Abstract
Endothelial dysfunction is implicated in increased cardiovascular risk in nondialyzed population. However, the prognostic impact of endothelial dysfunction on cardiovascular outcome has not been investigated in peritoneal dialysis (PD) patients.
We prospectively determined endothelial function by brachial artery endothelium-dependent vasodilation (flow-mediated dilation [FMD]) in 143 nondiabetic PD patients and 32 controls. Primary outcome was a major adverse cardiac and cerebrovascular event (MACCE).
Brachial FMD was significantly lower in PD patients than in controls (2.9% [1.3–4.7] vs 6.2% [5.4–8.3], P < 0.001). During a mean follow-up of 42 months, primary outcome was observed in 25 patients (17.5%). When patients were dichotomized by the median value of FMD (2.9%), incidence rates of MACCEs were significantly higher in the group with lower FMD compared with higher FMD (7.2 vs 3.0/100 person-years, P = 0.03). In multivariate Cox analysis, low FMD (≤2.9%) was a significant independent predictor of MACCEs (hazard ratio = 2.73, 95% confidence interval = 1.03–7.22, P = 0.04). Furthermore, multivariate fractional polynomial analysis showed that the risk of MACCE decreased steadily with higher FMD values.
Impaired brachial FMD was a significant independent predictor of MACCEs in PD patients. Estimating endothelial dysfunction by brachial FMD could be useful for stratifying cardiovascular risk in these patients.
INTRODUCTION
Cardiovascular disease is the most common cause of death in end-stage renal disease (ESRD) patients.1 In addition to traditional risk factors such as advanced age, hypertension, diabetes, smoking, and dyslipidemia, endothelial dysfunction was found to be implicated in the pathogenesis of cardiovascular events.2–4 Endothelial cells, which form the lining of blood vessels, play a crucial role in vascular homeostasis.5 By producing and releasing nitric oxide (NO), endothelial cells modulate vascular tone and structure.6 NO induces relaxation of smooth muscle cells with subsequent widening of the arteries.5,7 Therefore, loss of the normal homeostatic endothelial condition results in the activation of smooth muscle cells and intimal hypertrophy, which promote atherosclerosis or arteriosclerosis.5,8–10
Endothelial dysfunction can be assessed noninvasively as the degree of flow-mediated dilation (FMD) of the brachial artery.5 Lower brachial FMD was revealed to be significantly associated with increased cardiovascular events in patients with hypertension,11 coronary artery disease,2 and peripheral artery disease.12,13 Furthermore, FMD impairment was an independent predictor of cardiovascular outcome in patients with acute coronary syndrome (ACS).14–16 Fathi et al17 and a recent meta-analysis5 found that an inverse relationship between brachial FMD and future cardiovascular events was stronger in a diseased population than in asymptomatic populations. These results suggest that the prognostic value of endothelial dysfunction could be affected by underlying health status.
Because high mortality rates derived from cardiovascular disease in ESRD patients and renal dysfunction are an established cardiovascular risk factor, investigating the prognostic value of endothelial dysfunction has clinical relevance in these patients. However, to date, only a few studies have been conducted in ESRD patients, and results were conflicting.7,17–19 Two cohort studies in chronic hemodialysis (HD) patients found no significant differences in mortality between the high- and low-FMD groups.18,19 Although other studies demonstrated that endothelial dysfunction was associated with all-cause mortality in HD patients,7,17 study populations are clinically mixed with heterogeneity such as diabetes that significantly affects FMD, and assessment of venous dilation was performed by plethysmography, which is less NO dependent than arterial dilation. Moreover, the impact of endothelial dysfunction on cardiovascular outcome has never been explored in peritoneal dialysis (PD) patients. Therefore, we investigated whether endothelial dysfunction determined by brachial FMD was an independent predictor of cardiovascular outcome in ESRD patients on PD without a prior history of diabetes and cardiovascular diseases.
SUBJECTS AND METHODS
Ethics Statement
This study was carried out in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Yonsei University Health System (YUHS) Clinical Trial Center. We obtained informed written consent from all participants involved in the present study.
Study Participants
In this prospective study, all ESRD patients >20 years of age who underwent PD for >3 months at YUHS were initially screened for enrollment between January 2007 and December 2007. Patients were considered eligible if they had no history of malignancy or chronic inflammatory disease such as systemic lupus erythematosus and rheumatoid arthritis, and had no overt infection during the 3 months prior to study entry. We also excluded patients with a history of kidney transplantation, HD prior to PD, diabetes mellitus, previous cardiovascular disease, or any signs of volume overload such as jugular vein distension, weight gain >2 kg/d with peripheral pitting edema requiring frequent use of hypertonic PD solutions, or pulmonary congestion on chest x-ray.20 Cardiovascular disease was defined as a history of coronary, cerebrovascular, or peripheral vascular disease. Coronary artery disease was defined as a history of angioplasty, coronary artery bypass graft, myocardial infarction, or angina. Cerebrovascular disease was defined as previous transient ischemic attack, stroke, or carotid endarterectomy. Peripheral vascular disease was defined as a history of claudication, ischemic limb loss and/or ulceration or peripheral revascularization procedure. The final analysis included 143 patients. Controls were 32 age- and gender-matched healthy volunteers (Figure 1).
FIGURE 1.
