Abstract
Hyperammonemia has been reported to be associated with patients who receive valproic acid (VPA) therapy. This study aimed to determine the risk factors for hyperammonemia in patients with epilepsy treated with VPA. One hundred and fifty-eight adult patients with epilepsy aged older than 17 years who received VPA therapy were enrolled into this study. Blood samples were taken during the interictal state and analyzed for the blood level of ammonia. Statistical analysis was conducted between different groups of patients. The results showed that the frequency of hyperammonemia associated with VPA therapy was 27.8% (ammonia level >93 µg/dL), and 5.1% of the patients had severe hyperammonemia (ammonia level >150 µg/dL). The blood ammonia level was significantly correlated with the dosage of VPA and the plasma concentration of VPA. An increase of 1 mg in the dosage of VPA increased the risk of hyperammonemia by 0.1%. In addition, combination treatment with liver enzyme inducing antiepileptic drugs (AEDs) and antipsychotic drugs increased the risk of hyperammonemia. In conclusion, the use of VPA in adult patients with epilepsy was associated with a dose-dependent increase in blood concentrations of ammonia. Combination treatment with liver enzyme-inducing AEDs and antipsychotic drugs increased the risk of VPA-induced hyperammonemia. Most of the patients with VPA-induced hyperammonemia were asymptomatic; however, if patients taking VPA present with symptoms such as nausea, fatigue, somnolence, ataxia, and consciousness disturbance, the blood ammonia level should be measured.
INTRODUCTION
Valproic acid (VPA) is an antiepileptic drug (AED) that is widely used in the treatment of epilepsy, migraine, and psychiatric disorders. The most commonly reported adverse events associated with VPA include fatigue, gastrointestinal disturbances, weight gain, tremor, hair loss, thrombocytopenia, an increase in hepatic enzymes, and teratogenicity.1 Hyperammonemia has been reported in patients who receive VPA therapy.2–5 Although most patients are asymptomatic and the clinical significance of hyperammonemia associated with VPA therapy is still under debate,2 it may also rarely lead to hyperammonemic encephalopathy, which is associated with significant morbidity and central nervous system (CNS) damage.2,3,6
Ammonia is a product of the catabolism of proteins that contain nitrogen. It is normally converted to urea in the liver hepatocytes rendering it nontoxic, and it is then eliminated via the kidneys.7 Under normal conditions, the concentration of ammonia in the circulation remains low, typically less than 50 µmol/L (85 µg/dL). Studies have shown that a variety of environmental factors and medications may elevate blood ammonia levels leading to toxic effects on the CNS.7 The exact mechanism of VPA-induced hyperammonemia is still unknown.8 The possible mechanisms might be related to an imbalance between ammoniagenesis and ammonia disposal in the urea cycle that includes direct inhibition of the mitochondrial urea cycle enzyme, carbamoyl phosphate synthetase (CPS) I by VPA or its metabolites, an indirect effect on CPS I through interference in the synthesis of N-acetylglutamate, and inhibition of the mitochondrial fatty acid beta-oxidation pathway.9
The reported prevalence of hyperammonemia in patients receiving VPA therapy is highly variable, ranging from 2% to 80%.3–6,10–12 However, only a few studies have reported the possible risk factors associated with VPA-induced hyperammonemia. Two large-scale studies6,10 in Japan conducted on adult and pediatric patient groups reported the risk factors for hyperammonemia associated with VPA therapy, including VPA dose, female gender, and the concomitant use of phenytoin, phenobarbital, or topiramate. Although hyperammonemia induced by VPA therapy is mostly asymptomatic, it may be an unrecognized adverse effect in patients with epilepsy on VPA therapy. In this article, we analyzed the risk factors for hyperammonemia in patients with epilepsy who received VPA treatment for seizure control.
PATIENTS AND METHODS
Subjects
This was a single-center, prospective, observational study. From June 2012 to May 2013, 158 patients aged older than 17 years under VPA monotherapy or combination therapy for epilepsy were enrolled in this study. The study protocol was approved by the Ethics Committee of Chang Gung Memorial Hospital, Kaohsiung, Taiwan. Patients who had hyperammonemia due to any etiology except for VPA therapy and patients receiving VPA therapy for nonepileptic disorders were excluded from this study. All patients received a physical examination and interviews. The clinical records of the patients with epilepsy were reviewed and the age, gender, age at onset, duration of epilepsy, semiology, etiology, body height, weight, seizure control, dose and duration of VPA, medication regimens, ammonia level, comorbidities, and biochemistry results were analyzed.
