Abstract
Biliary strictures and bile leaks account for the majority of biliary complications after living donor liver transplantation (LDLT). The aim of this study was to examine differences in biliary complications after adult LDLTs were performed by an experienced senior surgeon and an inexperienced junior surgeon. Surgeries included bile duct reconstruction after adult LDLT using a right liver graft, and risk factors for biliary stricture were identified.
We retrospectively reviewed the medical records of 136 patients who underwent LDLT in order to identify patients who developed biliary complications.
The senior surgeon performed 102 surgeries and the junior surgeon performed 34 surgeries. The proportion of patients with biliary stricture was similar between the senior and the junior surgeons (27.5% vs 26.5%; P = 0.911). However, the incidence of biliary leakage was higher in patients of the junior surgeon than in those of the senior surgeon (23.5% vs 2.9%; P = 0.001). The frequency of percutaneous drainage was also higher for the junior surgeon than the senior surgeon because of the junior surgeon’s high leakage rate of the drainage. When the junior surgeon performed bile duct anastomosis, biliary leakage occurred in 7 patients between the 11th and 20th cases. However, biliary leakage occurred in only 1 case thereafter.
Bile duct reconstruction performed by beginner surgeons in LDLT using right lobe grafts should be cautiously monitored and observed by a senior surgeon until an inexperienced junior surgeon has performed at least 20 cases, because of the high incidence of biliary leakage related to surgeon’s inexperience in bile duct reconstructions in LDLT.
INTRODUCTION
Bile duct reconstruction in liver transplantation involves duct-to-duct (DD) anastomosis. The benefits of DD reconstruction include the preservation of physiological bilioenteric continuity and the sphincter of Oddi, less frequent colonization of the biliary tract, shorter operative time, fewer anastomoses,1 and the availability of endoscopic treatment as a salvage option if biliary complications develop.2 Roux-en-Y bilioenterostomy is currently used only under specific conditions, such as a gross disparity between the sizes of the ducts and diseased or unavailable ducts.
Living donor liver transplantation (LDLT) offers several advantages over deceased donor liver transplantation (DDLT): a shorter wait time, a shorter cold ischemic time (CIT), lower morbidity and mortality rates, lower overall costs with elective transplantation, good graft viability due to the absence of primary nonfunction, and theoretical immunological advantages.1,3 Despite these logistical and immunological advantages, biliary complications occur more frequently after LDLT versus DDLT because LDLT involves smaller caliber biliary and vascular structures.4 Biliary complications after LDLT include strictures (anastomotic and nonanastomotic), leaks (from anastomoses, cut surfaces, T-tube exit sites, and sinus tracts), choledocholithiasis, cholangitis, and more. Biliary strictures and bile leaks account for the majority of biliary complications after LDLT. Post-transplant biliary complications occur in 9%–40% of living donor liver transplant recipients, but the reported incidence of bile duct complications considerably differs between centers.5–9
Bile duct complications delay patient recovery from a transplant, reduce their quality of life, and may reduce the function and long-term survival of the allograft. This might reflect the physiological and technical nuances associated with partial liver grafts. Multiple tiny bile ducts, which often accompany partial liver grafts, and differential blood supplies to these ducts pose special challenges for LDLT programs.10,11
The aim of the present study was to examine differences in biliary complications after adult LDLT between experienced and inexperienced surgeons in bile duct reconstruction using right lobe graft, and to identify the risk factors for biliary stricture.
MATERIALS AND METHODS
Patients
Between January 2011 and December 2012, 162 adult patients underwent LDLT at Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. Medical records and the transplant database were collected from electronic medical records and retrospectively reviewed to identify patients who had developed biliary complications. Young patients (<18 years), patients with insufficient data, patients who died within 3 months of LDLT, and those who received left liver graft were excluded from the study. The population for this study included 136 patients who underwent primary liver transplantation at our institution and had at least 1 year of follow-up. The study was approved by the institutional review board of our center.
Preparation of the Liver Graft and Recipient Bile Duct
During donor surgeries, the portal veins and hepatic arteries were isolated without excessive periductal bile dissection. Preoperative magnetic resonance cholangiopancreatography showed the anatomy of bile ducts in the liver grafts. After the right bile duct was partially opened, probing of the bile duct directly through the opening site was routinely employed to accurately identify the location of the bile duct. This procedure minimizes the number of graft duct openings obtained from various donor bile duct anatomies. The liver parenchyma over the right hepatic ducts was fully preserved to prevent denudation. The recipients’ proximal bile duct was cut at the level of the hilar plate to create multiple bile duct openings.12 The recipients’ bile ducts were also dissected in a manner aimed to minimize damage, thus allowing most of the peritoneal coverage over the bile duct to be fully preserved.
