Abstract
The purpose of this study was to evaluate the diagnostic accuracy of multiparametric evaluation of breast lesions combining information of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), diffusion-weighted imaging (DWI), and 18F-fluoro-deoxi-glucose (18F-FDG) positron emission tomography/computed tomography (PET-CT). After approval of the institutional research ethics committee, 31 patients with suspicious breast lesions on MRI performed 18F-FDG PET-CT with a specific protocol for breast evaluation. Patients’ mean age was 47.8 years (range, 29–77 years). Positron emission tomography and magnetic resonance imaging (PET-MRI) images were fused. A lesion was considered positive on multiparametric evaluation if at least 1 of the following was present: washout/type 3 kinetic curve on DCE-MRI, restricted diffusion on DWI with minimum apparent diffusion coefficient value <1.00 × 10−3 mm2/s, and abnormal metabolism on 18F-FDG PET-CT (higher than the physiologic uptake of the normal breast parenchyma). Thirty-eight lesions with histologic correlation were evaluated on the 31 included patients, being 32 mass lesions (84.2%), and 6 nonmass lesions (15.8%). Lesions’ mean diameter was 31.1 mm (range, 8–94 mm). Multiparametric evaluation provided 100% sensitivity, 55.5% specificity, 87.9% positive predictive value, 100% negative predictive value, and 89.5% accuracy, with 29 true-positives results, 5 true-negatives, 4 false-positives, and no false-negative results. Multiparametric evaluation with PET-MRI functional data showed good diagnostic accuracy to differentiate benign from malignant breast lesions, reducing the number of unnecessary biopsies, without missing any diagnosis of cancer in our case series.
INTRODUCTION
Conventional imaging methods for breast evaluation, such as mammography and ultrasound provide many false-positive results, leading to a large number of unnecessary biopsies. Functional imaging methods show metabolic/biologic alterations in breast tissue that may be useful in further evaluation of suspicious lesions, providing greater confidence for choosing the appropriate management for each case.1,2 Most used functional imaging methods for breast cancer management are magnetic resonance imaging (MRI) and 18F-fluoro-deoxi-glucose (18F-FDG) positron emission tomography/computed tomography (PET-CT).
The main advantage of MRI is its high sensitivity because of the ability to assess the breast tissue vascularization after intravenous injection of the paramagnetic contrast. The enhancement pattern and morphology are the most important criteria used to identify suspicious lesions on breast MRI. However, despite showing a high sensitivity (90%) for the diagnosis of breast cancer, specificity of breast MRI is only moderate (72%) on most studies.3 Dynamic contrast-enhanced (DCE) MRI has been widely used to improve the specificity of MRI in characterizing breast lesions. DCE-MRI analysis provides the assessment of the type of time–signal intensity curve (kinetic curve), which is predictive of malignancy.4 Recently, diffusion-weighted imaging (DWI) has also being incorporated to breast MRI, with the potential to increase its specificity.5 DWI provides information about the free movement of water molecules in the tissue, which is mainly related to tissue cellularity and integrity of cell membranes. The high cell proliferation in malignant tumors causes more barriers to the diffusion of water molecules, resulting in high signal on DWI sequences. Instead, the benign tumors have a lower cell density and higher extracellular space, providing lower diffusion restriction of the water molecules. Because of these characteristics, the DWI appears to be a useful tool for differentiating between benign and malignant lesions. DWI can be quantified using the apparent diffusion coefficient (ADC), which is inversely proportional to the restriction on the movement of water molecules.6
18F-FDG PET-CT provides information related to tissue glucose metabolism and has been widely used for evaluation of different types of cancer. For patients with breast cancer, PET-CT has a proven role in detecting distant metastasis, recurrence, and evaluation of treatment response.7 For the diagnosis of primary breast lesions and locoregional staging, PET-CT alone has limited diagnostic value when compared with other imaging methods, mainly because of its low sensitivity for small and low-grade lesions.8 However, some authors have shown that the fusion of MRI and prone PET images had a good diagnostic accuracy to differentiate benign from malignant breast lesions, with potentially greater specificity than MRI images alone.9,10
The combination of PET and MRI offers multiple functional information that complement each other along with high-resolution anatomy.1,11 The aim of this study is to evaluate the diagnostic accuracy of multiparametric evaluation of breast lesions combining information of DCE-MRI, DWI, and dedicated 18F-FDG PET-CT. PET-MRI-fused images were evaluated to better locate the corresponding lesions in both methods.