The semiology and etiology of epilepsy was classified according to the recommendations of The International Classification of Epilepsies and Epileptic syndromes.13 Seizure free was defined as no seizure attacks for at least 12 months under current antiepileptic medication according to the consensus proposal of the International League Against Epilepsy.14 Liver enzyme inducers of AEDs included phenobarbital, phenytoin, and carbamazepine.15,16 Weak liver enzyme inducers included oxcarbazepine and topiramate.16 Levetiracetam and lamotrigine were classified as nonenzyme inducers.15,16
Assessment of Blood Ammonia Level
Blood samples were taken during the interictal state. As transient hyperammonemia has been reported to be associated with seizures,17 the blood samples collected from the patients with a recent seizure event were excluded from this study. In our clinical practice, blood samples are collected into a heparinized tube that is then immediately placed in ice water. Blood samples were centrifuged at 3000 rpm for 10 minutes to obtain serum samples. The plasma ammonia and VPA levels were rapidly analyzed by the central laboratory of Chang Gung Memorial Hospital. In brief, the level of ammonia was measured by a timed endpoint method using an ammonia reagent kit (Bechman Coulter, Brea, CA). The changes in the absorbance at 340 nm were monitored by a UniCel DxC 880i system (Bechman Coulter) to calculate the concentration of ammonia. A fluorescence polarization immunoassay was used to measure the level of VPA in conjunction with an AxSYM analyzer (Abbott Laboratories, Abbott Park, IL). The blood samples were sent to the central laboratory for the analysis of complete blood cell count and serum levels of creatinine, alanine aminotransferase, alkaline phosphatase, and gamma glutamyl transpeptidase (γGT). The cut-off value of the reference level of ammonia is 93 µg/dL. Therefore, we defined hyperammonemia as patients whose blood ammonia level was higher than 93 µg/dL.
Statistical Analyses
For categorical variables, the chi-square test or Fisher’s exact test was used as appropriate. Fisher’s exact test was used when 1 cell had an expected count of less than 5. Continuous variables such as age, age at onset, duration of epilepsy, body height, weight, body mass index, VPA dose, plasma VPA level, and biochemistry results were compared using the Mann–Whitney U test. Pearson correlations were performed to test the association between continuous variables, including the plasma level of VPA and ammonia. Logistic regression analysis was used to test the independent association between each risk variable as a predictor of hyperammonemia. Variables in the regression model included gender, dose of daily VPA, and combined medications including liver enzyme-inducing AEDs and antipsychotic drugs. The VPA dose and γGT between those taking and not taking liver enzyme inducers were compared by the Mann–Whitney test. All statistical analyses were performed using the Statistical Package for Social Science (SPSS, version 11.0 for Windows; Chicago, IL). A P value of <0.05 was considered to be statistically significant.
RESULTS
In total, 158 patients (54 females and 104 males) were enrolled in this study. The demographic data of the patients are listed in Table 1. The median age was 38 years (range: 18–88 y). Sixty-two (39.2%) patients suffered from idiopathic or cryptogenic epilepsy, whereas the remaining patients (60.8%) suffered from symptomatic etiologies including cerebrovascular accidents (N = 20), perinatal brain damage (N = 9), central nervous systemic infections (N = 11), head trauma (N = 36), neoplasms (N = 17), and immune diseases (N = 3). Of the 158 patients, 55 (35%) were seizure free and 103 patients were not seizure free in the past 1 year. The dosage of VPA used ranged from 300 to 2250 mg/day, and the blood level of VPA ranged from 3.21 µg/mL to 113.27 µg/mL. The level of ammonia ranged from 27 to 319 µg/dL. Thirty-one patients (19.6%) received VPA monotherapy and the other 127 patients (80.4%) received combination therapy with other AEDs. The frequency of hyperammonemia (ammonia level >93 µg/dL) associated with VPA therapy was estimated at 27.8% (N = 44) in all patients, 6.4% (2/31) in VPA monotherapy patients and 33.1% (42/127) in patients with combination therapy. The symptoms of VPA-induced hyperammonemia included nausea (N = 2), fatigue (N = 2), ataxia (N = 1), and consciousness disturbance (N = 1). One patient had encephalopathy with consciousness disturbance with an ammonia level of 319 µg/dL. None of the patients had seizure aggravation during the period when they had a higher ammonia level. The VPA dosage was reduced in 6 patients, and 3 patients gradually discontinued VPA treatment because of the adverse effects or the patient’s or family’s request. The level of ammonia returned to normal in all patients who reduced or discontinued VPA therapy.
TABLE 1.
