Abstract
The purpose of this study was to report the outcomes of patients with symptomatic locally advanced/recurrent gastric cancer treated with radiotherapy (RT) using modern 3-dimensional conformal techniques.
We retrospectively reviewed patients who had palliative RT for index symptoms of gastric bleeding, pain, and obstruction. Study endpoints included symptom response, median survival, and treatment toxicity.
Of 115 patients with median age of 77 years, 78 (67.8%) patients had metastatic disease at the time of treatment. Index symptoms were gastric bleeding, pain, and obstruction in 89.6%, 9.2%, and 14.3% of patients, respectively. Dose fractionation regimen ranged from 8-Gy single fraction to 40 Gy in 16 fractions. One hundred eleven patients (93.3%) were computed tomography (CT) planned. Median follow-up was 85 days. Response rates for bleeding, pain, and obstruction were 80.6% (83/103), 45.5% (5/11), and 52.9% (9/17), respectively, and median duration of response was 99 days, 233 days, and 97 days, respectively. Median survival was 85 days. Actuarial 12-month survival was 15.3%. There was no difference in response rates between low (≤39 Gy) and high (>39 Gy) biologically effective dose (BED) regimens (α/β ratio = 10). Median survival was significantly longer in patients who responded to RT compared with patients who did not (113.5 vs 47 days, P < 0.001). Three patients (2.6%) had grade 3 Common Toxicity Criteria equivalent toxicity (nausea/vomiting/anorexia).
External beam RT delivered using 3-dimensional conformal techniques is highly effective and well tolerated in the local palliation of gastric cancer, with palliation lasting the majority of patient’s lives. Short (≤39 Gy BED) RT schedules are adequate for effective symptom palliation. A phase II study of palliative gastric RT is ongoing.
INTRODUCTION
Gastric cancer is a common cancer in Southeast Asia and is the fourth most common cancer affecting men in Singapore. Even with improvements in treatment, prognosis remains poor with 5 year survival rates of approximately 20%.1
Patients with locally advanced disease with or without distant metastases can have significant local symptoms. Common symptoms from local extension are pain, bleeding (hematemesis, melena), and obstruction (dysphagia, vomiting). These symptoms can cause significant impact on a patient’s quality of life.
Options for palliation of local symptoms include palliative gastrectomy, surgical bypass, endoscopic stenting, palliative chemotherapy, and palliative radiotherapy (RT). RT is commonly used to control these symptoms as it is noninvasive and palliates symptoms.2–5 However, there are limited data on the actual benefits of RT used in the treatment of gastric cancer symptoms.
Most reported series have used chemoradiation for the palliation of localized gastric cancer symptoms.6–8 In these reports, local symptoms appear to be improved in about 50% to 86% of patients and the median duration of response ranges from 4 to 18 months.6–8
The problems in these older series included small patient numbers, older RT techniques being based on 2-dimensional (2D) planning, and the use of orthovoltage/cobalt machines.5,6 These results may not apply to current practice. In Singapore, majority of patients with locally advanced gastric cancer are treated with modern 3-dimensional conformal techniques.
The aim of this study was to report the outcome of palliative RT in patients with symptomatic locally advanced or recurrent gastric cancer managed with modern RT techniques.
MATERIALS AND METHODS
Patients
This review was approved by the institution review board. From September 1999 to December 2011, 115 patients with symptomatic locally advanced or recurrent gastric cancer, who were managed with palliative RT at the National Cancer Institute, Singapore (NCIS), were reviewed.
Information was obtained from patients’ medical and RT databases. Index symptoms were identified. Clinical factors reviewed included patient demographics (age, sex, performance status), tumor details (stage, size, histology), and RT parameters (field size, whether computed tomography [CT] planned/not CT planned, total dose, dose fractionation regimens, field arrangements).
Eligibility criteria included patients with symptomatic biopsy proven gastric cancer managed with palliative RT. Patients required at least 1 index symptom such as pain, bleeding, or obstruction. Patients who had previous irradiation to the stomach or who were treated with chemoradiotherapy were excluded.
Patient characteristics are outlined in Table 1 with a median age of 76 years (range, 39–95) and predominantly men. Thirty seven (32.2%) patients had locally advanced disease. Seventy eight (67.8%) patients had metastatic disease. One patient had a local recurrence after subtotal surgical resection. One hundred three patients presented with bleeding, 17 patients with obstruction, and 11 patients with pain as the index symptom. Six patients had a combination of these symptoms. All patients received RT only as the primary palliative treatment modality.