MATERIALS AND METHODS
After approval of the institution’s ethics review board and the National Committee for Research Ethics (CONEP), 31 patients with suspicious breast lesions on MRI and former indication of biopsy were included and performed 18F-FDG PET-CT with a specific protocol for breast evaluation. Patients’ mean age was 47.8 years (range, 29–77 years). All patients provided written informed consent to participate in the study and publish its results.
MRI was obtained with patient in prone position on a 1.5 T unit (SIGNA HDxt; GE Healthcare, Milwaukee, WI) using dedicated breast coil. Unenhanced sequences included axial T1-weighted 3-dimensional (3D) gradient echo (GRE) pulse sequence and sagittal T2-weighted short tau inversion recovery pulse sequence with fat signal suppression. The DWI sequences were performed with a 2-dimensional array spatial sensitivity encoding technique echo-planar imaging sequence in the axial plane. The sensitizing diffusion gradients were applied in 2 orthogonal planes with b values of 0 and 750 s/mm2. Dynamic evaluation included 5 axial T1-weighted 3D dynamic GRE pulse sequences, 1 precontrast, and 4 postcontrast, with fat signal suppression. The paramagnetic contrast used was Gd-DTPA (gadopentetate dimeglumine) at a dose of 20 mL (infusion rate of 3 mL/s), followed by bolus injection of 20 mL of saline. The final sequence consisted of a sagittal T1-weighted 3D GRE pulse sequence, with slice thickness of 1 mm and fat signal suppression. Breast MRI findings were interpreted by at least 2 radiologists with at least 8 years of experience on breast imaging, not blinded to the conventional imaging tests (mammography and ultrasound). The morphologic and kinetic characteristics of lesions evident on MRI were evaluated according to the Breat Imaging Report and Data System (BI-RADS) lexicon (2013).12 On DCE analysis, a small region of interest (ROI) was placed on the area of maximum enhancement within each lesion for curve analysis. Kinetic curves were classified as type I, persistently enhancing (progressive) type, which is suggestive of benignity; type II, plateau type, which has an intermediate probability for malignancy; and type III, washout type, which is indicative of malignancy. For DWI analysis, ADC maps were obtained using commercial software (Functool 7.4.01d; GE Healthcare, Milwaukee, WI), and the ROI was placed on the solid portion of the target lesion, avoiding necrotic or cystic areas. ADC values were calculated according to the following formula: ADC = 1/(b2 − b1) × ln(S1/S2), where S1 and S2 values were the signal intensities at the b values of 0 and 750 s/mm2, respectively.
PET-CT was performed on dedicated equipment (PET-CT Gemini; Philips Medical Systems, Cleveland, OH) after the administration of 0.154 mCi/kg of 18F-FDG in fasting, during muscle rest. Before the administration of 18F-FDG serum glucose levels were <150 mg/dL. The images were initiated between 60 and 120 minutes after the injection. Whole-body PET-CT examinations were performed on supine position, followed by dedicated images for breast evaluation on prone position, with the breasts set on a specially made device, which reproduces exactly the shape and position of the coil used in MRI. The cephalocaudal acquisition begins with contiguous CT slices of 2.5 mm thickness being conducted in 2-channel coil system without the use of intravenous contrast, followed by the acquisition of PET images. Each bed position of 15 cm field of view was acquired with acquisition time of 90 seconds. The reconstruction was performed in a 256 × 256 matrix, 60-cm field of view, and a section thickness of 2.5 mm. The interpretation and evaluation of PET-CT with 18F-FDG was performed by at least 2 nuclear medicine physicians, with at least 10 years of experience, blind to MR images. Lesions were considered positive on PET-CT visual analysis if its activity was greater than the adjacent parenchymal physiologic activity. The maximum SUV (standard uptake value) was calculated for each lesion after placement of the ROI covering the entire lesion. For data analysis, only the maximum SUV value measured in prone PET-CT images was considered.
PET and MRI (PET-MRI) images were fused on a dedicated workstation with Aquarius software, version 4.4 (Terarecon Inc, San Mateo, CA), by a radiologist with expertise in breast MRI, to better locate corresponding lesions. A lesion was considered positive on multiparametric evaluation if at least 1 of the following was present: washout/type 3 kinetic curve on DCE-MRI, restricted diffusion on DWI with minimum ADC value <1.00 × 10−3 mm2/s, and abnormal metabolism on 18F-FDG PET-CT (higher than the physiologic uptake of the normal breast parenchyma).
