Abstract
The impact of important preexisting comorbidities, such as liver and renal disease, on the outcome of liver resection remains unclear. Identification of patients at risk of mortality will aid in improving preoperative preparations. The purpose of this study is to develop and validate a population-based score based on available preoperative and predictable parameters predicting 90-day mortality after liver resection using data from a hepatitis endemic country.
We identified 13,159 patients who underwent liver resection between 2002 and 2006 in the Taiwan National Health Insurance Research Database. In a randomly selected half of the total patients, multivariate logistic regression analysis was used to develop a prediction score for estimating the risk of 90-day mortality by patient demographics, preoperative liver disease and comorbidities, indication for surgery, and procedure type. The score was validated with the remaining half of the patients.
Overall 90-day mortality was 3.9%. Predictive characteristics included in the model were age, preexisting cirrhosis-related complications, ischemic heart disease, heart failure, cerebrovascular disease, renal disease, malignancy, and procedure type. Four risk groups were stratified by mortality scores of 1.1%, 2.2%, 7.7%, and 15%. Preexisting renal disease and cirrhosis-related complications were the strongest predictors. The score discriminated well in both the derivation and validation sets with c-statistics of 0.75 and 0.75, respectively.
This population-based score could identify patients at risk of 90-day mortality before liver resection. Preexisting renal disease and cirrhosis-related complications had the strongest influence on mortality. This score enables preoperative risk stratification, decision-making, quality assessment, and counseling for individual patients.
INTRODUCTION
Liver surgery is a more common procedure that is performed at numerous hospitals worldwide.1 In Asia, the prevalence of hepatitis and hepatocellular carcinoma (HCC) is high.2 Because donor shortage remains a major problem in Asia, liver resection is considered the first-line treatment for some patients with HCC.3,4 In addition, intrahepatic cholangiocarcinoma, metastatic malignancies, and benign diseases such as trauma, intrahepatic bile duct stones, and benign tumors also require liver resection.1
With the advance in management of liver diseases and surgical techniques, the mortality rate of liver resection has decreased to <5% during the last 2 decades in most specialized centers5,6; however, patient safety remains of utmost concern. As the indications for liver resection expand and the number of liver resections increases worldwide, preoperative stratification of at-risk patients is needed to improve preoperative evaluation and preparation. Some investigators have developed scores to predict mortality after liver resection.7–11 However, a limitation of the available studies and those addressing prediction scores is that they included postoperative parameters and did not preoperatively stratify patients at risk of mortality. Furthermore, these studies were conducted in specialized centers.9,10 For scores derived from administrative data,7,8,11 the majority of their records indicated a diagnosis of metastatic disease, and noncirrhosis patients indicated for hepatic resection. It is not clear whether these scores are applicable to patients with hepatitis and cirrhosis in endemic areas.
Therefore, by accounting for prognostic preoperative factors, the purpose of this study was to develop and validate a simple, applicable score based on available preoperative and predictable parameters such as hepatitis/cirrhosis, preexisting cirrhosis-related complications, major comorbidities, and the magnitude of liver resection to predict 90-day mortality in patients scheduled for liver resection. Specifically, we sought to identify preoperative factors associated with 90-day mortality. To make the score as close as possible to the daily practice, we considered parameters readily available in the preoperative assessment. Unpredictable intraoperative factors such as duration of surgery, ischemic time, and blood loss were not considered. We used a population-based national database of Taiwanese patients who underwent liver resection between 2002 and 2006 to develop and validate this predictive model.
PATIENTS AND METHODS
Ethics Statements
This study was initiated after receiving approval from the Institutional Review Board of the Buddhist Dalin Tzu Chi General Hospital, Taiwan. Because the identification numbers and personal information of the individuals included in the study were not included in the secondary files, the review board stated that written patient consent was not required.
Patients and Study Design
We used data from 2002 to 2006 from the National Health Insurance Research Database (NHIRD) that covers medical benefit claims for >23 million people in Taiwan (approximately 99% of Taiwan’s population).12 Taiwan’s National Health Insurance (NHI) provides universal insurance coverage, comprehensive services, and is a single-payer system with the government as the sole insurer. The database was monitored for completeness and accuracy by Taiwan’s Department of Health. Patients who underwent liver resection for cancer disease (HCC, cholangiocarcinoma, and metastatic malignancy), and benign disease (eg, trauma, intrahepatic bile duct stones, and benign tumors) between 2002 and 2006 were included. A total of 13,159 patients were identified. Patient mortalities were identified from the National Register of Deaths Database.