Biliary Reconstruction Methods
Either DD anastomosis or hepaticojejunostomy (HJ) was selected for each patient according to which procedure would best allow reciprocal matching between the size of the graft and the number of recipient duct openings. DD was preferentially performed on single and snout graft ducts if a suitable recipient duct opening was available. When performing DD, continuous sutures using 6-0 absorbable or nonabsorbable monofilament were applied to the posterior and anterior anastomosis or continuous sutures using 6-0 absorbable or nonabsorbable monofilament were used in posterior anastomosis and interrupted sutures of 6-0 absorbable or nonabsorbable monofilament with 1-mm intervals were applied to the anterior anastomosis lines. A small internal stent was usually inserted in cases of small graft ducts (<2 mm) or an I-tube was inserted through the cystic duct for the purpose of decompression via bile drainage in the common bile duct. Anastomosis techniques for HJ were similar to those for DD. Intraoperative leak testing was performed using methylene blue solution through the cystic duct stump in patients with an I-tube. The experienced senior surgeon had performed bile duct reconstruction at least 600 times over a 7-year period by the end of the study. However, the junior surgeon had only recently begun bile duct reconstruction.
Management of Biliary Stricture
Liver dynamic computed tomography was routinely performed 2 weeks after LDLT, 2 or 3 times over the following year, and then once or twice per year after that. Diisopropyl iminodiacetic acid (DISIDA) scintigraphy, using technetium 99-m DISIDA, was also routinely performed in patients with elevated liver function tests. Endoscopic retrograde cholangiography (ERCP) was performed first if a DD recipient was suspected of having