Statistical analyses were performed using SPSS for Windows, version 17.0 (SPSS Inc, Chicago, IL). For descriptive analysis, absolute and relative frequencies were calculated for all variables. Continuous variables were expressed as mean and standard deviation when distribution is normal. Sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) were calculated for multiparametric PET-MRI evaluation, using histologic results as gold standard.
RESULTS
Thirty-eight lesions with histologic correlation were evaluated on the 31 included patients, 32 being mass lesions (84.2%), and 6 nonmass lesions (15.8%). Lesions’ mean diameter was 31.1 mm (range, 8–94 mm). Most mass lesions had irregular shape (n = 25, 78.1%) and heterogeneous enhancement (n = 27, 84.4%). Most nonmass lesions had a segmental distribution (n = 4, 66.7%). According to the BI-RADS lexicon, 2 lesions (5.3%) were classified as category 3, 13 (34.2%) as category 4, 14 (36.8%) as category 5, and 9 (23.7%) as category 6.
On DCE-MRI, type I kinetic curve was present in 9 mass lesions (28.2%), type II curve in 8 mass lesions (25.0%), and type III curve in 15 mass lesions (46.9%). On DWI, minimum ADC value ranged from 0.44 to 2.10 × 10−3 mm2/s (mean 1.02 ± 0.39 × 10−3 mm2/s), and 26 lesions (68.4%) showed restricted diffusion (ADC <0.00 × 10−3 mm2/s). PET-CT showed increased metabolic activity on 30 lesions (78.9%), with a maximum SUV ranging from 1.1 to 15.0 (mean, 4.8 ± 4.1). All lesions considered negative on PET-CT had maximum SUV value <1.0. On multiparametric evaluation, 33 (86.8%) lesions were considered positive and 5 (13.2%) lesions were negative.
Histologic results were obtained by ultrasound-guided percutaneous core needle biopsy in 21 lesions (55.3%) and surgical excision in 17 lesions (44.7%). Histologic evaluation showed 29 (76.3%) malignant lesions and 9 (23.7%) benign lesions. The most common benign lesion was fibroadenoma, found in 5 patients (55.6%). Invasive ductal carcinoma (IDC) was the most frequent malignant lesion, found in 26 cases (89.7%).
Table 1 shows comparison between histologic results and DCE-MRI, DWI, PET-CT, and multiparametric evaluation. All lesions with type III kinetic curve on DCE-MRI were malignant on histology, but there were 9 false-negative results. There were 4 false-negative results on DWI that showed increased 18F-FDG uptake on PET-CT, which included 1 IDC, 2 ductal carcinoma in situ (DCIS), and 1 mucinous carcinoma (Figure 1). There were 2 false-negative results on PET-CT that showed restricted diffusion on DWI, both were small low-grade IDC with positive hormone receptors expression and negative Her-2 status (Figure 2). Multiparametric evaluation provided 100% sensitivity, 55.5% specificity, 87.9% PPV, 100% NPV, and 89.5% accuracy, with 29 true-positive results, 5 true-negatives, 4 false-positives, and no false-negative results.
TABLE 1.
Comparison Between DCE-MRI, DWI, PET-CT, and Multiparametric Evaluation and Histologic Results of Breast Lesions (n = 38)
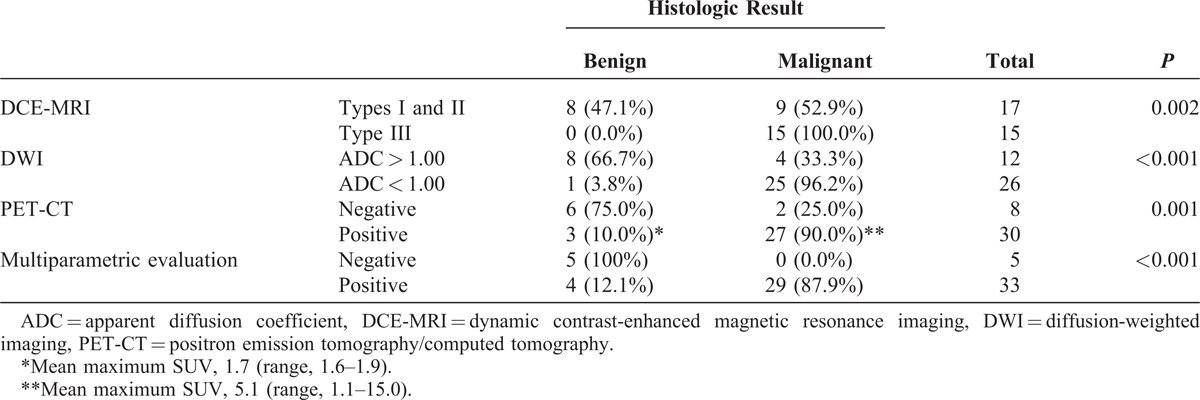
FIGURE 1.
