was used to separate the animals into large and small suppressors. The terminal trial for rats in the short access condition was Trial 18, while the terminal trial for rats in the extended access condition was Trial 16. Small suppressors were defined as rats that emitted >200 licks/5min during terminal saccharin intake, while large suppressors were defined as rats that emitted <200 licks/5min during terminal saccharin intake.
Drugs
Heroin HCl was generously provided by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, N.C., USA). Heroin was dissolved in sterile physiological saline to a concentration of 0.3 mg/ml.
Data Analysis
Subjects were excluded from the study if their catheters lost patency any time throughout the experiment. All behavioral data were analyzed using Statistica7 (StatSoft), utilizing 3 × 16 mixed factorial analyses of variance (ANOVA) varying group (Saline, Small Suppressors, and Large Suppressors) and trials. Post hoc tests were conducted, when appropriate, using Fisher’s least significant difference (LSD) tests, with αset at 0.05.
Results
Experiment 1 Short Access
Saccharin intake
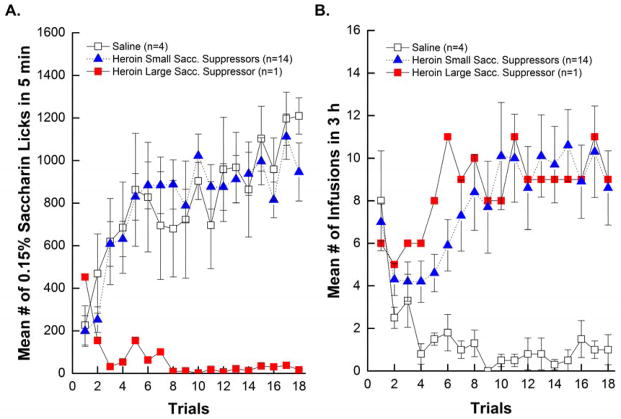
As shown in Figure 1A, the short access heroin rats could be separated into large and small saccharin suppressors based on terminal saccharin intake (Trial 18). That said, there was an absence of statistical significance between the two groups (p>0.05) because only 1 animal out of 15 met the criteria for a large suppressor.
Figure 1.
Short Access Addiction-like Behavior. Figure 1A. Mean (+/−SEM) number of licks/5 min of 0.15% saccharin across 18 trials for saline, large, and small suppressors. Figure 1B. Mean (+/−SEM) number of saline or heroin (.06 mg/0.2ml of heroin) infusions/3 h across 18 trials for the saline, large, and small suppressors.
Drug-taking behavior
Inspection of the drug taking behavior (Figure 1B) between the three groups revealed a significant Group x Trials interaction, F(34,255)=1.99; p<0.001, but post hoc tests determined no significant differences between large and small suppressors (p>0.05). Furthermore, regardless of saccharin avoidance, total heroin intake remained stable over the 18 taste drug trials when compared to Trial 1 intake (ps>0.05). There was, then, no reliable evidence for escalation across trials with short access.
Experiment 2 Extended Access
In the first replication of Experiment 2, catheter patency was lost during the course of the study for 4 saline and 5 heroin rats. In the second replication, the data from 2 saline rats and 5 heroin rats also were discarded due to a lack of catheter patency. Thus, the final number of subjects used for the extended access analysis was 11 saline subjects and 26 heroin subjects.
