Abstract
Infections by the protozoan enteroparasites Giardia duodenalis and Cryptosporidium spp are a major cause of morbidity in children attending day care facilities in developed countries. In this cross-sectional study, we aimed to estimate the occurrence and genotype frequencies of these pathogens in children attending day care centers in Majadahonda, Central Spain. To do so, single stool samples were obtained from 90 children and tested for the presence of G duodenalis and Cryptosporidium spp by conventional microscopy and immunochromatography. Positive results by these techniques were subsequently confirmed by immunofluorescence microscopy. G duodenalis-positive samples were subjected to molecular characterization studies by multilocus sequence-based genotyping of the glutamate dehydrogenase and β-giardin genes of the parasite. G duodenalis assemblages were confirmed by restriction fragment length polymorphism analyses and sequencing. A socioepidemiological questionnaire was used to identify variables potentially associated with giardiasis/cryptosporidiosis in the population of children under investigation. Overall, G duodenalis and Cryptosporidium spp were detected in 15.5% and 3.3% of stool samples, respectively. Giardiasis and cryptosporidiosis were found in 3/3 and 2/3 day care centers, respectively, affecting mainly infants aged 13 to 24 months. A total of 8 G duodenalis isolates were confirmed as subassemblage BIV, all of them belonging to asymptomatic children. Attempts to genotype Cryptosporidium isolates failed. None of the variables considered could be associated with higher risk of infection with giardiasis or cryptosporidiosis. These results clearly indicate that asymptomatic infections with G duodenalis and Cryptosporidium spp are frequent in <3-year-old children in Central Spain.
INTRODUCTION
The most common enteric protozoan pathogens affecting humans are Giardia duodenalis and Cryptosporidium species, which are major contributors to the burden of morbidity in the developed world.1 Direct person-to-person transmission of giardiasis and cryptosporidiosis is typically associated with poor fecal–oral sanitation and hygiene, although waterborne and foodborne transmission is also well documented worldwide.2,3 Additionally, international travelers returning from endemic areas and asymptomatic carriers may play an important role in the spreading of these infections.4
Although a significant percentage of cases of G duodenalis and Cryptosporidium infections may be asymptomatic, giardiasis and cryptosporidiosis typically result in diarrhea, with associated symptoms (eg, abdominal pain, nausea, vomiting, malabsorption, and weigh loss) ranging from acute to chronic.5,6 The severity of these diseases may be influenced by the parasite species/genotypes causing the infection and the age and immune status of the host. Therefore, in immunocompetent individuals, giardiasis is associated with intermittent symptomatology or even chronicity in many instances, whereas cryptosporidiosis is normally self-limiting and resolves spontaneously in 2 to 3 weeks. In immunocompromised subjects, cryptosporidiosis (but rarely giardiasis) may represent a life-threatening condition.7 This situation is further complicated by the fact that there is no vaccine or chemotherapeutical agent effective to prevent or treat cryptosporidiosis. Children attending day care settings and the elderly are among the most susceptible populations.
In Spain, Giardia and Cryptosporidium infections have been previously documented in a number of human, livestock, companion animal, and wild animal populations.8,9 However, reliable epidemiological information is restricted to certain geographical areas, whereas only incomplete or outdated information is currently available from most parts of the country. Molecular data regarding the species/genotypes circulating in Spain are even scarcer. Because of our limited knowledge on the frequency of giardiasis and cryptosporidiosis in populations of Spanish preschool children, the main goals of this study were to estimate the prevalence of Giardia and Cryptosporidium in children attending day care centers in Central Spain, molecularly characterize the parasites’ isolates obtained, and identify factors potentially associated with a higher risk of infection by these protozoan species.
MATERIALS AND METHODS
Area and Design of Study
The municipality of Majadahonda (Northwest of Madrid, Central Spain) has 70,198 inhabitants and extends >38.5 km2. Based on the 2012 census, there were 4664 (6.6%) children aged 0 to 4 years in its urban area. Migrant population accounted for 16.2% of the total population, with the highest proportion originating from South American and North-African countries. The municipality is endowed with 4 public day care centers located in districts of medium to high socioeconomic status, offering a total of 493 children’s places. Representatives of all 4 day care centers were personally contacted and, after holding informative meetings, asked for collaboration. Permission was obtained from 3 centers; the remaining one declined to participate in the study.
