Abstract
The purpose of this article is to perform a systematic review of the literature on the use of fecal microbiota transplantation (FMT) in inflammatory bowel disease (IBD).
There is an increasing interest of both physicians and patients in assessing the possible role of the FMT in the treatment of IBD.
Electronic and manual bibliographic searches were performed to identify original reports in which subjects with IBD were treated with FMT. Because of the scarcity of studies with adequate sample size, case series and case reports were also considered. A critical appraisal of the clinical research evidence on the effectiveness, safety, and other parameters related to FMT was made. Data extraction was independently performed by 2 reviewers.
We found a total of 31 publications on the use of FMT in IBD. The majority were case reports or case series, whereas 8 publications reported data from open-label trials including a very less number of patients. A total of 133 patients with IBD were managed with FMT. Of these, 57 subjects (43%) had a Clostridium difficile infection. A resolution or reduction of symptoms was reported in 80 of 113 (71%) patients with evaluable IBD. Moreover, FMT does not seem to provide the same safety profile showed for non-IBD individuals with C difficile infection.
The available evidence is limited and weak. FMT has the potential to be somehow of help in managing patients with IBD, but considerable further efforts are necessary to make this procedure a valid option for these subjects.
INTRODUCTION
Fecal microbiota transplantation (FMT), also known as “fecal bacteriotherapy” or “fecal infusion,” consists in the injection (by various routes: nasogastric or nasojejunal tube, upper endoscopy, retention enema, colonoscopy) of a liquid filtrate of feces from healthy donor into the gastrointestinal tract of recipient individual.1 The first modern use of FMT in humans was for the treatment of pseudomembranous colitis which at that time was believed to be caused by Micrococcus pyogenes (Staphylococcus). It was given as fecal enemas and was reported in 1958 in a 4-patient case series by Eiseman et al.2 Use of fecal transplantation for Clostridium difficile infection was also by enema and first reported in 1983 by Schwan et al.3
This procedure is now thought to at least, in part, restore the normal, functional intestinal microbiota in recipient patients with recurrent C difficile-related diarrhea. Because of this reason, the use of FMT against C difficile infection has raised worldwide, especially in developed countries, and its efficacy has been assessed by larger case series and also by a randomized controlled trial.4–7
Moreover, FMT has gained pathophysiological strength since the recently established concept of human gut microbiota and its significant role in health and disease has caught on in the medical scientific community. Considering gut microbiota as another organ of our body, the meaning of FMT changes from a simple infusion of stools to a kind of organ transplantation, that is, the transfer of microbial flora from a healthy donor to a patient with a disrupted one.8 This theoretical revolution has made FMT being experienced in several diseases related to gut microbiota imbalance, such as metabolic syndrome9 and inflammatory bowel disease (IBD).10
Although the etiology of IBD is unclear, both genetic and environmental factors are known to play a role in the pathogenesis of the disease. As shown by concordance studies in monozygotic twins, genetic factors have only a partial role in the development of the disease, and almost all genes linked to IBD are related to mucosal immunity.11,12 Among environmental factors, alteration of gut microbiota is known to be deeply involved in the pathogenesis of IBD, and the concept of an altered network between gut microbiome and host genetic factors, that leads to the loss of homeostasis, has been hypothesized.13,14 However, it is not yet clear if dysbiosis is a possible cause or an epiphenomenon of the disease.15 At present, almost all effective therapies (such as aminosalicylates, steroids, immunosuppressants, and biologics) are targeted toward the inflammatory and/or immunological component of the disease, and gut microbiota modulation through prebiotics and probiotics have shown uncertain results.16,17
FMT has been therefore attempted for the management of IBD, and there is a diffuse, increasing interest of both clinicians and patients in this issue.18 The first description on the use of FMT for the management of IBD dates back to 1989, when Justin D Bennet, suffering from ulcerative colitis (UC), self-administered a fecal infusion from a healthy donor.19 Bennet had active, severe UC, confirmed by endoscopy and histology and refractory to steroids and salicylates, for 7 years. The fecal transplant was carried out by large-volume retention enemas, and gave surprising results: Bennet kept himself symptom-free 6 months after the procedure, and biopsy samples of colonic mucosa revealed only signs of long-standing chronic inflammation, without features of acute inflammation. Afterward, other reports were published, most of which were case reports or case series and published only as abstracts at conferences. Moreover, many referred to patients with IBD treated with FMT for C difficile infection.