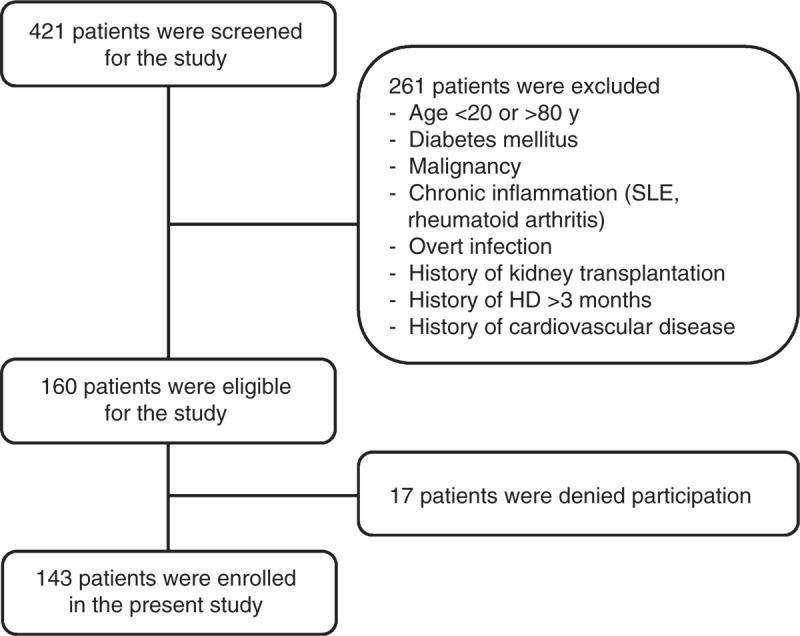
A flow diagram of the study. All ESRD patients >20 years of age who underwent PD for >3 months were initially screened for enrollment between January 2007 and December 2007. Excluding 278 patients, a total of 143 patients were enrolled. HD = hemodialysis, SLE = systemic lupus erythematosus.
Clinical and Biochemical Data Collection
Demographic and clinical data were recorded at study entry: age, gender, PD duration, primary renal disease, smoking status, and medication use. Body mass index (BMI) and biochemical data were measured at study enrollment. Patients were weighed in light clothing and their heights were measured without shoes on. BMI was calculated as weight/height2 (kg/m2). Blood was taken after a 12-hour overnight fast, and the following laboratory data were measured: hemoglobin, glucose, blood urea nitrogen, creatinine, albumin, iron profile, triglyceride, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, calcium, phosphorus, and intact parathyroid hormone concentrations. Insulin resistance was assessed using the homeostasis model assessment-insulin resistance (HOMA-IR) equation as follows: HOMA-IR = (fasting insulin in microunits per milliliter × fasting serum glucose in millimoles per liter/22.5). In addition, high-sensitivity C-reactive protein (hs-CRP) concentrations were determined by a latex-enhanced immunonephelometric method using a Behring Nephelometer II analyzer (Dade Behring, Newark, DE). Residual glomerular filtration rate was estimated by 24-hour urine collection.21 Kt/V urea was determined from the total loss of urea nitrogen in spent dialysate using PD Adequest 2.0 for Windows software (Baxter Healthcare, Deerfield, IL). A modified peritoneal equilibration test was performed with 4.25% glucose dialysis solution as described.22 Dialysate-to-plasma ratio of creatinine (D/P creatinine) and glucose (D/D0 glucose) concentrations at 4 hours of dwell time were used to describe the peritoneal small-solute transport rate.
Assessment of FMD
Endothelium-dependent vasodilation was assessed by determining brachial artery FMD, using high-resolution ultrasonography (LOGIQ 7; GE Medical Systems, Milwaukee, WI) as described.23 Subjects were informed to fast overnight; not to exercise; not to ingest substances that might affect FMD, such as caffeine, high-fat foods, or vitamin C; and not to use tobacco for at least 12 hours before measurement. On the day of FMD measurement, blood pressure was determined 3 times with a mercury sphygmomanometer. Baseline diameter (mean of 3 measurements) and peak flow velocity (mean of 2 measurements) of the brachial artery were determined. Thereafter, a pressure cuff on the forearm was inflated to at least 50 mm Hg above systolic blood pressure. The cuff was released to induce reactive hyperemia after 5 minutes, maximum peak flow velocity was measured within 15 seconds and diameter of brachial artery was measured between 45 and 60 seconds. Endothelium-independent vasodilation was tested 15 minutes after the FMD test and after obtaining a new baseline brachial artery diameter value. Changes in brachial artery diameter 5 minutes after administration of 0.4 mg sublingual nitroglycerine (nitroglycerin-mediated dilation [NMD]) were measured. FMD and NMD were calculated as percentage changes in the brachial artery diameter relative to the mean baseline diameter during reactive hyperemia and after administration of nitroglycerine, respectively. All ultrasonographic measurements were performed by a single observer who was blinded to patients’ clinical information.
Follow-Up and Endpoints
Patients were followed up at 3-month intervals through June 30, 2013. All deaths and hospitalizations were recorded in a serious adverse event database. All events were retrieved from the database and carefully reviewed to determine cardiovascular mortality and incident cardiovascular disease. Primary outcome was a major adverse cardiac and cerebrovascular event (MACCE). MACCEs were defined as death or hospitalization from an ACS, stable angina requiring coronary revascularization using percutaneous coronary intervention or coronary artery bypass grafting, new-onset congestive heart failure, or cerebrovascular attack. ACS was defined as presentation with chest pain and/or ischemic electrocardiographic changes and elevation of troponin T with evidence of infarct by stress imaging or coronary angiography and ventriculography. Cerebrovascular attack was defined as a transient or permanent neurologic deficit with computed tomography or magnetic resonance imaging evidence of cerebral ischemic infarct adjudicated by a neurologist. When a patient died within 60 days after transfer to HD, the death was regarded as a mortality event. Loss of follow-up, renal transplantation, and transfer to HD were censored at the end of PD treatment.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation, or median (interquartile range), and categorical variables as a number (percentage). Normality of distribution was ascertained by the Kolmogorov–Smirnov test. Subjects were divided into 2 groups according to the median value of brachial FMD. To compare the baseline characteristics, Student t test or Mann–Whitney U test and χ2 tests were used for continuous variables and categorical variables, respectively. Univariate and multivariate linear regression analyses were performed to determine significant factors associated with FMD. Cumulative survival curves were generated by the Kaplan–Meier method and between-group survival was compared by a log-rank test. Independent predictive value of FMD for primary outcome was ascertained by Cox proportional hazard regression models, which included significant variables in univariate Cox analysis. In addition, the prognostic value of FMD was also evaluated in a fractional polynomial model. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) for Windows version 18.0 (SPSS Inc, Chicago, IL) and Stata version 11.0 (StataCorp, College Station, TX). A P value <0.05 was considered statistically significant.