TABLE 1.
Patient Characteristics
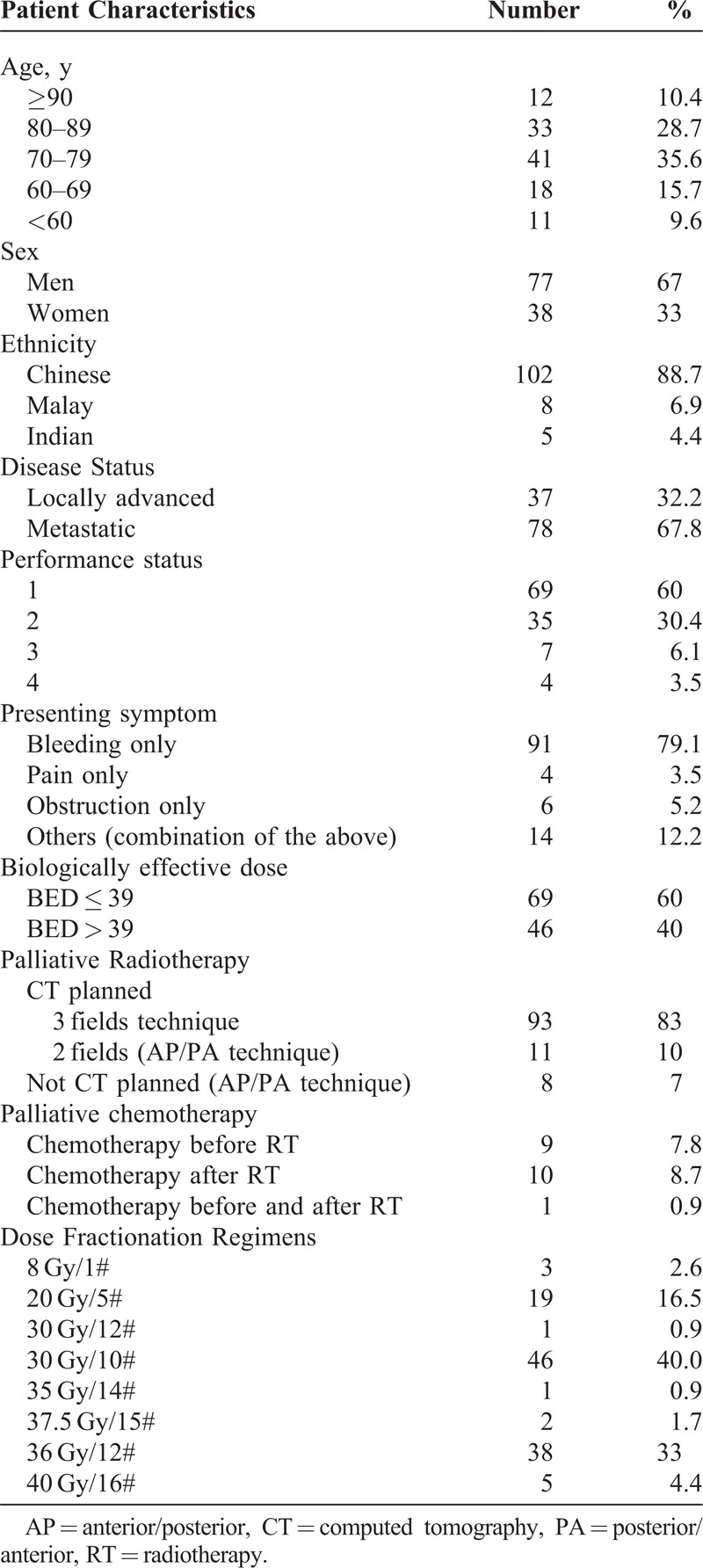
Ten patients received palliative chemotherapy before RT and 11 patients received palliative chemotherapy after completion of RT. The median follow-up was 85 days.
Pretreatment Evaluation
Pretreatment evaluation included recording of medical history, physical examination, and routine laboratory studies. Baseline staging investigations included abdominal and pelvic CT scan. Esophagogastroduodenoscopy was performed in all patients to confirm the diagnosis and also the presence of bleeding foci.