Measurements
The primary outcome measure was 90-day mortality, defined as death from all causes occurring after liver resection. The predictor variables were patient age, sex, indication for surgery, comorbidity, and surgical procedure (major, lobectomy or more; minor, less than lobectomy). The comorbidities included hypertension, ischemic heart disease, arrhythmia, cerebrovascular disease, chronic obstructive pulmonary disease, and associated conditions including chronic bronchitis and asthma, diabetes, hyperlipidemia, and renal diseases including acute and chronic renal failure, glomerulonephritis, and nephrotic syndrome. Patients’ severity of underlying liver disease was evaluated by the presence or absence of hepatitis/cirrhosis and cirrhosis-related complications. The cirrhosis-related complications included portal hypertension, esophageal/gastric variceal bleeding, ascites, pleural effusion, encephalopathy, and hepatorenal syndrome. We identified the presence or absence of these preexisting comorbidities and cirrhosis-related complications in each patient by querying the Taiwan NHI database using the International Classification of Diseases, Ninth Revision (ICD-9) codes. Any preoperatively existing comorbidity or cirrhosis-related complication was identified from the ICD-9 codes for inpatients within 6 months before surgery. For outpatients, the diseases that were coded 3 times or more in the records within 6 months before surgery were also identified from the ICD-9 codes.
Statistical Analysis
The SPSS (version 15; SPSS Inc, Chicago, IL) were used for data analysis. Univariate analyses were performed for the predictive variables. Those with statistical significance at the level of P < 0.1 were entered into a logistic regression model for odd ratios of 90-day mortality; multivariate logistic regression analysis was used to calculate the risk of mortality after adjustments for variables. Then, we developed a model to predict 90-day mortality in a randomly selected half of our study population (derivation population) and validated the model in the remaining half (validation population). For the random selection of patients for the derivation and validation populations, the incidences of mortality were comparable in both populations (defined as the incidence for the whole population). The β coefficients from the logistic regression model were used to develop an integer-based weighted point system for stratifying 90-day mortality risk. The referent for each variable was assigned a value of 0 and the coefficients for the others were adjusted proportionally, rounding to the nearest integer. Individual scores were assigned by summing the individual risk factor points. The risk scores were stratified into 4 risk groups as follows: score of 0 to 1, score of 2 to 3, score of 4 to 5, and score of 6 and above.
Within the derivation set, the risk score was calculated for each patient, and discrimination was assessed using the area under the receiver operating characteristic (ROC) curve. For validation of the risk score, the remaining half of the random sample was used. The risk score model was applied, and discrimination was assessed by ROC curve analysis.
RESULTS
A total of 13,159 patients who underwent liver resection between 2002 and 2006 were identified from the NHIRD. The patient characteristics are summarized in Table 1. The 90-day mortality rate for the overall cohort was 3.9%. Up to 39.2% of the patients’ records indicated a diagnosis of hepatitis and cirrhosis. The majority (61.2%) of liver resections were performed for HCC. Minor resection (less than lobectomy) was performed primarily (75.8%). After randomly sampling this group, the derivation set contained 6580 patients and the validation set contained 6579 patients.
TABLE 1.