Demographic Data of the 158 Patients Receiving Valproic Acid Therapy
Risk Factors of Hyperammonemia in the Patients With VPA Therapy
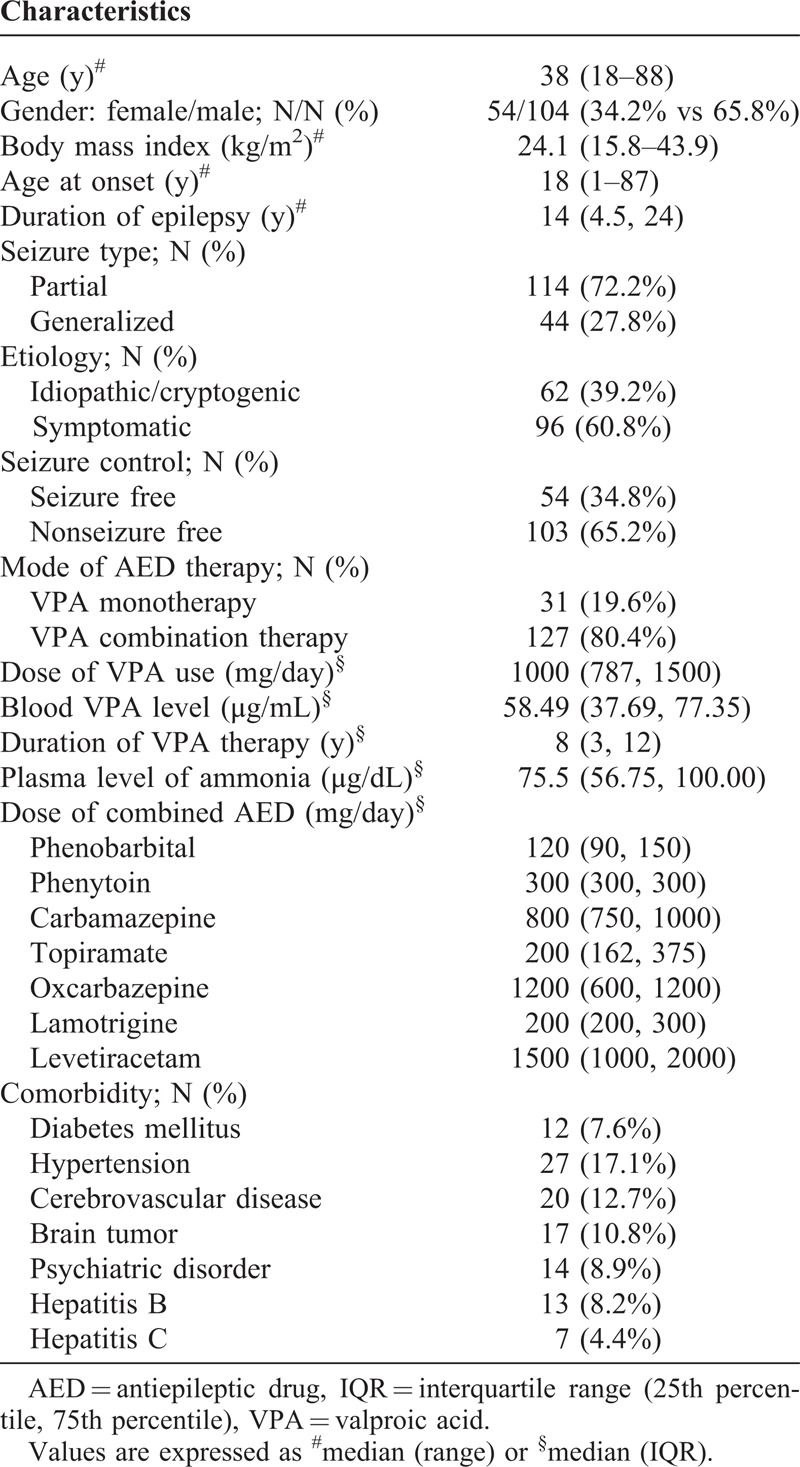
Comparisons of the clinical features between the patients with or without hyperammonemia are listed in Table 2. Statistical analysis between the 2 patient groups revealed that male gender, the dose of VPA, combination therapy, concomitant use with liver enzyme-inducing AEDs or antipsychotic drugs, comorbidity with brain tumor, and elevated serum γGT were significantly associated with VPA-induced hyperammonemia. After analyzing these variables, male gender (P < 0.001, odds ratio [OR] = 8.456, 95% confidence interval [CI] = 2.565–27.874), the dose of VPA (P = 0.029, OR = 1.001, 95% CI = 1.000–1.002), and combination therapy with liver enzyme-inducing AEDs (P = 0.014, OR = 2.834, 95% CI = 1.232–6.516) and antipsychotic drugs (P = 0.011, OR = 6.971, 95% CI = 1.559–31.160) were independently associated with hyperammonemia. There were no significant associations with the other variables including age, body mass index, semiology of epilepsy, duration of VPA therapy, etiology, associated medical diseases, and concomitant use of other medical medications (except for antipsychotic drugs). Among 10 patients who received combination therapy with antipsychotic drugs, 7 (70%) had hyperammonemia. The antipsychotic drugs included aripiprazole, sulpride, amisulpride, risperidone, paliperidone, and quetiapine.