a biliary stricture during the postoperative period. Percutaneous transhepatic biliary drainage (PTBD) was employed if patients with DD or HJ were not evaluated through ERCP. Stenoses were treated using sequential balloon dilatation, which was repeated every 3 months until complete resolution of the stricture. Detailed procedures for ERCP and PTBD are described elsewhere.2
Definition of Biliary Complications
Biliary stricture was defined as a segmental narrowing around the biliary anastomosis or splintage tube insertion demonstrated by ERCP, PTBD, or direct intraoperative confirmation. Biliary leakage was diagnosed on the basis of a bile leak through abdominal drains or a significant intra-abdominal collection of bile requiring ultrasound or radiological guided puncture. Alternatively, leakage was proven by ERCP or x-ray cholangiography via splinting tubes. Bile leakage at the cut liver surface was excluded using DISIDA.
Statistical Analysis
The continuous values are expressed as the median and range. Statistical analysis was performed using SPSS 21.0 (Chicago, IL) for Windows. Categorical variables were analyzed by χ2 test or Fisher’s exact test, as appropriate. Continuous variables were analyzed by the Mann–Whitney test, as appropriate. A P value of <0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
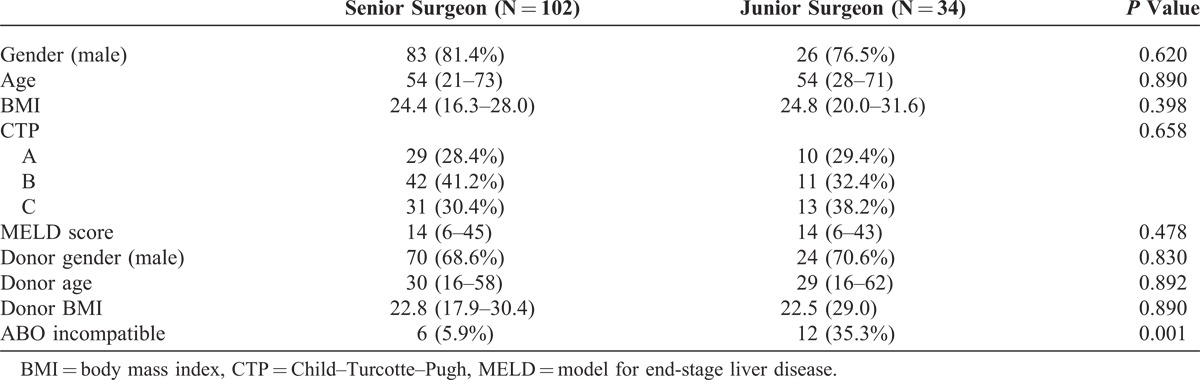
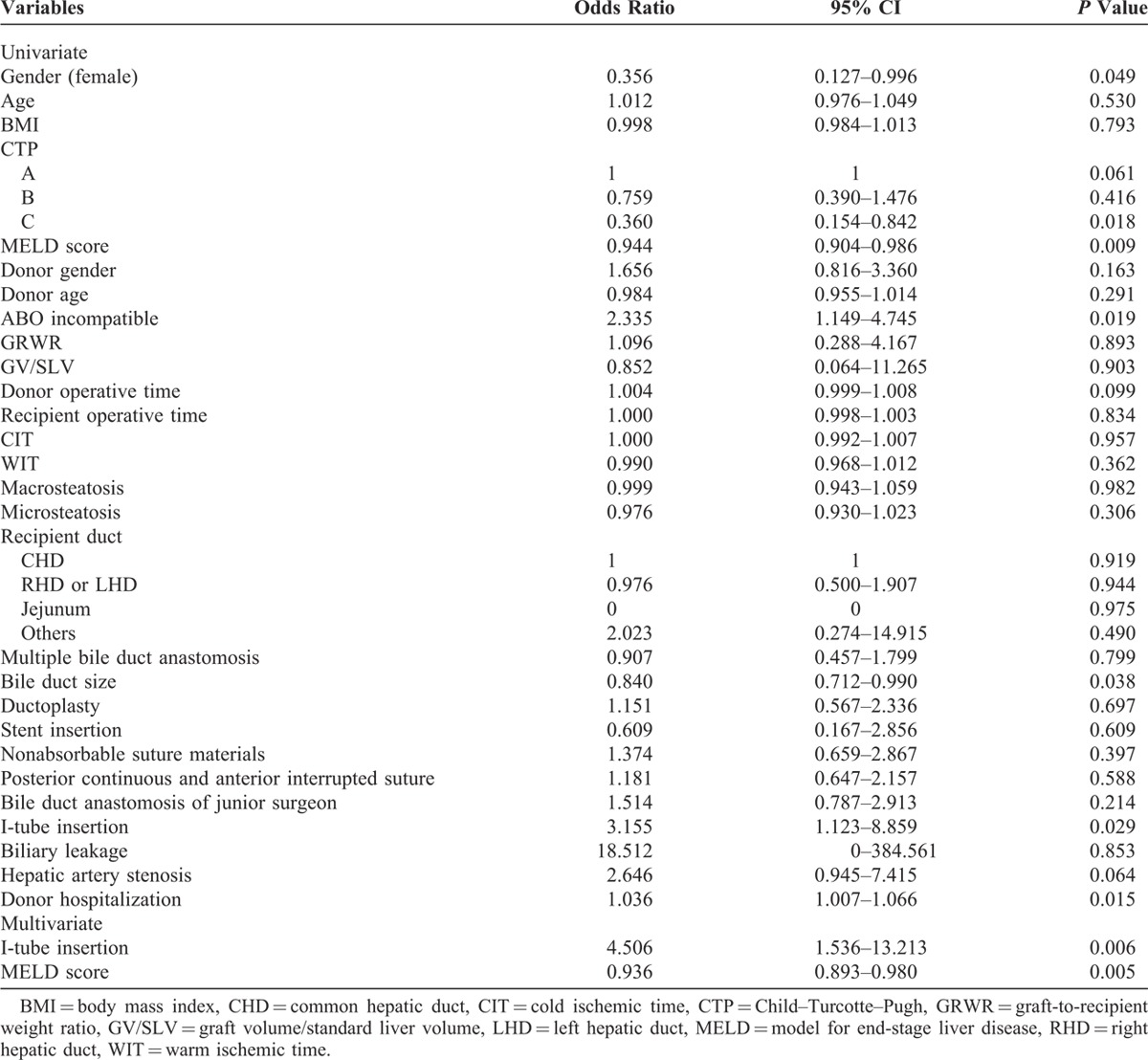
The senior surgeon performed 102 cases and the junior surgeon performed 34 cases within our study population. Gender, age, body mass index, Child–Pugh class, and model for end-stage liver disease (MELD) score did not significantly differ between the junior and the senior surgeon’s cases, but the proportion of ABO-incompatible LDLT was higher in patients of the junior surgeon (35.3% vs 5.9%; P < 0.001) (Table 1).
