Abstract
Peritoneal metastasis is the most frequent cause of death in patients with gastric cancer. Reverse transcriptase-polymerase chain reaction (RT-PCR) assay of peritoneal washes has been used to predict peritoneal metastasis of gastric carcinoma. We applied carcinoembryonic antigen (CEA) and melanoma-associated gene (MAGE) RT-PCR for the detection of peritoneal metastasis of gastric carcinoma after curative surgery and evaluated its clinical significance.
Peritoneal washes were obtained from 117 patients with gastric carcinoma. MAGE A1–A6 and CEA RT-PCR were performed, and the results were evaluated according to their clinicopathologic characteristics. Three-year follow-up clinical studies were periodically performed, and disease-free survival rates were retrospectively investigated using the medical records.
Among 117 peritoneal fluids, 11 cases (9.4%) revealed MAGE expression and 38 cases (32.5%) revealed CEA expression. When focusing on recurrence rates, RT-PCR-positive had much higher recurrence rates than RT-PCR-negative cases (32.5% vs 5.2%, P < 0.01). Univariate analysis revealed that depth of invasion, lymph node metastasis, tumor node metastasis (TNM) stage, Lauren classification, and MAGE and CEA expressions were independent prognostic factors for recurrence. In a multivariate analysis, MAGE expression and TNM stage were significantly and independently related to recurrence in patients who underwent curative resection. MAGE expression was determined to be the most important prognostic factor for recurrence (hazard ratio: 12.487, P < 0.01).
It is feasible to identify free cancer cells in peritoneal lavage by using a MAGE A1–A6 and CEA RT-PCR. MAGE RT-PCR results disclosed significant associations with peritoneal recurrence and proved to be the most important factor for the recurrence rate in patients with gastric carcinoma who had undergone radical resection.
INTRODUCTION
Despite the declining incidence in western countries, gastric cancer continues to be a worldwide health problem; it is still the fourth most common cancer worldwide and the second leading cause of cancer deaths.1 The most frequent cause of treatment failure following surgery for gastric cancer is peritoneal dissemination, mainly caused by the seeding of free cancer cells from the primary gastric cancer, which is the most common type of spread. Intraperitoneal free cancer cells isolated during peritoneal washing in patients with gastric cancer have been demonstrated to be significantly and independently related to the prognosis, influencing both recurrence and survival. Thus, the Japanese Classification of Gastric Carcinoma recommended peritoneal wash cytology in 1998.2 Even after curative resection, 98% of the patients with positive cytology diagnosed by intraoperative peritoneal lavage died of peritoneal recurrence.3
Recently, positive peritoneal cytology was classified as metastatic disease (M1) in the 7th edition of the American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) staging system for gastric cancer.1
Conventional cytology has been regarded as the only reliable method for detecting free cancer cells in the peritoneal wash and for predicting peritoneal metastasis,3,5 but it lacks sensitivity for the detection of residual cancer cells and prediction of peritoneal spread, and is not uniformly performed in practice.3–6 Furthermore, the sensitivity of conventional cytology has been reported to be very low (5%–15%) in cases without macroscopic peritoneal dissemination (P0) of the lavage fluid after curative resection.3–5
Recently, genetic detection using reverse transcriptase-polymerase chain reaction (RT-PCR) analysis has been used for the detection of free cancer micrometastases.7–9 The target genes of carcinoembryonic antigen (CEA), heparanase, matrix metalloproteinase-7, cytokeratin 20, telomerase, and melanoma-associated gene (MAGE) were used as potent molecular markers.10,11 The result of the RT-PCR of peritoneal washes is more sensitive than that of conventional cytology,12 and it strongly correlates with the peritoneal recurrence and prognosis after curative surgery.13,14
It is well known that CEA RT-PCR results for peritoneal fluid were proven to be a prognostic marker for the increased risk of death from cancer and peritoneal carcinomatosis. Recently, MAGE RT-PCR results have also disclosed a significant association with peritoneal recurrence of gastric carcinoma and were proven to be the most important prognostic factor for the overall survival rate in a previous study.11 But a trial on the comparison of the 2 markers after long-term follow-up has never been published in the literature review, so this study may be the first such trial.
This study was, therefore, conducted in order to investigate comparisons between CEA and MAGE for gastric free cancer cell detection in peritoneal washes without macroscopic peritoneal disseminations (P0), and to evaluate the clinical significance of the results through a 3-year follow-up clinical study after curative surgery.