Until now, only 1 systematic review has collected all pertinent reports up to 2011 on FMT in IBD, reporting a total of 41 patients with IBD who were treated with FMT10; a further narrative review on this topic reported a total of 8 patients with IBD treated with feces infusion.20
The aim of this systematic review is to analyze the potential role of FMT in the management of IBD, focusing on current pitfalls and available results.
METHODS
Our systematic review was conducted, when possible, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.21 Ethical approval was not necessary for this review study.
Eligibility Criteria
All original reports in which human subjects of any age with IBD were treated with FMT for the management of either IBD or C difficile infection in subjects with underlying IBD were considered for inclusion. Inclusion criteria also required the report of either safety or efficacy outcomes. Studies evaluating treatments other than FMT were excluded, as well as those including patients without IBD treated with FMT. In the case of mixed cohorts, only data from patients with IBD were taken into account. We did not include animal model studies or studies other than original reports (reviews, systematic reviews, meta-analyses, editorials, etc).
Because of the likely scarcity of studies with adequate sample size, case series with <10 patients and case reports were also considered, without year-span limits. Both pediatric and adult subjects were included. No language restriction was used in the search filter. We also included data that were presented only as abstracts at conferences.
Information Sources and Search Strategy
A literature search was performed using the following electronic databases: PubMed, SCOPUS, Web of Science (ISI), and the Cochrane Library. The last search was run on January 10, 2014. The terms “fecal microbiota,” “faecal microbiota,” “fecal” “faecal,” “feces,” “faeces,” “stool,” “microflora,” “fecal flora,” “faecal flora” were matched with the following words: “transplantation,” “transplant,” “bacteriotherapy,” “transfer,” “donation,” “donor,” “infusion,” “instillation,” “reconstitution,” “suspension,” “implant,” “implantation.” All the deriving terms were searched alone or in combination. They were then combined, by the Boolean operator “AND,” with the following terms: “IBD,” “inflammatory bowel disease,” “Crohn’s disease,” “ulcerative colitis,” “Clostridium difficile,” “Clostridium infection,” “pseudomembranous colitis.” The terms “Clostridium infection,” “Clostridium difficile,” and “pseudomembranous colitis” were considered in the search strategy to assure the identification of studies including patients with C difficile infection and underlying IBD.
All the terms were searched both as keywords and medical subject headings. The complete string used for the electronic search is shown in Table 1.
TABLE 1.
Complete String Used for the Electronic Search

The bibliographies of relevant (according to titles and abstracts) articles were hand-searched to provide additional references. Records from the following yearly symposia were hand-searched to find pertinent abstracts: United European Gastroenterology (former United European Gastroenterology Federation), 2008–2013; Digestive Disease Week, 2001–2013; European Crohn’s and Colitis Organization (ECCO) Congress, 2007–2012; and Crohn’s and Colitis Foundation of America (CCFA) Annual Scientific Meeting, 2003–2013. When necessary, the authors of the articles were also contacted for clarifications or missing information about their data.
Study Selection
Titles and abstracts were independently assessed by 2 reviewers (G.I. and G.C.) to determine the eligibility of the studies. Both investigators checked the fulfillment of inclusion and exclusion criteria; in the case of doubt, the full-text articles were retrieved and reviewed. A third author (S.B.) arbitrated in all the cases of a lack of agreement.
Data Collection Process and List of Items
Data extraction was performed independently by 2 reviewers (G.I. and G.C.), and were then cross-checked. Discrepancies were rectified by consensus. In the case of different reports from the same group of patients, the study with the most complete data was included. When articles grouped patients from a previous study and newly enrolled ones, only the latter were considered. In the case of mixed cohorts, including patients both with and without IBD (as in the case of subjects suffering from C difficile infection), only data from the former were included for the analysis.