Human Stool Samples and Questionnaires
A cross-sectional study was conducted in the spring of 2013 (April–June) among children (0–3 years old) attending 3 public day care centers in the municipality of Majadahonda. All children from each day care setting were invited to participate in the study. After informed consents were obtained from parents or legal guardians, recruited volunteers were provided with a prelabeled sampling kit including sterile polystyrene flasks for the recovery of stool samples, and instructions on how to take the sample safely. A standardized questionnaire covering demographic (age, sex, and day care center) data, clinical manifestations, contact with pet animals, visits to public parks or animal farms, and recent traveling abroad was also included. Collection of stool samples and epidemiological questionnaires were organized in collaboration with the day care centers at suitable times, transported to the laboratory at 4°C, and processed within 3 days after reception. No preservative solutions were added for long-term storage of stool samples.
For this study, cases were defined as children with stool samples positive for G duodenalis or Cryptosporidium by conventional microscopy and/or immunochromatography (ICT) and further confirmed by immunofluorescence microscopy. Symptoms considered compatible with giardiasis/cryptosporidiosis included diarrhea (defined as the occurrence of at least 3 watery stools within a 24-hour period), abdominal pain, constipation, nausea, and vomiting. This study has been approved by the research ethics committee of the Alfonso X El Sabio University.
Conventional Microscopy for the Detection of Giardia and/or Cryptosporidium
In order to increase microscopy sensitivity, aliquots (1 mg) of all stool samples were processed and concentrated using routine coprological procedures including the merthiolate–iodine–formaldehyde solution and the modified Telemann method.10 Examination was conducted at ×200 magnification switching to ×400 magnification when structures morphologically compatible with Giardia cysts or Cryptosporidium oocysts were suspected.
ICT Rapid Assay for the Detection of Giardia and/or Cryptosporidium
A strip of qualitative ICT commercial assay for the rapid simultaneous detection of Cryptosporidium and/or Giardia (Stick Crypto-Giardia; Operon, Zaragoza, Spain) was used in all stool samples. The tests were conducted at room temperature according to the recommended manufacturer’s instructions. Claimed diagnostic sensitivities and specificities of the test were 94% and 100% for Cryptosporidium and 100% and 95% for Giardia.
DFATs for the Detection of Giardia and/or Cryptosporidium
Stool samples of children, which tested positive or probable for Giardia and/or Cryptosporidium by conventional microscopy and/or ICT, were further assayed by direct fluorescent antibody test (DFAT) for confirmation. Briefly, 5 μL of concentrated (as described above) stool samples were placed on welled slides. Smears were air-dried, methanol fixed, and stained with fluorescein-labeled mouse monoclonal antibodies (Crypto/Giardia Cel; Cellabs, Sydney, Australia). Samples were examined on a Zeiss fluorescence microscopy (Carl Zeiss, Oberkochen, Germany) equipped with a MC63 camera system (Carl Zeiss, Oberkochen, Germany) at ×400 magnification. Known positive and negative controls from clinical specimens submitted to our laboratory for diagnosis were routinely included in each sample batch.
Total DNA Isolation
Total DNA was extracted from all DFAT-confirmed samples. A new, fresh aliquot (220 mg) of each stool sample was homogenized in stool lysis buffer and incubated at 95°C for 10 minutes. The DNA released from disrupted (oo)cysts was subsequently extracted using the QIAamp DNA Stool Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Purified DNA samples (200 µL) were stored at −20°C until further analysis.
Molecular Characterization of G duodenalis Assemblages
Extracted DNA from confirmed G duodenalis-positive samples were subsequently analyzed by multilocus sequence-based genotyping using 2 gene loci: glutamate dehydrogenase (GDH) and β-giardin (BG). The amplification of the GDH gene was performed by a seminested polymerase chain reaction (PCR) using the primer pairs GDHeF/GDHiF and GDHiF/GDHiF to yield a 432-bp fragment.11 The primary and secondary PCR reactions were carried out as follows: 1 step of 95°C for 3 minutes, followed by 35 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute. A final extension of 72°C for 7 minutes and a 4°C hold was used.