RESULTS
FMD in Controls and PD Patients
Baseline characteristics of the 143 patients on PD and 32 controls are shown in Table 1. The mean age was 47.2 ± 7.5 years in controls and 49.5 ± 11.2 years in PD patients. Fourteen patients (43.8%) were men among controls and 69 patients (48.3%) were men among PD patients. No significant differences between PD patients and controls were observed in age, sex, the proportion of smokers, diastolic blood pressure, or BMI. Compared with the control participants, PD patients showed significantly lower FMD (PD vs control, 2.9% [1.3–4.7] vs 6.2% [5.4–8.3], P < 0.001), whereas NMD was not different between the 2 groups (11.3% [9.3–14.6] vs 13.1% [11.7–14.2], P = 0.44) (Figure 2).
TABLE 1.
Baseline Characteristics in PD Patients and Controls
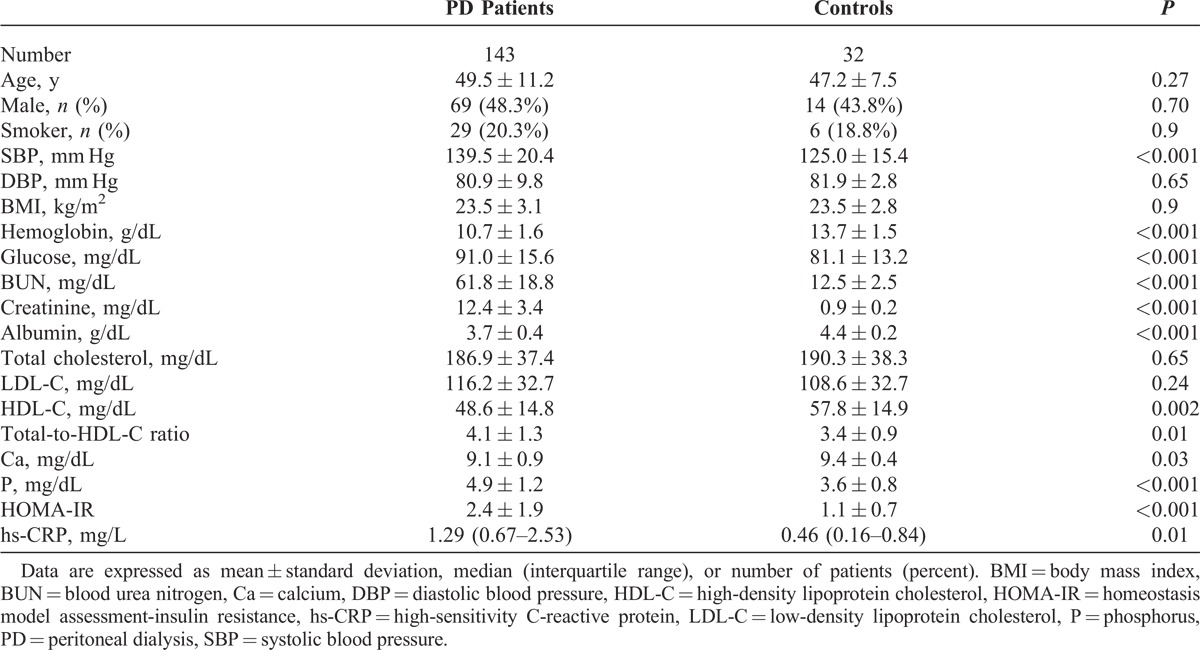
FIGURE 2.
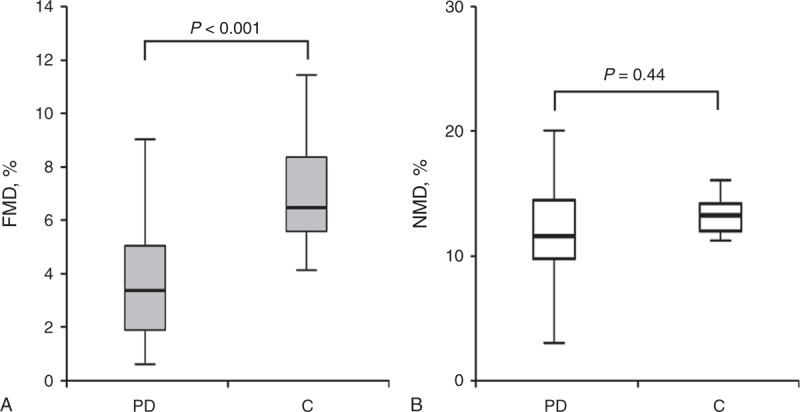
Endothelial function in PD patients and controls. Box plot of brachial artery FMD (A) and NMD (B) values in PD patients and controls. Boxes represent the interquartile range and the lines denote the median. Error bars are 95% confidence intervals. C = controls, FMD = flow-mediated dilation, NMD = nitroglycerin-mediated dilation, PD = peritoneal dialysis.