MATERIALS AND METHODS
Patients
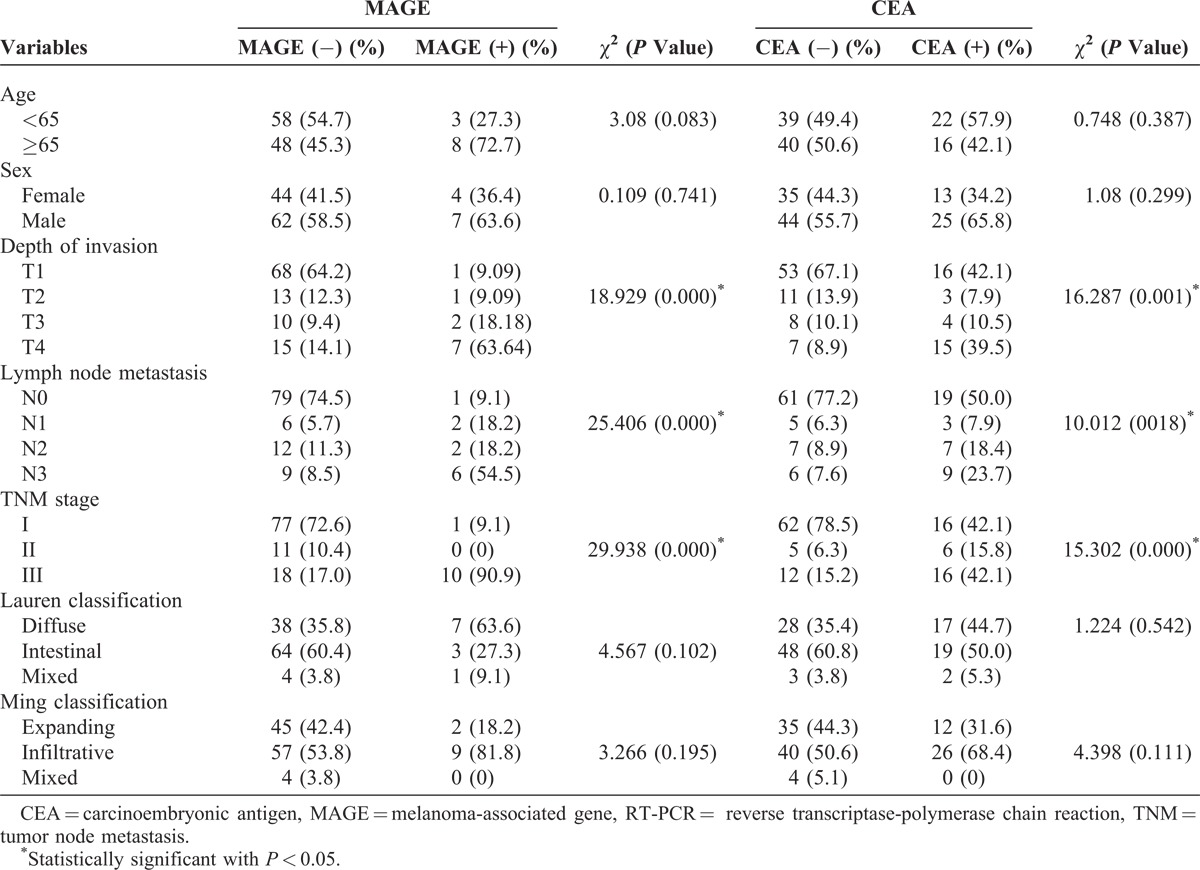
Peritoneal washing samples were collected from 117 consecutive patients who underwent operations for histologically proven gastric carcinoma at the Department of Surgery, Catholic University Medical Center of Daegu, Daegu, Korea, between January 2009 and January 2010. Patients who fulfilled the following criteria were eligible in this study: histologically confirmed gastric carcinoma using gastroduodenoscopy, no evidence of macroscopic peritoneal seeding or other distant metastasis on operation, potentially curative resection with complete macroscopic and histological removal of the tumor (R0 resection), and no treatment by neoadjuvant chemotherapy. The depth of cancer invasion (pT categories), lymph node metastasis (pN categories), and prognostic groups were histologically evaluated according to the 7th edition of the AJCC TNM staging system. Their clinicopathologic characteristics are summarized in Table 1.
TABLE 1.
Association Between RT-PCR Assay in Peritoneal Wash and Clinicopathologic Factors
The study design was approved by the Institutional Review Board of the Catholic University Medical Center of Daegu, and informed consent was obtained from all patients.
Peritoneal Fluid Collection and Processing
After laparotomy, the abdominal cavity was thoroughly examined for tumor metastasis. To obtain peritoneal wash fluids, we introduced 200 mL of saline solution into the peritoneal cavity at the beginning of the operation and carefully washed the cavity with gentle stirring. The wash fluids were collected at the drain site including the Douglas pouch using a catheter and a syringe. The fluids obtained were immediately transported to the laboratory and centrifuged, and 1 mL of TRIzol reagent (Invitrogen, Carlsbad, CA) was added to the pellets. The pellets were then stored in a deep freezer until RNA extraction.