TABLE 2.
Comparisons of the Demographic Data and Risk Factors Between Patients With Normal Ammonia Levels and Hyperammonemia
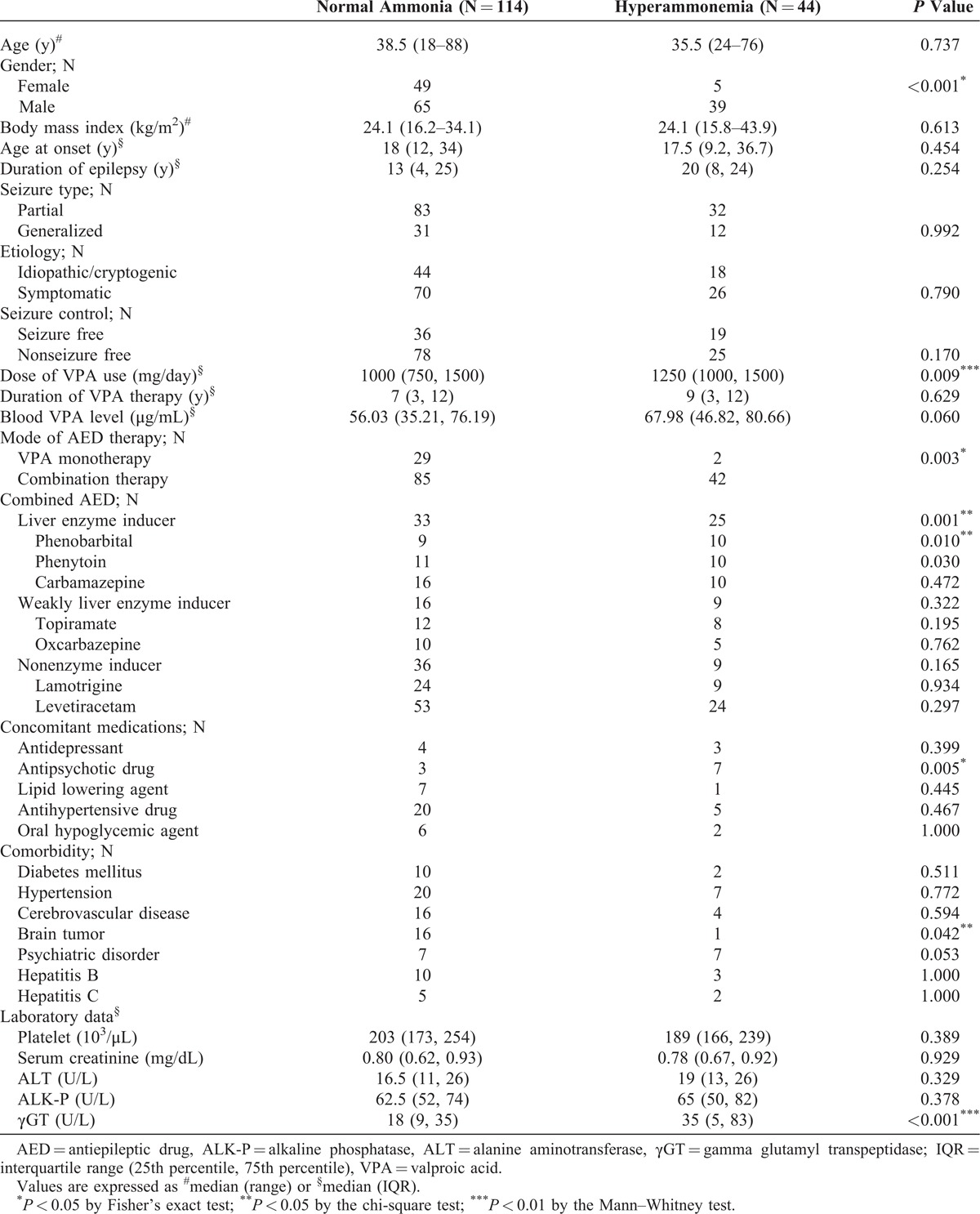
Based on Pearson correlation analysis, the plasma ammonia level showed a linear correlation with the blood concentration of VPA (r = 0.21, P = 0.008) (Figure 1). An increase of 1 mg in the dosage of VPA increased the risk of hyperammonemia by 0.1%.
FIGURE 1.