Saccharin intake
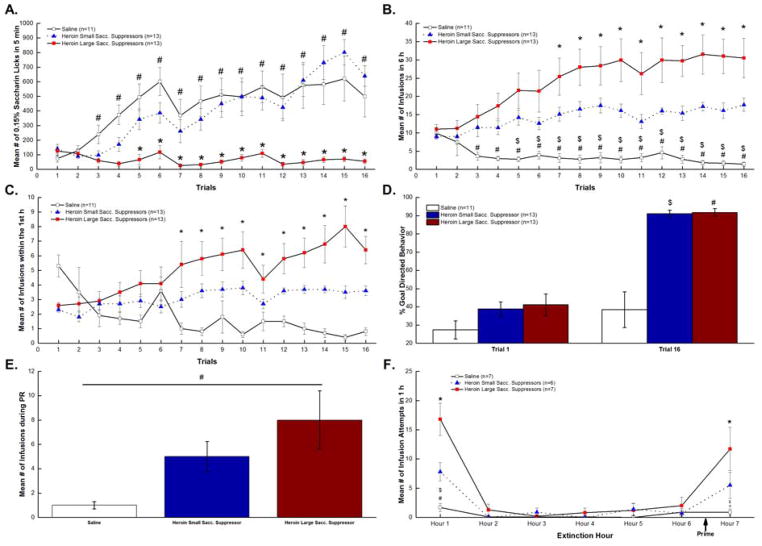
As seen in Figure 2A, rats in the extended access heroin condition were divided into large and small suppressors based on terminal saccharin intake (Trial 16). Unlike the short access procedure, extending the heroin access period from 3 h to 6 h yielded an increase in the number of heroin animals being classified as large suppressors (n=13) as opposed to small suppressors (n=13). As expected, the large suppressors from the extended access heroin condition showed significantly greater avoidance of the taste cue compared to both the extended access small suppressors and the saline controls. This conclusion was supported by post hoc tests of a significant Group x Trials interaction, F(30,510)=6.07, p<0.05. Extended access large suppressors showed a significant decrease in consumption of the drug-paired taste cue beginning with trial 3 compared to saline rats (ps<0.05) and beginning with trial 5 when compared to the extended access small suppressors (ps<0.05).
Figure 2.
Extended Access Addition-like Behavior. Figure 2A. Mean (+/−SEM) number of licks/5 min of 0.15% saccharin over 16 trials for saline, small, and large suppressors. Mean (+/−SEM) number of saline or heroin (.06 mg/0.2ml of heroin) infusions/6 h (Figure 2B) and within in the 1st h (Figure 2C) across 16 trials for saline, small, and large suppressors. Figure 2D. Percent goal directed behavior shown for Trial 1 and Terminal Trial 16 for saline, small, and large suppressors. Figure 2E. Mean (+/−SEM) number of saline or heroin infusions earned during progressive ratio testing for saline, small, and large suppressors. Figure 2F. Mean (+/−SEM) number of drug seeking behaviors/1 h exhibited during the 6 h extinction and 1 h heroin induced reinstatement tests for saline, small, and large suppressors. *p<0.05: large suppressors vs. small suppressors; #p<0.05: large suppressors vs. the saline controls; $p<0.05: small suppressors vs. the saline controls.
Drug-taking behavior
Figure 2B shows that heroin extended access large suppressor rats took more infusions over the 6 h period than did the small suppressor heroin rats or the saline self-administering controls. Support for this conclusion was provided by post hoc assessment of a significant Group x Trials interaction, F(30,510)=4.88, p<0.001. Regardless of avoidance of the drug-paired taste cue, both extended access large suppressors and extended access small suppressors showed increased infusions compared to saline rats by trial 3 and trial 5, respectively (ps<0.05). Further post hoc comparisons revealed that extended access large suppressors took more heroin infusions than extended access small suppressors, beginning with trial 7 (ps<0.05).
Drug escalation
According to Ahmed et al. (2000), escalation of drug intake marks the transition from controlled use to excessive use in humans and in rodent models. Here we employed a similar method by examining first hour drug intake in the extended access groups and by assessing the change in drug-taking behavior over time. As shown in Figure 2C, a mixed factorial ANOVA on first hour infusions revealed a significant Group x Trials interaction, F(30,510)=6.10; p<0.001. Post hoc analysis of this two-way interaction revealed that, compared to Trial 1 intake, both the extended access large and small suppressors showed an increase in drug intake in the 1st hour, beginning with Trial 7 (ps<0.05). However, closer inspection of the magnitude of escalation showed that, by Trial 7, extended access large suppressors exhibited a greater increase in their 1st hour intake than did the extended access small suppressors (ps<0.05). Ultimately, extended access large suppressors had a 2.46 fold increase in the number of infusions between Trial 1 and Terminal Trial 16, while the extended access small suppressors had only a 1.56 fold increase between Trial 1 and Terminal Trial 16.