RNA Extraction and RT-PCR
RNA extraction was performed as instructed by the manufacturer of TRIzol. Total RNA (1 μg) was reverse transcribed with ImProm-II Reverse Transcription System (Promega, Madison, WI). For individual PCR reactions, 4 μL of cDNA were amplified with gene-specific oligonucleotides at a concentration of 10 pmol per 50 μL reaction mixture using Solg h-Taq Polymerase (Solgent, Daejeon, Korea).
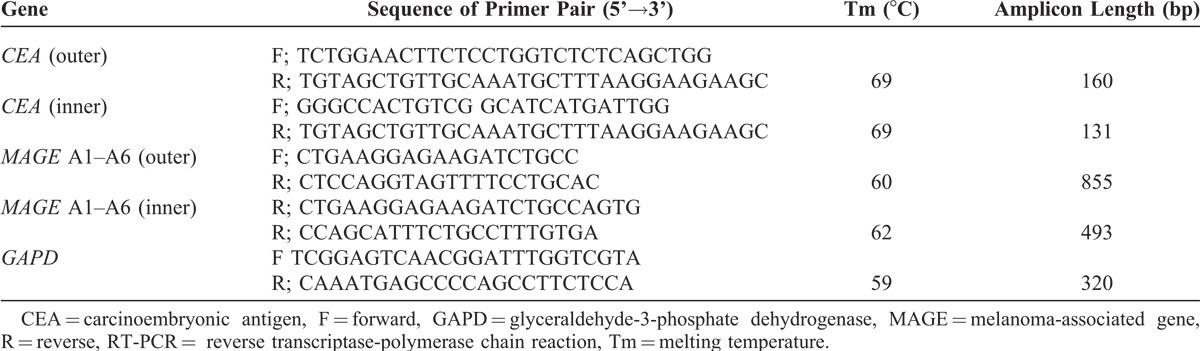
MAGE and CEA expressions were determined with nested PCR. The integrity of cDNA obtained was tested by amplifying glyceraldehyde-3-phosphate dehydrogenase transcripts. The used primers for gene-specific PCR are summarized in Table 2.
TABLE 2.
Primer Sequences Used for RT-PCR
Follow-Up Clinical Study
For the follow-up clinical studies, history taking, physical examination, serum tumor marker evaluation, simple chest x-ray, gastroduodenoscopy, and abdominal computed tomography (CT) scan were carried out at intervals of 3 to 4 months for advanced gastric carcinoma and biannually for early gastric carcinoma until the second year; later, biannual follow-up was performed until the third year. If necessary, abdominal ultrasonic examination, chest CT scan, whole body bone scan, and positron emission tomography scan were performed. Three-year disease-free survival (DFS) rates were retrospectively investigated using the medical records.
Statistical Analysis
Comparisons between groups were performed using the χ2 test for categorical variables. Survival rates were estimated and the Kaplan–Meier method and log–rank test were used for the comparisons between the survival curves with respect to the 3-year DFS, which was defined as no recurrence of the disease. Selected variables showing statistical significance in a univariate analysis were included as covariates in the multivariate analysis, using the Cox proportional hazard model. For the selection of optimal covariates in the Cox proportional hazard model, the forward conditional likelihood method was used. All tests were 2-sided and a P value of <0.05 was considered to indicate statistical significance. IBM SPSS ver. 19.0 and Medcalc ver. 12.5.0 were used for the analysis.
RESULTS
Patient Demographics
Of the 124 patients who were considered eligible, there were 7 cases of intraoperative macroscopic evidence of peritoneal seeding. These patients were excluded, and the remaining 117 patients underwent the survival analysis. There were 69 men and 44 women. Mean age (±standard deviation) of the patients was 61.1 ± 12.0 years. The number of patients according to the stage classification scheme of the 7th edition of the AJCC TNM staging system of gastric cancer were: 78 for stage I (IA/IB = 62/16), 11 for stage II (IIA/IIB = 3/8), and 28 for stage III (IIIA/IIIB/IIIC = 10/9/9).
Association Between RT-PCR Assay and Clinicopathologic Parameters
Clinicopathologic characteristics of the 117 patients studied are summarized in Table 1. Among 117 peritoneal fluids, 11 cases (9.4%) revealed MAGE expression, 38 cases (32.5%) revealed CEA expression, and 9 cases (7.7%) revealed both MAGE and CEA expressions. Compared with the MAGE-negative cases, MAGE-positive cases showed different lymph node metastasis (P < 0.001), depth of invasion (P < 0.001), and TNM stage (P < 0.001). MAGE expression was not associated with age, sex, or the Lauren and the Ming classifications. CEA-mRNA positivity was also correlated with the depth of tumor invasion (P = 0.001), lymph node metastasis (P = 0.018), and TNM stage (P < 0.001). Higher RT-PCR expression rates were observed in more extensive lymph node metastasis, deeper invasion, and advanced stages of tumor groups.
