Supplemental digital content is available in the text.
Abbreviations: CI = confidence interval, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, IE = infective endocarditis, OR = odds ratio
Abstract
Patients with diabetes mellitus (DM) have a higher incidence of infections, and those with bacteremia are more prone to develop sepsis and infective endocarditis (IE). Nevertheless, data concerning the impact of DM on the prognosis of patients with IE are limited and sometimes contradictory. We examined the impact of DM on the inhospital outcome of left-sided IE in a large cohort of patients. We studied 594 consecutive episodes of left-sided IE diagnosed at 3 tertiary care centers. They were divided into 2 groups: episodes in patients with DM (n = 114) and episodes in patients without DM (n = 480). We retrospectively analyzed the influence of DM therapy on patient outcome. Compared to patients without DM, patients with DM were older (67 ± 10 vs. 60 ± 15 yr; p < 0.001), less frequently male (53.5% vs. 67.9%; p = 0.004), and more commonly had chronic renal failure (23.9% vs. 6.9%; p < 0.001) and chronic obstructive pulmonary disease (14.6% vs. 7.8%; p = 0.019). Enterococcus (14.9% vs. 7.4%; p = 0.011) and Streptococcus bovis (8.8% vs. 3.8%; p = 0.024) were isolated more frequently. In the univariable analysis, septic shock (29.2% vs. 16.4%; p = 0.005) and mortality (43.5% vs. 30.0%; p = 0.008) were more common among patients with DM than in those without. Considering the different treatments for DM, septic shock (33.3%; p = 0.011) and death (50.8%; p = 0.012) were more frequent in patients receiving oral medication to treat diabetes than in patients with the other treatment modalities. However, multivariable analysis showed that DM had an independent association with development of septic shock (OR 2.282; 95% CI 1.186–4.393), but it was not a predictor of inhospital mortality.
Staphylococci were the most frequently involved microorganisms in all patients; however, Enterococcus and Streptococcus bovis were more frequently isolated from individuals with DM and left-sided IE, whereas viridans group streptococci were more commonly isolated from those with left-sided IE who did not have DM. DM was independently associated with the development of septic shock, but it was not an independent predictor of inhospital mortality in patients with left-sided IE.
INTRODUCTION
The prevalence of diabetes mellitus (DM) is progressively increasing in many regions of the world. Indeed, a demographic projection for 2030 is double the current number of patients with DM.24
Individuals with DM have a greater frequency and severity of infections.4,12 The reasons for this include incompletely defined abnormalities in cell-mediated immunity and phagocyte function associated with hyperglycemia, as well as diminished vascularization.4,12 Episodes of bacteremia occur more frequently in patients with DM, in part perhaps due to an increased rate of colonization of Staphylococcus aureus in the skin folds and nares of these individuals.5 Patients with DM and bacteremia are more prone to develop sepsis and infective endocarditis (IE).14 It has been well documented that patients with type 2 DM have a significantly higher prevalence of IE.20,23
In addition to cardiovascular disease, infection is one of the leading causes of death in hospitalized patients with DM. In fact, DM has been identified as a risk factor of poor prognosis in different bacterial infections including IE.6 Data concerning the impact of DM on clinical characteristics, microbiologic profile, and prognostic differences in patients with IE are scarce and sometimes contradictory.3,13 Other authors have found that only insulin-dependent DM is a predictor of poor inhospital outcome.7 Due to the conflicting results and the small number of patients with diabetes included in previous studies, we decided to assess the impact of DM on the clinical presentation and inhospital prognosis of a large series of patients with left-sided IE.
We conducted the present study to describe the differences in the epidemiology, clinical course, microbiology, and inhospital outcome between patients with DM and patients without DM who have left-sided IE, and to analyze the impact of DM therapy.
PATIENTS AND METHODS
Patient Population
We prospectively collected 724 episodes of IE diagnosed consecutively at 3 tertiary centers between 1996 and 2010 according to the Duke criteria (until 2002) or the modified Duke criteria (since 2003).17 Only definite cases of IE were included. Of these 724 episodes, 594 had left-sided IE and were evaluated to be included in the study. Episodes with right-sided IE were excluded because their epidemiology, clinical presentation, and prognosis are totally different. The diagnosis of DM was made by the physician in charge according to the guidelines criteria.22 Transient hyperglycemia during hospitalization was not considered. The 594 episodes of left-sided IE were analyzed and divided into 2 groups: episodes in patients with no DM (n = 480), and episodes in DM patients (n = 114). To ensure consecutive enrollment, all patients who underwent echocardiography to rule out IE were clinically followed until a diagnosis was established. Patients with a final diagnosis of IE were included in a multiproposal database. All patients underwent a detailed clinical history, standard physical examination, electrocardiography, blood analysis, urinalysis, a set of 3 blood cultures at admission and 3 additional blood cultures 48–72 hours later, and transthoracic and transesophageal echocardiography. Blood culture results were reported within 72 hours. Empiric antibiotic treatment was started when needed, and specific antibiotic treatment was initiated after the results of blood cultures were available. If blood cultures were negative after 72 hours, specific serologic tests were done for Chlamydia, Brucella, Q fever, Legionella, and Mycoplasma. For every patient, a standardized case report form with 18 epidemiologic, 8 clinical, 10 analytic, 4 radiographic, 6 electrocardiographic, 14 microbiologic, and 16 echocardiographic variables was completed and included in the database. This registry was approved by the local ethical committees.