Goal-directed behavior
To determine if the differences in drug taking behavior and drug escalation could be attributed to a difference in preference between the active and inactive spouts, goal-directed behavior was calculated. Goal-directed behavior was defined as the total number of licks on the active spout divided by the total number of licks on the active and inactive spouts combined. As shown in Figure 2D, on Trial 1 rats in the large and small suppressor groups showed no appreciable preference for either the active and inactive spouts (F(2,34)=1.35, p>0.05). However, by the terminal acquisition trial, a one-way ANOVA found a significant main effect of group (F(2,34)=26.3, p<0.001.) Both the large and small suppressors showed a robust preference for the active spout compared to the inactive spout. Large suppressors goal-directed behavior preference ratio was 91.88%±2.06 and small suppressors goal-directed behavior preference ratio was 91.18%±1.92. Post hoc tests showed that this preference was significantly different from saline subjects (ps<0.05), while comparison between the large and small suppressors found no difference between the two (ps>0.05). Thus, while the large suppressors made numerically many more licks on the active spout than the small suppressors, the ratio of responding on the active vs. the inactive spout was essentially identical between the two groups.
Motivation
To determine the rats’ willingness to work for drug, a single progressive ratio test was conducted after the terminal day of acquisition (Figure 2E). A one-way ANOVA revealed differences in the motivation to work for drug among the three groups (F(2,34)=4.45, p<0.05). Post hoc analysis confirmed that large suppressors worked significantly harder than saline controls (p<0.05), while small suppressors did not (p>0.05). There also was a tendency for large suppressors to work harder than small suppressors during the PR test, but this pattern of behavior did not attain statistical significance (p>0.05).
Extinction and reinstatement
Following re-establishment of FR responding during the three-day maintenance phase, persistence during extinction and drug-induced reinstatement was assessed using rats from the 2nd replication (n=7 saline, n=6 small suppressors, and n=7 large suppressors). As shown in Figure 2F, both sets of rats in the heroin condition increased drug seeking relative to the saline controls across the extinction and reinstatement phases and the magnitude of this effect was greatest in the large suppressors. This conclusion was supported by post hoc tests of a significant Group x Time interaction, F(12,102)=4.02; p<0.001. Specifically, post hoc tests of this two-way interaction confirmed that the large suppressors showed significantly more drug seeking behavior within the first hour than the small suppressors (p<0.05). Importantly, drug-seeking behavior was not significantly different across groups between hours 2 and 6 of the extinction component of the test. Following the non-contingent iv infusion of heroin, however, post hoc analysis revealed a robust resumption of drug seeking behavior in the large suppressors compared to the small suppressors and in comparison to the saline controls (ps<0.05).
Comparison between short 3 h vs. extended 6 h access
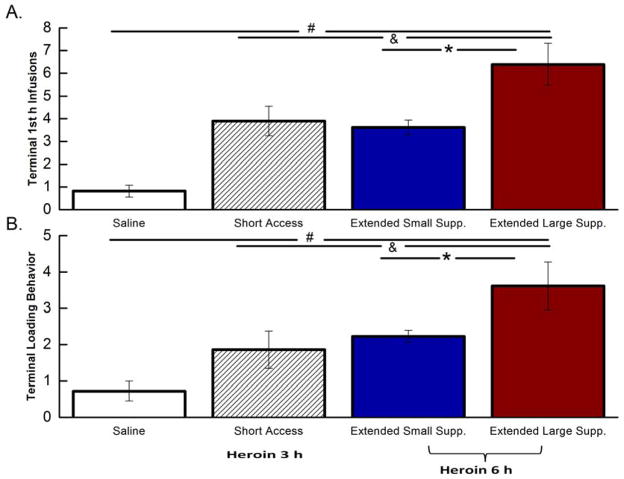
To test for differences in drug taking behavior between short and extended access procedures, the small (n=14) and the large (n=1) suppressors in the short access condition were combined, given that all rats self-administered about the same number of infusions of heroin/3 h. The terminal number of 1st hour infusions (measured during Trial 18 for short access and Trial 16 for extended access rats) was compared between saline controls (combined from short and extended access procedures, n=15), rats in the short access condition, extended access large, and extended access small, suppressors. A one-way ANOVA revealed a significant main effect of group in the number of 1st hour infusions (F(3,52)=13.10; p<0.001). Post hoc analysis revealed that extended access large suppressors showed the greatest number of infusions within the first hour compared to the other 3 groups (Figure 3A). Interestingly extended access small suppressors exhibited a similar number of 1st hour infusions as the short access rats (p>0.05). Short access, then, promoted behavior akin to that elicited by the extended access small suppressors.