Recurrence Rate for 3 Years and Sites According to RT-PCR Expression
When focusing on recurrence rates, RT-PCR-positive cases had much higher recurrence rates than RT-PCR-negative cases (13/40, 32.5% vs 4/77, 5.2%, P < 0.01; Table 3).
TABLE 3.
Recurrent Sites and Rate According to RT-PCR (CEA or MAGE) Expression in Peritoneal Washes
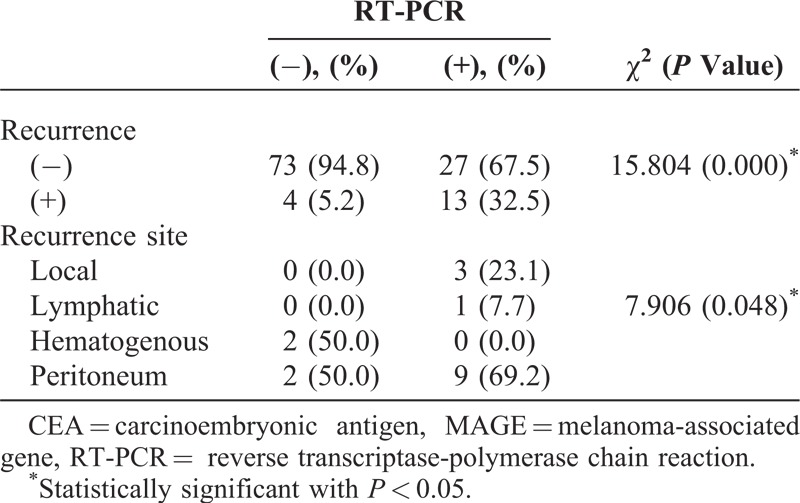
Thirteen RT-PCR-positive recurrent patients showed metastasis: 3 in local recurrences, 1 in lymphatics, and 9 in peritoneums, whereas RT-PCR-negative recurrent patients had 4 recurrences: 2 in hematogenous spreads and 2 in peritoneums. There was a significant correlation between peritoneal recurrence and RT-PCR expression in peritoneal wash fluid (P = 0.048). A higher number of patients with a positive RT-PCR assay revealed peritoneal recurrence than those with a negative RT-PCR assay. Of the 9 patients with a positive RT-PCR assay and peritoneal recurrence, 8 (88.9%) patients died of peritoneal recurrence. One patient with recurrence is being treated with chemotherapy and is being followed up.
Prognosis (3-Year DFS) and RT-PCR Assay
We further investigated whether the RT-PCR assay could be a predictor of the DFS in patients with gastric cancer. Survival curves for the 4 patient groups classified on the basis of the mRNA expressions are shown in Figure 1.
FIGURE 1.
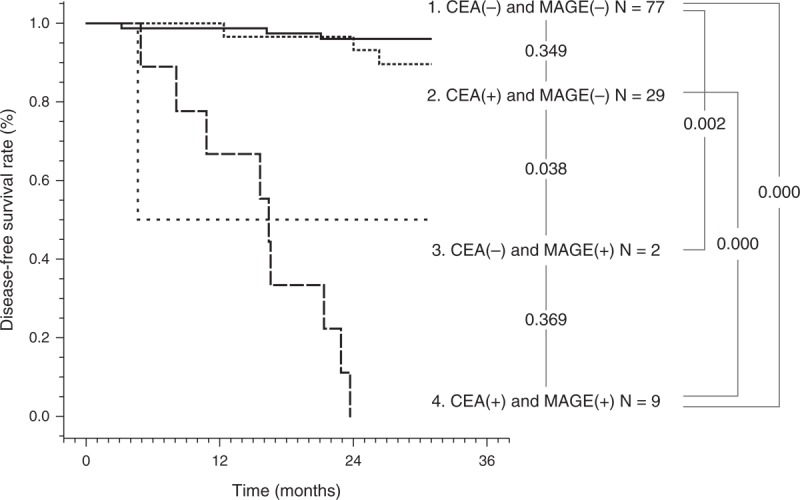
Three-year DFS curves according to mRNA expression of MAGE and CEA in peritoneal washes in patients with gastric cancer after curative surgery. A difference in the 3-year DFS rates between the 4 groups was observed. CEA = carcinoembryonic antigen, DFS = disease-free survival, MAGE = melanoma-associated gene.