Baseline Patient Characteristics
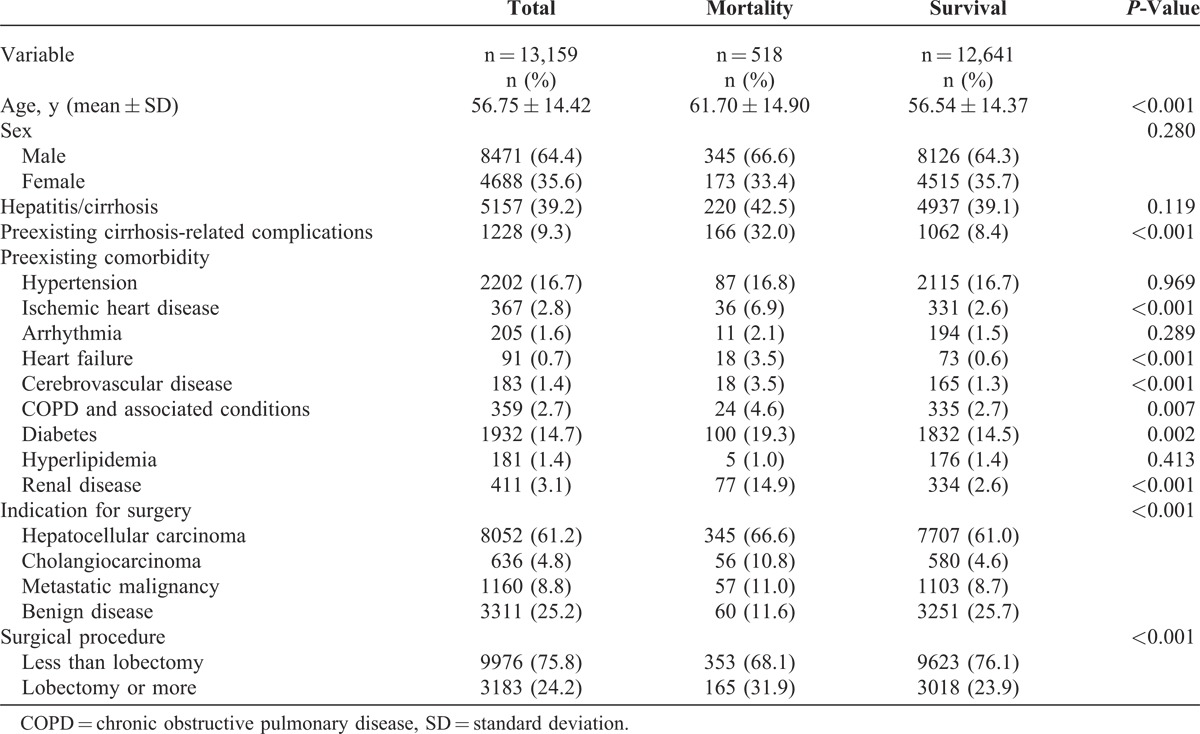
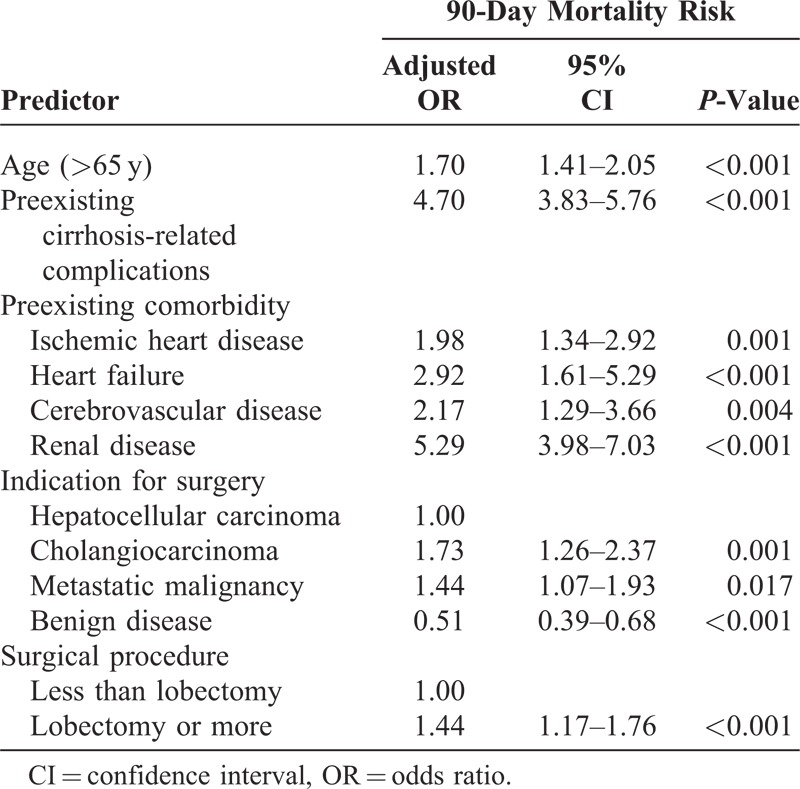
Table 2 shows the results of the multivariate logistic regression analysis. After adjusting for other factors, among those preexisting comorbidities, renal disease showed the strongest association with 90-day mortality (adjusted odds ratio, 5.29; 95% confidence interval, 3.98–7.03; P < 0.001), followed by cirrhosis-related complications (adjusted odds ratio, 4.70; 95% confidence interval, 3.83–5.76; P < 0.001), heart failure (adjusted odds ratio, 2.92; 95% confidence interval, 1.61–5.29; P < 0.001), cerebrovascular disease (adjusted odds ratio, 2.17; 95% confidence interval, 1.29–3.66 ; P = 0.004), and ischemic heart disease (adjusted odds ratio, 1.98; 95% confidence interval, 1.34–2.92; P = 0.001). Cholangiocarcinoma (adjusted odds ratio, 1.73; 95% confidence interval, 1.26–2.37; P = 0.001) and metastatic malignancy (adjusted odds ratio, 1.44; 95% confidence interval, 1.07–1.93; P = 0.017) showed stronger association with 90-day mortality than HCC. Old age (adjusted odds ratio, 1.70; 95% confidence interval, 1.41–2.05; P < 0.001) and major procedure (lobectomy or more) (adjusted odds ratio, 1.44; 95% confidence interval, 1.17–1.76; P < 0.001) were also associated with 90-day mortality.