Definition of Terms
Early prosthetic valve IE was defined as occurring within the first year after surgery, and late prosthetic valve IE, beyond 1 year.19 Acute-onset IE was considered to exist when the time between the appearance of symptoms and hospital admission was less than 15 days.21 Renal insufficiency was defined as the presence of a serum creatinine concentration higher than 2 mg/dL. Heart failure was diagnosed on the basis of established criteria.11 Previous valvulopathy was defined as a structural abnormality of the heart valves. From an echocardiographic perspective, vegetation was defined as a thrombus-like mass with shaggy echoes and erratic motion independent of that of the valve. The vegetation was measured in various planes. The maximal diameter and area were used for subsequent analysis. In case of multiple vegetations, the largest was measured. Perivalvular complications were defined as follows: abscess, well-delineated perivalvular area of reduced echodensity with no flow; pseudoaneurysm, echolucent perivalvular pouch with flow in its interior; and fistula, a narrow communication between 2 adjacent cavities.1,9 Adequate antibiotic treatment was defined when high-dose intravenous antibiotic combinations known to be bactericidal in vitro against the isolated microorganisms were used. Empiric antibiotic regimens were chosen for culture-negative cases according to established guidelines.2,10 Urgent surgery, defined as surgery done before antibiotic regimen was completed, was performed when any of the following occurred: heart failure unresponsive to medication, pulmonary edema, persistent signs of infection, and repeat embolism despite appropriate antibiotic treatment, with persistence of vegetations on echocardiography. The initial presence of perivalvular complications in patients with a favorable clinical course was not considered an indication for urgent surgery, although enlargement of pseudoaneurysms and abscesses or progression to a fistula were considered indications.
Nosocomial and community-acquired IE were defined as in the literature.10 Persistent signs of infection were defined as persistent bacteremia or fever after 7 days of appropriate antibiotic treatment, once other possible foci of infection had been ruled out.10
Sepsis was defined as the presence of infection and systemic inflammatory response. Severe sepsis was considered when sepsis was associated with organ dysfunction, hypoperfusion, or hypotension that can be reversible by administering fluids. Septic shock was defined as the presence of an acute circulatory failure in sepsis, characterized by persistent arterial hypotension (systolic pressure <90 mm Hg) that could not be reversed by administration of intravenous fluids.16
Definition of Events
Death and septic shock during hospitalization were regarded as events. Any inhospital death was considered an event regardless of its cause.
Statistical Analysis
The database was established with a protocol and procedures manual that was periodically revised in consensus meetings with all the investigators. Continuous variables were reported as mean value (standard deviation) or median (25th–75th percentiles). For dichotomous variables, the groups were compared by a 2-tailed Student t-test or Mann-Whitney U test as appropriate. In the presence of multiple categories, ANOVA or the Kruskal-Wallis test was used when appropriate. Categorical variables, expressed as a frequency and a percentage, were compared with the chi-square test and the Fisher exact test as necessary. Prognostic influence of DM therapy on inhospital mortality and septic shock was first tested in a univariable analysis (Pearson chi-square test or ANOVA, with post-hoc Bonferroni test).
Multivariable analysis, considering events as the dependent variable, was performed with a logistic regression model by means of a backward stepwise method. In consecutive steps, variables that were statistically significant in the univariable analysis, and others considered clinically relevant, were included in the logistic regression. The adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for each variable were calculated. When a change in OR >10% was found, the variable was considered clinically relevant. A stratified analysis of mortality by diabetes status was performed.
All tests were 2-sided, and differences were considered statistically significant at p values < 0.05. Statistical analysis was performed with PASW Statistics v 17.0 (SPSS Inc., Chicago, IL).