The amplification of the BG gene was performed using a nested PCR using the primer pairs G7_F/G759_R and G99_F/G609_R to yield a 511-bp fragment.12 The primary PCR reaction was carried out with the following amplification condition: 1 step of 95°C for 7 minutes, followed by 35 cycles of 95°C for 30 seconds, 65°C for 30 seconds, and 72°C for 1 minute. A final extension of 72°C for 7 minutes and a 4°C hold was used. Cycling parameters for the secondary PCR reaction were the same as above except that the annealing temperature was 55°C. The list of oligonucleotide primers for the detection of G duodenalis at the GDH and BG loci are detailed in Supplemental Content 1 (http://links.lww.com/MD/A41).
PCR products were resolved on 2% D5 agarose gels (Conda, Madrid, Spain) stained with Pronasafe nucleic acid staining solution (Conda, Madrid, Spain). Amplicons of the expected size for GDH or BG were subsequently purified using the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). In order to confirm G duodenalis assemblages, all purified amplicons were sequenced in both directions using the same internal primer sets as in their respective PCR assays.
PCR-RFLP of the GDH and BG
Restriction digests were carried out on gel-purified PCR products in 20 µL reactions. Ten microliters of purified PCR product was added to 1× FastDigest buffer and 0.6 µL of FastDigest NlaIV and FastDigest RsaI (for GDH), or 0.6 µL of FastDigest HindIII (for BG). All restriction enzymes were purchased from Thermo Fisher Scientific (Waltham, MA). Digestion was performed at 37°C for 4 hours or overnight. Restriction profiles were visualized on 2% agarose gels.
Molecular Characterization of Cryptosporidium Species
Identification of Cryptosporidium species/genotypes was attempted by multilocus sequence-based genotyping using 2 gene loci: the small subunit (SSU) ribosomal RNA gene of Cryptosporidium hominis and the Cryptosporidium oocyst wall protein (COWP). Two nested PCR protocols were used to amplify the SSU and COWP as described elsewhere.13,14 The list of oligonucleotide primers for the detection of Cryptosporidium spp used are shown in Supplemental Content 1 (http://links.lww.com/MD/A41).
All Giardia and Cryptosporidium PCR reactions were carried out using BIOTAQ DNA polymerase (Bioline GmbH, Luckenwalde, Germany) on a 2720 thermal cycler (Applied Biosystems, Pleasanton, CA). Appropriate positive and negative controls were routinely included in each round of PCR.
Sequence Analyses
Chromatograms and sequences were examined using the BioEdit sequence analysis program (http://www.mbio.ncsu.edu/BioEdit/page2.html). The BLAST tool (http://www.ncbi.nlm.nih.gov/blast/) was used to compare nucleotide sequences with sequences in the NCBI and GiardiaDB (http://giardiadb.org/giardiadb/) databases. Representative sequences for each G duodenalis assemblage at the GDH (GenBank accession numbers are L40509 (AI), L40510 (AII), AF069059 (BIII), L40508 (BIV), U60984 (C), U60986 (D), U47632 (E), and AF069057 (F)) and the BG (GenBank accession numbers are AY655702 (AI), AY072723 (AII), AY072724 (AIII), AY072727 (B), AY545646 (C), AY545647 (D), AY072729 (EI), AY545650 (EII), AY653159 (EIII), and AY647264 (F)) loci were used. Sequence alignments were conducted in MEGA 6 free software (http://www.megasoftware.net/).
Data Analysis
To assess associations between possible risk factors measured in the questionnaire to children and infection with either G duodenalis or Cryptosporidium spp, data were analyzed with simple tails and prevalence odds ratios, and their 95% CI were calculated. Statistical power, defined as the probability of rejecting the null hypothesis while the alternative hypothesis is true, was calculated to determine the adequacy of the sample size used. Minimum power level was set at 0.80. Descriptive statistical analyses were conducted in OpenEpi, a free software (http://openepi.com/v37/Menu/OE_Menu.htm).
RESULTS
A single stool sample was obtained from each of the 90 recruited children for a mean return rate of 20.9% (range, 19.2–25.2; SD, 3.4). Conventional microscopy, ICT, and DFAT showed matching positive results for G duodenalis in 14 (15.6%) samples, and for Cryptosporidium spp in 3 (3.3%) samples, respectively. Epidemiological questionnaires were satisfactorily completed and returned by 88 participants (response rate, 97.8%) and considered in the analyses (Table 1). The male:female ratio was 0.91. The age range was from 10 to 36 months (mean, 24.6; SD, 7.8), with 13 to 24 month children accounting for 60.2% of the total. G duodenalis and Cryptosporidium spp infections were detected in 3/3 and 2/3 day care centers (Figure 1A). Both G duodenalis and Cryptosporidium spp affected children of all age ranges, with the former presenting higher prevalence in infants of 13 to 36 months of age (Figure 1B). Children of both sexes were similarly affected by cryptosporidiosis or giardiasis (Figure 1C). Interestingly, only 3/17 (17.6%) cases infected with G duodenalis or Cryptosporidium spp presented symptoms clinically compatible with infections caused by these pathogens (Figure 1D).