Figure 3.
Comparisons between Short Access and Extended Access groups. Mean (+/−SEM) number of terminal 1st hour infusions (Figure 3A) and terminal loading behaviors (Figure 3B) for saline-treated rats, rats in the short access group, extended access small suppressors, and extended access large suppressors. *p<0.05: Extended access large suppressors vs. extended access small suppressors; #p<0.05: Extended access large suppressors vs. the saline group; &p<0.05: Extended access large suppressors vs. the short access group.
Terminal Log10 latency (in seconds) to 1st infusion was also examined among the 4 groups. A one-way ANOVA revealed a significant main effect of group on their latency to 1st infusion (F(3,52)=15.20; p<0.001). Post hoc tests revealed that all 3 heroin groups regardless of length of drug access had a shorter log10 latency to 1st infusion compared saline rats (ps<0.05). However, there was a lack of statistical significance in the latency to 1st heroin infusion among the 3 drug conditions (ps>0.05), possibly due to a floor effect (saline log10 latency: 3.3±0.3; short access: 1.7±0.3; extended access small suppressors: 1.4±0.2; extended access large suppressors: 1.1±0.1). To further study the temporal dynamics of drug taking terminal drug loading behavior also was examined to see if rats infuse a different amount of drug during the start of the final acquisition session. Using the Ahmed et al. (2000) definition for loading behavior, we examined the first 20 minutes of self-administration on Terminal Trial 16. The first 20 minutes were used to compare loading behavior across the 4 groups. As seen in Figure 3B, a one-way ANOVA revealed a significant main effect of group (F(3,52)=6.79; p<0.001). As expected regardless of length of access, all heroin rats showed increased loading behavior when compared to saline rats from both procedures. However, additional post hoc tests confirmed that extended access large suppressors exhibited the greatest loading behavior of the 4 groups (ps<0.05). Also of note is that extended access small suppressors showed a similar loading behavior to that of rats in the short access condition. Thus, significant load up behavior was found during the terminal trial for all rats self-administering heroin, with the effect being most marked in the extended access large suppressors.
Extended access drug escalation relationships
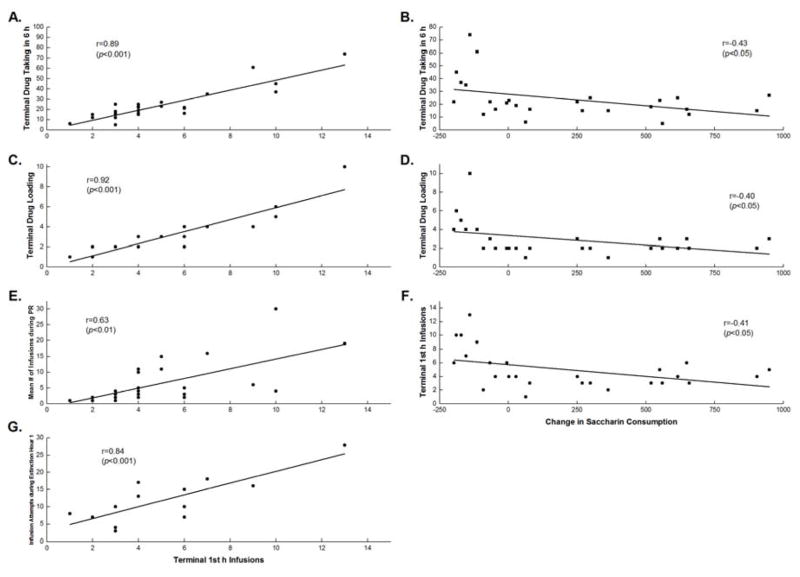
To determine the relationship between drug escalation and other addiction-like behaviors in the extended access groups, correlational analyses were conducted. As shown in Figure 4 left panels, the results showed that greater Terminal Trial 16 drug escalation was associated with greater terminal drug intake (r=0.89; p<0.001), greater drug loading (r=0.92; p<0.001), a greater motivation to work for drug (r=0.63; p<0.01), and greater 1st h drug seeking during extinction (r=0.84; p<0.001).