RESULTS
Univariable Analysis of Clinical, Echocardiographic, and Prognostic Characteristics in Patients With DM
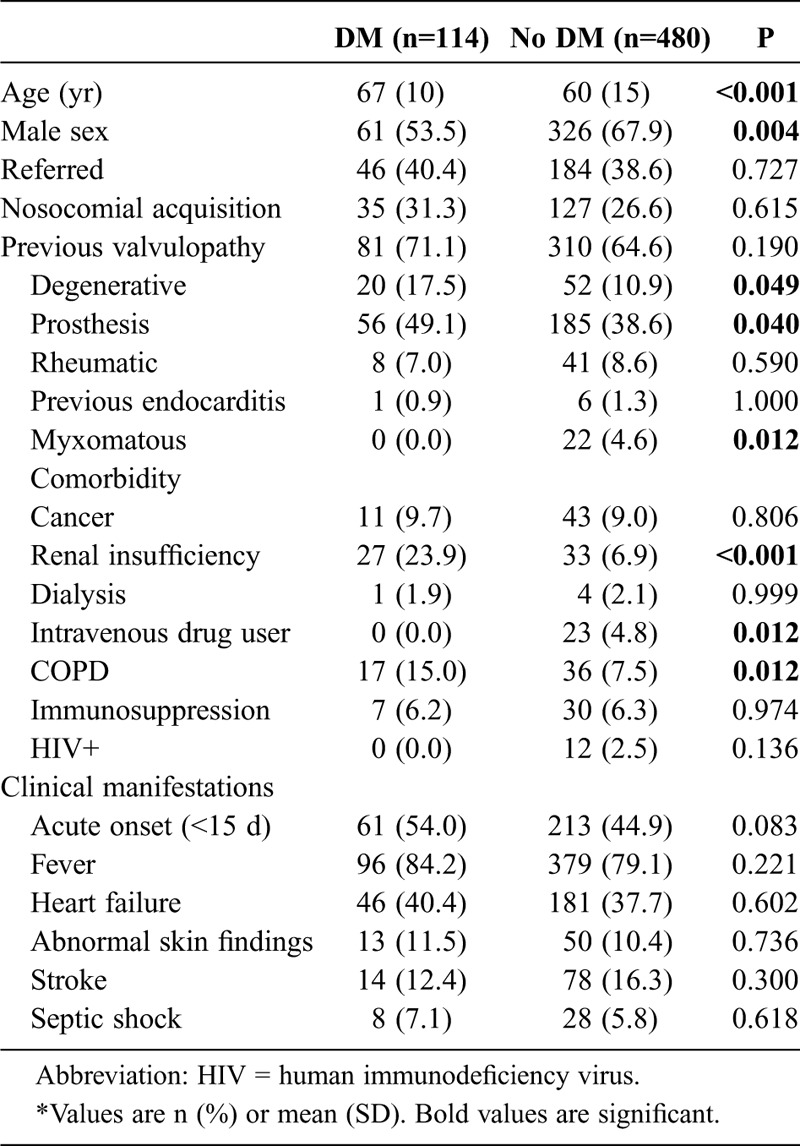
Mean age of our patient population (n = 594) was 62 ± 15 years. About two-thirds of the cases (60.8%) had community-acquired infection, and 230 (38.7%) were referred from another hospital. Table 1 shows demographic features, previous cardiac conditions, comorbidities, and clinical presentation comparisons between episodes that occurred in patients with and without DM. Patients with DM were older than those without DM, and were less frequently male. Regarding comorbidity, chronic renal failure and chronic obstructive pulmonary disease (COPD) were more common in patients with DM, while intravenous drug addiction was more frequent among patients without DM. Degenerative valvulopathy and valvular prosthesis as predisposing cardiac conditions were more frequent in patients with DM, whereas mitral valve prolapse was more common in patients without DM. Among electrocardiographic features at admission, it is noteworthy that the presence of a left-bundle branch block was more frequent in patients with DM.
TABLE 1.
Epidemiologic Characteristics, Comorbidities and Clinical Presentation at Admission*
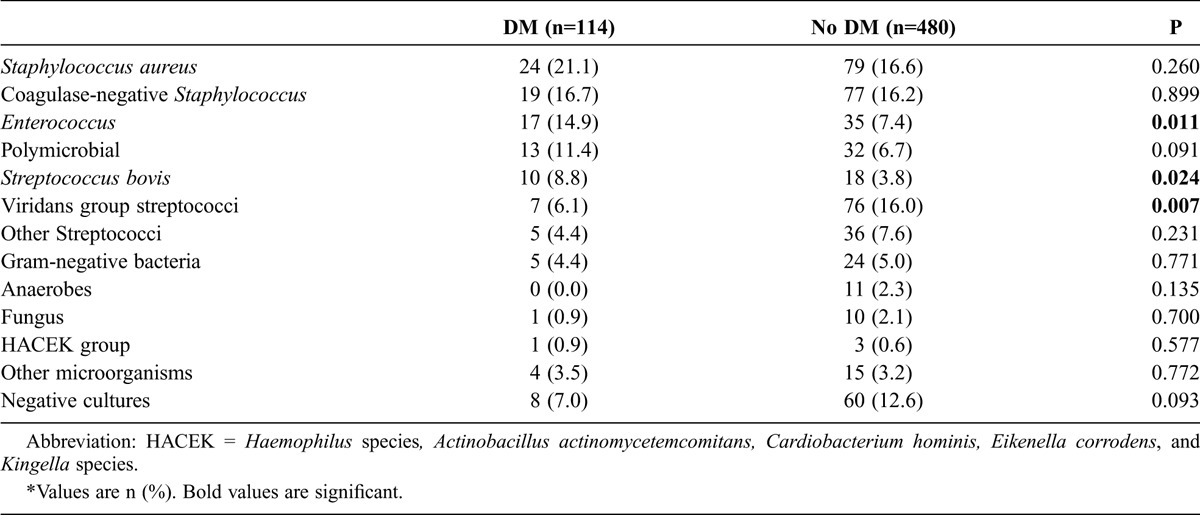
The microbiologic profile is shown in Table 2. Enterococcus and S. bovis were more frequent in patients with DM, whereas the viridans group streptococci were more commonly isolated in those without DM.