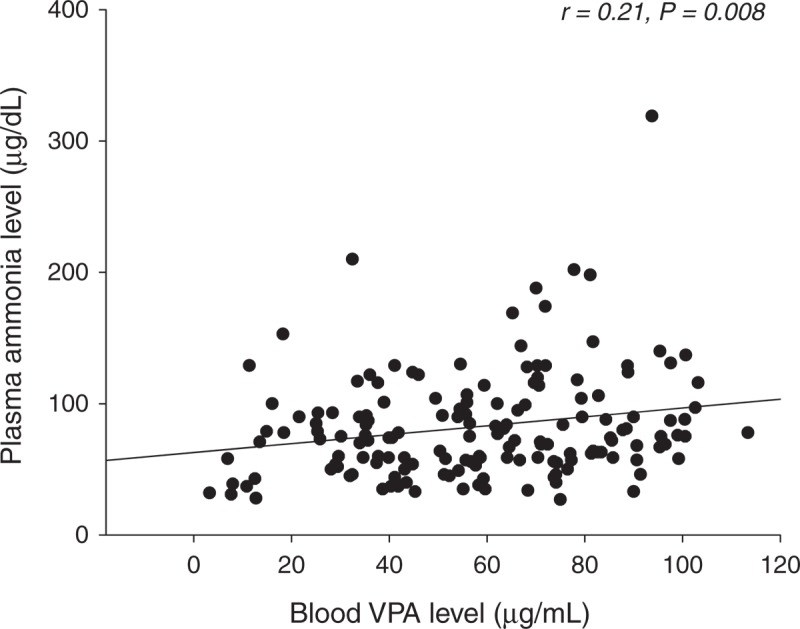
Relationship between the blood ammonia level and the blood concentration of valproic acid in 158 patients with epilepsy.
Relationship of Combination Therapy With Enzyme-Inducing AEDs in VPA-Induced Hyperammonemia
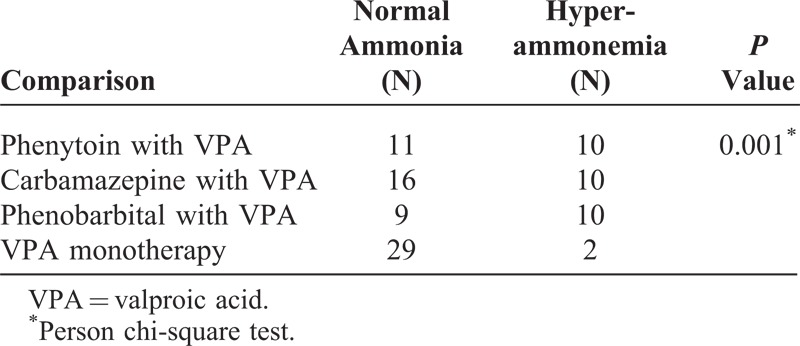
The regimens of combined AEDs are listed in Table 2. Levetiracetam (N = 77) was most frequently used in combination therapy, followed by lamotrigine (N = 33), carbamazepine (N = 26), phenytoin (N = 21), topiramate (N = 20), phenobarbital (N = 19), and oxcarbazepine (N = 15). Among the 58 patients who received combination therapy with liver enzyme-inducing AEDs, 25 had hyperammonemia. However, the patients undergoing combination therapy with the weak enzyme inducers (topiramate and oxcarbazepine) or nonenzyme inducers (levetiracetam and lamotrigine) did not showed significantly increased plasma levels of ammonia. Table 3 shows the contribution of 3 liver enzyme-inducing AEDs (carbamazepine, phenytoin, and phenobarbital) in VPA-induced hyperammonemia. The results showed that the patients receiving combination therapy with phenytoin, carbamazepine, or phenobarbital had a higher percentage of hyperammonemia. In addition, the patients who received combination therapy with liver enzyme-inducing AEDs had significantly increased plasma ammonia levels compared with the patients who received VPA monotherapy. However, there were no significant differences between these 3 patient groups with enzyme-inducing combination therapy.
TABLE 3.
Comparison of VPA Monotherapy and VPA Combination Therapy With Liver Enzyme Inducers Between Patients With Normal Blood Ammonia Levels and Hyperammonemia
DISCUSSION
In the present study, we confirmed that hyperammonemia is often an asymptomatic adverse effect in patients with epilepsy receiving VPA therapy. Furthermore, we demonstrated the novel observation that the blood level of ammonia was significantly correlated with the dosage of VPA and the plasma concentration of VPA. An increase of 1 mg in the dosage of VPA increased the risk of hyperammonemia by 0.1%. In addition, combination treatment with liver enzyme-inducing AEDs and antipsychotic drugs increased the risk of hyperammonemia.
In this article, the patients who had hyperammonemia related to any etiology except for VPA therapy were excluded. Therefore, no patient had preexisting hyperammonemia before VPA therapy. In addition, the dose of VPA was not adjusted according to the blood ammonia level before treatment. In the patients with hyperammonemia, reduced or discontinued VPA led to the blood ammonia level returning to a normal range, indicating the causality of hyperammonemia with VPA therapy in patients with epilepsy.