TABLE 1.
Analysis of the Variables Included in the Epidemiological Questionnaire Potentially Involved in the Transmission of Giardia and/or Cryptosporidium
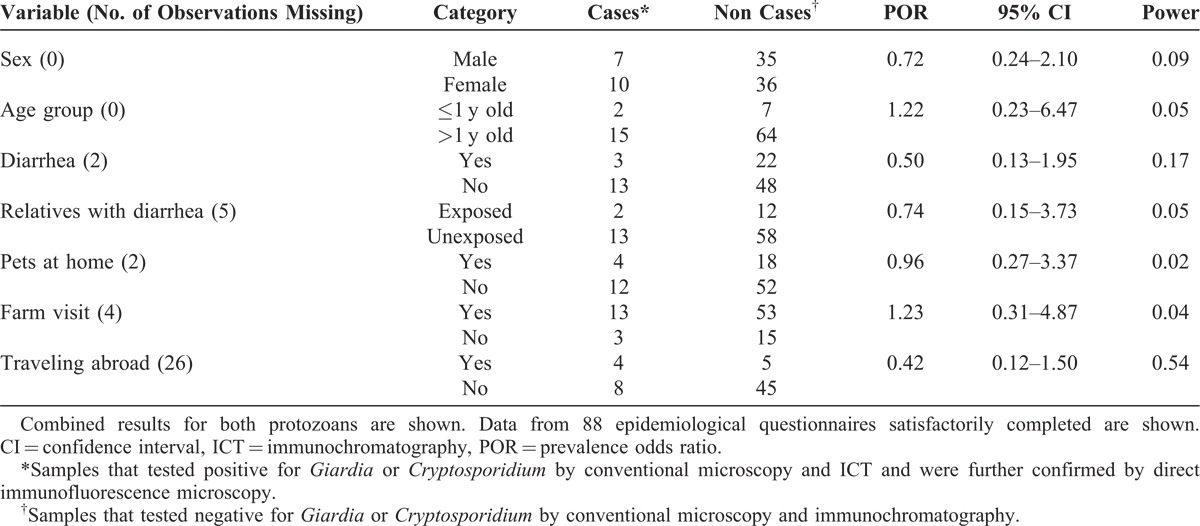
FIGURE 1.
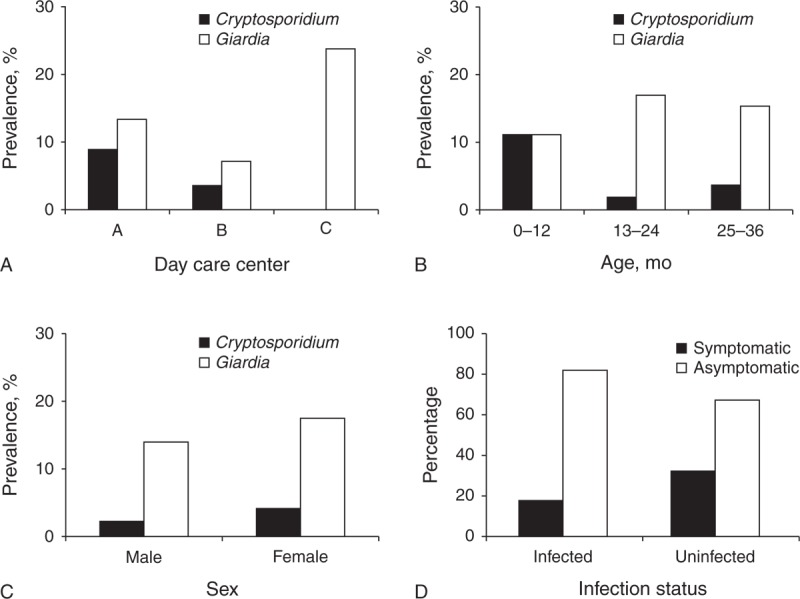
Presence of G duodenalis and Cryptosporidium spp infections in children by day care facility (A), group of age (B), sex (C), and presence/absence of symptoms compatible with cryptosporidiosis/giardiasis (D), Majadahonda, Central Spain.