TABLE 2.
Microbiologic Profile*
Regarding echocardiographic findings, no differences were found in the location of the infection, the type of valve affected (native vs. prosthetic), or the presence of periannular complications. Vegetation detection rate and vegetation size were similar in both patients with and without DM. Moderate or severe valve insufficiency was more frequent among patients without DM.
Clinical events during inhospital evolution are summarized in Table 3. Heart failure and the need for cardiac surgery were equally frequent in both groups, patients with and without DM.
TABLE 3.
Clinical Events During Inhospital Evolution*
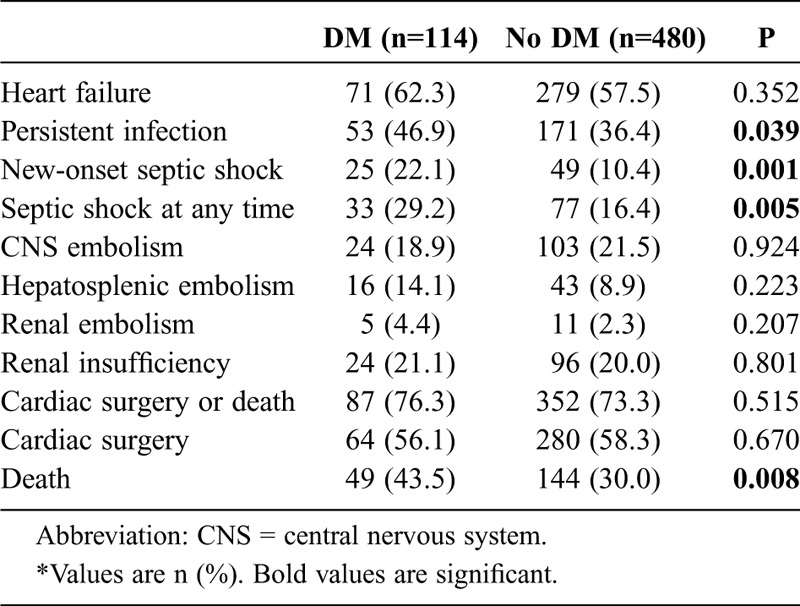
In those episodes that occurred in patients with DM, the main indications for surgery were heart failure (n = 31; 48.4%) and persistent signs of infection (n = 22; 34.4%). These percentages are similar to those seen in patients without diabetes. Remarkably enough, persistent signs of infection (46.9% vs. 36.4%; p = 0.039) and septic shock (27.4% vs. 16.0%; p = 0.005) occurred more often in patients with DM. The median time at which new-onset septic shock occurred was 1.5 weeks; 32.1% of all episodes of septic shock occurred in the postoperative period. No differences were found in the timing of presentation of this severe complication between the groups.
Mortality was higher in patients with diabetes (43.5% vs. 30.0%; p = 0.008). The main cause of death among patients with and without DM was septic shock, followed by multiorgan failure and heart failure, with no significant differences between the 2 groups.
Age did not influence the relationship between microbiologic findings and the presence or absence of DM. The interaction between age and the microorganisms found to be more frequent among patients with DM was not significant (p = 0.964 for Enterococcus and p = 0.208 for S. bovis). There was also no significant interaction effect of age on the development of septic shock (p = 0.223) or mortality (p = 0.472).