Radiotherapy
All patients received external beam RT. Radiation was administered with photons of 6 or 10 MV (megavoltage) photons from a linear accelerator. The majority of patients were CT planned (107 patients) although 8 patients were conventionally planned. The planning target volume was the stomach/partial stomach with a 1 to 2 cm margin. The organs of interest that were contoured included the liver, kidney, and spinal cord. Dose–volume histograms were reviewed, ensuring that 50% of the total renal volume did not receive a dose of 20 Gy or more. Stomach motion during respiration was included in volume expansion to plan target volume.
Of 107 patients who were CT planned, 96 were treated with a conformal multifield technique. The most commonly used technique was a single anterior/posterior (AP) field with parallel opposing lateral fields. Eleven patients who were CT planned and all 8 patients who were conventionally planned were treated with AP and posterior/anterior (PA) fields.
Radiation was delivered to the whole stomach in 109 patients and the partial stomach in 6 patients. The median field size was 18.3 × 14.7 cm (range, 7.5 × 8.5 to 27 × 23 cm).
RT regimens ranged from an 8-Gy single fraction to 40 Gy/16 fractions. The majority of patients (46/115) received 30 Gy/10 fractions delivered at 3 Gy/fraction, 5 fractions/wk. The distribution of patients by dose and fractionation is shown in Table 1.
A biologically effective dose (BED) of ≤39 Gy was received by 46/69 (67%) of patients with Eastern Cooperative Oncology Group (ECOG) 1, 14/35 (40%) with ECOG 2, 5/7 (71%) with ECOG 3, and 4/4 (100%) with ECOG 4.
A BED of >39 Gy was received by 23/69 (33%) of patients with ECOG 1, 21/35 (60%) with ECOG 2, 2/5 (29%) with ECOG, and 0/4 (0%) with ECOG 4.
Response Evaluation
Patients were reviewed 1-month after treatment for the assessment of treatment response. For patients with bleeding as the index symptom, a response to RT was scored when they did not require further blood transfusions or gastroscopy for hemostasis after treatment. For patients with pain as the index symptom, a partial response to RT was scored when they had decreased pain or decreased analgesia, same pain but decreased analgesia, and a complete response was scored if their pain resolved after treatment. Obstruction was defined in 3 categories: patients requiring parenteral nutrition, patients tolerating liquids, and patients tolerating solids. An improvement upward of 1 category quantified as a partial response. Resolution of obstructive symptoms was scored as a complete response. Duration of response was defined as the time from response in patients who achieved satisfactory palliation until symptom recurrence/progression or death. The duration of index-symptom relief and survival time from RT was determined for each patient. The ratio between this duration of symptom relief and survival multiplied by 100 was called “percent net symptom relief.”9 This represented the percentage of the remaining patients’ life after RT that was spent with relief of index symptom and without need for further treatment. Toxicity was retrospectively scored using the Common Toxicity Criteria version 3.0.
Biologically Effective Dose
BED is an approximate quantity by which different RT fractionation regimens are compared. It is given by BED = nD(1 + [D/{α/β}]), where n = number of fractions, D = dose/fraction, nD = total dose, and α/β is the alpha/beta ratio and is taken to be 10 for adenocarcinomas.
Statistical Analysis
Survival time after treatment was also recorded. Statistical analyses were performed using SPSS version 15.0 (SPSS Inc, Chicago, IL). Study end points included symptom response (including response rates and duration of response), median survival, and treatment toxicity. To investigate for a dose–response relationship, patients were grouped according to RT doses. BED was calculated using a tumor α/β ratio of 10. The median BED was 39 Gy, which corresponded to a dose fractionation regimen of 30 Gy in 10 fractions. Patients were divided into 2 subgroups distributed around the median BED. Subgroup analysis performed on patients according to BED was via Fisher exact test. The Kaplan–Meier method was used to draw the time to event curve. A P value of <0.05 (2-sided) was considered to be statistically significant.
RESULTS
Treatment Response
Relief From Bleeding
Eighty three of 103 (80.6%) patients with bleeding as the index symptom responded to RT. The median duration of response was 99 days. The mean percent net relief was 92.5%. Twenty patients with bleeding did not respond to treatment.
Relief From Pain
Five of 11 (45.5%) patients with pain as the index symptom had a partial response to RT. The median duration of response was 233 days. The mean percent net relief was 91.3%. This meant that for patients with pain, who responded to RT, the pain relief lasted without need for further treatment for 91.3% of their remaining life. Of the responders, 4 patients received 30 Gy/10# and 1 patient received 20 Gy/5#. The remaining 6 patients did not benefit from treatment.
