Abstract
Treatment for recurrent hepatocellular carcinoma (RHCC) remains controversial. This study tried to compare survival benefits between radiofrequency ablation (RFA) and reresection for RHCC patients following curative surgical treatments.
Databases were searched for comparative studies published from 2008 to 2014 on RFA versus reresection in treating RHCC. Meta-analysis was performed using a random or fixed-effect model to compare the overall survivals (OSs) and disease-free survivals (DFSs) between RFA and reresection. Begg funnel plot and Egger test were performed to assess the publication bias.
Six retrospective comparative studies fulfilled our criteria and were included. For patients with RHCC, RFA was equivalent to reresection in 1-year OSs (odds ratio [OR] 0.86; 95% confidence interval [CI], 0.50–1.49; P = 0.587), 3-year OSs (OR 0.91; 95% CI, 0.64–1.28; P = 0.581), and 5-year OSs (OR 0.97; 95% CI, 0.69–1.36; P = 0.846). However, reresection was superior to RFA in 3-year DFSs (OR 2.25; 95% CI, 1.37–3.68; P = 0.001) and 5-year DFSs (OR 3.70; 95% CI, 1.98–6.93; P = 0.000). The outcome of 1-year DFSs was unstable with statistical heterogeneity among studies included in meta-analysis (I2 = 77.4%). No evidence of publication bias was found. RFA was considered as a less invasive modality for RHCC patients.
RFA achieves comparable OSs as reresection in the treatment of RHCC, with lower postoperative complications.
BACKGROUND
Hepatocellular carcinoma (HCC) is the seventh most common malignant tumor and the third leading cause of cancer deaths in the world. It is the third most frequent cancer in China and is increasing in western countries.1–3 Liver transplantation, surgical resection, and radiofrequency ablation (RFA) are curative treatment modalities for HCC according to the guidelines of the American Association for the Study of Liver Diseases.4 However, the prognosis of curative treatments for HCC is rather disappointing because of a high recurrence rate. A 5-year recurrence rate of >50% has been reported after surgical resection,5–8 which is even higher after curative RFA treatment.9,10 Even in patients undergoing liver transplantation, the postoperative recurrence rate is between 15% and 20%.11 Therefore, the treatment of recurrent hepatocellular carcinoma (RHCC) is critical in improving the survival of HCC patients. For most patients with intrahepatic recurrence without general metastasis and macrovessel invasion, there are still chances to achieve a curative treatment, with liver transplantation, reresection, or RFA. Though liver transplantation is recognized as the best choice for RHCC,12 the shortage of donor livers has limited its wide application. As to the choice of reresection and RFA, no consensus has yet been reached. Though surgical resection is considered the gold standard for the treatment of HCC,4 there are drawbacks such as excessive damage to the liver function and more difficulty in a second open surgery for RHCC patients.13 RFA, as a less invasive and repeatable modality, is more feasible in RHCC patients with impaired liver function and unresectable tumor lesions.14,15 In order to prove that RFA may be a promising alternative to reresection, we conducted this evidence-based research to compare the treatment efficacy between RFA and reresection in RHCC patients.
METHODS
Search Strategy and Data Extraction
All studies we needed were retrieved by searching databases including Cochrane library, PubMed, and EMBASE using the following keywords: “recurrent hepatocellular carcinoma,” “radiofrequency ablation,” and “re-resection/repeated resection/surgical resection.” In order to cover all relevant studies, reference lists of all these retrieved articles were manually reviewed for more possibly useful information. No language limitation was set. All studies we needed should fulfill the following criteria:
Controlled trials directly comparing RFA with reresection for clinically or pathologically confirmed RHCC;
All RHCC patients received curative treatments before and were scanned by computed tomography (CT) or magnetic resonance imaging (MRI) to confirm complete clearance or ablation of tumors;
RHCC without macrovessel invasion and general metastasis;
Data including 1-, 3-, and 5-year overall survival (OS) and disease-free survival (DFS) and local recurrence rate were given in detail.
Articles were excluded when patients with severely impaired liver function or poor general health conditions or patients with tumor residual or insufficient ablation after initial curative treatments were included.
Two trained reviewers (H.C. and W.K, both doctors experienced in the procedure of liver cancer treatment) extracted the data independently on a specially designed form. Qualities of the retrieved studies were assessed according to the Jadad scale. The 2 reviewers were blinded to each other about data extraction and quality assessment result. Data needed in this study included the name of the first author, publishing year, time to recurrence, number of patients, age, sex, tumor size, tumor number, Child-Pugh stage, and long-term survival rates. Any discrepancies were resolved by discussion.