Data related to the study characteristics (design, Country, year of publication, length of follow-up) and outcomes (rates of clinical remission, suspension of drugs, resolution of symptoms, adverse events, use of objective, validated scores), the patients (number, presence of C difficile infection, prior IBD therapy), and the FMT procedure (donor relationship, patient preparation, weight of infused stools, route of administration, number of infusions), respectively, were extracted from each primary study. Study references and citations were collected in Endnote software application version 6.0 (Thomson Reuters, New York, NY). A data collection form was designed in Microsoft Excel 2007 (Microsoft, Redmond, WA).
Quality Assessment
Methodological quality of studies included for the analysis was assessed by one reviewer (G.I.). The 8 quality items provided by the Centre for Reviews and Dissemination checklist for appraising the quality of case series studies were used to examine each study (Table 2).22 We considered all reports including at least 3 patients treated with FMT as “case series.” Only case series that met all 8 criteria were rated as “good” quality. Results of methodological quality assessment did not influence the eligibility of the studies.
TABLE 2.
Centre for Reviews and Dissemination Checklist for Appraising the Quality of Case Series Studies22

RESULTS
Study Selection and Included Studies
Our search identified a total of 2752 articles (after removing the duplicates). According to the titles and abstracts, 159 of them were considered for further assessment. After review of full text, 31 of them fulfilled our eligibility criteria and were included in the final analysis19,23–52 (see PRISMA flow diagram in the online supporting documents; http://links.lww.com/MD/A58 http://links.lww.com/MD/A59).