Figure 4.
Terminal 1st h Infusions as a function of Terminal Drug Taking (A), Terminal Drug Loading (C), Mean Number of Infusions during PR (E), and Mean Attempts During Extinction Hour 1 (G). Change in Saccharin Consumption (Trial 16 – Trail 1) as a function of Terminal Drug taking in 6 h (B), Terminal Drug Loading (D), and Terminal 1st h Infusions (F).
Additional correlational analyses were conducted to further understand the relationship between the change in saccharin consumption over the drug acquisition phase and the addiction-like behaviors measured in this study. The change in saccharin consumption over the course of the acquisition phase was calculated by taking the difference between the Terminal Trial 16 and Trial 1 saccharin licks/5 min. As seen in Figure 4 right panels, a significant correlation was seen whereby greater avoidance of the heroin-paired saccharin cue at the end of acquisition (i.e., lower saccharin intake) was associated with greater Terminal Trial 16 drug taking (r=−0.43; p<0.05), larger Terminal Trial 16 drug escalation (r=−0.41; p<0.05), and greater Terminal Trial 16 drug loading (r=−0.40; p<0.05). A trend towards significance was seen between suppression of the drug-paired taste cue and greater 1st h extinction behaviors (r=−0.51; p=0.07) and greater reinstatement behavior following the drug prime (r=−0.50; p=0.08). These latter two correlations may have missed statistical significance partially due the reduced sample size used for extinction/reinstatement testing.
Discussion
Rats avoid intake of a taste cue when paired with experimenter-administered drugs of abuse including morphine, cocaine, ethanol, amphetamine, and heroin (Cappell & LeBlanc, 1971; Gomez et al., 2000; Grigson, Twining, & Carelli, 2000; Grigson, Wheeler, Wheeler, & Ballard, 2001; Liu, Showalter, & Grigson, 2009). They also avoid intake when paired with the opportunity to self-administer the drug. This was first shown with amphetamine and later with cocaine (Grigson & Twining, 2002; Wise, Yokel, & DeWit, 1976). Here, we extend this story by showing that rats also avoid intake of a saccharin cue when paired with the opportunity to self-administer heroin using extended, but not short access to drug.
Earlier studies with self-administered amphetamine and cocaine showed that greater avoidance of the saccharin cue was highly correlated with greater drug-taking behavior (Grigson & Twining, 2002; Wise et al., 1976). In the cocaine studies, greater avoidance of the taste-cue was linked to greater motivation for drug, as large suppressors also exhibited a shorter latency to take drug, a greater willingness to work for drug, and greater seeking for cocaine following an extended period of abstinence (Grigson & Twining, 2002; Twining et al., 2009). The present study extended these findings by showing that, like cocaine, greater avoidance of a heroin-paired saccharin cue under extended access conditions also is accompanied by greater drug intake, larger drug loading behavior at the start of the session, greater seeking during extinction, and greater drug-induced reinstatement.