The reported prevalence of hyperammonemia under VPA therapy is highly variable, ranging from 2% to 80%.3–6,10–12,18 In the current study, the frequency of hyperammonemia associated with VPA therapy was 27.8%. Among these patients, 5.1% had severe hyperammonemia (>150 µg/dL). The incidence of hyperammonemia has been reported to be 0%–56% in patients receiving VPA monotherapy,3–6,10–12,18,19 and 2%–80% in patients with combination therapy.3–6,10–12,18,19 The high variability in incidence may be related to the different definitions of cut-off values for hyperammonemia in these studies. Furthermore, these studies were mostly conducted on pediatric patients. In the current study, hyperammonemia was found in 6.4% of the patients with VPA monotherapy and in 33.1% of the patients with combination therapy. Although a few studies20–22 have reported that some patients with advanced hyperammonemia developed encephalopathy and status epileptics, most of our patients with hyperammonemia were asymptomatic. Moreover, the patients with symptomatic hyperammonemia usually had a benign course and rapidly recovered after correction of blood ammonia with treatment such as lactulose therapy. In patients with severe and symptomatic hyperammonemia, it may be necessary to decrease the VPA dosage or to discontinue the drug completely.
Previous studies have identified diverse risk factors for VPA-induced hyperammonemia associated with VPA therapy, including age,18 dose of VPA,4,6,10,11 and concomitant use of drugs such as phenytoin, phenobarbital, topiramate, and risperidone.5,6,10,23,24 Whether the degree of the decrease in blood ammonia level is related to the dosage of VPA is controversial. Emerging evidence has shown that the increase in ammonia concentration induced by VPA is dose dependent. Sharma et al11 reported that a higher incidence of hyperammonemia was noted in pediatric patients receiving a high dose of VPA (40–60 mg/kg/day) compared with those receiving a low dose (20–39 mg/kg/day). Recently, 2 large-scale studies6,10 in Japan including 1 adult patient group and 1 pediatric patient group reported that the ammonia level was thought to be VPA dose dependent in both patient groups. In the present study, we also noted that hyperammonemia was significantly correlated with the dosage of VPA and the blood concentration of VPA.
The concomitant use of VPA with liver enzyme-inducing AEDs has been reported to be an important risk factor for VPA-induced hyperammonemia,5,6,10,23 particularly in combination with phenobarbital and phenytoin, and to a lesser extent with carbamazepine.6 The present study showed that the concomitant use of VPA with liver enzyme-inducing AEDs, including phenytoin, carbamazepine, and phenobarbital, also led to a higher incidence of hyperammonemia than VPA monotherapy. Among these 3 liver enzyme-inducing AEDs, phenobarbital had the strongest significant effect on VPA-induced hyperammonemia (P < 0.001), followed by phenytoin (P = 0.002), and carbamazepine (P = 0.007) compared with VPA monotherapy. However, the mechanism involved in hyperammonemia with the concomitant use of VPA with liver enzyme-inducing AEDs remains unclear. Several mechanisms have been proposed. Liver enzyme-inducing AEDs may increase the activity of cytochrome P450 enzymes, resulting in a decrease in the blood level of VPA.6,10 This interaction may lead physicians to increase the dose of VPA to maintain the therapeutic concentration, resulting in hyperammonemia.6,10 We found that the patients with hyperammonemia under combination therapy with enzyme-inducing AEDs also received significantly higher doses of VPA (P = 0.002). Another possible mechanism is that enzyme-inducing AEDs may activate the cytochrome P450 2A6, 2C9, 2C19, and 3A4 enzymes6,25 resulting in the rapid metabolism of VPA to metabolic compounds such as propionate and 4-en-VPA, which inhibit CPS-1 activity and cause an increase in the blood ammonia level. Moreover, different genetic polymorphisms may also influence the effect of enzyme-inducing AEDs on VPA-induced hyperammonemia.26
In the present study, the combination with newer generation AEDs including topiramate, oxcarbazepine, levetiracetam, and lamotrigine did not show significant effects on the increase in blood ammonia level. Topiramate is classified as a weak enzyme-inducing AED and carbonic anhydrase inhibitor.16 Combination therapy with topiramate and VPA has been reported to be associated with an increased risk of hyperammonemia.23,27,28 However, it was difficult to identify the risk of topiramate in VPA-induced hyperammonemia that may be because of the small number of patients in each group in this study, and further studies are needed to confirm this observation.