Most of these were case reports or case series, whereas 8 publications reported data from open-label trial with a small number of patients treated.23,31,37,38,43,46,49,50 Really, from the study of Grehan et al,23 only 1 patient had IBD of a total of 10 patients treated in a small open-label trial.
Quality Assessment
Eight open-label trials23,31,37,38,43,46,49,50 and 15 case series24,27,28,30–32,37,38,41,43,45,46,49,50,52 were considered for the methodological quality assessment. According to the criteria of the Centre for Reviews and Dissemination checklist for appraising the quality of case series studies,22 all studies achieved a rating of “poor” (Table 2).
Patients’ Characteristics
A total of 133 patients (77 affected by UC, of whom 8 had pouchitis; 53 were affected by CD; and 3 had an undefined IBD) were managed with FMT, the most of which because of resistance to therapy or dependence on medications (Table 3 ). Of these, 57 (25 UC, 31 CD, and 1 undefined IBD) subjects (43%) had a C difficile infection. In a study by Borody et al,27an additional undefined number of subjects with IBD among a total of 55 were treated with FMT. From this study, however, it was not possible to deduce the number of subjects with IBD who were treated and therefore they were not included in the sum of total patients with IBD.
TABLE 3.
FMT for the Management of IBD
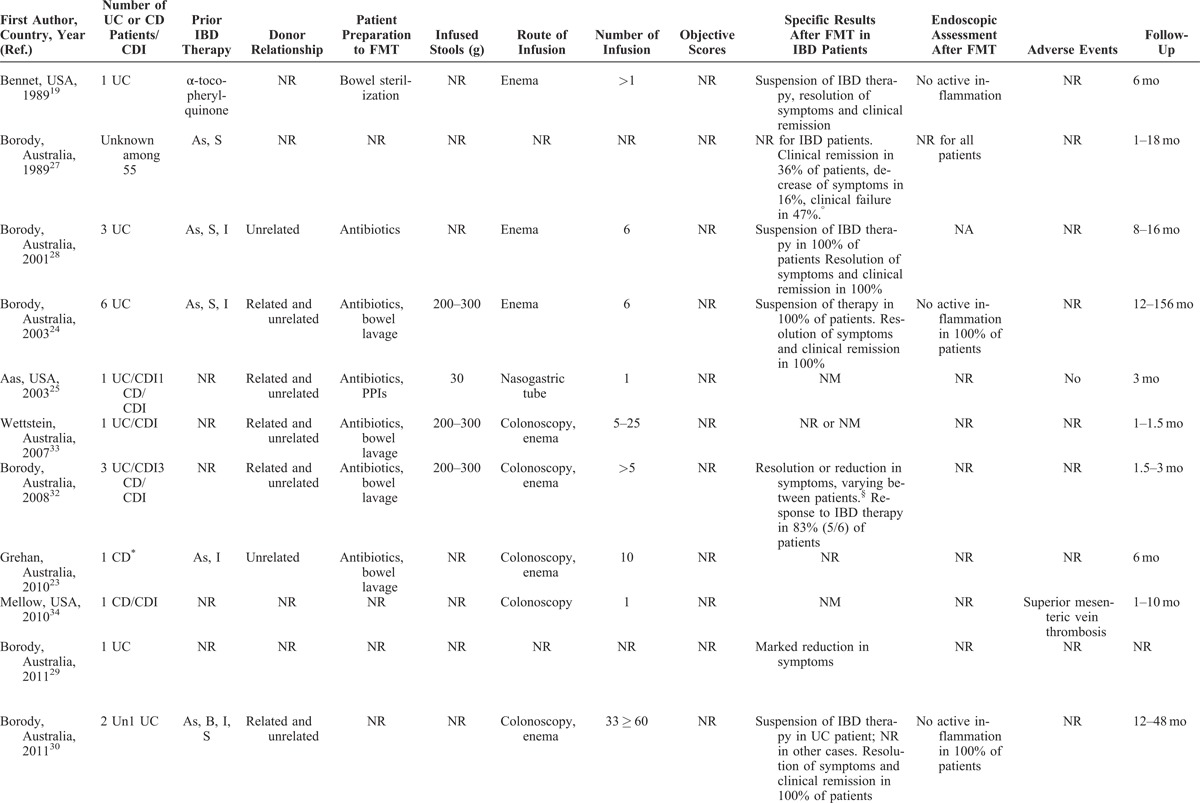
TABLE 3 (Continued).
FMT for the Management of IBD
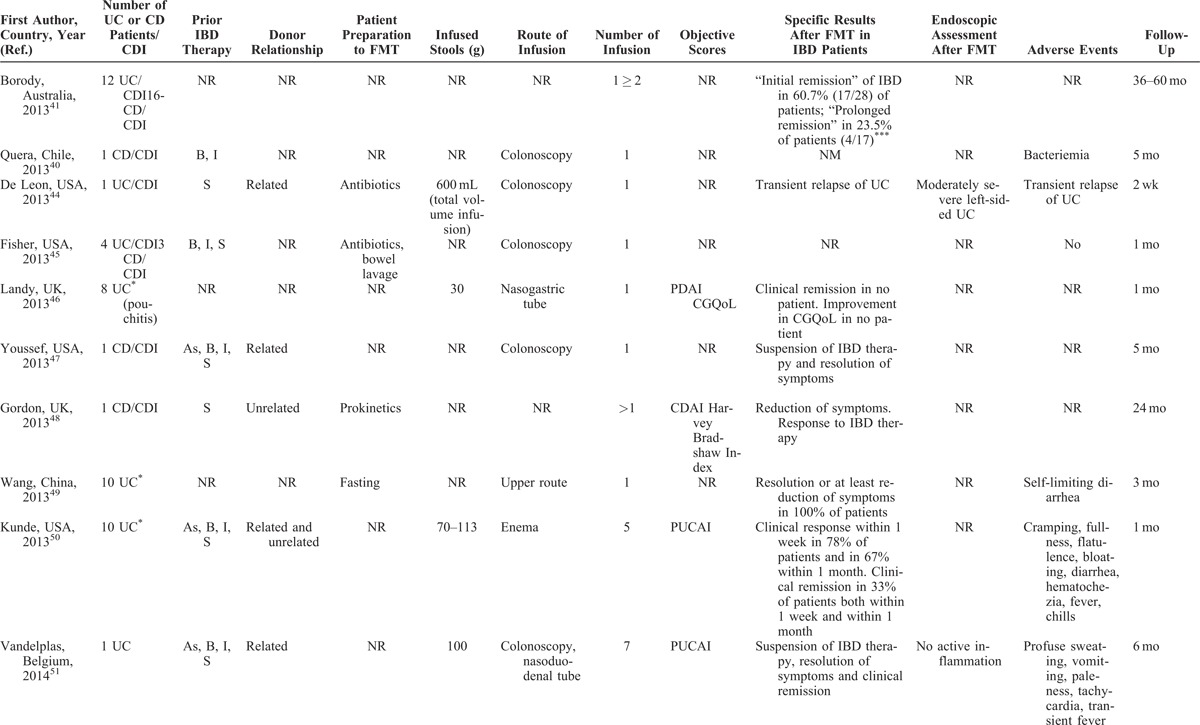
TABLE 3 (Continued).