These findings are consistent with the cocaine data. An additional question asked here, however, was whether these individual differences in responsiveness for the heroin-paired saccharin cue also would be accompanied by greater escalation of drug-taking behavior in the extended access model. As stated, escalation of drug-taking is considered a hallmark of addiction in rats and humans (Ahmed et al., 2000; Ahmed & Koob, 1998; O’Brien, Ehrman, & Ternes, 1984). The results were affirmative. Large suppressors in the extended access study also exhibited the greatest escalation of drug self-administration behavior across training. When looking only at 1st h intake, both the large and the small suppressors exhibited an escalation of heroin self-administration across trials, but the effect was greater in the large suppressors. Although reports are relatively few, other laboratories have noted that not all animals escalate their pattern of intake, even with extended access (Mantsch, Yuferov, Mathieu-Kia, Ho, & Kreek, 2004; Willuhn, Burgeno, Groblewski, & Phillips, 2014). Here we show marked individual differences in escalation of heroin self-administration behavior and link greater escalation with a number of other indices of substance use disorder and addiction. Escalation of heroin intake was robustly and significantly correlated with drug taking, drug loading, motivation under progressive ratio testing, and drug seeking behaviors during extinction.
Relative to the extended access condition, the short access procedure has been thought to elicit behaviors that are consistent with drug taking as opposed to drug addiction. In particular, limited access to drugs of abuse such as cocaine and heroin generally support stable and consistent drug taking over time (Ahmed et al., 2000; Ahmed & Koob, 1998). Consistent with these observations, our rats in the short access heroin group showed steady drug intake over the 18 sessions, with no clear individual differences in responding. Further, unlike previous findings with brief 1 h access to cocaine (Grigson & Twining, 2002), short 3 h access to heroin also did not support large individual differences in avoidance of the drug-paired taste cue. The failure to produce individual differences in avoidance of the drug paired taste cue during the short access experiment was not likely due to the use of short access alone. As noted earlier, our laboratory has found that individual differences in saccharin avoidance following pairings with short 1 h access to cocaine self-administration and these individual differences are accompanied by individual differences in drug seeking and taking (Grigson & Twining, 2002). Likewise Deroche-Gamonet et al. (2004) found individual differences in addiction-like behavior in a short access procedure when cocaine availability was presented intermittently during each drug session. Using a similar paradigm, we also found individual differences in addiction-like behavior for heroin with intermittent daily 2 h access to heroin (Tacelosky et al., Submitted). Therefore it is possible to obtain individual differences in addiction-like behavior for either cocaine or heroin using a short access procedure.
Why, then, did we not find evidence of individual differences in the short access procedure here? Of course, the finding could be an anomaly. Another possibility is that the failure to find individual differences in either saccharin or drug intake in experiment 1 may have been due to an interaction involving the use of heroin, the use of a sweet cue, and the shorter access period employed. In support, the human and rodent literature has shown that opiates have a direct effect on the perceived value of sweets. Methadone maintained patients exhibit an increased preference for sugary foods over other sources of nutrition (Nolan & Scagnelli, 2007; Zador, Lyons Wall, & Webster, 1996). Treatment of rats with morphine can enhance the amount of ingestive behaviors emitted towards sucrose indicating an increase in perceived hedonic value of the sweet solution (Rideout & Parker, 1996). Furthermore direct infusion of opiate agonists into the nucleus accumbens of rats accentuates the value of sucrose (Zhang & Kelley, 1997). Taken together, these data suggest that while anticipation of access to drug can devalue a sweet cue, the sweet cue also may mitigate against this devaluation when the anticipated drug is an opiate. Finally, the length of access also may play a role. Kenny et al. (2006) demonstrated that 1 h access to heroin could lower the intracranial self-stimulation (ICSS) threshold of rats indicating a heightened sensitivity to rewards. Only when the rats underwent extended access (23 h) to heroin was there an elevation in ICSS threshold. Thus, by increasing their sensitivity to rewards and the increased palatability of sweets due to heroin, both these factors could have prevented the saccharin avoidance behavior seen previously with cocaine in the short access condition. However by extending the length of access to drug, we may override these effects.