Clinical Characteristics According to FMD Groups
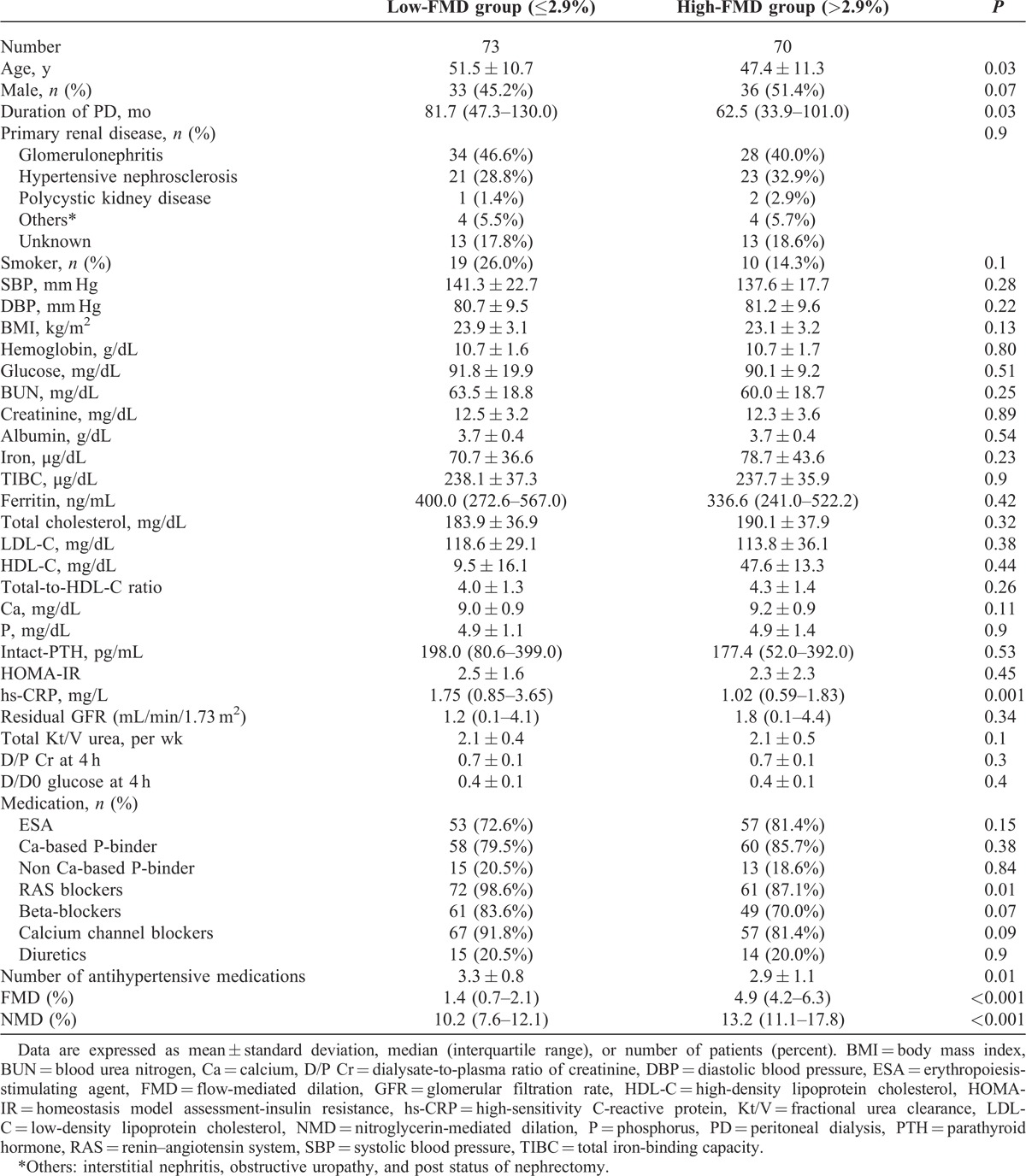
Clinical characteristics of PD patients by FMD groups are shown in Table 2. Patients were dichotomized as above or below the median value of FMD (2.9%). Compared with the higher FMD groups, the mean age, PD duration, hs-CRP, the proportion of patients taking renin–angiotensin system blockers, and the number of antihypertensive medications were significantly higher, whereas NMDs were significantly lower in the low-FMD groups. No significant differences were seen in sex, primary renal disease, blood pressure, and biochemical variables between the 2 groups.
TABLE 2.
Baseline Characteristics of the PD Subjects According to FMD Group
Association of FMD With Clinical and Biochemical Parameters in PD Patients
In univariate analysis, FMD was negatively associated with age, men, PD duration, BMI, and log hs-CRP concentrations. Multivariate linear regression analysis revealed that age and log hs-CRP levels had independent inverse association with FMD in PD patients (Table 3).
TABLE 3.
Univariate and Multivariate Linear Regression Analysis of Clinical and Biochemical Variables for FMD

FMD as an Independent Predictor of MACCEs
During a mean follow-up duration of 42.4 ± 21.6 months, 10 patients (7.0%) died from cardiovascular diseases, and the primary outcome was observed in 25 patients (17.5%). Compared with the group with higher FMD values, incidence rates of primary outcome were higher in the group with low FMD values (7.2 and 3.0 per 100 person-years, respectively, P = 0.03) (Table 4). The Kaplan–Meier plot showed that event-free survival rates were significantly lower in the low-FMD group than in high-FMD group (log-rank test, P = 0.04) (Figure 3). In univariate Cox proportional hazard analysis, older age, higher BMI, and higher HOMA-IR levels as well as low-FMD groups were significant risk factors for primary outcome. In multivariate Cox analysis, low FMD (≤2.9%) was a significant independent predictor of MACCEs (hazard ratio = 2.73, 95% confidence interval = 1.03–7.22, P = 0.04) after adjustment for age, sex, PD duration, BMI, HOMA-IR, albumin, and log hs-CRP levels (Table 5). Furthermore, when FMD was evaluated in fractional polynomial analysis, the risk of MACCE decreased steadily with higher FMD values (Figure 4).