FMT for the Management of IBD
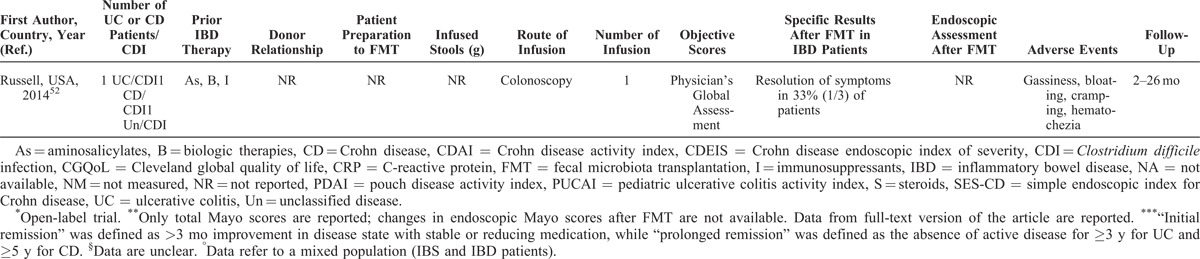
Most articles did not completely report data of patients with IBD, such as disease activity, distribution and duration of disease, or ongoing therapy. Objective scores (Crohn disease activity index [CDAI], Crohn disease endoscopic index of severity [CDEIS], simple endoscopic index for Crohn disease [SES-CD], Mayo, pouch disease activity index [PDAI], pediatric ulcerative colitis activity index [PUCAI], and others) were evaluated in a total of 55 (41%) patients, while an endoscopic assessment was made only in 21 (16%) (Table 3 ).
The duration of the disease was reported in about a half of overall patients, ranging from 6 months to more than 30 years, with a mean of 11 years. The location of the disease was quite equally distributed among patients, including proctitis, left-sided colitis, and pancolitis in UC, and ileal, ileocolonic, and isolated colonic CD, respectively. Eight patients had a pouchitis.46 Prior IBD-specific medications were described in more than half of reports (Table 3 ). When described, most patients with C difficile infection and underlying IBD were refractory to specific IBD medications or unable to undergo immunosuppressive therapy because of infection.
Patients’ Preparation, Route of Administration, and Volume of Infusion
When reported, most patients were prepared to FMT with bowel lavage (polyethylene glycol) or with unspecified antibiotics. Moreover, none of the 31 included articles reported the complete methodology of FMT procedure. In most studies, patients received multiple fecal infusions, ranging from 1 to 10 (Table 3 ); a very high number of infusion were performed in 2 studies from Australia (until 25 and >60, respectively).30,33
When reported, 20 patients received FMT by enema, 23 patients by colonoscopy, 11 by a combination of colonoscopy and enema, and 42 by upper route (nasogastric or nasojejunal tube, gastroscopy), whereas 6 patients experienced FMT infusion by both upper and lower route (Table 3 ). Fecal quantity varied from 30 to 300 g, diluted in saline solution (until 600 mL of suspension); in general, higher quantities of stools were infused by lower route as compared to the upper route.
Donors
In most studies, fecal donors included healthy related (relatives) and unrelated individuals (including nonrelative family members or friends and unknown subjects). However, in few studies, first-degree relatives or hospital and health care workers were expressly excluded. When reported, most donors underwent a viral screening (including hepatitis A virus, hepatitis B virus, hepatitis C virus, Epstein–Barr virus, human immunodeficiency virus, and cytomegalovirus), as well as stool tests for C difficile toxin, parasites, ova, and bacterial pathogens.