Concomitant use of VPA with antipsychotic drugs was also considered to be a risk factor for VPA-induced hyperammonemia in the presented study. VPA has been reported to have a drug interaction with risperidone, possibly through competition for protein-binding sites in the blood, and thus increases the risk of hyperammonemia.24,29,30 However, the number of patients receiving antipsychotic medications was small in this study. Further large-scale studies are necessary to analyze this risk factor.
Female gender has also been reported to be a risk factor for hyperammonemia.6,10 However, in the current study, the male patients had a higher frequency of hyperammonemia than the female patients. This may be partially related to the high percentage of male patients in the current study (66%) compared with the previous studies (45%–54%), and differences in diet between males and females.6,10
Whether abnormal liver function is related to an increase of blood ammonia level in patients under VPA therapy is controversial.23,29 In the present study, coexisting chronic hepatitis or abnormal alanine aminotransferase and alkaline phosphatase levels were not significantly associated with VPA-induced hyperammonemia. However, we noted that the patients with hyperammonemia had significantly higher γGT levels. Based on the Mann–Whitney test results, we found that the level of γGT was significantly related to concomitant use of VPA with liver enzyme-inducing AEDs (P < 0.001). While liver enzyme-inducing AEDs may increase the blood level of γGT,31,32 we, thus, suggest that an increased level of γGT may be related to the concomitant use of liver enzyme-inducing AEDs.
In conclusion, the use of VPA in adult patients with epilepsy was associated with a dose-dependent increase in blood ammonia levels. In addition, combination treatment with liver enzyme-inducing AEDs and antipsychotic drugs increased the risk of VPA-induced hyperammonemia. Although most of the patients with VPA-induced hyperammonemia were asymptomatic, some were symptomatic. If any patient taking VPA presents with symptoms such as nausea, fatigue, somnolence, ataxia, and consciousness disturbance, the blood ammonia level should be measured promptly, and if hyperammonemia is confirmed, the VPA dosage and concomitant use of enzyme-inducing AEDs should be decreased. In the patients who present with severe hepatic encephalopathy, it may be necessary to discontinue VPA altogether.
Footnotes
Abbreviations: AED = antiepileptic drug, ALK-P = alkaline phosphatase, ALT = alanine aminotransferase, CI = confidence interval, CNS = central nervous system, CPS = carbamoyl phosphate synthetase, γGT = γGTgamma glutamyl transpeptidase, ILAE = International League Against Epilepsy, IQR = interquartile range, OR = odds ratio, VPA = valproic acid.
Y-LT and C-RH contributed equally in writing of the manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Dreifuss FE, Langer DH. Side effects of valproate. Am J Med. 1988;84:34–41. [DOI] [PubMed] [Google Scholar]
- 2.Nicolai J, Carr RB. The measurement of ammonia blood levels in patients taking valproic acid: looking for problems where they do not exist? Epilepsy Behav. 2008;12:494–496. [DOI] [PubMed] [Google Scholar]
- 3.Murphy JV, Marquardt K. Asymptomatic hyperammonemia in patients receiving valproic acid. Arch Neurol. 1982;39:591–592. [DOI] [PubMed] [Google Scholar]
- 4.Haidukewych D, John G, Zielinski JJ, et al. Chronic valproic acid therapy and incidence of increases in venous plasma ammonia. Ther Drug Monit. 1985;7:290–294. [DOI] [PubMed] [Google Scholar]
- 5.Kugoh T, Yamamoto M, Hosokawa K. Blood ammonia level during valproic acid therapy. Jpn J Psychiatry Neurol. 1986;40:663–668. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto Y, Takahashi Y, Suzuki E, et al. Risk factors for hyperammonemia associated with valproic acid therapy in adult epilepsy patients. Epilepsy Res. 2012;101:202–209. [DOI] [PubMed] [Google Scholar]
- 7.Auron A, Brophy PD. Hyperammonemia in review: pathophysiology, diagnosis, and treatment. Pediatr Nephrol. 2012;27:207–222. [DOI] [PubMed] [Google Scholar]
- 8.Hung CC, Li TM, Wei IH, et al. The real mechanism of VPA-induced hyperammonemia remains unknown. Gen Hosp Psychiatry. 2011;33:84. [DOI] [PubMed] [Google Scholar]