TABLE 2.
Multivariate Logistic Regression Analysis
We further grouped the indications for surgery into benign and malignant diseases to reduce the number of variables for the prediction model. Table 3 shows the results of the multivariate model with the predictors and risk scores from the 6580 patients randomly selected for the development of the model. Grouping the scores into 4 risk groups resulted in a gradient for mortality estimates. The mortality rates of each group were 1.1%, 2.2%, 7.7%, and 15% (Figure 1). The model discriminated the derivation set well, with an area under the ROC curve of 0.75. When applied to the validation set, the model continued to discriminate well, with an area under the ROC curve of 0.75.
TABLE 3.
Development of a Predictor Score Based on Multivariate Logistic Regression Analysis of the Derivation Set
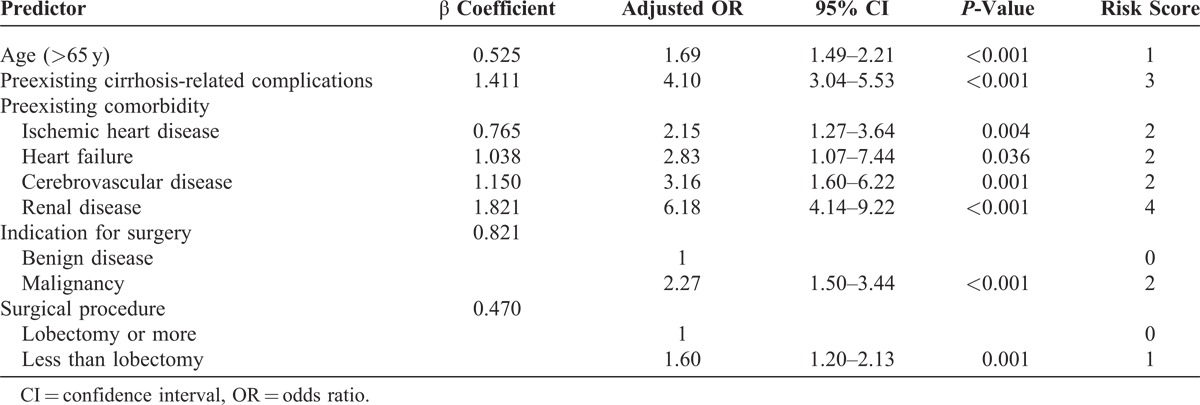
FIGURE 1.
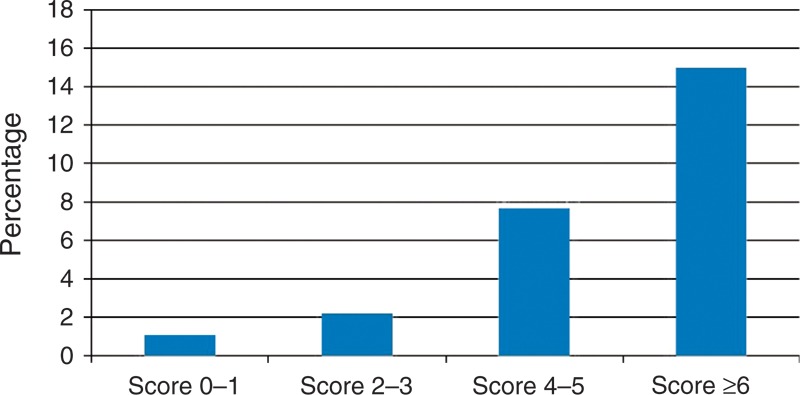
Estimated risk of 90-day mortality by prediction score group.