TABLE 4.
Study Outcomes According to FMD Group
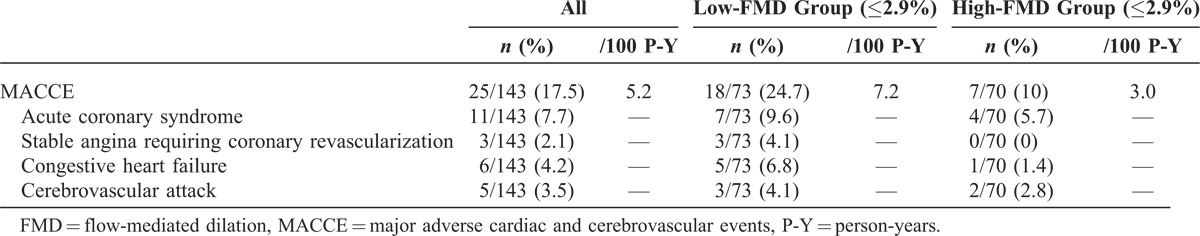
FIGURE 3.
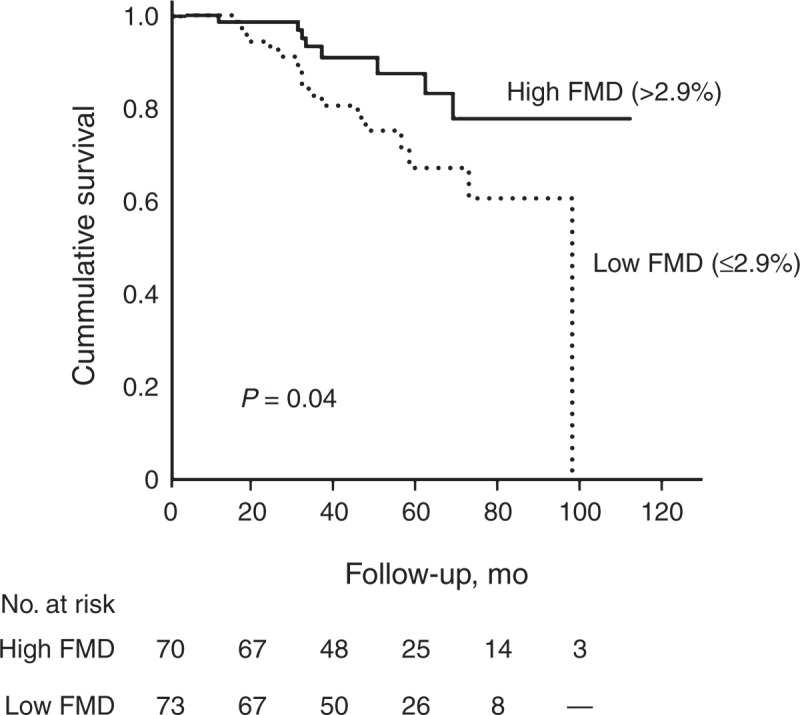
Kaplan–Meier plots for primary outcome according to FMD groups. The low-FMD group had a significantly higher risk of MACCEs compared with the high-FMD group (log-rank test, P = 0.04). FMD = flow-mediated dilation.
TABLE 5.
Multivariate Cox Proportional Hazard Models of FMD Group for MACCEs
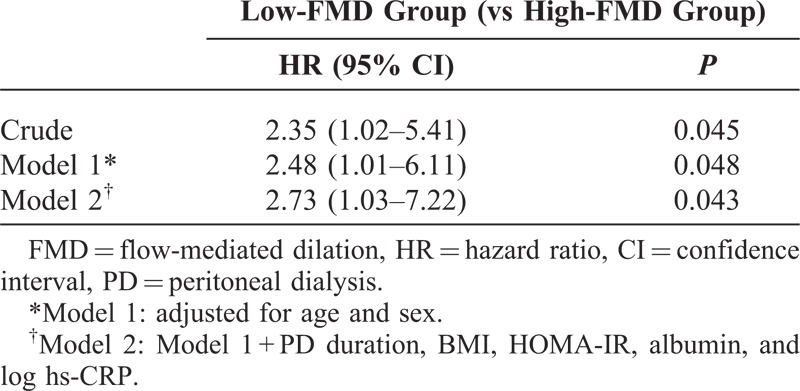
FIGURE 4.
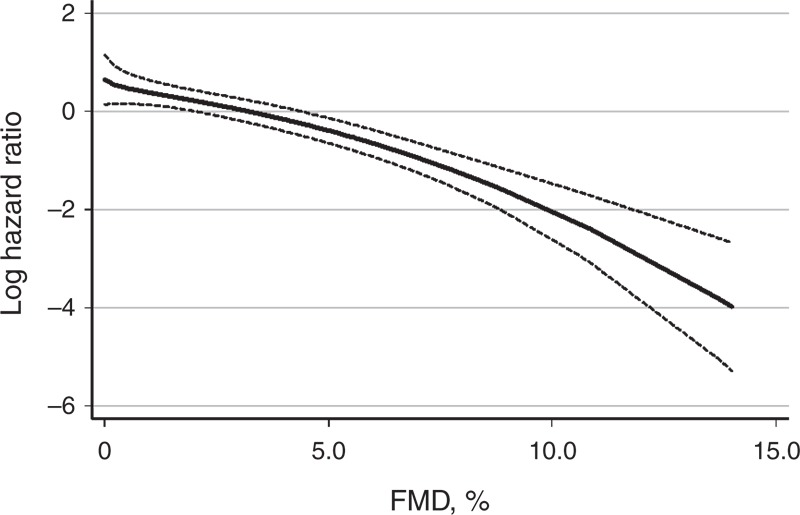
Multivariate fractional polynomial graphs for association between FMD and MACCEs. Hazard ratios were calculated after adjustment for age, sex, PD duration, BMI, HOMA-IR, albumin, and hs-CRP concentrations. Shaded areas indicate 95% confidence limits. Abbreviation: FMD = flow-mediated dilation.