Relief From Obstruction
Nine of 17 patients (52.9%) with obstruction as the index symptom had a partial response to RT. The median duration of response was 97 days. The mean percent net relief was 85.6%. Of the responders, 4 patients received 30 Gy/10#, 3 patients received 36 Gy/12#, and 2 patients received 20 Gy/5#. The remaining 8 patients did not benefit from treatment.
Survival
The median survival for our cohort of patients was 85 days with a 12 month actuarial survival of 15.3%.
Median survival of patients who responded to radiotherapy was 113.5 days (0–1225 days). The median survival of patients who did not respond to radiotherapy was 47 days (2–390 days). The median survival of patients who responded to radiotherapy was significantly longer compared with patients who did not respond to radiotherapy (P = <0.001) (Figure 1).
FIGURE 1.
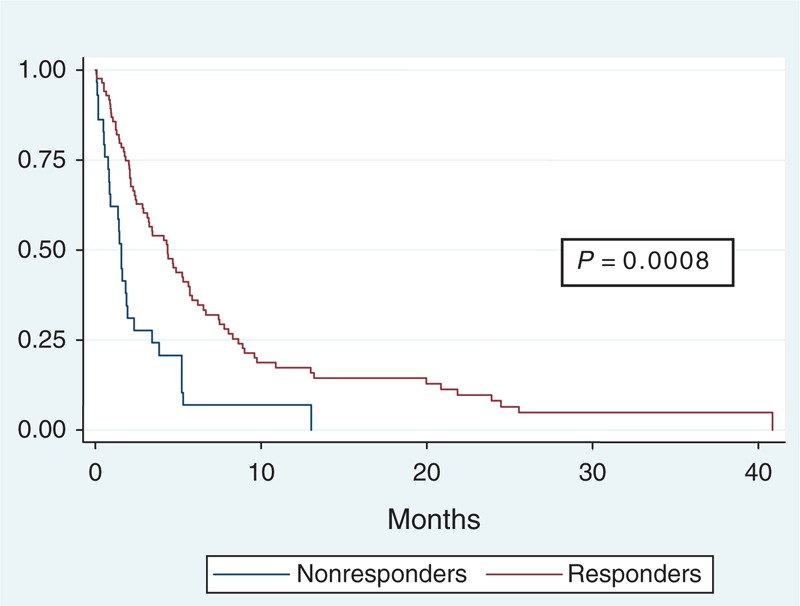
Kaplan–Meier survival curve patients who responded to RT versus patients who did not. RT = radiotherapy.
The planned treatment was completed by 112 (97.3%) patients. One died after 5 fractions. Two other patients did not continue after completing 8 and 10 fractions, respectively, because of deterioration of condition. Eight patients died during the first 4 weeks after the end of radiotherapy. They were scored as not having a response to radiotherapy.
Symptom Response According to BED
Forty four of 103 of patients with bleeding, 2 of 11 patients with pain, and 7 of 17 of patients with obstruction received a BED of >39 Gy. (Table 2).
TABLE 2.
Symptom Response and Symptom Recurrence According to BED
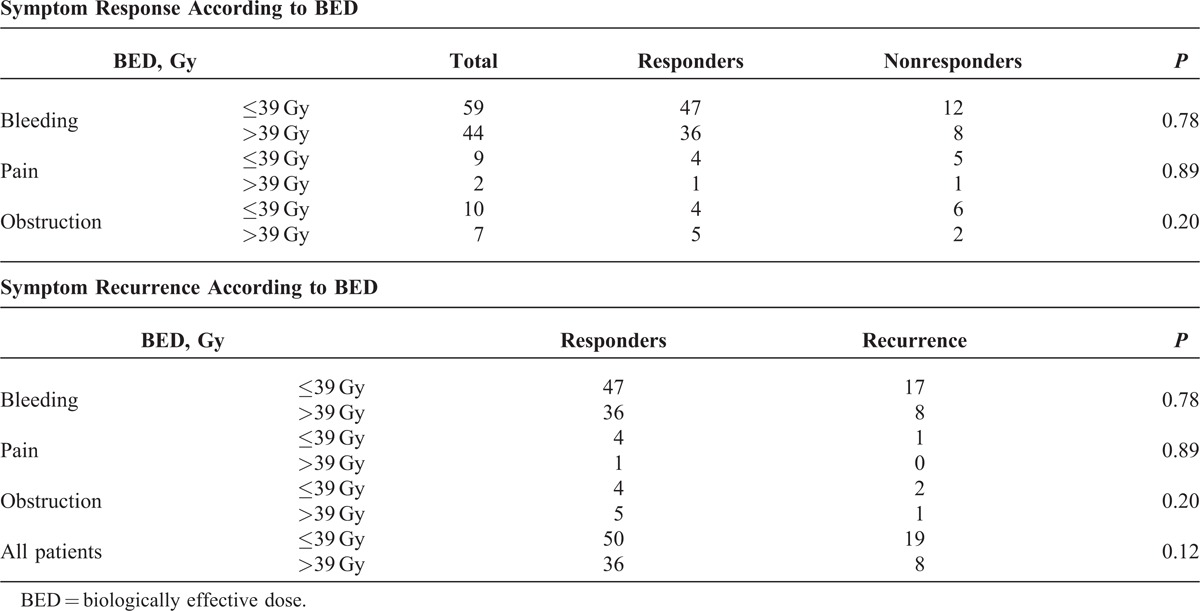
No obvious dose response was evident for symptom response using a cutoff median BED of 39 Gy.