Statistical Analysis
Meta-analysis was performed with the commercially available software STATA, version 12.0 (STATA, College Station, TX). Dichotomous variables were described as relative frequency and were compared by χ2 test. Odds ratio (OR) with 95% confidence interval (CI) was calculated to compare the primary treatment efficacy between 2 groups. Either a fixed-effect model or a random-effect model was used according to heterogeneity among trials when the meta-analysis was performed. Statistical heterogeneity among the studies was assessed by χ2 and I2 test. I2 ≥ 50% was thought to be with large inconsistency, and a random-effect model was used. Begg funnel plot and Egger test were performed to assess the publication bias. Significance was defined when P < 0.05.
RESULTS
Description of Studies Selection
Six retrospective comparative studies16–21 published from 2008 to 2014 were included in our analysis, which were all nonrandomized controlled trials (NRCTs) with low Jadad scores. Chan et al22 conducted similar studies in 2012 and 2013. However, the latter was excluded for lack of information of DFS.22 All these retrieved studies involved RHCC patients with previous curative treatments of surgical resection or RFA. In total, there were 642 patients, 314 for RFA and 328 for reresection. There were no statistical differences between the 2 treatment groups at previous treatment stage in age, sex, tumor size, tumor number, and liver function. All these patients were scanned by contrast-enhanced CT or MRI after initial treatments to be confirmed “curative treated.” Baseline information was shown in Table 1.
TABLE 1.
Baseline Demographic and Clinical Information of Retrieved Comparative Studies
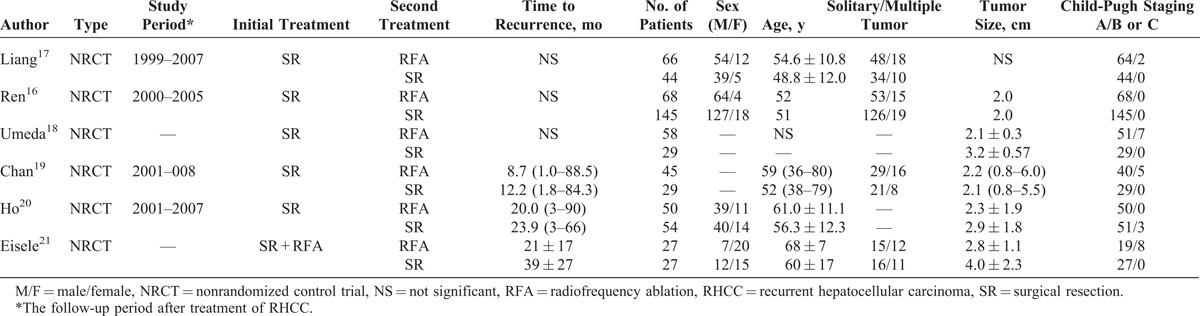
OSs for RHCC Treated by RFA or Reresection
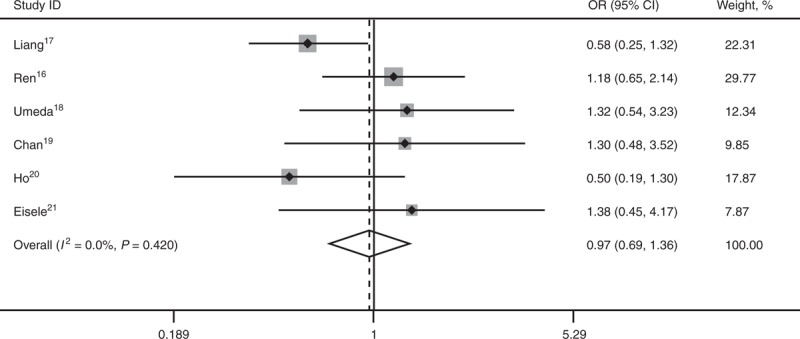
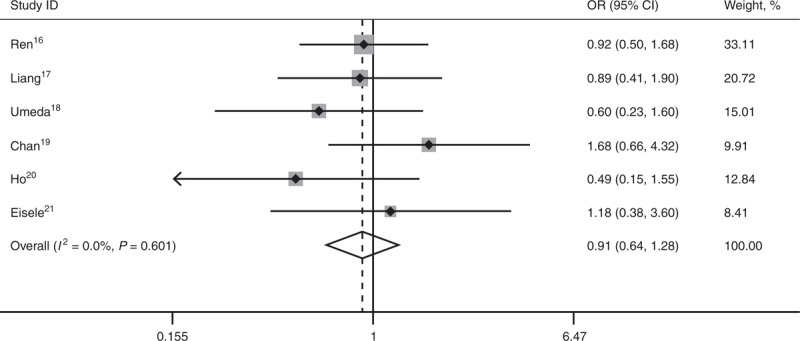
All 6 studies16–21 reported long-term OS. No statistical difference existed between the 2 comparative groups in 1-year OS (OR 0.86; 95% CI, 0.50–1.49; P = 0.587), 3-year OS (OR 0.91; 95% CI, 0.64–1.28; P = 0.581), and 5-year OS (OR 0.97; 95% CI, 0.69–1.36; P = 0.846). There’s no heterogeneity among the 6 studies, and a fixed-effect model was used (Figures 1–3).