Univariable Analysis of Inhospital Outcome Related to DM Therapy
We analyzed IE episodes in relation to DM therapy and divided the study cases into 4 groups: patients without DM (n = 480), patients under only dietetic treatment (n = 24), patients treated with oral medications for DM (n = 61), and patients receiving insulin (n = 29). (See data comparing these 4 groups in the Appendix provided as Supplemental Digital Content. http://links.lww.com/MD/A26) Of note, renal insufficiency was most frequent in patients receiving insulin therapy (35.7%; p < 0.001), and coagulase-negative staphylococci were most prevalent in patients treated with oral medications for diabetes (24.6%; p = 0.048). Regarding clinical events during inhospital evolution, septic shock was most common in patients treated with oral medications for DM (26.7%; p = 0.003), and death was highest in this group (50.8%; p = 0.012).
Multivariable Analysis of New-Onset Septic Shock
To define the variables independently associated with the development of septic shock during hospitalization, we included the variables that were significant in the univariable analysis and those that were considered clinically relevant in a multivariable logistic regression analysis. Variables that were considered for the model were DM, previous valvulopathy, S. aureus, viridans group streptococci, acute clinical onset, periannular complications, vegetation detection, persistent signs of infection, hepatic embolism, acute renal failure, heart failure, and death.
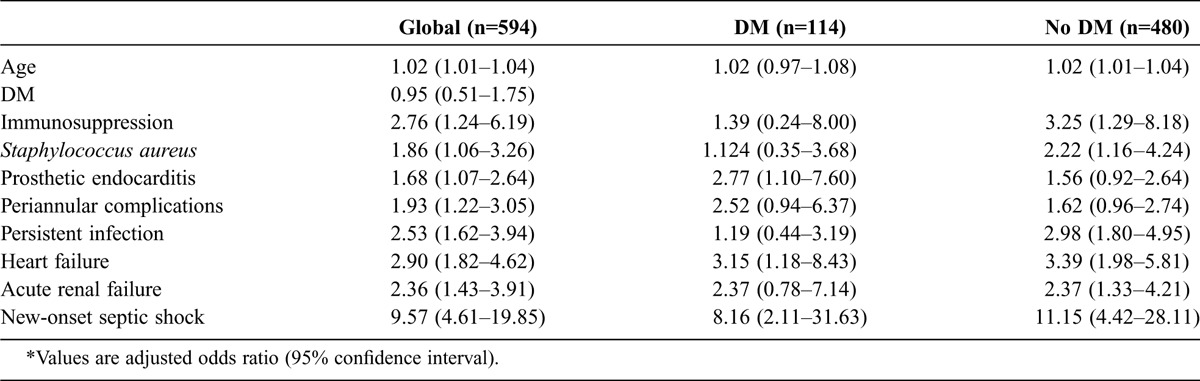
In the multivariable analysis the variables independently associated with septic shock were DM (OR, 2.282; 95% CI, 1.186–4.393), S. aureus (OR, 2.288; 95% CI, 1.207–4.337), persistent signs of infection (OR, 5.068; 95% CI, 2.598–9.888), and death (OR, 9.252; 95% CI, 4.636–18.463).
Multivariable Analysis of Inhospital Mortality Stratified by Diabetes Status
To identify independent predictors of inhospital mortality, we performed a multivariable logistic regression analysis stratified by diabetes status. We included in the model all the variables with statistical significance or clinical relevance: DM, age, sex, nosocomial infection acquisition, immunosuppression, COPD, chronic renal failure, acute clinical onset, S. aureus, prosthetic valve IE, vegetation detection, periannular complications, persistent signs of infection, heart failure, central nervous system embolism, acute renal failure, new-onset septic shock, and cardiac surgery. Independent predictors of inhospital death are shown in Table 4.
TABLE 4.
Inhospital Mortality Logistic Model for All Patients and Stratified by DM Status*
DISCUSSION
We studied the impact of DM on the inhospital outcome of patients with IE. We selected episodes of IE involving only left-sided valves due to the well-known differences with the right-sided isolated infection,10 and so we could study a more homogeneous population. The present group of patients is one of the largest series of episodes of IE with DM. The DM rate (19.2%) we identified is consistent with the rate reported in previous studies.7,13
As in other investigations,3,13 patients with DM in our cohort were older, were more often female, and had more comorbidities (chronic renal failure and COPD) than patients without DM. As pointed out by Kourany et al,13 these patient characteristics should be considered when looking at the impact of DM on short- and long-term outcomes of IE. In the present study, staphylococci were the most common microorganisms in patients with DM, but their incidence was similar to that seen in episodes that occurred in patients without DM. Enterococcus and S. bovis were more frequently isolated in patients with DM than in those without DM. Previous studies18 have suggested that the older age of patients with diabetes could be related to a higher prevalence of Enterococcus and S. bovis, but the findings in the present study were independent of age.
Other authors have found a similar microbiologic profile.5 The presence of heart failure, detection of vegetations, rate of embolism, and periannular complications were similar in both groups. Consistent with previous studies,3,5,7,13 the need for valve surgery was similar in both groups.