All children had been visiting parks and public playgrounds regularly before the survey. Twenty two children including 3 G duodenalis cases and 1 Cryptosporidium case reported having pets (dogs, cats, turtles, canaries, and parakeets) at home. Only 9 children (5.7%) traveled to a foreign country during the past 6 months, mainly in Europe (France, Italy, Portugal, and Romania) and South America (Brazil). None of the variables considered in this study (day care center, sex, age, contact with relatives experiencing diarrheal episodes, contact with pets, farm visits, and traveling abroad) could be associated with higher prevalence odds of infection with cryptosporidiosis or giardiasis (Table 1). The low statistical power of the analyses suggests our data may not detect such associations due to small sample size and, in the case of Cryptosporidium, small number of positive results.
Out of the 14 samples positive for G duodenalis, 8 isolates were successfully amplified at the GDH locus. Digestion of purified PCR products with NlaIV resulted in identical profiles, suggestive of subassemblage BIII or BIV11 (Supplemental Content 3, panel A, http://links.lww.com/MD/A43). Failure to cleave the original PCR products with RsaI (Supplemental Content 3, panel B, http://links.lww.com/MD/A43) confirmed all 8 isolates as subassemblage BIV.11 A 366-pb fragment, equivalent to positions 103 and 468 of the subassemblage BIV reference sequence (GenBank accession number, L40508), were cleanly read from the raw sequence data for each sequence. Alignment analyses revealed 100% similarity among 6 of our isolates (isolates 143, 150, 156, 157, 160, and 167) and the reference sequence L40508. The remaining 2 sequences (isolates 145 and 158) presented 4-point mutations at positions 183 (T to C), 387 (T to C), 396 (C to T), and 423 (C to T).
The BG gene was successfully amplified in 7/8 isolates already amplified at the GDH locus. Digestion of purified PCR products with HaeIII resulted in identical profiles (Supplemental Content 3, panel C, http://links.lww.com/MD/A43) compatible with assemblage B.11 Alignment analyses of the BG sequences for these samples demonstrated that all 7 isolates (isolates 145, 150, 156, 157, 158, 160, and 167) had identical sequences, but revealed 6-point mutations at position 159 (G to A), 165 (C to T), 309 (C to T), 324 (C to T), 393 (C to T), and 471 (T to C) when compared with a 437-pb fragment equivalent to positions 137 and 573 of the corresponding assemblage B reference sequence (GenBank accession number, AY072727).
Representative sequences from each different isolate found in this study were submitted to GenBank, including isolate 158 (accession number, KJ645940) and isolate 145 (accession number, KJ645941) for GDH, and isolate 150 (accession number, KJ645942) for BG, respectively.
Unfortunately, attempts to amplify the SSU and COWP genes in all 3 samples positive for Cryptosporidium spp failed repeatedly.
DISCUSSION
Infants and toddlers are particularly susceptible to oral–fecal transmitted infectious diseases, presumably because of their immature and inexperienced immune systems, high hand-to-mouth activity, and undeveloped hygienic habits. Childcare facilities might provide adequate environments for the fast spread of enteric infections with children confined within limited spaces, particularly if appropriate sanitation and hygiene standards are not fulfilled.15 It is, therefore, not surprising that day care center attendants have increased risk of acquiring childhood diseases.16,17 Among them, both symptomatic and asymptomatic infections by G duodenalis and Cryptosporidium spp have been frequently reported in day care centers in developed countries including Germany, The Netherlands, and the United Kingdom.18–20
In Spain, previous epidemiological studies in pediatric populations have demonstrated the presence of G duodenalis and Cryptosporidium spp infections in 3% to 25% and 1% to 10% of the children analyzed, respectively.8,9,21,22 Therefore, the frequency of G duodenalis (15.6%) and Cryptosporidium spp (3.3%) found in the present study falls well within the prevalence ranges documented in previous national surveys. However, these data should be interpreted with caution due to the comparatively small sample size of this study. Additionally, it is important to take into consideration that these figures may underestimate the actual prevalence of these pathogens because of 2 potential drawbacks. First, the screening tests used in our study (conventional microscopy and ICT) may fail to detect infections at very low intensities. Second, because G duodenalis and Cryptosporidium spp shed (oo)cysts intermittently and only a single stool specimen was examined per child, it is likely that a number of infections had been missed.