FIGURE 1.
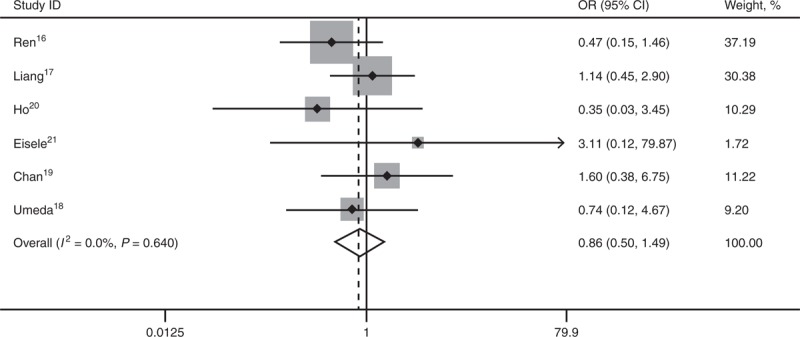
Forest plots showing the pooled result of 1-year OS. CI = confidence interval, OR = odds ratio, OS = overall survival.
FIGURE 3.
Forest plots showing the pooled result of 5-year overall survival. CI = confidence interval, OR = odds ratio, OS = overall survival.
FIGURE 2.
Forest plots showing the pooled result of 3-year overall survival. CI = confidence interval, OR = odds ratio, OS = overall survival.
DFSs for RHCC Treated by RFA or Reresection
Three studies16,19,21 reported 1-, 3-, and 5-year DFS. SR is superior to RFA for 3-year DFS (OR 2.25; 95% CI, 1.37–3.68; P = 0.001) and 5-year DFS (OR 3.70; 95% CI, 1.98–6.93; P = 0.000). There was statistical significant heterogeneity among the 3 studies for 1-year DFS (I2 = 77.4%), and a random-effect model was used (OR 1.56; 95% CI, 0.35–6.89; P = 0.560) (Figures 4–6).
FIGURE 4.
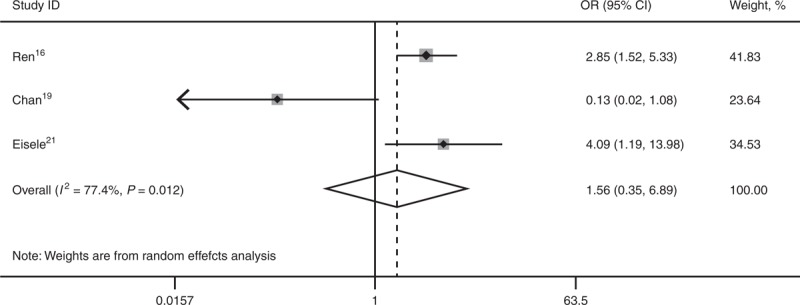
Forest plots showing the pooled result of 1-year disease-free survival. CI = confidence interval, OR = odds ratio.
FIGURE 6.
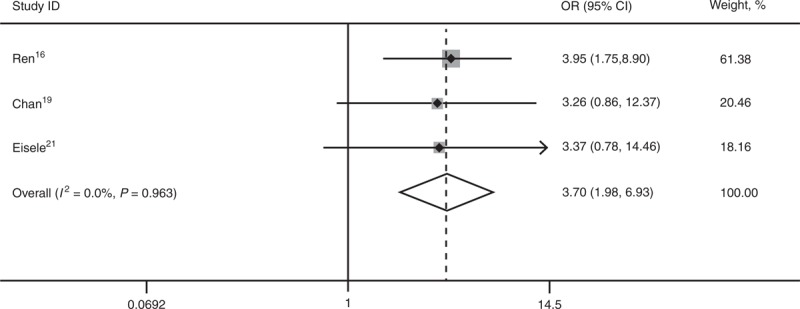
Forest plots showing the pooled result of 5-year disease-free survival. CI = confidence interval, OR = odds ratio.