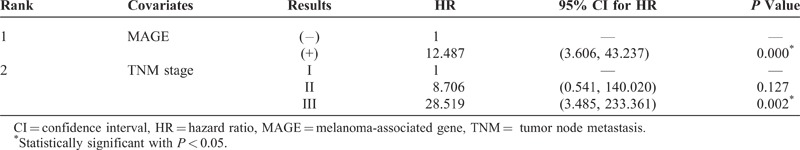
The clinicopathologic variables analyzed by the univariate analysis with binary multiple logistic regression were as follows: age, sex, depth of invasion, lymph node metastasis, TNM stage, CEA expression, MAGE expression, Lauren classification, and Ming classification. Six variables, namely, depth of invasion, lymph node metastasis, TNM stage, CEA expression, MAGE expression, and Lauren classification, were found to affect the patient outcome on the univariate analysis (Table 4). Multivariate analysis of variance was performed using the Cox stepwise regression method with the conditional forward method to identify individual variables. Among these 6 factors, MAGE expression and TNM stage were found to be statistically significant independent prognostic factors by the multivariate analysis. The MAGE RT-PCR results proved to be the most important factor for recurrence in patients with gastric carcinoma who had undergone radical resection (hazard ratio [HR]: 12.487, P < 0.01; Table 5).
TABLE 4.
Univariate Analysis of Factors Affecting Recurrence Using the Binary Multiple Logistic Regression Method
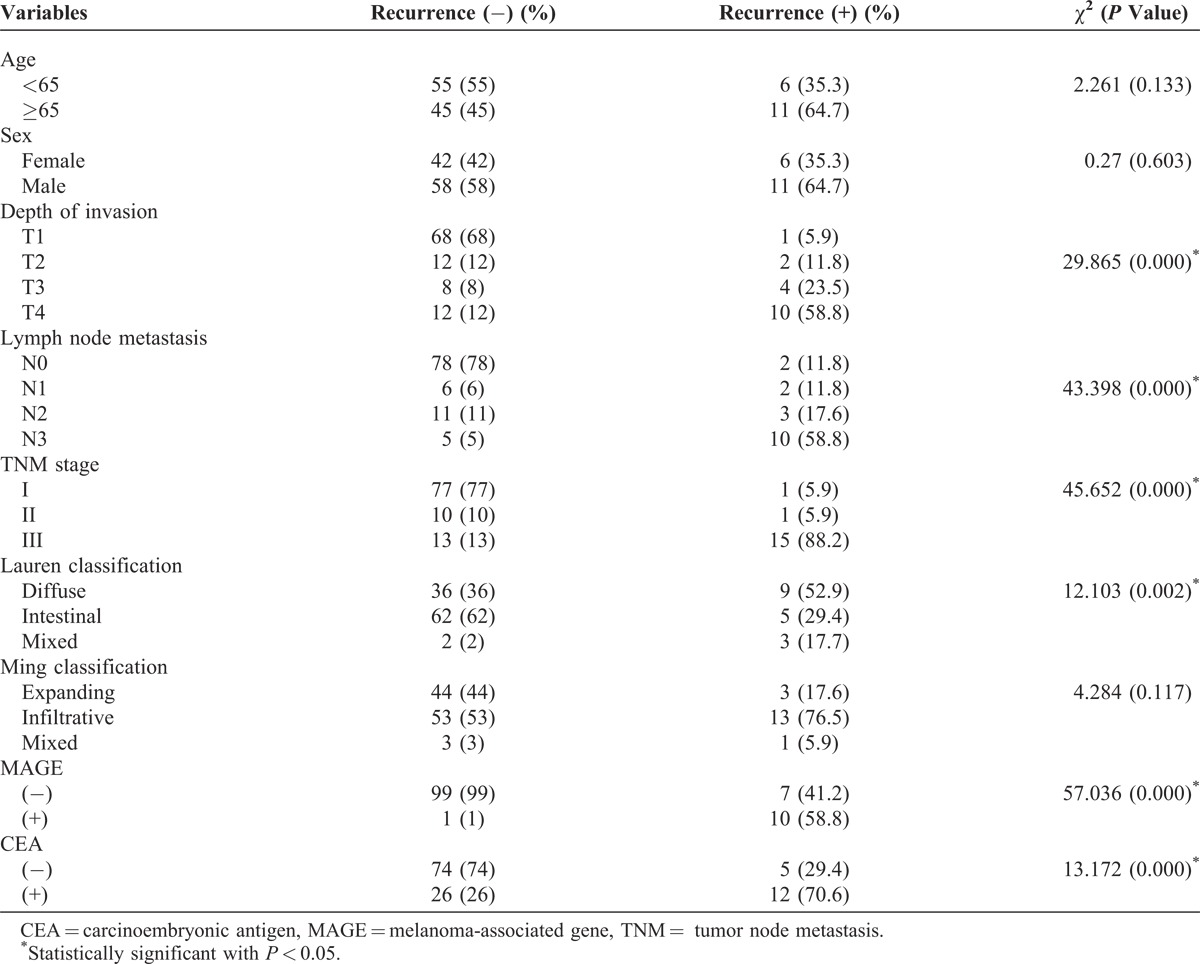
TABLE 5.