Importantly, most (82.4%) of the children with giardiasis or cryptosporidiosis did not show any clinical manifestation of illness. This finding is in agreement with the data obtained in similar studies in other European countries.19,20 Because asymptomatic carriers serve as a community reservoir of disease, they are potential contributors to the spreading of the infection to healthy subjects, including adults. Indeed, child-to-adult infection transmission of G duodenalis seems plausible as housewives and nursing mothers changing nappies have been demonstrated to have a 4-fold increased risk of giardiasis.17
Although not completely elucidated, it seems clear now that the pathology and virulence of G duodenalis and Cryptosporidium spp infections are the consequence of a multifactorial process involving both host (age, immune system status, and nutritional status) and parasite (strain genotype, infectious dose, and coinfections) features.6,7,23 In recent years, a number of studies have attempted to correlate the presence of clinical manifestations (mainly diarrhea) with the genotype of the parasite causing the infection. In the case of G duodenalis, an early study carried out in The Netherlands found that G duodenalis assemblage A isolates were more prevalent in asymptomatic infected individuals, whereas assemblage B isolates were more frequently found in infected subjects with persistent diarrhea.24 Opposite results have been consistently reported in surveys carried out in Australia, Bangladesh, Portugal, Spain, and Turkey.25–29 Inconclusive data or no correlation between genotypes and symptoms were obtained in other studies performed in Brazil, India, and Iran.30–32 Molecular information from Spanish pediatric populations have shown that, in patients <5 years old, symptomatic giardiasis was present in 81.2% of assemblage AII infections but only in 34.6% of assemblage B cases.28 In another study, assemblage B was the genotype chiefly found (57.1%) in apparently healthy children of 1 to 12 years.33 In the present survey, all 8 isolates of G duodenalis from 8 different asymptomatic children were conclusively assigned to subassemblage BIV. The fact that subassemblage BIV was found circulating in the 3 day care centers under study indicates that this may be the most prevalent G duodenalis genotype in children in this geographical area. Although limited, our results also support the hypothesis that assemblage B isolates are predominantly found in infected subjects without clinical manifestations.
This is the first study describing G duodenalis assemblage B subtypes in Spanish human isolates. Based on sequence analyses of the GDH gene, a genetic variant (accession number, KJ645941) was identified in 2/8 isolates characterized as subassemblage BIV. This variant had 100% similarity with partial sequences reported in human isolates from Holland (accession number, AY826197), Norway (accession number, DQ923581), and Thailand (accession number, HM747963). Similarly, based on sequence analyses of the BG gene, all 7 isolates characterized were assigned to the same G duodenalis assemblage B variant (accession number, KJ645942). This variant showed 100% similarity with partial sequences reported in human isolates from Belgium (accession number, EU881698), Sweden (accession number, HM165208), Egypt (accession number, HM171691), Australia (accession number, HQ179581), and New Zealand (accession number, EU274397). Taken together, these data seem to suggest that the G duodenalis assemblage B genotypes/subtypes described in the present study have very likely a global distribution.
Regarding Cryptosporidium infections, among the 173 Cryptosporidium isolates characterized in Spanish children to date, C hominis (62.4%) was the Cryptosporidium species more frequently identified, followed by C parvum (35.3%), C meleagridis (1.7%), and C felis (0.6%).34,35 Unfortunately, in this study, amplification of Cryptosporidium-specific DNA from infected children failed and no genotyping could be carried out. The fact that very low numbers of oocysts were detected by DFAT in the 3 Cryptosporidium-positive samples suggests that very likely the amount of purified DNA was beyond the detection limit of the PCR methods used. Therefore, the Cryptosporidium species currently circulating in this pediatric population remains unknown.