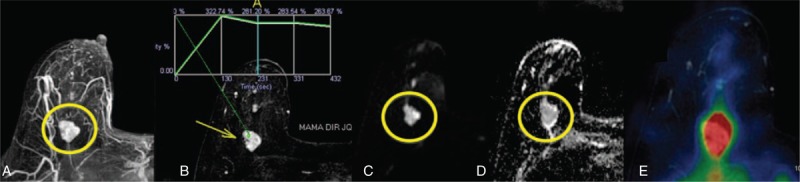
A 63-year-old female patient with a probably benign mass on conventional breast examinations. MRI showed a lobulated mass in the right breast (A). DCE-MRI showed an indeterminate plateau-type kinetic curve (B). The lesion showed high signal on DWI (C) and a small decrease in signal intensity on ADC map (D), ADC = 1.50 × 10−3 mm2/s, suggestive of benign lesion. PET-MRI fusion showed increased 18F-FDG uptake (E), suggestive of malignant lesion. Percutaneous biopsy was compatible with mucinous carcinoma. 18F-FDG = 18F-fluoro-deoxi-glucose, ADC = apparent diffusion coefficient, DCE-MRI = dynamic contrast-enhanced magnetic resonance imaging, DWI = diffusion-weighted imaging, PET-MRI = positron emission tomography and magnetic resonance imaging.
FIGURE 2.
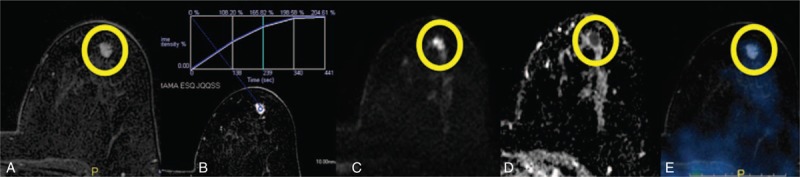
A 57-year-old female patient with a proven IDC in the right breast. Preoperative MRI showed a small mass on the left breast (A). DCE-MRI showed a progressive-type kinetic curve (B), suggestive of benign lesion. The lesions showed high signal on DWI (C) and low signal on ADC map (D), ADC = 0.84 × 10−3 mm2/s, suggestive of malignant lesion. PET-MRI fusion showed no increase of 18F-FDG uptake on the lesion (E), suggestive of benign lesion. After surgical resection, the histologic diagnosis was low-grade IDC. 18F-FDG = 18F-fluoro-deoxi-glucose, ADC = apparent diffusion coefficient, DCE-MRI = dynamic contrast-enhanced magnetic resonance imaging, DWI = diffusion-weighted imaging, IDC = invasive ductal carcinoma, PET-MRI = positron emission tomography and magnetic resonance imaging.
DISCUSSION
Functional imaging has evolved significantly in recent years, improving the diagnostic yield of breast lesions suspicious for malignancy. In the modern concept of target therapy, PET-MRI seems to be an advantageous alternative for breast cancer management. The results of the present study show that this methodology is promising and has a good diagnostic accuracy to differentiate benign from malignant breast lesions, reducing the number of false-positive results found on MRI.