Multivariate Analysis of Factors Affecting Recurrence Using Cox Proportional Hazards Regression Model With Conditional Forward Method
DISCUSSION
Peritoneal metastasis is the most frequent pattern of recurrence in patients with gastric carcinoma, so diagnostic methods for the prediction of peritoneal dissemination are needed. Free cancer cells in the abdominal cavity, considered to be responsible for peritoneal carcinomatosis, have been detected in peritoneal washes through cytologic examination with Papanicolaou staining, ThinPrep cytology,15 immunohistochemistry, and the RT-PCR. Conventional cytology has been regarded as the gold standard for detecting free cancer cells in the peritoneal lavage, but it is often criticized for its relatively low sensitivity, ranging from 10% to 32.9% in gastric carcinoma invading the serosa.16–19 Furthermore, it is much lower (5%–15%) when there are no macroscopic peritoneal disseminations (P0) of the lavage fluid after curative resection.3–5
Over the last 2 decades, RT-PCR that targets cancer cell-related mRNA has been developed and used in clinical practice, but based on several studies, the results of RT-PCR of peritoneal lavage fluid strongly correlate with peritoneal recurrence and prognosis after curative surgery.13,14,20–23 In this study, the incidences of positive MAGE and CEA expressions were 9.4% and 32.5%, respectively, regardless of the depth of invasion. In invasion of the serosa, these 2 investigations had excellent sensitivity for the detection of peritoneal free cancer cells, with sensitivities of 31.8% (7/22) and 68.1% (15/22), respectively (Table 1). Although we could not conclude the incremental yield and clinical significance of RT-PCR positivity over the conventional Papanicolaou stain because of not performing conventional cytology (CY), these results might show that RT-PCR of peritoneal washes is more sensitive than conventional cytology.
In the past, several studies regarding cytologic examination have been performed only for patients with serosal involvement.16,24,25 However, the detection of the free cancer cells has been reported in a small number of patients with nonserosal involvement.18,26 In this study, there were recurrences in 7 patients with pT1–3 tumor not invading the serosa (Table 4). Some of these patients with no invaded serosa were known to have free cancer cells in the peritoneal cavity, and the detection of these cells was a useful means of predicting recurrence.
The sensitivity and specificity of the CEA RT-PCR assay for recurrence in this study were 70.6% (12/17) and 74.0% (74/100), respectively (Table 4), which are comparable results with previously published articles.23,27–30 Kodera et al23 suggested that the possibility that the false-positive rate of CEA RT-PCR may have been influenced by the expression rate in both gastric carcinoma cells and nontumor cells may exceed 10%, and that this apparently had a negative influence on the specificity of this technique. MAGE could be expressed in gastric carcinoma, but there is no expression of MAGE in normal gastric tissue31; no significant correlation was observed between the expression of MAGE and clinicopathologic factors.11 In this study, the sensitivity and specificity of MAGE expression for recurrence were 58.8% (10/17) and 99.0% (99/100), respectively (Table 4), and there was an improvement of specificity in the MAGE expression compared with the CEA expression.
As a diagnostic modality for detecting free cancer cells, high specificity of recurrence is very important to reduce false enrollment of patients for further evaluation and treatment. Also, our previous study showed that MAGE expression may disclose a significant association with peritoneal recurrence of gastric carcinoma.11 The influence of CEA RT-PCR on peritoneal recurrence for patients with gastric cancer has been reported by other centers. Wang et al27 reported that the technique of CEA RT-PCR was more sensitive than conventional cytological examination and plasma CEA levels in the detection of peritoneal free cancer cells as well as in the prediction of peritoneal recurrence. They also reported that CEA RT-PCR assay was an independent and most significant predictor for peritoneal recurrence (odds ratio: 4.082, P = 0.001) among these 3 diagnostic modalities in a multivariate model using logistic stepwise regression analysis. Kodera et al6 also reported similar results that a positive CEA RT-PCR result was the most important independent variable associated with peritoneal recurrence among 242 patients without peritoneal seeding at operation (HR: 1.57, P = 0.0203). Although other investigators found that the CEA RT-PCR status was the most important factor for peritoneal recurrence, on multivariate analysis, our study revealed that MAGE expression was determined to be the most important prognostic factor for peritoneal recurrence among 117 patients without peritoneal metastasis at operation (HR: 12.487, P < 0.001) (Table 5). It was similar to the findings of our previous study, in which it was determined without performing CEA RT-PCR expression, that MAGE expression was the most important prognostic factor for the overall survival rate.11 We believe that this difference was mainly because of the lower specificity of CEA RT-PCR compared with MAGE RT-PCR.