In summary, our epidemiological and molecular data reveal that both G duodenalis and Cryptosporidium spp infections are frequent in children attending day care facilities in Central Spain. The finding that 3 quarter of the children infected with these pathogens did not have clinical manifestations raises important public health concerns, as asymptomatic carriers may inadvertently spread these diseases to other community members. This fact highlights the convenience of carrying out universal screening programs aiming to detect asymptomatic giardiasis and cryptosporidiosis in order to minimize the possibility of child-to-child or child-to-adult transmission. Finally, well-designed case–control studies are needed to undoubtedly demonstrate the potential correlation between a specific G duodenalis assemblage and the development of associated clinical features in infected individuals.
ACKNOWLEDGMENT
The authors thank Dr Israel Cruz (National Centre for Microbiology, Majadahonda, Spain) for assisting with sequence analyses.
Footnotes
Abbreviations: BG = BGβ-giardin, COWP = COWPCryptosporidium oocyst wall protein, GDH = GDHglutamate dehydrogenase, ICT = immunochromatography, DFAT = direct fluorescent antibody test, PCR = polymerase chain reaction, SSU = SSUsmall subunit.
This work has been funded by grants 1.010.422 from Fundación Universidad Alfonso X El Sabio-Santander and Proyecto de Investigación 10/01240 from Fondo de Investigación Sanitaria, Spanish Ministry of Economy and Competitiveness.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- 1.Fletcher SM, Stark D, Harkness J, et al. Enteric protozoa in the developed world: a public health perspective. Clin Microbiol Rev. 2012;25:420–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmena D. Waterborne transmission of Cryptosporidium and Giardia: detection, surveillance and implications for public health. In: Mendez-Vilas A, ed. Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Badajoz: Formatex; 2010:3–14. [Google Scholar]
- 3.Dawson D. Foodborne protozoan parasites. Int J Food Microbiol. 2005;103:207–227. [DOI] [PubMed] [Google Scholar]
- 4.ten Hove RJ, van Esbroeck M, Vervoort T, et al. Molecular diagnostics of intestinal parasites in returning travellers. European J Clin Microbiol Infect Dis. 2009;28:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leitch GJ, He Q. Cryptosporidiosis—an overview. J Biomed Res. 2012;25:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson LJ, Hanevik K, Escobedo AA, et al. Giardiasis—why do the symptoms sometimes never stop? Trends Parasitol. 2010;26:75–82. [DOI] [PubMed] [Google Scholar]
- 7.Bouzid M, Hunter PR, Chalmers RM, et al. Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev. 2013;26:115–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarro-i-Martinez L, del Águila C, Bornay-Llinares FJ. Cryptosporidium: a genus in revision. The situation in Spain. Enferm Infecc Microbiol Clin. 2011;29:135–143. [DOI] [PubMed] [Google Scholar]
- 9.Carmena D, Cardona GA, Sánchez-Serrano LP. Current situation of Giardia infection in Spain: implications for public health. World J Clin Infect Dis. 2012;2:1–12. [Google Scholar]
- 10.Thiepont D, Rochette F, Vanparijs F. Diagnostic De Verminose Par Examen Coprologique. Beerse: Janssen Research Foundation; 1979. [Google Scholar]
- 11.Read CM, Monis PT, Thompson RC. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect Genet Evol. 2004;4:125–130. [DOI] [PubMed] [Google Scholar]
- 12.Lalle M, Pozio E, Capelli G, et al. Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol. 2005;35:207–213. [DOI] [PubMed] [Google Scholar]
- 13.Jongwutiwes S, Tiangtip R. Molecular analysis of Cryptosporidium species isolated from HIV-infected patients in Thailand. Trop Med Int Health. 2002;7:357–364. [DOI] [PubMed] [Google Scholar]
- 14.Pedraza-Díaz S, Amar C, Nichols G, et al. Nested polymerase chain reaction for amplification of the Cryptosporidium oocyst wall protein gene. Emerg Infect Dis. 2001;7:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MB, Greig JD. A review of enteric outbreaks in child care centers: effective infection control recommendations. J Environ Health. 2008;71:24–32. [PubMed] [Google Scholar]