DISCUSSION
In this study from a national database identifying 13,159 liver resections between 2002 and 2006 in Taiwan, we developed and validated an easily applicable score to predict the risk of perioperative mortality in patients scheduled for hepatic resection. The overall perioperative mortality was 3.9%, whereas the mortality according to score groups ranged from 1.1% to 15%. Factors most strongly associated with 90-day mortality were preexisting renal disease and cirrhosis-related complications.
The strength of this study is that it was a nationwide population-based study including almost all the patients undergoing hepatic resection in Taiwan. By the end of 2006, the NHI covered 99.0% of Taiwan’s population,12 with nearly complete follow-up information of mortality among the entire study population, and the dataset was routinely monitored for diagnostic accuracy by the NHI Bureau of Taiwan. The present score is unlike the population-based score published by Simons et al7,11 using the Charlson Comorbidity Index score to classify patients. Their data were queried from patient discharges for hepatic resection. It was unclear whether these comorbidities, particularly liver and renal disease, the 2 main concerns influencing the outcome of hepatic resection, were present before or occurred after the surgery. In our study, the entire dataset was queried from preoperative inpatient and outpatient records, using only the clinical factors that were widely available before surgery. Moreover, the aforementioned previous study focused on predicting inhospital mortality as the outcome measure, which does not reflect all deaths that occur after discharge. In contrast, we measured 90-day mortality to account for all risk during the perioperative period.
Increasingly, more liver resections are being performed on patients who may be at higher risk given the nature of liver diseases such as cirrhosis, and previous investigators have consistently suggested that careful patient selection is crucial for achieving better outcomes.13,14 The main preoperative factors that predict postoperative liver dysfunction include the presence of chronic liver disease, extensive liver resection, and the presence of a small liver remnant. It has been reported that portal hypertension is not a contraindication for hepatic surgery in properly selected patients, who had similar survival compared with patients without portal hypertension.15,16 However, the present population-based findings derived from patients who underwent liver resection supported the notion that preexisting cirrhosis-related complications remain a significant influence on increasing the risk of perioperative mortality.
Acute renal failure is a major complication after abdominal surgery that is associated with major morbidity and mortality.17,18 However, very limited data exist regarding the impact of renal disease on the outcomes of liver resection.19,20 Some single-center studies showed that end-stage renal disease patients undergoing liver resection did not have higher perioperative mortality compared with patients without end-stage renal disease.21–23 Our findings revealed that among the factors associated with perioperative mortality because of liver resection, preexisting renal disease was the strongest, which was also in agreement with previous studies of other procedures showing that postoperative acute renal failure significantly impaired the outcomes after major surgery.24,25 This result indicates that when treating patients with renal disease, careful surgical techniques and perioperative management are crucial for lower mortality risk.
There are some limitations in this study. First, patient diagnosis and identification of comorbidities were completely dependent on ICD-9 codes, and any coding errors in patients’ underlying diseases could lead to disparities in preexisting comorbidities and cirrhosis-related complications. Nonetheless, the NHI Bureau of Taiwan randomly reviews the charts and interviews patients in order to verify diagnostic accuracy.26 Second, the NHIRD is an administrative database and lacks certain clinical information such as the subtleties of liver dysfunction, the glomerular filtration rate, and the creatinine for renal disease that may not be completely captured using ICD-9 codes alone. However, the diagnosis of hepatitis/cirrhosis, preexisting cirrhosis-related complications, and renal disease represented the comorbidity status of liver and renal disease before surgery. Further studies with primary data might be conducted to reveal a deeper insight about the severity of these preexisting diseases and their relationship with perioperative mortality. Third, instead of surgical mortality, all-cause mortality was used. However, the short-term surgical and all-cause mortality differed only to a small degree.
Despite these limitations, our score is an easy to use tool for clinical application with good discriminatory ability. The use of a national database that included almost all liver resections increased its generalizability over many single-institution case series. As the experience in liver resections increases, the number of patients with more and worse comorbidities being considered for liver resection may increase. In addition to the widely used Child–Turcotte–Pugh score in liver surgery, the incorporation of preexisting comorbidities and cirrhosis-related complications in the present tool might be helpful for patient counseling and informed consent. This type of risk stratification also allows for different treatment options to be considered in patients with certain comorbidities.
In summary, our results demonstrated that this score could be used to identify patients at higher risk of 90-day mortality before liver resection. This population-based risk score also revealed that preexisting renal and cirrhosis-related complications had the strongest influence on 90-day mortality in liver resection. This score may be useful for preoperative risk stratification, decision-making, quality assessment, and counseling of individual patients.