Symptom Recurrence According to BED
There was a trend for poorer local control with a BED for ≤39 Gy (P = 0.12) (Table 2).
Recurrent bleeding occurred in 17/47 patients who received a BED of ≤39 Gy and 8/36 patients who received a BED of >39 Gy.
Recurrence/progression of pain occurred in 1/4 patients who received a BED of ≤39 Gy and 0/1 patient who received a BED of >39 Gy.
Recurrence/progression obstruction occurred in 2/4 patients who received a BED of ≤39 Gy and 1/5 patients who received a BED of >39 Gy.
Table 3 shows symptom response and symptom recurrence for selected group of variables.
TABLE 3.
Symptom Response and Symptom Recurrence for Selected Group of Variables
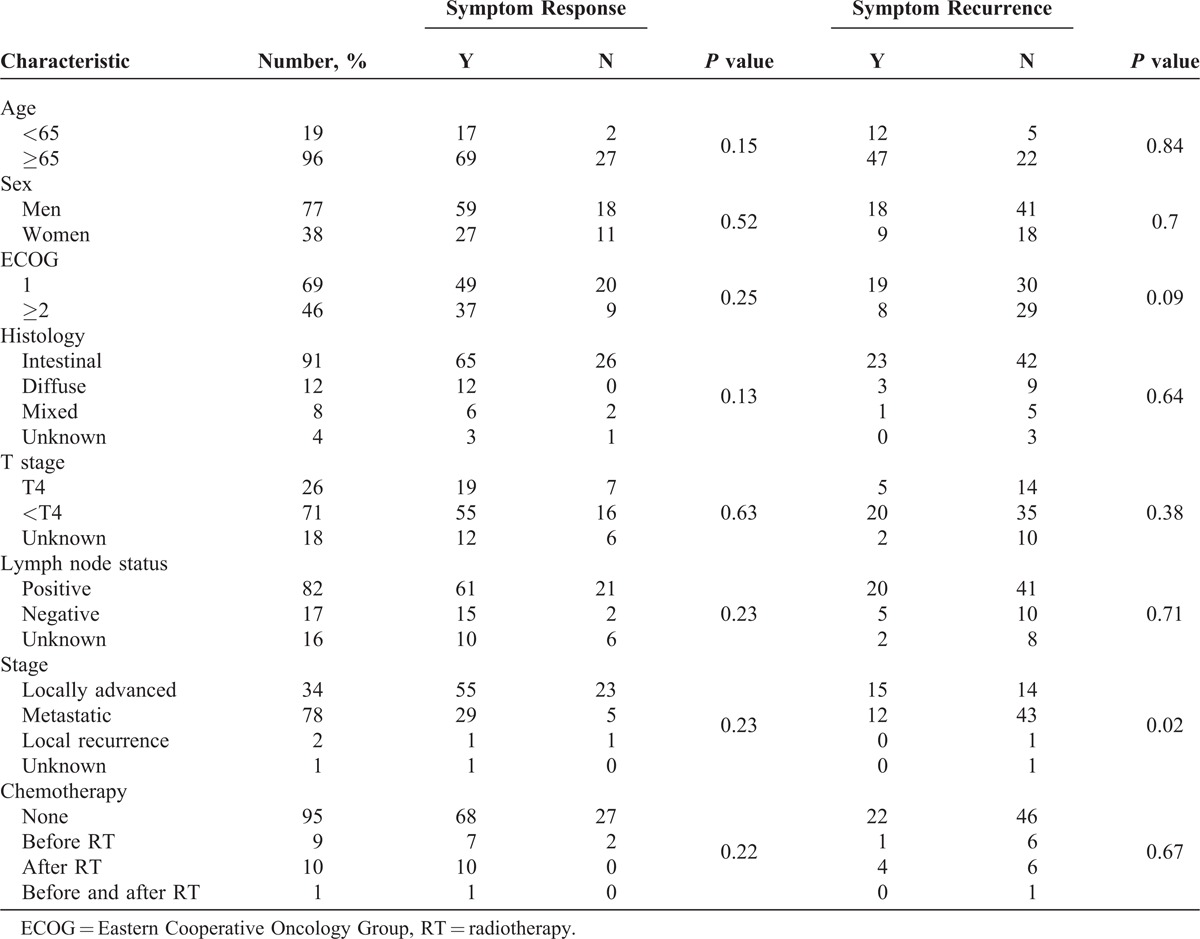
On univariate analysis, patients with locally advanced nonmetastatic disease had significantly poorer local control compared with patients with metastatic disease (P = 0.02).
Toxicity
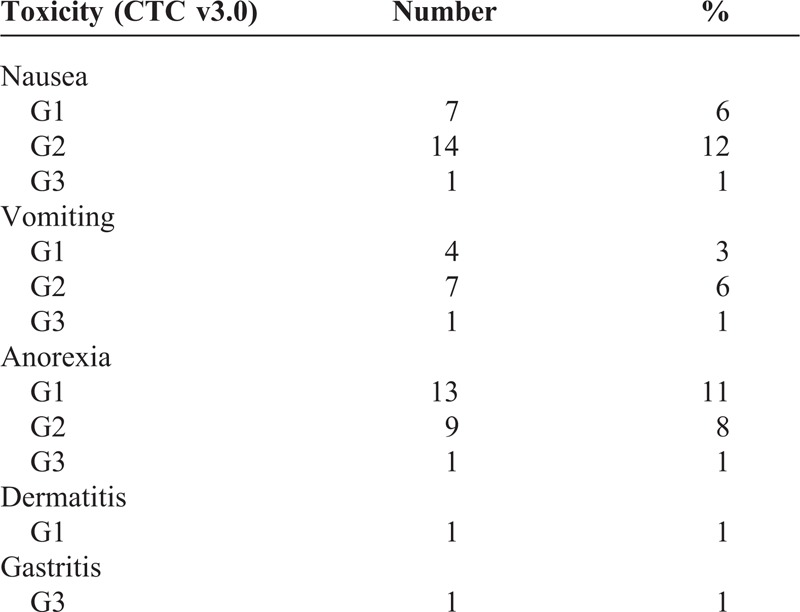
Treatment toxicity is displayed in Table 4. Three (3%) grade 3 Common Toxicity Criteria equivalent toxicity was recorded. One patient had nausea and vomiting and had to be admitted for intravenous fluids and antiemetics. One patient had gastritis and 1 patient had anorexia and had to be admitted for intravenous fluids. All 3 patients had transient symptoms that resolved after approximately 1 week’s stay in hospital. There were no grade 4 or 5 toxicities.
TABLE 4.
Toxicity for All Patients
DISCUSSION
This study demonstrates that palliative radiotherapy is highly effective and tolerable in the palliation of localized gastric cancer symptoms, with palliation lasting >85% of a patient’s life. It is the largest study that we know of evaluating the efficacy of palliative RT, delivered using modern radiotherapy techniques in gastric cancer palliation.
Palliative chemotherapy has improved the survival and quality of life of patients with advanced gastric cancer. Unfortunately, patients may be unfit to undergo these intensive regimens because of age or coexisting medical conditions. Our study included a predominantly elderly cohort of patients. The median age of our patients was 77 years (range, 39–95). Seventy five percent of our cohort of patients were aged 70 years and above, and >80% of patients were judged too frail to undergo chemotherapy.