Using the International Collaboration on Endocarditis-Merged Database (ICE-MD) multicenter international database, Kourany et al13 found that the inhospital mortality was significantly higher in patients with DM, and they underwent surgery less frequently than those without DM in spite of a similar complication rate (heart failure, valvular regurgitation, detection of vegetations, and intracardiac abscesses). No clear reason for the lower occurrence of surgery in patients with DM was given. In addition, they suggested that DM per se has no impact on infectious complications of IE.13 Their findings should be interpreted with caution due to their heterogeneity and the lack of systematized data collection; in fact, in 928 of 2212 patients included in the registry, no information on the presence or absence of DM was available.
Chirillo et al5 analyzed 309 episodes of IE, of whom 70 had DM. As in our cohort, compared to patients without diabetes, patients with DM were older, and Enterococcus was more commonly isolated (Table 5). Mortality was higher among patients with DM, and DM was an independent predictor of inhospital mortality.
TABLE 5.
Comparison Between 2 Cohorts of Patients With DM and IE*
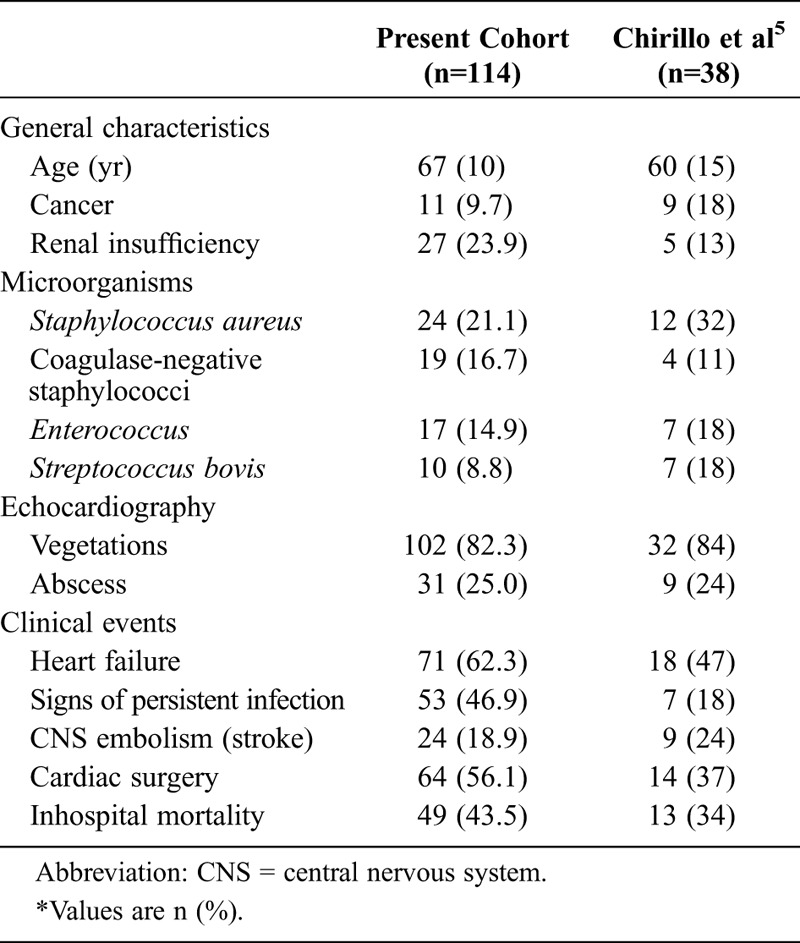
Chu et al6 found that DM was an independent predictor of inhospital mortality. Surprisingly, in that series overall mortality was extremely high (56%). Bishara et al3 reported that DM per se did not affect mortality on multivariable analysis, and Duval et al,7 assessing 75 patients with DM, reported that insulin-treated DM was a predictor of inhospital death, whereas DM treated with oral medications for diabetes was not. These inconsistencies indicate that the impact of DM on the outcome of patients with IE is uncertain.
Overall, in the present study, 32.5% of patients died during hospitalization. On univariable analysis, the presence of DM was associated with higher inhospital mortality compared with patients without DM (43.5% vs. 30%; p = 0.008), and yet, on multivariable analysis, we did not find DM to be an independent predictor of inhospital mortality. Similarly, in the Italian Study on Endocarditis,15 DM was a significant predictor of inhospital mortality in the univariable but not in the multivariable analysis. Importantly, DM had a strong independent association with the development of septic shock (OR, 2.282; 95% CI, 1.186–4.393). In contrast to the present study, other studies that considered the impact of DM on inhospital mortality did not include septic shock in the multivariable analysis.5–7,13
Chirillo et al5 found that the cause of death among patients with DM was mostly related to infection, contrary to what Kourany et al13 observed. It is well known that individuals with DM have a greater frequency and severity of infections,4,12,14 and infection is one of the main causes of death in hospitalized patients with diabetes. Among the reasons for this susceptibility to severe infections are abnormalities in cell-mediated immunity and phagocyte function, diminished vascularization, and increased rate of colonization of S. aureus.4,12
The findings of the present study might reinforce a strategy of closer monitoring and perhaps anticipated surgery in patients with DM and IE, considering their trend to develop septic shock.