FIGURE 5.
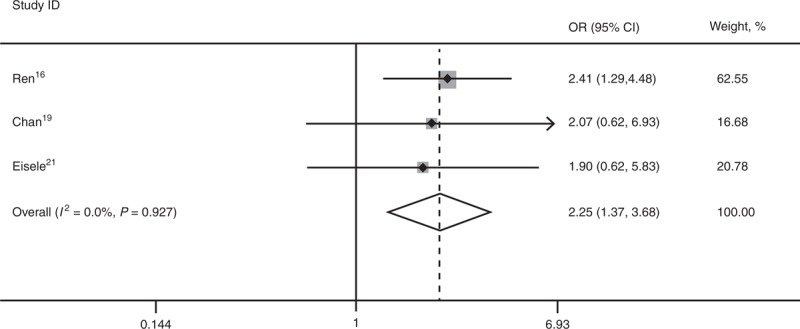
Forest plots showing the pooled result of 3-year disease-free survival. CI = confidence interval, OR = odds ratio.
Sensitivity Analysis and Bias Analysis
In order to evaluate the stability of the result of meta-analysis, we conducted a sensitivity analysis. The corresponding pooled ORs were not materially altered except for the meta-analysis of 1-year DFS. The difference is statistically significant when a fixed-effect model was used. The meta-analyses of 1-, 3-, and 5-year OS and 3- and 5-year DFS were relatively stable and credible.
No obvious asymmetry was shown on Begg funnel plot, and Egger test did not show any evidence of publication bias in all comparisons (1-year OS, P = 0.926; 3-year OS, P = 0.789; 5-year OS, P = 0.821; 1-year DFS, P = 0.503; 3-year DFS, 0.231; 5-year DFS, 0.163).
DISCUSSION
Intrahepatic recurrence of HCC is not uncommon after curative surgical treatments, and an appropriate treatment of RHCC is critical in improving long-term outcomes after initial therapies. Liver transplantation, reresection, and RFA were 3 potential curative treatment options.4 However, there is no consensus on the treatment strategy for RHCC. It is currently accepted that liver transplantation is the best treatment option for HCC as it removes not only tumor lesions but also concurrent cirrhosis, blocking the underlying process of carcinogenesis.23,24 However, limited liver donor resources make it difficult for patients to be treated on time before tumor progression. Generally speaking, for patients with resectable tumor lesions and well-preserved liver function, reresection is usually considered.25,26 Nevertheless, only a small proportion of patients with RHCC are amenable to liver transplantation (10%) or reresection (12%).27 RFA is offered as an alternative for those with poor liver function or unresectable tumor lesions, which are not suitable for reresection.15 Several retrospective controlled studies compared RFA with reresection in the treatment of RHCC.16–22 This study was a systematic review and meta-analysis of these important clinical literature.
In our study, baseline demographic data between the 2 groups did not have any statistical difference at initial treatment stage. Patients developing intrahepatic recurrence were treated with either RFA or reresection. It showed that RFA was comparable to reresection for long-term OSs. However, reresection was superior to RFA for long-term disease-free survivals. Ren et al16 and Liang et al17 reported significantly lower complication rate after treatment of RFA. There are some advantages of RFA when compared with reresection in treating RHCC. First, as a minimally invasive treatment modality, RFA can be performed percutaneously, therefore avoiding a second laparotomy. Second, in patients with small and cirrhotic liver remnant, RFA may be the only choice for conservation as much of the limited nontumorous liver parenchyma as possible.28,29 Therefore, for patients with well preserved liver function and resectable tumor lesions, reresection is no doubt preferred to RFA. However, for those who are not candidates for reresection, RFA would be a better choice, which shows comparable OSs as reresection. Though with higher recurrence rate, RFA serves as an ideal treatment choice for unresectable RHCC for its advantage of less invasiveness and repeatability.