Although DWI and PET reflect different tissue properties, previous studies have shown the association between their measures in different organs, most probably because increased cellularity is related to increased glucose metabolism in many malignant tumors.13–15 However, some articles that compared both methods in the evaluation of breast lesions have shown that the correlation between these 2 biomarkers is relatively weak.16,17 Given that both DWI and PET have similar clinical applications, ADC and SUV values may offer complementary information to aid in determination of diagnosis and prognosis of breast tumors.18
Previous studies have shown that the combined analysis of functional imaging data, such as DCE-MRI, DWI, and 18F-FDG PET-CT, significantly improves the diagnostic accuracy of breast MRI.19–22 However, none of these methods is 100% sensitive or specific because there is a considerable overlap of benign and malignant lesions, resulting in both false-negative and false-positive results. False-negative results are the most relevant for breast cancer and must be avoided because they can delay proper diagnosis and treatment.
On DCE-MRI, washout type kinetic curve is very suggestive of breast cancer; however, this enhancement pattern has been found in a small proportion of malignant lesions. Most authors suggest that both plateau or washout types should be suggestive of malignancy; however, we considered only the second in our study to reduce the number of false-positive results. Bluemke et al23 found a 20.5% sensitivity and 90.4% specificity for washout type, and 63.2% sensitivity and 65.4% specificity when using either plateau or washout types as an indicator of malignancy. In our case series, washout type was present in 62.5% of malignant mass lesions.
On DWI, false-negative values can be obtained in cystic/necrotic malignancies, low-grade tumors such as DCIS and malignant lesions with lower cellularity, such as mucinous carcinoma.24 We found four false-negative results on DWI. Because of the presence of mucin, mucinous carcinomas typically show high signal on T2 sequences and high ADC values, as shown in one of our cases.25 Cheng et al26 recently showed higher ADC values for DCIS, when compared with IDC, and for nonmass lesions, when compared with mass lesions.
False-negative results can also occur in 18F-FDG PET-CT. In our sample, there were 2 false-negative results on PET-CT that showed restricted diffusion on DWI, both were small low-grade IDC with positive hormone receptors expression and negative Her-2 status. Previous studies have demonstrated the lower sensitivity of PET-CT for low-grade tumors and lesions <10 mm because of the limited spatial resolution of PET scanners.8,27,28 In addition, 18F-FDG uptake is lower on tumors with positive hormone receptor expression (luminal A and luminal B subtypes), when compared with less-differentiated tumors with negative hormone receptor expression (Her-2 and triple-negative subtypes).29,30 Several methods have been developed to improve the current results of PET-CT for the diagnosis of breast lesions. Recently, a research group has been developing a dedicated simultaneous PET-MRI breast imaging system, which allows a combination of a high-resolution PET scan with morphologic and functional MRI data in a single study.31 In addition to increased spatial resolution, more specific markers for breast cancer have been developed to overcome the results of the PET with 18F-FDG, which is a nonspecific marker. Among these new markers, we highlight the 18F-16-α-17-β-fluoroestradiol and 68Ga-trastuzumab, which can portray noninvasively tumor expression of estrogen receptors and Her-2, respectively, with the potential to assist in the treatment planning and response evaluation.1
We believe that the combination of multiple functional methods with high specificity for the diagnosis of breast cancer can reduce the number of false-negative results found on these methods alone and yet reduce the number of false-positive results of conventional MRI. However, most studies that evaluated PET-MRI for breast evaluation combined functional information of 18F-FDG PET-CT only with the morphological information of MRI. Pinker et al32 recently published the initial results of a study on multiparametric 18F-FDG PET-MRI, and their results were consistent with the findings of the present study. According to the data presented, when several MRI and PET parameters are combined, the sensibility and specificity for differentiation of benign and malignant breast tumors are higher than when only 1 or 2 parameters are used. In addition, multiparametric 18F-FDG PET-MRI may lead to a reduction of the unnecessary breast biopsies recommended by MRI only.32
There are no studies evaluating the costs of adding PET/CT to MRI in the evaluation of breast lesions. Currently, performing a combined PET-MRI is less cost-effective than existing breast imaging methods, and this approach would not be practical in a clinical setting until larger studies are developed. However, a significant reduction in unnecessary breast biopsies by using this combined method may improve its cost-effectiveness.
The results of this study should be considered in the context of some limitations. Because of the small size and high number of malignant cases in our sample, it is difficult to generalize the results to the general population. The inclusion of lesions with prior malignant diagnosis (BI-RADS 6 lesions) in this study could be considered a potential confounding bias; however, all these lesions were considered suspicious on MRI analysis blind to previous clinical data and were submitted to new biopsy or surgical resection after the PET-CT. In these cases, PET-CT was performed at least 15 days after the procedure to reduce interference of the inflammatory process in the SUV values. Finally, although we use a positioning device on the PET with the same model of the coil used in breast MRI, breast structures might be in a slightly different position in both tests. To minimize possible incompatibilities, we performed a manually adjusted alignment using identifiable landmarks in both the PET and MR images, as described in the methodology.