In recent years a number of laboratories have begun to develop animal models in an effort to mimic the human transition from use to abuse and addiction. In the taste drug model presented here, the proportion of extended access heroin rats that met the criteria for large suppressors was 50%. This percentage is similar to that reported by Lenoir et al. (2013) where 51% of rats with a history of extended access to heroin preferred heroin over saccharin when challenged with concurrent choices. The other half of the rats in the saccharin-heroin condition, on the other hand, may have been protected by a stronger preference for the natural saccharin reward and, thereby, consumed more of the sweet cue and less of the drug. The behavior of this subset of the extended access rats paralleled the behavior of rats tested in the short access saccharin-heroin condition. Thus, when comparing drug-taking behavior in both short and extended access rats, the extended access small suppressors and the short access rats showed similar 1st hour drug taking and drug loading behaviors.
Based on these results, it can be argued that the other 50% of the rats in the extended access condition were more vulnerable to the addictive properties of heroin. As summarized, these rats avoided the saccharin cue and then responded more for the drug of abuse. Indeed, they exhibited all indices of ‘addiction-like’ behavior. They took more drug, exhibited greater load up at the start of the session, escalated heroin intake across trials, tended to work harder for heroin when placed on the progressive ratio schedule of reinforcement, exhibited greater seeking during extinction, and greater heroin-induced reinstatement. The percentage of rats that were classified as large suppressors in the extended access conditioned is similar to those humans who engage in heroin use but transition to drug dependence (SAMHSA, 2012). As such, the taste-drug extended access model appears to have good face validity, and, therefore, may prove useful to better understand the transition from opiate use to abuse and addiction.
Acknowledgments
The authors thank the National Institute on Drug Abuse for generously providing heroin. Support for this research was provided by NIH grants DA009815 (to PSG) and DA036322 (to CGI). Support also was provided by a grant from the Pennsylvania Department of Health, Commonwealth Universal Research Enhancements SAP# 4100055576 (to PSG). We thank Elizabeth Colechio and Danielle Alexander for their technical assistance.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22(4):413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology. 1994;2(3):244. [Google Scholar]
- Cappell H, LeBlanc AE. Conditioned aversion to saccharin by single administrations of mescaline and d-amphetamine. Psychopharmacologia. 1971;22(4):352–356. doi: 10.1007/BF00406873. [DOI] [PubMed] [Google Scholar]
- Cason AM, Grigson PS. Prior access to a sweet is more protective against cocaine self-administration in female rats than in male rats. Physiology & Behavior. 2013;112–113:96–103. doi: 10.1016/j.physbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science (New York, NY) 2004;305(5686):1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Gomez F, Leo NA, Grigson PS. Morphine-induced suppression of saccharin intake is correlated with elevated corticosterone levels. Brain Research. 2000;863(1–2):52–58. doi: 10.1016/s0006-8993(00)02093-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1998;10(2):163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Hajnal A. Once is too much: Conditioned changes in accumbens dopamine following a single saccharin-morphine pairing. Behavioral Neuroscience. 2007;121(6):1234–1242. doi: 10.1037/0735-7044.121.6.1234. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: A model of drug-induced devaluation of natural rewards. Behavioral Neuroscience. 2002;116(2):321–333. doi: 10.1037//0735-7044.116.2.321. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC, Carelli RM. Heroin-induced suppression of saccharin intake in water-deprived and water-replete rats. Pharmacology, Biochemistry, and Behavior. 2000;66(3):603–608. doi: 10.1016/s0091-3057(00)00253-7. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Wheeler RA, Wheeler DS, Ballard SM. Chronic morphine treatment exaggerates the suppressive effects of sucrose and cocaine, but not lithium chloride, on saccharin intake in Sprague-Dawley rats. Behavioral Neuroscience. 