Although some patients may have derived additional benefits from subsequent chemotherapy, the number of patients who received palliative chemotherapy following radiotherapy was small. Only 7.8% (9 patients) patients were eligible for chemotherapy before radiotherapy and 8.7% (10 patients) patients were eligible for chemotherapy after palliative radiotherapy. The impact of chemotherapy on the outcomes of patients is likely to be negligible given the small numbers of patients who received chemotherapy. No patient received concurrent chemotherapy together with radiotherapy.
The results from our review are comparable with older studies using RT alone or chemoradiation. Most data for palliative gastric treatments were based on chemoradiation. Median duration of responses has been shown to range from 4 to 18 months.6–8 However, the patient numbers were small and techniques used were old and based on 2D simulation in these reports. In a previous report, we documented the efficacy and tolerability of palliative radiotherapy in a small cohort of patients. Among 33 patients with locally advanced or recurrent gastric cancer treated with palliative radiotherapy, symptom relief was noted in 54.3% of patients (13/24) with bleeding, 25% of patients (2/8) with obstruction, and 25% of patients with pain (2/8). This palliation was durable and lasted for a median duration of 140, 102, and 105 days for dysphagia, bleeding, and pain, respectively. However, patient numbers were small and a correlation between radiation dose and symptom relief was not established.10 More recently, Kim et al11 reported their experience of 37 patients treated with palliative radiotherapy with or without chemotherapy. The rates of control for bleeding, dysphagia/obstruction, and pain were 70% (14/20), 81% (13/16), and 86% (6/7), respectively. These symptoms were controlled without additional interventions for a median of 70%, 81%, and 49% of the patients’ remaining life, respectively. Lower (<41 Gy) BED predicted poorer local control, whereas T4 tumors had a trend toward inferior local control. However, only 13 patients received radiotherapy alone and nearly two-thirds of patients received concurrent chemotherapy.
Concurrent chemoradiotherapy may increase symptom response rate when used for palliation of local symptoms but at the cost of higher treatment toxicity. Asakura et al12 reported a palliative benefit of 73% in 30 patients treated with palliative radiotherapy with or without chemotherapy for gastric cancer bleeding. Compared with radiotherapy alone, chemoradiotherapy patients had a significantly lower rebleed rate (P < 0.01) but at considerably increased toxicity. Among patients receiving chemoradiotherapy, 1 patient had grade 3 nonhematological toxicity and 5 patients had grade 3 and 4 hematological toxicity. There were no grade 3 or higher adverse events in patients treated with radiation alone.
Most reports on palliative radiotherapy for localized gastric cancer are based on 2D simulation and AP/PA techniques. The advent of megavoltage treatment machines with the use of CT planning has led to the development of new radiotherapy techniques with which cancer patients can be treated. The use of 3-dimensional information derived from CT scans allows us to visualize the tumor and organs at risk (such as the liver, kidney, and spinal cord) more accurately compared with 2D planning that relied on information from x-rays and fluoroscopy. This allows us to design multifield techniques to enable us to deliver a higher radiation dose to the tumor bed with greater conformality, while at the same time minimizing the dose to surrounding soft tissue, bone, liver, kidneys, and small bowel. We can thus treat to higher doses and larger field sizes, with minimal side effects throughout the range of doses used. There were very low rates of acute toxicities, despite some patients being treated with relatively large field sizes. The median field size was 18.3 × 14.7 cm (range, 7.5 × 8.5 to 27 × 23 cm). The low rates of severe toxicity (grade 3 and 4) may be because of the use of CT planning and hence reduced volume of normal tissue irradiated. Ninety three percent (107/115) of our patients were CT planned. Of patients who were CT planned, 90% (96/107) were treated with a multifield conformal technique.
A wide range of radiation dose/fractionation regimens were used and standardization of doses delivered was done by calculating the BED. Our results show that radiotherapy is effective throughout the dose fractionation regimens outlined, with a high response rate of 80% for bleeding and response rates of approximately 50% for pain and obstruction. Furthermore, response is durable and continues for >90% of patients’ remaining life. The median survival of patients who responded to radiotherapy was significantly longer than patients who did not respond (47 vs 113.5 days, P < 0.001). This suggests that local therapeutic effect may translate into longer survival. No obvious dose response was evident for symptom response and symptom recurrence using a cutoff median BED of 39 Gy. Similar results are seen for palliative radiotherapy for other tumor sites. Duchesne et al13 compared in a prospective randomized trial 2 fractionation regimens in the palliation of locally advanced symptomatic bladder cancer. There was no significant difference in efficacy for symptom palliation between the high BED regimen (35 Gy in 10 fractions) and the low BED regimen (21 Gy in 3 fractions). This suggests that a higher BED regimen may not be essential for effective palliation of local symptoms. Given that there was no obvious dose response between higher and lower BED regimens, individualization of treatment regimens based on patient’s performance status, disease burden may be important. It may be prudent to prescribe a short fractionation regimen with high doses/fraction to patients with poor performance status/widely metastatic disease, limited life expectancy, and those who require urgent symptom control. In addition, we found that patients with locally advanced nonmetastatic disease had significantly poorer local control than patients with metastatic disease (P = 0.02).