DISCUSSION
Endothelial dysfunction is closely associated with increased cardiovascular risk.2–4 Because cardiovascular disease is the most common cause of death in ESRD patients,1 investigating the association between endothelial dysfunction and cardiovascular outcome might have a clinical importance in these patients. However, to date, the prognostic value of endothelial dysfunction has not been explored in PD patients. In the present study, brachial FMD was found to be significantly lower in PD patients compared with controls. Furthermore, we demonstrated for the first time that endothelial dysfunction determined by brachial FMD was a significant independent predictor of MACCEs in nondiabetic ESRD patients undergoing PD.
Endothelial cells regulate vascular tone and function by releasing NO, which creates an antiatherogenic milieu by inhibition of platelet aggregation, smooth muscle cell proliferation, and leukocyte adhesion.24 Therefore, malfunction of endothelial cells begins prior to overt atherosclerosis.9 Endothelial dysfunction has been investigated first in coronary arteries.2,25 Cardiovascular events were greater in patients with an abnormal coronary vascular response to acetylcholine or nitroglycerin.2,25 However, the technique used for assessment of coronary artery reactivity is invasive and thus limited to clinical applications.17 Other studies assessed peripheral vascular response by forearm venous plethysmography,3,4,7 in the belief that vascular disease is a systemic process.10 Although patients with decreased response had more adverse cardiovascular events, venous dilation during plethysmography is less NO dependent than arterial dilation.17 In this regard, brachial artery FMD is a useful method to examine vascular reactivity noninvasively and to reflect NO-dependent vascular response in consistency with coronary reactivity.9
A recent meta-analysis of 23 studies of 14,753 subjects revealed that low FMD was significantly associated with increased cardiovascular risk.5 Of note, this inverse relationship between FMD and cardiovascular disease was strengthened in diseased subjects and Asian populations, suggesting that the prognostic significance of FMD was affected by underlying health status and ethnicity.5 In support, studies on asymptomatic general populations did not find an independent prognostic value of FMD.26–28 Similar results were also found in nondiabetic, intermediate-risk (5% < Framingham risk score < 20%) patients.29 In contrast, low-brachial FMD was significantly associated with increased cardiovascular events in patients with hypertension,11 coronary artery disease,2 or peripheral artery disease.12,13 In a comparative study of non-ST-segment elevation-ACS and stable coronary artery disease, FMD was significantly impaired in the acute stage of non-ST-segment elevation-ACS, compared with a stable coronary artery disease group. Moreover, FMD at 3 months after the acute event was an independent predictor of cardiac death or readmission for new ACS or angina pectoris recurrence.16
Because uremia has been considered a nontraditional cardiovascular risk factor,30 a significant association between endothelial dysfunction and increased cardiovascular risk might be expected in ESRD patients. Previous studies indicated that endothelial function was impaired in ESRD patients compared with controls.7,31,32 Furthermore, brachial FMD was significantly lower in HD patients than predialysis chronic kidney disease and kidney transplant patients.33 In this study, FMD was significantly decreased in PD patients compared with controls in accordance with prior studies, and NMD was comparable between PD patients and controls. Although all subjects did not meet the diagnostic criteria for diabetes,34 fasting plasma glucose and HOMA-IR were significantly higher in PD patients as compared with controls. Glucose absorption via glucose-based dialysate has been known to result in hyperglycemia and hyperinsulinemia in PD.35 In a study of 252 nondiabetic patients who newly started PD, impaired fasting glucose (IFG) was observed in 138 (54.8%) patients after 1 month of PD therapy.36 In the present study, our PD subjects were prevalent patients with median dialysis vintage of 71 months and the proportion of IFG were higher in the PD group (PD vs controls, 29 [20.3%] vs 2 [6.2%], P = 0.06). Because the impairment of brachial FMD might be attributed to higher fasting plasma glucose and HOMA-IR levels, we excluded 31 patients with IFG to mitigate the confounding effect of hyperglycemia. Brachial FMD was significantly impaired in the PD group even after excluding subjects with IFG (PD vs control, 3.1% [1.3–5.2] vs 6.7% [5.3–8.4], P < 0.001). Meanwhile, PD patients had significantly elevated systolic blood pressure compared with controls in our study. Even though hypertension is known to be implicated in increased arterial stiffness leading to endothelial dysfunction,37,38 systolic blood pressure (β = −0.070, P = 0.408) and diastolic blood pressure (β = 0.223, P = 0.056) had no associations with FMD in PD patients in univariate linear regression analysis. However, the proportion of taking renin–angiotensin system blockers and the number of antihypertensive medications were higher in the low-FMD group. Therefore, we inferred that the condition requiring more antihypertensive medication, rather than the actual value of blood pressure, might contribute to endothelial dysfunction.
To date, few studies investigated the prognostic impact of endothelial dysfunction on clinical outcome in ESRD patients. Furthermore, the association between endothelial dysfunction and clinical outcomes has not been explored in ESRD patients on PD. London et al7 have shown that decreased flow debt repayment was independently associated with all-cause mortality in 78 prevalent HD patients. However, they evaluated forearm postischemic vasodilation by venous plethysmography to assess endothelial function. In the study of Fathi et al,17 even though FMD is lower in patients who have experienced cardiovascular events, FMD was not of independent prognostic value in these patients. Study participants had preexisting multiple risk factors such as diabetes or previous coronary artery diseases. We included selective PD patients without a prior history of diabetes and cardiovascular diseases to reach homogeneity in the study population. In the present study, we showed for the first time that low brachial FMD was a significant independent predictor of MACCEs in PD patients.