- 16.Lu N, Samuels ME, Shi L, et al. Child day care risks of common infectious diseases revisited. Child Care Health Dev. 2004;30:361–368. [DOI] [PubMed] [Google Scholar]
- 17.Hoque ME, Hope VT, Scragg R, et al. Nappy handling and risk of giardiasis. Lancet. 2001;357:1017–1018. [DOI] [PubMed] [Google Scholar]
- 18.Sagebiel D, Weitzel T, Stark K, et al. Giardiasis in kindergartens: prevalence study in Berlin, Germany, 2006. Parasitol Res. 2009;105:681–687. [DOI] [PubMed] [Google Scholar]
- 19.Davies AP, Campbell B, Evans MR, et al. Asymptomatic carriage of protozoan parasites in children in day care centers in the United Kingdom. Pediatr Infect Dis J. 2009;28:838–840. [DOI] [PubMed] [Google Scholar]
- 20.Enserink R, Scholts R, Bruijning-Verhagen P, et al. High detection rates of enteropathogens in asymptomatic children attending day care. PLoS One. 2014;9:e89496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artieda J, Basterrechea M, Arriola L, et al. Outbreak of cryptosporidiosis in a child day care centre in Gipuzkoa, Spain, October to December 2011. Euro Surveill. 2012;17:pii: 20070. [DOI] [PubMed] [Google Scholar]
- 22.Fuentes I, Martín C, Beristain X, et al. Cryptosporidium hominis genotypes involved in increased incidence and clusters of cases/outbreaks, Navarra, Spain, 2012. Epidemiol Infect. 2014. In press. http://dx.doi.org/10.1017/S0950268814001836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalmers RM, Davies AP. Minireview: clinical cryptosporidiosis. Exp Parasitol. 2010;124:138–146. [DOI] [PubMed] [Google Scholar]
- 24.Homan WL, Mank TG. Human giardiasis: genotype linked differences in clinical symptomatology. Int J Parasitol. 2001;31:822–826. [DOI] [PubMed] [Google Scholar]
- 25.Read C, Walters J, Robertson ID. et al. Correlation between genotype of Giardia duodenalis and diarrhoea. Int J Parasitol. 2002;32:229–231. [DOI] [PubMed] [Google Scholar]
- 26.Haque R, Mondal D, Karim A, et al. Prospective case–control study of the association between common enteric protozoal parasites and diarrhea in Bangladesh. Clin Infect Dis. 2009;48:1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeida AA, Delgado ML, Soares SC, et al. Genotype analysis of Giardia isolated from asymptomatic children in northern Portugal. J Eukaryot Microbiol. 2006;53:S177–S178. [DOI] [PubMed] [Google Scholar]
- 28.Sahagún J, Clavel A, Goñi P, et al. Correlation between the presence of symptoms and the Giardia duodenalis genotype. Eur J Clin Microbiol Infect Dis. 2008;27:81–83. [DOI] [PubMed] [Google Scholar]
- 29.Aydin AF, Besirbellioglu BA, Avci IY, et al. Classification of Giardia duodenalis parasites in Turkey into groups A and B using restriction fragment length polymorphism. Diagn Microbiol Infect Dis. 2004;50:147–151. [DOI] [PubMed] [Google Scholar]
- 30.Kohli A, Bushen OY, Pinkerton RC, et al. Giardia duodenalis assemblage, clinical presentation and markers of intestinal inflammation in Brazilian children. Trans R Soc Trop Med Hyg. 2008;102:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ajjampur SS, Sankaran P, Kannan A, et al. Giardia duodenalis assemblages associated with diarrhea in children in South India identified by PCR-RFLP. Am J Trop Med Hyg. 2009;80:16–19. [PMC free article] [PubMed] [Google Scholar]
- 32.Abdollah R, Roointan ES, Samarbafzadeh AR, et al. Investigation of possible correlation between Giardia duodenalis genotypes and clinical symptoms in Southwest of Iran. Iran J Parasitol. 2013;8:389–395. [PMC free article] [PubMed] [Google Scholar]
- 33.Cardona GA, Carabin H, Goñi P, et al. Identification and molecular characterization of Cryptosporidium and Giardia in children and cattle populations from the province of Álava, North of Spain. Sci Total Environ. 2011;412–413:101–108. [DOI] [PubMed] [Google Scholar]
- 34.Llorente MT, Clavel A, Goñi MP, et al. Genetic characterization of Cryptosporidium species from humans in Spain. Parasitol Int. 2007;56:201–205. [DOI] [PubMed] [Google Scholar]
- 35.Abreu-Acosta N, Quispe MA, Foronda-Rodríguez P, et al. Cryptosporidium in patients with diarrhoea, on Tenerife, Canary Islands, Spain. Ann Trop Med Parasitol. 2007;101:539–545. [DOI] [PubMed] [Google Scholar]