We have provided evidence for treatment doses and field sizes for effective gastric cancer palliation with minimal toxicity. However, there are limitations to this study. First, this study is retrospective in nature and subjected to reviewer bias. Second, our dose fractionation regimens were heterogeneous, including 8 different fractionation regimens. Prospective randomized trials are warranted to delineate the most appropriate fractionation schedules for effective and durable symptom palliation.
CONCLUSION
We conclude that external beam radiation therapy, delivered using modern RT techniques, is an effective and well-tolerated modality in the local palliation of gastric cancer, with lasting palliation of local symptoms. A high proportion of patients with bleeding achieve palliation with radiotherapy. Durable responses are achieved irrespective of the presenting symptom. Median duration of response is in the order of 99 days or more. This is close to the median survival of patients, which is 85 days. Symptom response is a surrogate for improved survival.
The optimal dose/fractionation regimen for palliation of local symptoms, however, remains to be established in prospective randomized trials.
Footnotes
Abbreviations: AP = anterior/posterior, BED = biologically effective dose, CT = computed tomography, PA = posterior/anterior, RT = radiotherapy.
Manuscript presented in part at the American Society of Therapeutic Radiation Oncology 2012 Annual Scientific Meeting.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Hundahl SA, Philips JL, Menck HR. The National Cancer Data Base report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: fifth edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 2.Mantell BS. Radiotherapy for dysphagia due to gastric carcinoma. Br J Surg. 1982;69:69. [DOI] [PubMed] [Google Scholar]
- 3.Myint AS. The role of radiotherapy in the palliative treatment of gastrointestinal cancer. Eur J Gastroenterol Hepatol. 2000;12:381–390. [DOI] [PubMed] [Google Scholar]
- 4.Tsukiyama I, Akine Y, Kajiura Y, et al. Radiation therapy for advanced gastric cancer. Int J Radiat Oncol Biol Phys. 1988;15:123–127. [DOI] [PubMed] [Google Scholar]
- 5.Nordman E. The value of megavolt therapy in gastric carcinoma. Bull Cancer. 1976;63:217–222. [PubMed] [Google Scholar]
- 6.Henderson IWD, Lipowska B, Lougheed MN, et al. Clinical evaluation of combined radiation and chemotherapy in gastrointestinal malignancies. Am J Roentgenol Radium Ther Nucl Med. 1968;102:545. [DOI] [PubMed] [Google Scholar]
- 7.Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373–378. [DOI] [PubMed] [Google Scholar]
- 8.Falkson G, Van Eden EB, Sandison AG. A controlled clinical trial of fluorouracil plus imidazole carboxamide dimethyl triazeno plus vincristine plus bis-chloroethyl nitrosourea plus radiotherapy in stomach cancer. Med Pediatr Oncol. 1976;2:111. [DOI] [PubMed] [Google Scholar]
- 9.Salazar OM, Rubin P, Hendrickson FR, et al. Single-dose half-body irradiation for the palliation of multiple bone metastases from solid tumor: Final RTOG report. Cancer. 1986;58:29–36. [DOI] [PubMed] [Google Scholar]
- 10.Tey J, Back MF, Shakespeare TP, et al. The role of palliative radiation therapy in symptomatic locally advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2007;67:385–388. [DOI] [PubMed] [Google Scholar]
- 11.Kim MM, Rana V, Janjan NA, et al. Clinical benefit of palliative radiation therapy in advanced gastric cancer. Acta Oncol. 2008;47:421–427. [DOI] [PubMed] [Google Scholar]
- 12.Asakura H, Hashimoto T, Harada H, et al. Palliative radiotherapy for bleeding from advanced gastric cancer. Is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol. 2011;137:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47:379–388. [DOI] [PubMed] [Google Scholar]