Recently, the 7th edition of the AJCC TNM staging system for gastric cancer emphasized that positive peritoneal cytology should be classified as M1.1 This study showed compelling evidence for the utility of molecular markers in the diagnosis of peritoneal metastasis and recurrence. Using genetic techniques, we can select patients at high risk as well as those at low risk for peritoneal recurrence. For high-risk patients, more aggressive adjuvant chemotherapy may be justifiable to prevent peritoneal recurrence, and for low-risk patients, based on the genetic diagnosis, chemotherapy may be omitted after curative resection.
In conclusion, identifying free cancer cells in the peritoneal lavage using a gene amplification technique (mRNA-specific RT-PCR method) is a viable technique. MAGE RT-PCR results disclosed significant associations with peritoneal recurrence and proved to be the most important prognostic factor for recurrence in patients with gastric carcinoma who had undergone radical resection. We believe that this information could contribute to determining the optimal preoperative and postoperative adjuvant therapeutic modality of gastric cancer after curative resection without peritoneal metastasis.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, CT = computed tomography, GAPD = glyceraldehyde-3-phosphate dehydrogenase, HR = hazard ratio, IRB = Institutional Review Board, M1 = metastatic disease, MAGE = melanoma-associated gene, MMP-7 = matrix metalloproteinase-7, PET = positron emission tomography, RT-PCR = reverse transcriptase-polymerase chain reaction.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Washington K. 7th edition of the AJCC Cancer Staging Manual: Stomach. Ann Surg Oncol. 2010;17:3077–3079. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 2nd English edition. Gastric Cancer. 1998;1:10–24. [DOI] [PubMed] [Google Scholar]
- 3.Bando E, Yonemura Y, Takeshita Y, et al. Intraoperative lavage for cytological examination in 1297 patients with gastric carcinoma. Am J Surg. 1999;178:256–262. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Zhang X, Xu H, et al. Detection of peritoneal micrometastasis by reverse transcriptase-polymerase chain reaction for heparanase mRNA and cytology in peritoneal wash samples. J Surg Oncol. 2005;90:59–65. [DOI] [PubMed] [Google Scholar]
- 5.Bentrem D, Wilton A, Mazumdar M, et al. The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol. 2005;12:347–353. [DOI] [PubMed] [Google Scholar]
- 6.Kodera Y, Nakanishi H, Ito S, et al. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: analysis of real time reverse transcriptase-polymerase chain reaction after 5 years of follow up. J Am Coll Surg. 2006;202:231–236. [DOI] [PubMed] [Google Scholar]
- 7.Noura S, Yamamoto H, Ohnishi T, et al. Comparative detection of lymph node micrometastases of stage II colorectal cancer by reverse transcriptase polymerase chain reaction and immunohistochemistry. J Clin Oncol. 2002;20:4232–4241. [DOI] [PubMed] [Google Scholar]
- 8.Yoshioka S, Fujiwara Y, Sugita Y, et al. Real-time rapid reverse transcriptase-polymerase chain reaction for intraoperative diagnosis of lymph node micrometastasis: clinical application for cervical lymph node dissection in esophageal cancers. Surgery. 2002;132:34–40. [DOI] [PubMed] [Google Scholar]
- 9.Okada Y, Fujiwara Y, Yamamoto H, et al. Genetic detection of lymph node micrometastases in patients with gastric carcinoma by multiple-marker reverse transcriptase-polymerase chain reaction assay. Cancer. 2001;92:2056–2064. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara Y, Doki Y, Taniguchi H, et al. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer. 2007;10:197–204. [DOI] [PubMed] [Google Scholar]
- 11.Jeon CH, Shin IH, Park JB, et al. Prognostic significance of MAGE in peritoneal washes in gastric carcinoma patients without peritoneal metastasis results of a 5-year follow-up study. J Clin Gastroenterol. 2010;44:682–686. [DOI] [PubMed] [Google Scholar]