TABLE 1.
Baseline Characteristics Prior to Living Donor Liver Transplantation
Perioperative Characteristics
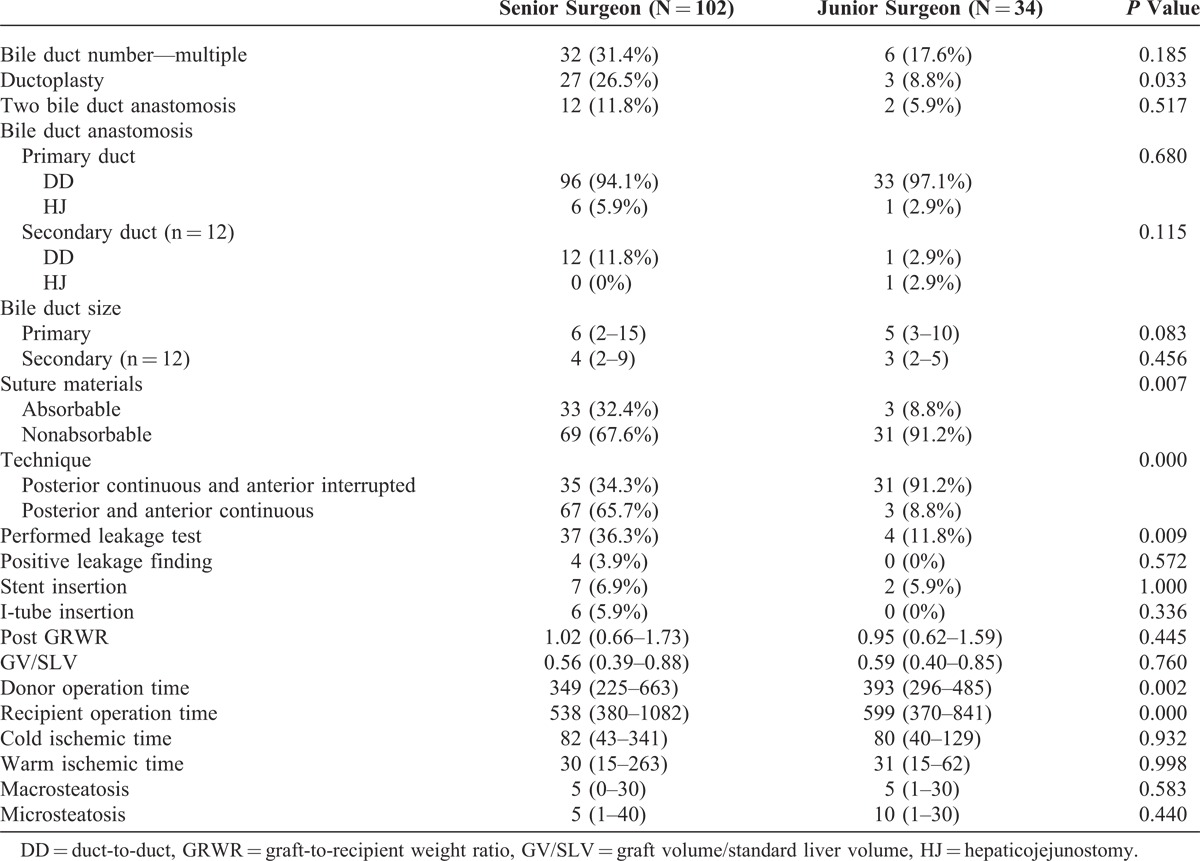
The proportion of patients with multiple bile ducts was higher for the senior surgeon than the junior surgeon, but there was no statistically significant difference between the 2 groups. The presence of ductoplasty was therefore more common in the senior surgeon’s cases because of the number of patients with multiple bile ducts. There were no statistically significant differences in suture materials or suture technique between the senior and the junior surgeons. Stent insertion, I-tube insertion, graft-to-recipient weight ratio, graft volume/standard liver volume, warm ischemic time (WIT), CIT, macrosteatosis, and microsteatosis did not significantly differ. However, both donor and recipient operative times were longer in surgeries performed by the junior surgeon than by the senior surgeon (Table 2).