Time to recurrence, which is thought to be an independent prognostic factor of RHCC,30 was comparable between the 2 groups in all studies except that of Eisele et al.21 It is reported that tumor recurrence within 1 year is more likely to develop an intrahepatic metastasis, with relatively poor prognosis, unlike tumor recurrence after 1 year, which is prone to be a multicentric occurrence.30 However, even in the study of Eisele et al,21 the average time to recurrence was >1 year in either group. Besides, it should be noted that in the study of Umeda et al18 and Eisele et al,21 patients who received reresection seemed to get better liver function and larger tumor size than those receiving RFA therapy. Also in the study of Umeda et al,18 patients in the RFA group seemed to get more multiple lesions at the time of recurrence. However, no statistical evidence was provided. It is reported that liver function, tumor number, and tumor size were important prognostic factors for either RFA or surgical resection.31–34 However, the effect of these factors still remains uncertain. In order to rule out the influence of these factors, further randomized controlled trials (RCTs) would be necessary.
There are some limitations in our study. First, all the included studies in meta-analysis were retrospective controlled studies, which contributed low evidence level to our study. Selection bias existed that patients with better general health condition are more likely to be allocated to reresection group, which may explain the better prognosis of reresection. Second, only a small number of institutions researched on the treatment options for RHCC. In total, 6 studies published results on this aspect and only 3 of them reported DFSs. The total sample size of our study is small because of lack of qualified studies. There is statistical heterogeneity among 3 studies reporting 1-year DFS (I2 = 77.4%), making the pooled outcome unstable and less convincing. More studies of high quality are needed to be done in future. Third, as we mentioned above, intrahepatic recurrence of HCC is complex on the mechanism, as it can be derived from intrahepatic metastasis or multicentric occurrence.35 Different patterns of recurrence is accompanied with different prognosis.36 However, it is difficult to tell them apart in clinical practice.
To date, no RCTs have been published to compare the treatment efficacy of RFA and reresection for RHCC. Still, the result of our study is referential to clinical practice, which may give some advice to hepatobiliary surgeons in the decision-making process for a proper treatment of RHCC. Recent studies reported that RFA, in combination with other treatment modalities such as TACE or molecular targeted therapy, showed better results than RFA treatment alone.37,38 We believe that with the development of technology and advances in treatment strategy, lower recurrence, and better outcome will be achieved for RHCC patients receiving RFA.
CONCLUSION
RFA achieves comparable OSs as reresection in the treatment of RHCC, with lower complications. However, reresection is superior to RFA in long-term DFSs.
ACKNOWLEDGMENT
The authors thank Xu Fu, who is an expert in English, for his assistance in the writing of this article in English.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, HCC = hepatocellular carcinoma, NRCT = nonrandomized controlled trial, OR = odds ratio, OS = overall survival, RCT = randomized controlled trial, RFA = radiofrequency ablation, RHCC = recurrent hepatocellular carcinoma.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. http://globocan.iarc.fr Accessed February 1, 2014. [Google Scholar]
- 2.Taylor-Robinson SD, Foster GR, Arora S, et al. Increase in primary liver cancer in the UK. Lancet. 1997;350:1142–1143. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roayaie S, Obeidat K, Sposito C, et al. Resection of hepatocellular cancer </=2 cm: results from two Western centers. Hepatology. 2013;57:1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung TT, Poon RT, Yuen WK, et al. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg. 2013;257:506–511. [DOI] [PubMed] [Google Scholar]
- 7.Shrager B, Jibara G, Schwartz M, et al. Resection of hepatocellular carcinoma without cirrhosis. Ann Surg. 2012;255:1135–1143. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa K, Kokudo N, Imamura H, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lencioni R, Cioni D, Crocetti L, et al. Early stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967. [DOI] [PubMed] [Google Scholar]
- 10.N’Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475–1483. [DOI] [PubMed] [Google Scholar]
- 11.Welker MW, Bechstein WO, Zeuzem S, et al. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int. 2013;26:109–118. [DOI] [PubMed] [Google Scholar]