Decreased FMD is associated with cardiovascular end-organ damage including left ventricular hypertrophy, vascular calcification, increased arterial stiffness, and carotid atherosclerosis.37,38 The mechanism by which FMD affects cardiovascular outcome in PD patients has several possibilities. An increase in serum mediators of endothelial function, such as asymmetric dimethylarginine (ADMA) and endothelin-1 (ET-1), is a candidate. ADMA, a natural inhibitor of endothelial NO synthase, has been known to increase according to the decline of residual renal function.39,40 ET-1, a vasoconstrictor produced by endothelial cells, is found to be elevated in dialysis patients, resulting in suppression of endothelial-dependent dilation.39,41 These impaired vascular responses are suggested to lead to dysregulation of coronary blood flow, limiting the ability of the myocardium to increase cardiac output and leading to ischemia and stunning.39,41 In addition, chronic inflammation is closely associated with abnormal endothelial function in dialysis patients.20,42–44 In this study, brachial FMD had a significant inverse relationship with hs-CRP levels, consistent with prior studies.20,42–44 Based on evidences that chronic inflammation plays a crucial role in the development of atherosclerosis,45 we surmise that endothelial dysfunction leads to unfavorable cardiovascular outcomes in association with inflammation.
The current study has several limitations. First, the study subjects were all Korean prevalent PD patients in a single center, with a relatively long duration of PD. Although the prognostic impact of residual renal function has been demonstrated in several prospective studies,46–49 residual renal function was not significantly associated with the primary outcome in this study. This finding can be explained by the characteristics of our subjects, long-term maintenance PD patients with deteriorated residual renal function. Thus, selection bias is possible and the results might not be generalized to other populations. Our findings need to be confirmed in a long-term study with a large number of subjects. Second, the cardiovascular mortality was relatively low compared with mortality in previous studies on Western ESRD patients.50 We infer that ethnicity and patient characteristics contribute to this difference in mortality. Because we enrolled patients who were nondiabetic and had no previous history of cardiovascular disease, it is possible that patients with severe endothelial dysfunction were not included in this study. However, our enrollment criteria allowed us to focus on the independent association between endothelial dysfunction per se and cardiovascular outcome in PD patients and exclude the confounding effects of diabetes and comorbid disease. Third, other methods representing vascular function such as pulse wave velocity and carotid intima medial thickness were not determined. Lastly, we cannot exclude the effect of residual and unmeasured confounders due to the limitation of observational study. Nevertheless, our results support that FMD is a surrogate marker of vascular health and a widely used noninvasive and reliable measurement of endothelial function.5,20
In conclusion, this study demonstrated for the first time that impairment of FMD is a significant independent predictor of MACCEs in PD patients. Considering the significant association between endothelial dysfunction and cardiovascular risk, estimating endothelial function by brachial FMD could be a useful tool to stratify cardiovascular risk in PD patients.
Footnotes
Abbreviations: ACS = acute coronary syndrome, ADMA = asymmetric dimethylarginine, BMI = body mass index, ESRD = end-stage renal disease, ET-1 = endothelin-1, FMD = flow-mediated dilation, HD = hemodialysis, HOMA-IR = homeostasis model assessment-insulin resistance, hs-CRP = high-sensitivity C-reactive protein, IFG = impaired fasting glucose, MACCE = major adverse cardiac and cerebrovascular event, NMD = nitroglycerin-mediated dilation, NO = nitric oxide, PD = peritoneal dialysis.
This work was supported by the Brain Korea 21 PLUS Project for Medical Science, Yonsei University, the National Research Foundation of Korea grant funded by the Ministry of Education, Science and Technology of the Korean government (No. 2011-0030711), and a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020).
The authors have no conflicts of interest to disclose.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32suppl 3:S112–S119. [DOI] [PubMed] [Google Scholar]
- 2.Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 3.Heitzer T, Schlinzig T, Krohn K, et al. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. [DOI] [PubMed] [Google Scholar]
- 4.Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. [DOI] [PubMed] [Google Scholar]
- 5.Ras RT, Streppel MT, Draijer R, et al. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168:344–351. [DOI] [PubMed] [Google Scholar]
- 6.Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. [DOI] [PubMed] [Google Scholar]
- 7.London GM, Pannier B, Agharazii M, et al. Forearm reactive hyperemia and mortality in end-stage renal disease. Kidney Int. 2004;65:700–704. [DOI] [PubMed] [Google Scholar]
- 8.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 9.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. [DOI] [PubMed] [Google Scholar]
- 11.Muiesan ML, Salvetti M, Paini A, et al. Prognostic role of flow-mediated dilatation of the brachial artery in hypertensive patients. J Hypertens. 2008;26:1612–1618. [DOI] [PubMed] [Google Scholar]
- 12.Beohar N, Flaherty JD, Davidson CJ, et al. Antirestenotic effects of a locally delivered caspase inhibitor in a balloon injury model. Circulation. 2004;109:108–113. [DOI] [PubMed] [Google Scholar]
- 13.Gokce N, Keaney JF, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. [DOI] [PubMed] [Google Scholar]
- 14.Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the “vulnerable” patient. Circulation. 2004;110:1926–1932. [DOI] [PubMed] [Google Scholar]