- 9.Aires CC, van Cruchten A, Ijlst L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55:426–434. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Takahashi Y, Imai K, et al. Risk factors for hyperammonemia in pediatric patients with epilepsy. Epilepsia. 2013;54:983–989. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Gulati S, Kabra M, et al. Blood ammonia levels in epileptic children on 2 dose ranges of valproic acid monotherapy: a cross-sectional study. J Child Neurol. 2011;26:109–112. [DOI] [PubMed] [Google Scholar]
- 12.Hamed SA, Abdella MM. The risk of asymptomatic hyperammonemia in children with idiopathic epilepsy treated with valproate: relationship to blood carnitine status. Epilepsy Res. 2009;86:32–41. [DOI] [PubMed] [Google Scholar]
- 13. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. [DOI] [PubMed] [Google Scholar]
- 14.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. [DOI] [PubMed] [Google Scholar]
- 15.Chuang YC, Chuang HY, Lin TK, et al. Effects of long-term antiepileptic drug monotherapy on vascular risk factors and atherosclerosis. Epilepsia. 2012;53:120–128. [DOI] [PubMed] [Google Scholar]
- 16.Belcastro V, Striano P, Gorgone G, et al. Hyperhomocysteinemia in epileptic patients on new antiepileptic drugs. Epilepsia. 2010;51:274–279. [DOI] [PubMed] [Google Scholar]
- 17.Liu KT, Yang SC, Yeh IJ, et al. Transient hyperammonemia associated with postictal state in generalized convulsion. Kaohsiung J Med Sci. 2011;27:453–456. [DOI] [PubMed] [Google Scholar]
- 18.Altunbasak S, Baytok V, Tasouji M, et al. Asymptomatic hyperammonemia in children treated with valproic acid. J Child Neurol. 1997;12:461–463. [DOI] [PubMed] [Google Scholar]
- 19.Wyllie E, Wyllie R, Rothner AD, et al. Valproate-induced hyperammonemia in asymptomatic children. Cleve Clin Q. 1983;50:275–277. [DOI] [PubMed] [Google Scholar]
- 20.van den Broek MP, Sikma MA, Ververs TF, et al. Severe valproic acid intoxication: case study on the unbound fraction and the applicability of extracorporeal elimination. Eur J Emerg Med. 2009;16:330–332. [DOI] [PubMed] [Google Scholar]
- 21.Velioglu SK, Gazioglu S. Non-convulsive status epilepticus secondary to valproic acid-induced hyperammonemic encephalopathy. Acta Neurol Scand. 2007;116:128–132. [DOI] [PubMed] [Google Scholar]
- 22.Kifune A, Kubota F, Shibata N, et al. Valproic acid-induced hyperammonemic encephalopathy with triphasic waves. Epilepsia. 2000;41:909–912. [DOI] [PubMed] [Google Scholar]
- 23.Hamer HM, Knake S, Schomburg U, Rosenow F. Valproate-induced hyperammonemic encephalopathy in the presence of topiramate. Neurology. 2000;54:230–232. [DOI] [PubMed] [Google Scholar]
- 24.Carlson T, Reynolds CA, Caplan R. Case report: valproic acid and risperidone treatment leading to development of hyperammonemia and mania. J Am Acad Child Adolesc Psychiatry. 2007;46:356–361. [DOI] [PubMed] [Google Scholar]
- 25.Patsalos PN, Froscher W, Pisani F, van Rijn CM. The importance of drug interactions in epilepsy therapy. Epilepsia. 2002;43:365–385. [DOI] [PubMed] [Google Scholar]
- 26.Yagi M, Nakamura T, Okizuka Y, et al. Effect of CPS14217C>A genotype on valproic-acid-induced hyperammonemia. Pediatr Int. 2010;52:744–748. [DOI] [PubMed] [Google Scholar]
- 27.Knudsen JF, Sokol GH, Flowers CM. Adjunctive topiramate enhances the risk of hypothermia associated with valproic acid therapy. J Clin Pharm Ther. 2008;33:513–519. [DOI] [PubMed] [Google Scholar]
- 28.Longin E, Teich M, Koelfen W, Konig S. Topiramate enhances the risk of valproate-associated side effects in three children. Epilepsia. 2002;43:451–454. [DOI] [PubMed] [Google Scholar]
- 29.Carr RB, Shrewsbury K. Hyperammonemia due to valproic acid in the psychiatric setting. Am J Psychiatry. 2007;164:1020–1027. [DOI] [PubMed] [Google Scholar]
- 30.Holroyd S, Overdyke JT. Hyperammonemia associated with valproic acid use in elderly psychiatric patients. J Neuropsychiatry Clin Neurosci. 2012;24:372–374. [DOI] [PubMed] [Google Scholar]
- 31.Lippi G, Montagnana M, Salvagno GL, Guidi GC. Influence of stable, long-term treatment with phenobarbital on the activity of serum alanine aminotransferase and gamma-glutamyltransferase. Br J Biomed Sci. 2008;65:132–135. [DOI] [PubMed] [Google Scholar]
- 32.Aldenhovel HG. The influence of long-term anticonvulsant therapy with diphenylhydantoin and carbamazepine on serum gamma-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase. Eur Arch Psychiatry Neurol Sci. 1988;237:312–316. [DOI] [PubMed] [Google Scholar]