- 12.Ng KK, Lo CM, Liu CL, et al. Survival analysis of patients with transplantable recurrent hepatocellular carcinoma: implications for salvage liver transplant. Arch Surg. 2008;143:68–74. [DOI] [PubMed] [Google Scholar]
- 13.Itamoto T, Nakahara H, Amano H, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery. 2007;141:589–597. [DOI] [PubMed] [Google Scholar]
- 14.Guan YS, Liu Y. Interventional treatments for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2006;5:495–500. [PubMed] [Google Scholar]
- 15.Wu JY, Yang W, Cui M, et al. Efficacy and feasibility of radiofrequency ablation for decompensated cirrhotic patients with hepatocellular carcinoma. Chin Med J (Engl). 2010;123:1967–1972. [PubMed] [Google Scholar]
- 16.Ren ZG, Gan YH, Fan J, et al. Treatment of postoperative recurrence of hepatocellular carcinoma with radiofrequency ablation comparing with repeated surgical resection. Zhonghua wai ke za zhi. 2008;46:1614–1616. [PubMed] [Google Scholar]
- 17.Liang HH, Chen MS, Peng ZW, et al. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol. 2008;15:3484–3493. [DOI] [PubMed] [Google Scholar]
- 18.Umeda Y, Matsuda H, Sadamori H, et al. A prognostic model and treatment strategy for intrahepatic recurrence of hepatocellular carcinoma after curative resection. World J Surg. 2011;35:170–177. [DOI] [PubMed] [Google Scholar]
- 19.Chan AC, Poon RT, Cheung TT, et al. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg. 2012;36:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho CM, Lee PH, Shau WY, et al. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery. 2012;151:700–709. [DOI] [PubMed] [Google Scholar]
- 21.Eisele RM, Chopra SS, Lock JF, et al. Treatment of recurrent hepatocellular carcinoma confined to the liver with repeated resection and radiofrequency ablation: a single center experience. Technol Health Care. 2013;21:9–18. [DOI] [PubMed] [Google Scholar]
- 22.Chan AC, Chan SC, Chok KS, et al. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl. 2013;19:411–419. [DOI] [PubMed] [Google Scholar]
- 23.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. [DOI] [PubMed] [Google Scholar]
- 24.Bigourdan JM, Jaeck D, Meyer N, et al. Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transpl. 2003;9:513–520. [DOI] [PubMed] [Google Scholar]
- 25.Cherqui D, Laurent A, Mocellin N, et al. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250:738–746. [DOI] [PubMed] [Google Scholar]
- 26.Berry K, Ioannou GN. Are patients with Child’s A cirrhosis and hepatocellular carcinoma appropriate candidates for liver transplantation? Am J Transplant. 2012;12:706–717. [DOI] [PubMed] [Google Scholar]
- 27.Tranchart H, Chirica M, Sepulveda A, et al. Long-term outcomes following aggressive management of recurrent hepatocellular carcinoma after upfront liver resection. World J Surg. 2012;36:2684–2691. [DOI] [PubMed] [Google Scholar]
- 28.Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249:20–25. [DOI] [PubMed] [Google Scholar]
- 29.Rossi L, Zoratto F, Papa A, et al. Current approach in the treatment of hepatocellular carcinoma. World J Gastrointest Oncol. 2010;2:348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minagawa M, Makuuchi M, Takayama T, et al. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann surg. 2003;238:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulier S, Ni Y, Jamart J, et al. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nouso K, Matsumoto E, Kobayashi Y, et al. Risk factors for local and distant recurrence of hepatocellular carcinomas after local ablation therapies. J Gastroenterol Hepatol. 2008;23:453–458. [DOI] [PubMed] [Google Scholar]
- 33.Ballem N, Berber E, Pitt T, et al. Laparoscopic radiofrequency ablation of unresectable hepatocellular carcinoma: long-term follow-up. HPB (Oxford). 2008;10:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faber W, Sharafi S, Stockmann M, et al. Long-term results of liver resection for hepatocellular carcinoma in noncirrhotic liver. Surgery. 2013;153:510–517. [DOI] [PubMed] [Google Scholar]
- 35.Sakon M, Umeshita K, Nagano H, et al. Clinical significance of hepatic resection in hepatocellular carcinoma: analysis by disease-free survival curves. Arch Surg. 2000;135:1456–1459. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda M, Fujii H, Kono H, et al. Surgical treatment of recurrent hepatocellular carcinoma based on the mode of recurrence: repeat hepatic resection or ablation are good choices for patients with recurrent multicentric cancer. J Hepatobiliary Pancreat Surg. 2001;8:353–359. [DOI] [PubMed] [Google Scholar]
- 37.Mertens JC, Martin IV, Schmitt J, et al. Multikinase inhibitor sorafenib transiently promotes necrosis after radiofrequency ablation in rat liver but activates growth signals. Eur J Radiol. 2012;81:1601–1606. [DOI] [PubMed] [Google Scholar]
- 38.Kagawa T, Koizumi J, Kojima S, et al. ; Tokai RFA Study Group. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer. 2010;116:3638–3644. [DOI] [PubMed] [Google Scholar]