- 15.Karatzis EN, Ikonomidis I, Vamvakou GD, et al. Long-term prognostic role of flow-mediated dilatation of the brachial artery after acute coronary syndromes without ST elevation. Am J Cardiol. 2006;98:1424–1428. [DOI] [PubMed] [Google Scholar]
- 16.Careri G, Nerla R, Di Monaco A, et al. Clinical correlates and prognostic value of flow mediated dilation in patients with non-ST segment elevation acute coronary syndromes. Am J Cardiol. 2013;111:51–57. [DOI] [PubMed] [Google Scholar]
- 17.Fathi R, Haluska B, Isbel N, et al. The relative importance of vascular structure and function in predicting cardiovascular events. J Am Coll Cardiol. 2004;43:616–623. [DOI] [PubMed] [Google Scholar]
- 18.Morimoto S, Yurugi T, Aota Y, et al. Prognostic significance of ankle-brachial index, brachial-ankle pulse wave velocity, flow-mediated dilation, and nitroglycerin-mediated dilation in end-stage renal disease. Am J Nephrol. 2009;30:55–63. [DOI] [PubMed] [Google Scholar]
- 19.Dalton BS, Fassett RG, Geraghty DP, et al. Vascular function and mortality in haemodialysis patients: a pilot study. Arch Cardiovasc Dis. 2011;104:518–523. [DOI] [PubMed] [Google Scholar]
- 20.Han SH, Lee SC, Kang EW, et al. Reduced residual renal function is associated with endothelial dysfunction in patients receiving peritoneal dialysis. Perit Dial Int. 2012;32:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Olden RW, Krediet RT, Struijk DG, et al. Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 1996;7:745–750. [DOI] [PubMed] [Google Scholar]
- 22.Pride ET, Gustafson J, Graham A, et al. Comparison of a 2.5% and a 4.25% dextrose peritoneal equilibration test. Perit Dial Int. 2002;22:365–370. [PubMed] [Google Scholar]
- 23.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 24.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. [DOI] [PubMed] [Google Scholar]
- 25.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. [DOI] [PubMed] [Google Scholar]
- 26.Anderson TJ, Charbonneau F, Title LM, et al. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–169. [DOI] [PubMed] [Google Scholar]
- 27.Lind L, Berglund L, Larsson A, et al. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011;123:1545–1551. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T, Hirata K, Elkind MS, et al. Metabolic syndrome, endothelial dysfunction, and risk of cardiovascular events: the Northern Manhattan Study (NOMAS). Am Heart J. 2008;156:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 31.van Guldener C, Janssen MJ, Lambert J, et al. Endothelium-dependent vasodilatation is impaired in peritoneal dialysis patients. Nephrol Dial Transplant. 1998;13:1782–1786. [DOI] [PubMed] [Google Scholar]
- 32.Kocak H, Gumuslu S, Sahin E, et al. Relationship between carotid artery intima-media thickness and brachial artery flow-mediated dilation in peritoneal dialysis patients. Int Urol Nephrol. 2009;41:409–416. [DOI] [PubMed] [Google Scholar]
- 33.Recio-Mayoral A, Banerjee D, Streather C, et al. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease—a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis. 2011;216:446–451. [DOI] [PubMed] [Google Scholar]
- 34.American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37suppl 1:S14–S80. [DOI] [PubMed] [Google Scholar]
- 35.Holmes CJ, Shockley TR. Strategies to reduce glucose exposure in peritoneal dialysis patients. Perit Dial Int. 2000;20suppl 2:S37–S41. [PubMed] [Google Scholar]
- 36.Szeto CC, Chow KM, Kwan BC, et al. New-onset hyperglycemia in nondiabetic Chinese patients started on peritoneal dialysis. Am J Kidney Dis. 2007;49:524–532. [DOI] [PubMed] [Google Scholar]
- 37.Joannides R, Bakkali EH, Le Roy F, et al. Altered flow-dependent vasodilatation of conduit arteries in maintenance haemodialysis. Nephrol Dial Transplant. 1997;12:2623–2628. [DOI] [PubMed] [Google Scholar]
- 38.Pannier B, Guerin AP, Marchais SJ, et al. Postischemic vasodilation, endothelial activation, and cardiovascular remodeling in end-stage renal disease. Kidney Int. 2000;57:1091–1099. [DOI] [PubMed] [Google Scholar]
- 39.Dubin R, Owens C, Gasper W, et al. Associations of endothelial dysfunction and arterial stiffness with intradialytic hypotension and hypertension. Hemodial Int. 2011;15:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacAllister RJ, Rambausek MH, Vallance P, et al. Concentration of dimethyl—arginine in the plasma of patients with end-stage renal failure. Nephrol Dial Transplant. 1996;11:2449–2452. [DOI] [PubMed] [Google Scholar]
- 41.Burton JO, Jefferies HJ, Selby NM, et al. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dogra G, Irish A, Chan D, et al. Insulin resistance, inflammation, and blood pressure determine vascular dysfunction in CKD. Am J Kidney Dis. 2006;48:926–934. [DOI] [PubMed] [Google Scholar]
- 43.Cheng LT, Gao YL, Qin C, et al. Volume overhydration is related to endothelial dysfunction in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 2008;28:397–402. [PubMed] [Google Scholar]
- 44.Yilmaz MI, Saglam M, Carrero JJ, et al. Serum visfatin concentration and endothelial dysfunction in chronic kidney disease. Nephrol Dial Transplant. 2008;23:959–965. [DOI] [PubMed] [Google Scholar]
- 45.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. [DOI] [PubMed] [Google Scholar]
- 46.Bargman JM, Thorpe KE, Churchill DN, et al. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162. [DOI] [PubMed] [Google Scholar]
- 47.Paniagua R, Amato D, Vonesh E, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13:1307–1320. [DOI] [PubMed] [Google Scholar]
- 48.Termorshuizen F, Korevaar JC, Dekker FW, et al. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of The Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis. 2003;41:1293–1302. [DOI] [PubMed] [Google Scholar]
- 49.Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53:1068–1081. [DOI] [PubMed] [Google Scholar]
- 50.Robinson BM, Port FK. International hemodialysis patient outcomes comparisons revisited: the role of practice patterns and other factors. Clin J Am Soc Nephrol. 2009;4suppl 1:S12–S17. [DOI] [PubMed] [Google Scholar]