Outcomes
Overall, outcome data were incompletely provided, and there was a considerable variability among studies in the parameters that were measured. Most of declared outcomes were the resolution or reduction of symptoms (Table 3 ). Other parameters, such as “clinical remission,” “suspension of therapy,” and others, were not evaluable. In 10 reports, including a total of 55 (41%) objective scores were used (CDAI, CDEIS, SES-CD, Mayo, PDAI, Cleveland global quality of life, Harvay Bradshaw Index, PUCAI, and Physician’s Global Assessment).31,37–39,43,46,48,50–52 In 7 studies, including a total of 21 subjects, an adjunctive reported outcome was the amelioration of the endoscopic picture.19,24,30,31,38,44,51
Where it is possible to analyze data, the “resolution” or “reduction” of symptoms was reported in 80 of 113 (71%) patients, with success rates similar among patients with UC and CD. Thirty-four of 55 (62%) subjects with evaluable IBD had a reduction or resolution of symptoms when some kind of objective score was used. When we considered only patients with evaluable IBD (n = 77) who did not have C difficile infection, the rate of amelioration of symptoms was 69% (55 subjects). Twenty-five of 36 (70%) subjects with evaluable IBD having C difficile infection had a reduction or resolution of symptoms following FMT. None of the 8 patients with pouchitis had an amelioration of symptoms after FMT.46 Endoscopic assessment after FMT showed an amelioration of endoscopic picture in 12 of 21 (57%); this rate was of 20% (2 of 10 subjects) when endoscopic assessment was made by an objective score (Table 3 ).
Recently, in 2 small prospective studies37,38 investigating the use of FMT in patients with UC refractory to medical therapy, mucosal and stool samples were collected for the assessment of gut microbiota changes prior and after FMT. None of enrolled patients achieved clinical remission, but a short-term improvement of symptoms was reported in 7 of the total 11 subjects. Changes of gut microbiota toward the donor microbiota occurred in 3 patients without any correlation with clinical response. In the study by Angelberger et al,38 baseline Mayo scores correlated positively with Enterobacteriaceae abundance and negatively with Lachnospiraceae abundance. FMT provided a temporary increase of phylotype richness that was similar to donor microbiota. In only 1 patient, an improvement in both endoscopic and total Mayo score was observed; interestingly, its microbiota remained similar to donor microbiota for a longer time than other patients, maintaining 4 stable abundant donor phylotypes (C spiroforme, Faecalibacterium prausnitzii, Rosebura faecis, and Bacteroides ovatus) over time.
Adverse Events
Generally, FMT is considered a safe procedure. Most common adverse events include diarrhea on the day of administration, abdominal pain, belching, or constipation.53 A recent meta-analysis on the use of FMT for C difficile infection reported neither serious adverse events nor deaths attributed to feces infusion.54 Nevertheless, the administration of FMT for IBD does not seem to provide the same safety profile as for C difficile infection (Table 3 ). When reported, the most common adverse events related to the feces infusion were high fever, a temporary increase in C-reactive protein (CRP), diarrhea, vomiting, and other symptoms (Table 3 ). Notably, serious adverse events, such as bacteremia and transient relapse of previously quiescent UC, were reported in patients with IBD undergoing FMT for C difficile infection.40,44
DISCUSSION
FMT represents a promising therapeutic option for the restoration of disrupted intestinal microbiota, although the studies in the literature we assessed are poor in quality and proper clinical trials with rigorous inclusion criteria and outcome end points have not been performed.