TABLE 2.
Perioperative Characteristics
Outcomes of Biliary Reconstruction
Initial biliary complications are summarized in Table 3. The proportion of patients with biliary stricture were similar between the senior and the junior surgeons (27.5% vs 26.5%; P = 0.911). However, the incidence of biliary leakage was more common in the cases of the junior surgeon than the senior surgeon (23.5% vs 2.9%; P = 0.001). Thus, the number of percutaneous drainage cases was higher for the junior surgeon than the senior surgeon. In bile duct anastomosis performed by the junior surgeon, biliary leakage occurred in 7 patients between the 11th and 20th cases. However, biliary leakage occurred in only 1 case thereafter (Figure 1).
TABLE 3.
Biliary Complications after Living Donor Liver Transplantation
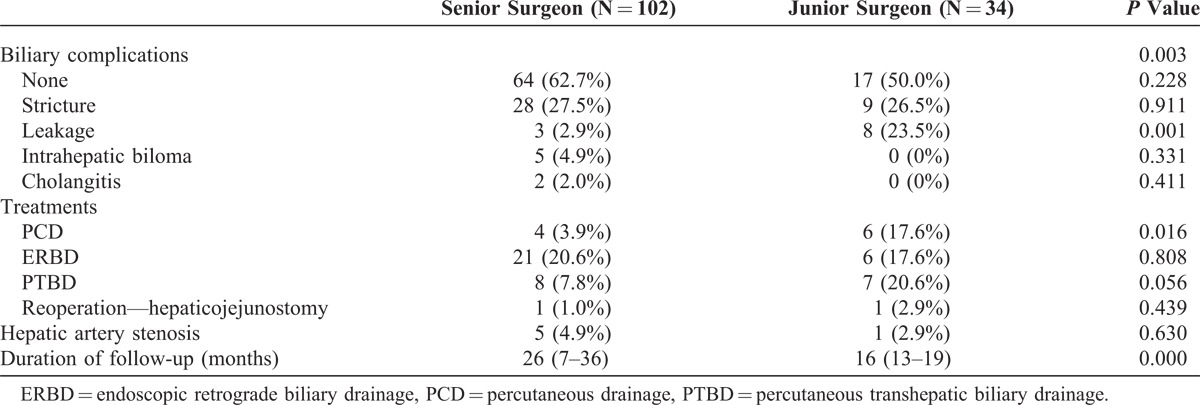
FIGURE 1.
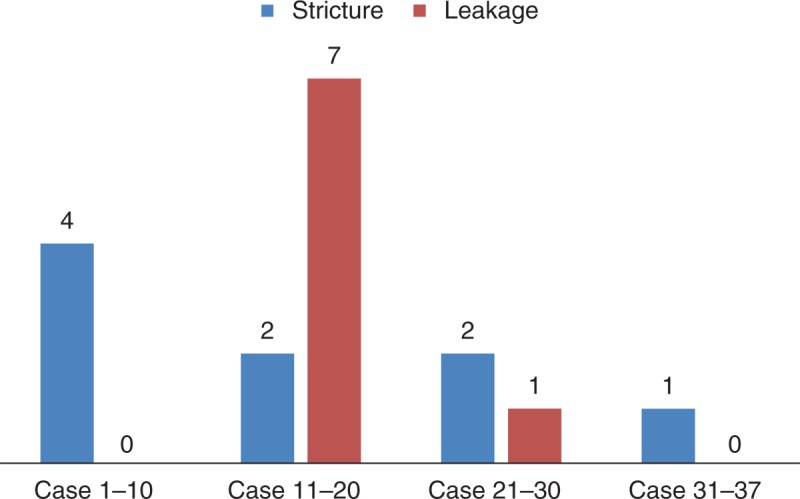
Biliary complications in LDLT performed by the junior surgeon using a right liver graft. LDLT = living donor liver transplantation.
Risk Factors for Biliary Stricture
During post-transplantation follow-up, the incidence of biliary stenosis in cases performed by the senior and the junior surgeon was 29.4% (N = 30) and 38.2% (N = 13), respectively. There was no statistically significant difference between the 2 groups (P = 0.396). The 1 and 2-year biliary stricture-free rates were 75.7% and 67.5%, respectively. Univariate analysis indicated that risk factors closely associated with biliary stricture included female gender, decreased MELD score, ABO incompatibility, decreased bile duct size, I-tube insertion through the cystic duct, and increased donor hospitalization. Multivariate analysis revealed that I-tube insertion and decreased MELD score were predisposing factors for biliary stricture (Table 4).