ACKNOWLEDGMENTS
This study is based in part on data from the NHIRD provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes (registry number 101115). The interpretation and conclusions contained herein do not represent the opinions of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
Footnotes
Abbreviations: HCC = hepatocellular carcinoma, ICD-9 = International Classification of Diseases, Ninth Revision, NHI, National Health Insurance, NHIRD = National Health Insurance Research Database, ROC = receiver operating characteristic.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Justin B, Dimick M, Reid M, et al. National trends in the use and outcomes of hepatic resection. J Am Coll Surg. 2004;199:31–38. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka M, Katayama F, Kato H, et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol. 21:401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. [DOI] [PubMed] [Google Scholar]
- 4.Bigourdan JM, Jaeck D, Meyer N, et al. Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transpl. 2003;9:513–520. [DOI] [PubMed] [Google Scholar]
- 5.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. [DOI] [PubMed] [Google Scholar]
- 6.Dimick JB, Cowan JA, Jr, Knol JA, et al. Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg. 2003;138:185–191. [DOI] [PubMed] [Google Scholar]
- 7.Simons JP, Hill JS, Ng SC, et al. Perioperative mortality for management of hepatic neoplasm: a simple risk score. Ann Surg. 2009;250:929–934. [DOI] [PubMed] [Google Scholar]
- 8.Simons JP, Ng SC, Hill JS, et al. In-hospital mortality for liver resection for metastases: a simple risk score. J Surg Res. 2009;156:21–25. [DOI] [PubMed] [Google Scholar]
- 9.Balzan S, Belghiti J, Farges O, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyder O, Pulitano C, Firoozmand A, et al. A risk model to predict 90-day mortality among patients undergoing hepatic resection. J Am Coll Surg. 2013;216:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simons JP, Ng SC, Hill JS, et al. In-hospital mortality from liver resection for hepatocellular carcinoma: a simple risk score. Cancer. 2010;116:1733–1738. [DOI] [PubMed] [Google Scholar]
- 12. National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. http://www.nhi.gov.tw/webdata/webdata.aspx?menu=17&menu_id=1023&WD_ID=1023&webdata_id=815 Accessed May 15, 2014. [Google Scholar]
- 13.Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127(5 suppl 1):S248–S260. [DOI] [PubMed] [Google Scholar]
- 14.Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253:453–469. [DOI] [PubMed] [Google Scholar]
- 15.Cucchetti A, Ercolani G, Vivarelli M, et al. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922–928. [DOI] [PubMed] [Google Scholar]
- 16.Santambrogio R, Kluger MD, Costa M, et al. Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh’s A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford). 2013;15:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107:892–902. [DOI] [PubMed] [Google Scholar]
- 18.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858. [DOI] [PubMed] [Google Scholar]
- 19.Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698––708.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saner F. Kidney failure following liver resection. Transplant Proc. 2008;40:1221–1224. [DOI] [PubMed] [Google Scholar]
- 21.Cheng SB, Wu CC, Shu KH, et al. Liver resection for hepatocellular carcinoma in patients with end-stage renal failure. J Surg Oncol. 2001;78:241–246. [DOI] [PubMed] [Google Scholar]
- 22.Yeh CN, Lee WC, Chen MF. Hepatic resection for hepatocellular carcinoma in end-stage renal disease patients: two decades of experience at Chang Gung Memorial Hospital. World J Gastroenterol. 2005;11:2067–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orii T, Takayama T, Haga I, et al. Efficacy of a liver resection for hepatocellular carcinoma in patients with chronic renal failure. Surg Today. 2008;38:329–334. [DOI] [PubMed] [Google Scholar]
- 24.Kim CS, Oak CY, Kim HY, et al. Incidence, predictive factors, and clinical outcomes of acute kidney injury after gastric surgery for gastric cancer. PLoS One. 2013;8:e82289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mooney JF, Ranasinghe I, Chow CK, et al. Preoperative estimates of glomerular filtration rate as predictors of outcome after surgery: a systematic review and meta-analysis. Anesthesiology. 2013;118:809–824. [DOI] [PubMed] [Google Scholar]
- 26. National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. http://www.nhi.gov.tw/webdata/webdata.aspx?menu=17&menu_id=659&webdata_id=4524 Accessed May 15, 2014. [Google Scholar]