- 12.Kodera Y, Nakanishi H, Ito S, et al. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer. 2005;8:142–148. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi H, Kodera Y, Torii A, et al. Detection of carcinoembryonic antigen-expressing free tumor cells in peritoneal washes from patients with gastric carcinoma by polymerase chain reaction. Jpn J Cancer Res. 1997;88:687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakanishi H, Kodera Y, Yamamura Y, et al. Rapid quantitative detection of carcinoembryonic antigenexpressing free tumor cells in the peritoneal cavity of gastriccancer patients with real-time RT-PCR on the LightCycler. Int J Cancer. 2000;89:411–417. [DOI] [PubMed] [Google Scholar]
- 15.Ryu CK, Park JI, Min JS, et al. The clinical significance and detection of intraperitoneal micrometastases by ThinPrepⓇ cytology with peritoneal lavage fluid in patients with advanced gastric cancer. J Korean Gastric Cancer Assoc. 2008;8:189–197. [Google Scholar]
- 16.Ikeguchi M, Oka A, Tsujitani S, et al. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994;14:2131–2134. [PubMed] [Google Scholar]
- 17.Burke EC, Karpeh MS, Jr, Conlon KC, Brennan MF. Peritoneal lavage cytology in gastric cancer: an independent predictor of outcome. Ann Surg Oncol. 1998;5:411–415. [DOI] [PubMed] [Google Scholar]
- 18.Kodera Y, Yamamura Y, Shimizu Y, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999;72:60–64. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T, Ochiai T, Hayashi H, et al. Importance of positive peritoneal lavage cytology findings in the stage grouping of gastric cancer. Surg Today. 1999;29:111–115. [DOI] [PubMed] [Google Scholar]
- 20.Yonemura Y, Fujimura T, Ninomiya I, et al. Prediction of peritoneal micrometastasis by peritoneal lavaged cytology and reverse transcriptase-polymerase chain reaction for matrix metalloproteinase-7 mRNA. Clin Cancer Res. 2001;7:1647–1653. [PubMed] [Google Scholar]
- 21.Schmidt P, Thiele M, Rudroff C, et al. Detection of tumor cells in peritoneal lavages from patients with gastrointestinal cancer by multiplex reverse transcriptase PCR. Hepatogastroenterology. 2001;48:1675–1679. [PubMed] [Google Scholar]
- 22.Kodera Y, Nakanishi H, Yamamura Y, et al. Prognostic value and clinical implications of disseminated cancer cells in the peritoneal cavity detected by reverse transcriptase-polymerase chain reaction and cytology. Int J Cancer. 1998;79:429–433. [DOI] [PubMed] [Google Scholar]
- 23.Kodera Y, Nakanishi H, Yamamura Y, et al. Quantitative detection of disseminated free cancer in peritoneal washes with real-time reverse transcriptase polymerase chain reaction. Ann Surg. 2002;235:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe S, Yoshimura H, Tabara H, et al. Curative resection of gastric cancer: limitation of peritoneal lavage cytology in predicting the outcome. J Surg Oncol. 1995;59:226–229. [DOI] [PubMed] [Google Scholar]
- 25.Wu CC, Chen JT, Chang MC, et al. Optimal surgical strategy for potentially curable serosa-involved gastric carcinoma with intraperitoneal free cancer cells. J Am Coll Surg. 1997;184:611–617. [PubMed] [Google Scholar]
- 26.Koga S, Kaibara N, Iitsuka Y, et al. Prognostic significance of intraperitoneal free cancer cells in gastric cancer patients. J Cancer Res Clin Oncol. 1984;108:236–238. [DOI] [PubMed] [Google Scholar]
- 27.Wang JY, Lin SR, Lu CY, et al. Gastric cancer cell detection in peritoneal lavage: RT-PCR for carcinoembryonic antigen transcripts versus the combined cytology with peritoneal carcinoembryonic antigen levels. Cancer Lett. 2005;223:129–135. [DOI] [PubMed] [Google Scholar]
- 28.Kodera Y, Nakanishi H, Ito S, et al. Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer. 2002;5:69–76. [DOI] [PubMed] [Google Scholar]
- 29.Shimomura K, Sakakura C, Takemura M, et al. Combination of L-3-phosphoserine phosphatase and CEA using real-time RT-PCR improves accuracy in detection of peritoneal micrometastasis of gastric cancer. Anticancer Res. 2004;24:1113–1120. [PubMed] [Google Scholar]
- 30.Oyama K, Terashima M, Takagane A, Maesawa C. Prognostic significance of peritoneal minimal residual disease in gastric cancer detected by reverse transcription-polymerase chain reaction. Br J Surg. 2004;91:435–443. [DOI] [PubMed] [Google Scholar]
- 31.Park JW, Kwon TK, Kim IH, et al. A new strategy for the diagnosis of MAGE-expressing cancers. J Immunol Methods. 2002;266:79–86. [DOI] [PubMed] [Google Scholar]