TABLE 4.
Risk Factors for Biliary Stricture
DISCUSSION
The development of biliary complications after LDLT is correlated with biliary anatomy, and has been significantly influenced by technical aspects such as reconstruction design and type of anastomosis. However, the rate of bile duct complications during the learning curve of inexperienced surgeons has not yet been investigated. The present study demonstrated that the biliary leakage rate was higher than for an inexperienced surgeon than for an experienced surgeon (23.5% vs 2.9%; P = 0.001), but the incidence of biliary stricture in cases performed by the junior surgeon was similar to that of the senior surgeon (26.5% vs 27.5%; P = 0.911). Biliary stricture is the most common complication of adult LDLT. Biliary stricture-free survival rates at 1 and 2 years post-transplant were 75.7% and 67.5%, respectively.
The role of simulation in surgical training has grown dramatically. Recently, some studies have reported the benefits of simulation-based training in terms of technical and nontechnical skills.13 The combination of simulation and teaching has the potential to impove patient care and safety. However, the incidence of bile duct anastomosis in LDLT is rare. The exploration of a simulation program is not cost effective. Rather, junior surgeons can acquire the technical skills needed for bile duct anastomosis through training in animal models such as pigs.
The majority of biliary leakages can be conservatively managed with indwelling abdominal drains, splinting bile drains, or percutaneous radiology guided drainage.14,15 In the present study, biliary leakage was also controlled with percutaneous biliary drainage, endoscopic, and/or transhepatic biliary drainage. However, major problems associated with mortality (eg, sepsis, hemorrhage, and pseudoaneurysms) can occur even after these active treatments. Fortunately, for patients, biliary leakage was not associated with mortality in our study. The initial 20 cases performed by the junior surgeon showed high leakage rates compared with those of the senior surgeon, and bile duct anastomosis in LDLT was cautiously employed until the initial 20 cases had been completed. Additionally, the senior surgeon checked for biliary leakage after bile duct reconstruction, and therefore the biliary leakage rate in the senior surgeon was low compared with that of the junior surgeon.
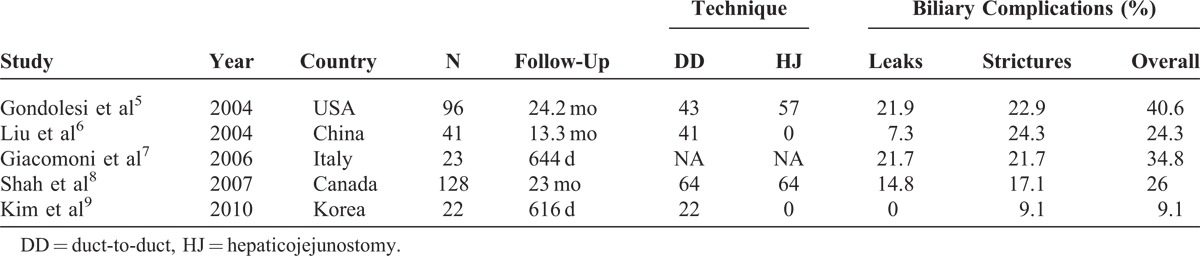
Anastomotic biliary stricture was the most common complication associated with liver transplant (Table 5). The incidence of biliary stricture is higher in LDLT than in DDLT,4,16 which might be explained by devascularization of the bile duct at the hilar dissection of the graft and the technical challenges of biliary reconstruction (eg, multiple-duct orifice and small ducts). All strictured bile duct cases in our study were successfully treated with endoscopic retrograde biliary drainage or PTBD.
TABLE 5.
Biliary Complications in LDLT With a Right Liver Graft Recipients
Multiple bile ducts in right liver grafts added to the challenge of bile duct reconstruction. Reconstruction of double graft ducts can be achieved using several approaches, such as unification ductoplasty, double DD, double HJ, or a combination of these.5,17,18 Where the 2 ducts are adjacent to each other, unification ductoplasty with or without septotomy may be applicable. Although this approach can facilitate the feasibility of a single anastomosis, such artificial manipulation also increases the risk of bile duct stump ischemia.17 Our study showed that multiple bile duct anastomosis and ductoplasty were not predisposing factors for biliary stricture. Analysis, according to the number of biliary reconstructions, did not show any group to be more vulnerable to biliary stricture.