In conclusion, multiparametric evaluation with PET-MRI functional data showed good diagnostic accuracy to differentiate benign from malignant breast lesions, reducing the number of unnecessary biopsies, without missing any diagnosis of cancer in our case series. These initial results confirm the potential of multiparametric evaluation with PET-MRI in the differential diagnosis of breast lesions, which should be the subject of future studies to confirm these findings.
Footnotes
Abbreviations: 18F-FDG = 18F-FDG18F-fluoro-deoxi-glucose, ADC = apparent diffusion coefficient, BI-RADS = Breat Imaging Report and Data System, DCE = dynamic contrast enhanced, DCIS = ductal carcinoma in situ, DWI = diffusion-weighted imaging, Gd-DTPA = gadopentetate dimeglumine, GRE = gradient echo, IDC = invasive ductal carcinoma, MRI = magnetic resonance imaging, NPV = negative predictive value, PET-CT = positron emission tomography/computed tomography, PPV = positive predictive value, ROI = region of interest, SUV = standard uptake value.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Pinker K, Bogner W, Gruber S, et al. Molecular imaging in breast cancer—potential future aspects. Breast Care (Basel). 2011;6:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler. AM. A molecular approach to breast imaging. J Nucl Med. 2014;55:177–180. [DOI] [PubMed] [Google Scholar]
- 3.Peters NHGM, Borel Rinkes IHM, Zuithoff NPA, et al. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246:116–124. [DOI] [PubMed] [Google Scholar]
- 4.El Khouli RH, Macura KJ, Jacobs MA, et al. Dynamic contrast-enhanced MRI of the breast: quantitative method for kinetic curve type assessment. AJR Am J Roentgenol. 2009;193:W295–W300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandão AC, Lehman CD, Partridge SC. Breast magnetic resonance imaging: diffusion-weighted imaging. Magn Reson Imaging Clin N Am. 2013;21:321–336. [DOI] [PubMed] [Google Scholar]
- 6.Sahin C, Aribal E. The role of apparent diffusion coefficient values in the differential diagnosis of breast lesions in diffusion-weighted MRI. Diagn Interv Radiol. 2013;19:457–462. [DOI] [PubMed] [Google Scholar]
- 7.Lavayssière R, Cabée A-E, Filmont J-E. Positron emission tomography (PET) and breast cancer in clinical practice. Eur J Radiol. 2009;69:50–58. [DOI] [PubMed] [Google Scholar]
- 8.Groheux D, Espié M, Giacchetti S, et al. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388–405. [DOI] [PubMed] [Google Scholar]
- 9.Bitencourt A, Lima E, Chojniak R, et al. PET-MRI fusion in the diagnosis of breast lesions. Cancer Imaging. 2012;12:44. [Google Scholar]
- 10.Moy L, Ponzo F, Noz ME, et al. Improving specificity of breast MRI using prone PET and fused MRI and PET 3D volume datasets. J Nucl Med. 2007;48:528–537. [DOI] [PubMed] [Google Scholar]
- 11.Antoch G, Bockisch A. Combined PET/MRI: a new dimension in whole-body oncology imaging? Eur J Nucl Med Mol Imaging. 2009;36suppl 1:S113–S120. [DOI] [PubMed] [Google Scholar]
- 12.Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® magnetic resonance imaging. In: ACR BI-RADS® Atlas, Breast Imaging Report Data System. 5th ed Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 13.Schmidt H, Brendle C, Schraml C, et al. Correlation of simultaneously acquired diffusion-weighted imaging and 2-deoxy-[18F] fluoro-2-d-glucose positron emission tomography of pulmonary lesions in a dedicated whole-body magnetic resonance/positron emission tomography system. Invest Radiol. 2013;48:247–255. [DOI] [PubMed] [Google Scholar]
- 14.Schwenzer NF, Schmidt H, Gatidis S, et al. Measurement of apparent diffusion coefficient with simultaneous MR/positron emission tomography in patients with peritoneal carcinomatosis: comparison with 18F-FDG-PET. J Magn Reson Imaging. 2013. 10.1002/jmri.24497. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo B, Angotti F, Marano GD. Relationship between PET-FDG and MRI apparent diffusion coefficients in brain tumors. Q J Nucl Med Mol imaging. 2009;53:17–22. [PubMed] [Google Scholar]