In the current cohort, we found that patients treated with oral medications for DM, compared to other patients with DM and compared to patients without DM, had more infections due to coagulase-negative staphylococci, higher rate of septic shock, and higher mortality. These results are markedly different from those of Duval et al,7 who found that staphylococci and inhospital death were more frequent in insulin-treated patients with DM, and failed to elucidate the reasons why insulin-treated patients with DM were at higher risk of mortality. We do not have a clear explanation for these divergent results. It is well known that the prognosis of patients with DM when affected by other conditions is more determined by an optimal glycemic control than by the type of DM therapy.8 Unfortunately, data on glycemic control (glucose levels, glycated hemoglobin) are not available in our database, and we can not evaluate their impact on patient outcome. Nonetheless, in the series of Chirillo et al,5 glycated hemoglobin levels were similar in survivors and nonsurvivors. In addition, insulin therapy to maintain adequate glycemic control was administered in the same proportion of patients, and did not have a clear positive effect on patient outcome. A larger, prospective, and more detailed (DM therapy, glycemic control, etc.) study is needed.
The present study has several limitations. It is part of a multiproposal prospective collection of data with a large number of cases, but it has the potential for referral bias because all the participating hospital were tertiary care centers. In addition, as this registry began in 1996, when glycated hemoglobin was not used in clinical practice, data on previous glycemic control were not available, and they might influence patient outcome.
We conclude that patients with left-sided IE and DM are older and have more comorbidities (especially renal insufficiency) than those without DM. Staphylococci are the most frequently involved microorganisms in all patients; however, Enterococcus and S. bovis are more frequently isolated from individuals with left-sided IE and DM, whereas viridans group streptococci are more commonly isolated from those with left-sided IE who do not have DM. Gut enteric pathogens were more frequent in patients with diabetes. DM was independently associated with septic shock, but not with inhospital mortality. Therefore, we consider the prognostic relevance of DM in patients with IE to be due to the relationship of DM with septic shock.
ACKNOWLEDGMENTS
The authors thank María del Trigo, MD, and María del Carmen Manzano, MD, for their collaboration in collecting data for the study.
Abbreviations
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- DM
diabetes mellitus
- IE
infective endocarditis
- OR
odds ratio
Footnotes
Financial support and conflicts of interest: The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citation is provided in the HTML and PDF versions of this article on the journal’s website (www.md-journal.com).
REFERENCES
- 1.Anguera I, Miro JM, Vilacosta I, Almirante B, Anguita M, Munoz P, San Roman JA, de Alarcon A, Ripoll T, Navas E, Gonzalez-Juanatey C, Cabell CH, Sarria C, Garcia-Bolao I, Farinas MC, Leta R, Rufi G, Miralles F, Pare C, Evangelista A, Fowler VG Jr, Mestres CA, de Lazzari E, Guma JR. Aorto-cavitary fistulous tract formation in infective endocarditis: clinical and echocardiographic features of 76 cases and risk factors for mortality. Eur Heart J. 2005;26:288–297. [DOI] [PubMed] [Google Scholar]
- 2.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME, Ferrieri P, Gerber MA, Tani LY, Gewitz MH, Tong DC, Steckelberg JM, Baltimore RS, Shulman ST, Burns JC, Falace DA, Newburger JW, Pallasch TJ, Takahashi M, Taubert KA. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394–e434. [DOI] [PubMed] [Google Scholar]
- 3.Bishara J, Peled N, Samra Z, Sagie A, Leibovici L, Pitlik S. Infective endocarditis in diabetic and non-diabetic patients. Scand J Infect Dis. 2004;36:795–798. [DOI] [PubMed] [Google Scholar]
- 4.Calvet HM, Yoshikawa TT. Infections in diabetes. Infect Dis Clin North Am. 2001;15:407–421. [DOI] [PubMed] [Google Scholar]
- 5.Chirillo F, Bacchion F, Pedrocco A, Scotton P, De Leo A, Rocco F, Valfre C, Olivari Z. Infective endocarditis in patients with diabetes mellitus. J Heart Valve Dis. 2010;19:312–320. [PubMed] [Google Scholar]