Among the preoperative recipient factors, advanced recipient age and severely impaired liver function seemed to be associated with an increased risk of developing biliary stricture.6,19 Also, ischemia-reperfusion injury with prolonged WIT/CIT and differences in preservation methods/solutions have been previously reported to be risk factors,20,21 but these factors were not associated with biliary stricture in our study. However, a low MELD score was a predisposing factor for biliary stricture. Hepatic artery thrombosis22 and ABO incompatibility23 have been reported to be possible risk factors for biliary complications. Our study revealed a trend between these complications and biliary stricture, but a statistically significant difference was not reached in our study, possibly because events such as ABO incompatibility and hepatic artery thrombosis are infrequent. Other studies have reported bile leakage and multiple bile ducts as predisposing factors for biliary stricture,24 but our study did not show an association between these factors.
The method and materials used for suturing are significantly associated with the incidence of biliary complications. A higher incidence of biliary stricture (43.1%) was reported after biliary reconstruction was performed using 6-0 prolene when either interrupted suture or posterior continuous and anterior interrupted suture. When using continuous suture with 7-0 prolene, the incidence of biliary stricture significantly decreased to 4.7%, but the incidence of bile leakage rose to 23.1%.25 Recently, tailored telescopic reconstruction using the inner layer of the bile duct epithelium in DD anastomosis was found to lower biliary complication rates compared with the conventional method using the whole layer (9.1% vs 43.5%).9 The present study showed that suture method and the use of nonabsorbable suture materials were not associated with biliary stricture. Stenting at the anastomotic site was used to prevent accidental catching of the posterior wall and post-transplant anastomotic stricture in grafts with small ducts.17,19 The rationale of the I-tube includes protecting the anastomosis from leakage by lowering biliary pressure in the distal bile duct, easy access to the biliary tree, monitoring the quality of output bile, and allowing the cholangiographic assessment of biliary anatomy.
This study has several limitations. First, this was a retrospective study, and randomization and matching were not used. Second, results from our study were not generalized. This study reported on the effect of the learning curve in surgeons who are beginners at bile duct reconstruction in LDLT using a right liver graft and showed that biliary stricture was not associated with suture method and materials.
In conclusion, bile duct reconstruction performed by beginner surgeons in LDLT using a right liver graft should be cautiously monitored and observed by experienced senior surgeons until at least 20 cases have been performed due to the high incidence of biliary leakage after LDLT related to surgeon inexperience. However, it is important to note that there was no statistical difference in biliary stricture between the 2 groups. I-tube insertion through cystic duct and low MELD score were predisposing factors for biliary stricture, but surgeon experience level, suture method, and suture materials were not.
Footnotes
Abbreviations: DD = duct-to-duct, DDLT = deceased donor liver transplantation, DISIDA = diisopropyl iminodiacetic acid, ERCP = endoscopic retrograde cholangiography, GRWR = graft-to-recipient weight ratio, GV/SLV = graft volume/standard liver volume, HJ = hepaticojejunostomy, LDLT = living donor liver transplantation, MELD = model for end-stage liver disease, PCD = percutaneous drainage, PTBD = percutaneous transhepatic biliary drainage.
JMK and WC equally contributed to this manuscript as co-first authors.