- 16.Choi BB, Kim SH, Kang BJ, et al. Diffusion-weighted imaging and FDG PET/CT: predicting the prognoses with apparent diffusion coefficient values and maximum standardized uptake values in patients with invasive ductal carcinoma. World J Surg Oncol. 2012;10:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baba S, Isoda T, Maruoka Y, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med. 2014;55:736–742. [DOI] [PubMed] [Google Scholar]
- 18.Rakheja R, Chandarana H, DeMello L, et al. Correlation between standardized uptake value and apparent diffusion coefficient of neoplastic lesions evaluated with whole-body simultaneous hybrid PET/MRI. AJR Am J Roentgenol. 2013;201:1115–1119. [DOI] [PubMed] [Google Scholar]
- 19.Schnall MD, Rosten S, Englander S, et al. A combined architectural and kinetic interpretation model for breast MR images. Acad Radiol. 2001;8:591–597. [DOI] [PubMed] [Google Scholar]
- 20.Yabuuchi H, Matsuo Y, Okafuji T, et al. Enhanced mass on contrast-enhanced breast MR imaging: lesion characterization using combination of dynamic contrast-enhanced and diffusion-weighted MR images. J Magn Reson Imaging. 2008;28:1157–1165. [DOI] [PubMed] [Google Scholar]
- 21.Partridge SC, DeMartini WB, Kurland BF, et al. Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. AJR Am J Roentgenol. 2009;193:1716–1722. [DOI] [PubMed] [Google Scholar]
- 22.Moy L, Noz ME, Maguire GQ, et al. Role of fusion of prone FDG-PET and magnetic resonance imaging of the breasts in the evaluation of breast cancer. Breast J. 2010;16:369–376. [DOI] [PubMed] [Google Scholar]
- 23.Bluemke DA, Gatsonis CA, Chen MH, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA. 2004;292:2735–2742. [DOI] [PubMed] [Google Scholar]
- 24.Bitencourt A, Meyrellis L, Souza J, et al. Features of breast lesions on diffusion-weighted imaging. Cancer Imaging. 2012;12:195. [Google Scholar]
- 25.Woodhams R, Kakita S, Hata H, et al. Diffusion-weighted imaging of mucinous carcinoma of the breast: evaluation of apparent diffusion coefficient and signal intensity in correlation with histologic findings. AJR Am J Roentgenol. 2009;193:260–266. [DOI] [PubMed] [Google Scholar]
- 26.Cheng L, Bai Y, Zhang J, et al. Optimization of apparent diffusion coefficient measured by diffusion-weighted MRI for diagnosis of breast lesions presenting as mass and non-mass-like enhancement. Tumour Biol. 2013;34:1537–1545. [DOI] [PubMed] [Google Scholar]
- 27.Kumar R, Chauhan A, Zhuang H, et al. Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat. 2006;98:267–274. [DOI] [PubMed] [Google Scholar]
- 28.Bitencourt AGV, Lima ENP, Chojniak R, et al. Can 18F-FDG PET improve the evaluation of suspicious breast lesions on MRI? Eur J Radiol. 2014;83:1381–1386. [DOI] [PubMed] [Google Scholar]
- 29.Basu S, Chen W, Tchou J, et al. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: a potentially useful method for d. Cancer. 2008;112:995–1000. [DOI] [PubMed] [Google Scholar]
- 30.Bitencourt AGV, Lima ENP, Chojniak R, et al. Correlation between PET-CT results and histological and immunohistochemical findings in breast carcinomas. Radiol Bras. 2014;47:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravindranath B, Huang PJ, Junnarkar S, et al. Quantitative clinical evaluation of a simultaneous PET/MRI breast imaging system. J Nucl Med. 2012;53:1217. [Google Scholar]
- 32.Pinker K, Bogner W, Baltzer P, et al. Improved differentiation of benign and malignant breast tumors with multiparametric 18fluorodeoxyglucose positron emission tomography magnetic resonance imaging: a feasibility study. Clin Cancer Res. 2014;20:3540–3549. [DOI] [PubMed] [Google Scholar]