2001;115(2):403–416. doi: 10.1037//0735-7044.115.2.403. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. Journal of Neuroscience. 2006;26(22):5894–5900. doi: 10.1523/jneurosci.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz KL, Twining RC, Baldwin AE, Vrana KE, Grigson PS. Heroin self-administration: I. Incubation of goal-directed behavior in rats. Pharmacology, Biochemistry, and Behavior. 2008;90(3):344–348. doi: 10.1016/j.pbb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. The International Journal on Drug Policy. 2012;23(1):37–44. doi: 10.1016/j.drugpo.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended Heroin Access Increases Heroin Choices Over a Potent Nondrug Alternative. Neuropsychopharmacology. 2013;38(7):1209–1220. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Showalter J, Grigson PS. Ethanol-Induced Conditioned Taste Avoidance: Reward or Aversion? Alcoholism, Clinical and Experimental Research. 2009;33(3):522–530. doi: 10.1111/j.1530-0277.2008.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology. 2004;175(1):26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Nolan LJ, Scagnelli LM. Preference for Sweet Foods and Higher Body Mass Index in Patients Being Treated in Long-Term Methadone Maintenance. Substance Use & Misuse. 2007;42(10):1555–1566. doi: 10.1080/10826080701517727. [DOI] [PubMed] [Google Scholar]
- Nyland JE, Grigson PS. A drug-paired taste cue elicits withdrawal and predicts cocaine self-administration. Behavioural Brain Research. 2013;240:87–90. doi: 10.1016/j.bbr.2012.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Ehrman RN, Ternes JW. Classical conditioning in opiate dependence. NIDA Research Monograph. 1984;49:35–46. [PubMed] [Google Scholar]
- Peavy KM, Banta-Green CJ, Kingston S, Hanrahan M, Merrill JO, Coffin PO. “Hooked on” Prescription-Type Opiates Prior to Using Heroin: Results from a Survey of Syringe Exchange Clients. Journal of Psychoactive Drugs. 2012;44(3):259–265. doi: 10.1080/02791072.2012.704591. [DOI] [PubMed] [Google Scholar]
- Puhl MD, Blum JS, Acosta-Torres S, Grigson PS. Environmental enrichment protects against the acquisition of cocaine self-administration in adult male rats, but does not eliminate avoidance of a drug-associated saccharin cue. Behavioural Pharmacology. 2012;23(1):43–53. doi: 10.1097/FBP.0b013e32834eb060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Boisvert M, Guan Z, Fang J, Grigson PS. A Novel Model of Chronic Sleep Restriction Reveals an Increase in the Perceived Incentive Reward Value of Cocaine in High Drug-Taking Rats. Pharmacology, Biochemistry, and Behavior. 2013;109:8–15. doi: 10.1016/j.pbb.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Fang J, Grigson PS. Pharmacology, Biochemistry and Behavior. Pharmacology, Biochemistry, and Behavior. 2009;94(2):262–270. doi: 10.1016/j.pbb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout HJ, Parker LA. Morphine enhancement of sucrose palatability: analysis by the taste reactivity test. Pharmacology, Biochemistry, and Behavior. 1996;53(3):731–734. doi: 10.1016/0091-3057(95)02077-2. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A Cocaine Cue Acts as an Incentive Stimulus in Some but not Others: Implications for Addiction. Bps. 2010;67(8):730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National Findings. Office of Applied Studies. NSDUH Series H-44. Rockville, MD: 2012. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. HHS Publication No. SMA 12–4713. [Google Scholar]
- Tacelosky DM, Alexander DN, Morse M, Hajnal A, Berg A, Levenson R, Grigson PS. Low Expression of D2R and Wntless Correlates with High Motivation for Heroin. 2015. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behavioral Neuroscience. 2009;123(4):913–925. doi: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Groblewski PA, Phillips PEM. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nature Neuroscience. 2014;17(5):704–709. doi: 10.1038/nn.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Yokel RA, DeWit H. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science. 1976;191(4233):1273–1275. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]
- Zador D, Lyons Wall PM, Webster I. High sugar intake in a group of women on methadone maintenance in South Western Sydney, Australia. Addiction. 1996;91(7):1053–1061. [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology. 1997;132(4):350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]