Writing assistance was provided in the production of this manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Wang SF, Huang ZY, Chen XP. Biliary complications after living donor liver transplantation. Liver Transpl. 2011;17:1127–1136. [DOI] [PubMed] [Google Scholar]
- 2.Lee YY, Gwak GY, Lee KH, et al. Predictors of the feasibility of primary endoscopic management of biliary strictures after adult living donor liver transplantation. Liver Transpl. 2011;17:1467–1473. [DOI] [PubMed] [Google Scholar]
- 3.Maluf DG, Stravitz RT, Cotterell AH, et al. Adult living donor versus deceased donor liver transplantation: a 6-year single center experience. Am J Transplant. 2005;5:149–156. [DOI] [PubMed] [Google Scholar]
- 4.Freise CE, Gillespie BW, Koffron AJ, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant. 2008;8:2569–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gondolesi GE, Varotti G, Florman SS, et al. Biliary complications in 96 consecutive right lobe living donor transplant recipients. Transplantation. 2004;77:1842–1848. [DOI] [PubMed] [Google Scholar]
- 6.Liu CL, Lo CM, Chan SC, Fan ST. Safety of duct-to-duct biliary reconstruction in right-lobe live-donor liver transplantation without biliary drainage. Transplantation. 2004;77:726–732. [DOI] [PubMed] [Google Scholar]
- 7.Giacomoni A, Lauterio A, Slim AO, et al. Biliary complications after living donor adult liver transplantation. Transpl Int. 2006;19:466–473. [DOI] [PubMed] [Google Scholar]
- 8.Shah SA, Grant DR, McGilvray ID, et al. Biliary strictures in 130 consecutive right lobe living donor liver transplant recipients: results of a Western center. Am J Transplant. 2007;7:161–167. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Lee KW, Kim YK, et al. Tailored telescopic reconstruction of the bile duct in living donor liver transplantation. Liver Transpl. 2010;16:1069–1074. [DOI] [PubMed] [Google Scholar]
- 10.Ohkubo M, Nagino M, Kamiya J, et al. Surgical anatomy of the bile ducts at the hepatic hilum as applied to living donor liver transplantation. Ann Surg. 2004;239:82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunji H, Cho A, Tohma T, et al. The blood supply of the hilar bile duct and its relationship to the communicating arcade located between the right and left hepatic arteries. Am J Surg. 2006;192:276–280. [DOI] [PubMed] [Google Scholar]
- 12.Lee KW, Joh JW, Kim SJ, et al. High hilar dissection: new technique to reduce biliary complication in living donor liver transplantation. Liver Transpl. 2004;10:1158–1162. [DOI] [PubMed] [Google Scholar]
- 13.Seymour NE. VR to OR: a review of the evidence that virtual reality simulation improves operating room performance. World J Surg. 2008;32:182–188. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759–769. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Ko GY, Sung KB, et al. Bile leak following living donor liver transplantation: clinical efficacy of percutaneous transhepatic treatment. Liver Transpl. 2008;14:1142–1149. [DOI] [PubMed] [Google Scholar]
- 16.Gomez CM, Dumonceau JM, Marcolongo M, et al. Endoscopic management of biliary complications after adult living-donor versus deceased-donor liver transplantation. Transplantation. 2009;88:1280–1285. [DOI] [PubMed] [Google Scholar]
- 17.Fan ST, Lo CM, Liu CL, et al. Biliary reconstruction and complications of right lobe live donor liver transplantation. Ann Surg. 2002;236:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazumi S, Chiba T. Biliary complications after a right-lobe living donor liver transplantation. J Gastroenterol. 2005;40:861–865. [DOI] [PubMed] [Google Scholar]
- 19.Qian YB, Liu CL, Lo CM, et al. Risk factors for biliary complications after liver transplantation. Arch Surg. 2004;139:1101–1105. [DOI] [PubMed] [Google Scholar]
- 20.Welling TH, Heidt DG, Englesbe MJ, et al. Biliary complications following liver transplantation in the model for end-stage liver disease era: effect of donor, recipient, and technical factors. Liver Transpl. 2008;14:73–80. [DOI] [PubMed] [Google Scholar]
- 21.Park JB, Kwon CH, Choi GS, et al. Prolonged cold ischemic time is a risk factor for biliary strictures in duct-to-duct biliary reconstruction in living donor liver transplantation. Transplantation. 2008;86:1536–1542. [DOI] [PubMed] [Google Scholar]
- 22.Seo JK, Ryu JK, Lee SH, et al. Endoscopic treatment for biliary stricture after adult living donor liver transplantation. Liver Transpl. 2009;15:369–380. [DOI] [PubMed] [Google Scholar]
- 23.Lo CM, Shaked A, Busuttil RW. Risk factors for liver transplantation across the ABO barrier. Transplantation. 1994;58:543–547. [DOI] [PubMed] [Google Scholar]
- 24.Marubashi S, Dono K, Nagano H, et al. Biliary reconstruction in living donor liver transplantation: technical invention and risk factor analysis for anastomotic stricture. Transplantation. 2009;88:1123–1130. [DOI] [PubMed] [Google Scholar]
- 25.Kim BW, Bae BK, Lee JM, et al. Duct-to-duct biliary reconstructions and complications in 100 living donor liver transplantations. Transplant Proc. 2009;41:1749–1755. [DOI] [PubMed] [Google Scholar]