- 6.Chu VH, Cabell CH, Benjamin DK Jr, Kuniholm EF, Fowler VG Jr, Engemann J, Sexton DJ, Corey GR, Wang A. Early predictors of in-hospital death in infective endocarditis. Circulation. 2004;109:1745–1749. [DOI] [PubMed] [Google Scholar]
- 7.Duval X, Alla F, Doco-Lecompte T, Le Moing V, Delahaye F, Mainardi J-L, Plesiat P, Celard M, Hoen B, Leport C. Diabetes mellitus and infective endocarditis: the insulin factor in patient morbidity and mortality. Eur Heart J. 2007;28:59–64. [DOI] [PubMed] [Google Scholar]
- 8.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. [DOI] [PubMed] [Google Scholar]
- 9.Graupner C, Vilacosta I, San Roman JA, Ronderos R, Sarria C, Fernandez C, Mujica R, Sanz O, Sanmartin JV, Pinto AG. Periannular extension of infective endocarditis. J Am Coll Cardiol. 2002;39:1204–1211. [DOI] [PubMed] [Google Scholar]
- 10.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Moreillon P, Antunes MJ, Thilen U, Lekakis J, Lengyel M, Muller L, Naber CK, Nihoyannopoulos P, Moritz A, Zamorano JL. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009). The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and by the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30:2369–2413. [DOI] [PubMed] [Google Scholar]
- 11.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol. 1993;22(Suppl A):6A–13A. [DOI] [PubMed] [Google Scholar]
- 12.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–1912. [DOI] [PubMed] [Google Scholar]
- 13.Kourany WM, Miro JM, Moreno A, Corey GR, Pappas PA, Abrutyn E, Hoen B, Habib G, Fowler VG Jr, Sexton DJ, Olaison L, Cabell CH. Influence of diabetes mellitus on the clinical manifestations and prognosis of infective endocarditis: a report from the International Collaboration on Endocarditis—Merged Database. Scand J Infect Dis. 2006;38:613–619. [DOI] [PubMed] [Google Scholar]
- 14.Laupland KB, Gregson DB, Zygun DA, Doig CJ, Mortis G, Church DL. Severe bloodstream infections: a population-based assessment. Crit Care Med. 2004;32:992–997. [DOI] [PubMed] [Google Scholar]
- 15.Leone S, Ravasio V, Durante-Mangoni E, Crapis M, Carosi G, Scotton PG, Barzaghi N, Falcone M, Chinello P, Pasticci MB, Grossi P, Utili R, Viale P, Rizzi M, Suter F. Epidemiology, characteristics, and outcome of infective endocarditis in Italy: the Italian Study on Endocarditis. Infection. 2012;40:527–535. [DOI] [PubMed] [Google Scholar]
- 16.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 17.Li JS, Sexton DJ, Mick N, Nettles R, Fowler WG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. [DOI] [PubMed] [Google Scholar]
- 18.Lopez J, Revilla A, Vilacosta I, Sevilla T, Villacorta E, Sarria C, Pozo E, Rollan MJ, Gomez I, Mota P, San Roman JA. Age-dependent profile of left-sided infective endocarditis: a 3-center experience. Circulation. 2010;12:892–897. [DOI] [PubMed] [Google Scholar]
- 19.Lopez J, Revilla A, Vilacosta I, Villacorta E, Gonzalez-Juanatey C, Gomez I, Rollan MJ, San Roman JA. Definition, clinical profile, microbiological spectrum, and prognostic factors of early-onset prosthetic valve endocarditis. Eur Heart J. 2007;28:760–765. [DOI] [PubMed] [Google Scholar]
- 20.Movahed MR, Hashemzadeh M, Jamal MM. Increased prevalence of infectious endocarditis in patients with type II diabetes mellitus. J Diabetes Complications. 2007;21:403–406. [DOI] [PubMed] [Google Scholar]
- 21.Revilla A, Lopez J, Vilacosta I, Villacorta E, Rollan MJ, Echevarria JR, Carrascal Y, Di Stefano S, Fulquet E, Rodriguez E, Fiz L, San Roman JA. Clinical and prognostic profile of patients with infective endocarditis who need urgent surgery. Eur Heart J. 2007;28:65–71. [DOI] [PubMed] [Google Scholar]
- 22.Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, Hellman R, Jellinger PS, Jovanovic LG, Levy P, Mechanick JI, Zangeneh F. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl 1):1–68. [DOI] [PubMed] [Google Scholar]
- 23.Strom BL, Abrutyn E, Berlin JA, Kinman JL, Feldman RS, Stolley PD, Levison ME, Korzeniowski OM, Kaye D. Risk factors for infective endocarditis: oral hygiene and nondental exposures. Circulation. 2000;102:2842–2848. [DOI] [PubMed] [Google Scholar]
- 24.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. [DOI] [PubMed] [Google Scholar]