With an average 91% success rate, FMT is now considered an effective intervention for recurrent infection by C difficile.55 At present, however, a number of attempts to use FMT in the therapeutic algorithm of patients with IBD does not seem capable to achieve the same outstanding results obtained in the management of C difficile infection, neither in terms of effectiveness nor with regard to safety. From our data, FMT achieved a “resolution” or “reduction” of symptoms in 71% of patients with IBD, whereas this rate was lower (62%) when some kind of objective score was used. If we exclude IBD subjects with C difficile infection from our analysis, the rate of amelioration of symptoms remained consistent (69%). An amelioration of endoscopic picture was reported in 57% of subjects investigated, whereas this rate drops to 20% when an objective score was used for the endoscopic assessment after FMT. Finally, IBD subjects with C difficile infection had a reduction or resolution of symptoms in 70% of cases after FMT. This finding seems to be well below than that found in the non-IBD individuals with C difficile infection (92%).7,56 The evaluation of the impact of FMT on other outcomes, such as the achievement of clinical remission or its effect on IBD-specific treatment, as well as a distinction of UC and CD, is not currently possible because available studies are plagued by several methodological pitfalls. Most reports consist in case reports or case series, whereas 7 published open-label trials included only a very small cohort of patients. Moreover, in the various studies published so far, there is a great heterogeneity of the enrolled population, in terms of disease typology, disease activity, and treatment. It is principally not clear to what level of the therapeutic algorithm FMT should fit. Based on the current published literature, we cannot make any conclusions as to which type of FMT in patients with IBD may benefit the most.
It is also plausible that both IBD severity and duration may influence the outcome, and therefore not all patients with IBD will benefit from transfer of microbiota. Patients with long-standing severe disease, refractory to medical therapy, might be difficult to treat because of their chronic, powerful immune up-regulation.57–59 On the contrary, gut microbiota impairment is not the unique pathophysiological factor leading to IBD, whereas genetic and environmental inputs have also been shown to influence the composition of gut microbiota itself.57–59 Therefore, the restoration of gut microbiota imbalance through FMT very likely does not represent the ultimate therapeutic solution for the disease.
It is also conceivable, as observed in the trial by Angelberger et al,38 that the composition of the donor’s gut microbiota may influence the clinical outcome. New tools for the investigation of microbiota composition, such as target gene sequencing and metagenomics, may give a great contribution in this field, and their application for the selection of donors represents an intriguing perspective.
Also FMT does not seem to offer the same safety profile as for C difficile infection (Table 3 ), although the quality of the reported literature in this matter is poor so that it is hard to draw any conclusions. Our data show that most common adverse events after FMT were fever, increase in CRP, diarrhea, vomiting, in some cases, bacteremia, and relapse of previously quiescent UC.40–44 Presumably, in the contest of a patient with impaired mucosal immunity and severe inflammation, with the addition of the defection of specific components of the gut barrier, such as mucus layer or enterocyte junctions, this kind of adverse events may occur more easily.60
Another important key point is that the FMT procedure for patients with IBD is far from being standardized. Key concerns regarding donor selection, patient preparation, volume of infusion, and route of administration have not yet been defined. From the literature data, close relatives have been frequently selected as donors of stool in FMT protocols against C difficile infection.55,56 Actually, we do not know whether the same criteria for donor selection are the right ones also for the use of FMT in patients with IBD. Recent evidences show that relatives of IBD-affected patients have an altered microbiota, and that dysbiosis may itself play a role for an increased risk of IBD.61,62 On the contrary, IBD subjects could have a hypothetical specific virus/bacterial susceptibility as a function of any immunosuppressive therapy. Therefore, the donor selection protocol used for C difficile infection (including collection of medical history and screening of both donor and patient for common viruses and enteric pathogens) could be not adequate when dealing with IBD subjects.
The route of fecal infusion may also constitute an additional concern of the FMT procedure in IBD. With regard to C difficile infection, lower gastrointestinal route seems to achieve higher eradication rates than upper delivery.54,56 Considering the specific alterations of gut microbiota composition found in patients with IBD, this issue could acquire even more relevance in the management of the disease. For example, Bacteroidetes can be damaged by gastric acid secretion, therefore a lower route may be preferable; on the other side, many spore-forming Firmicutes require transit through the upper gastrointestinal tract to be effective.20,54
Last, but not in terms of importance, is the issue concerning the number of fecal infusions that are needed to obtain a clinical benefit in patients with IBD. In most reports, for the treatment of C difficile infection, the protocol considered a single administration of stools.54 When FMT is applied to IBD, the idea is widely recognized that patients with long-standing disease may require several infusion of feces in order to maintain the infused microbiota in recipients after transplantation stable.38 Our data show that multiple infusions (ranging from >1 to >60) have been used in most studies (Table 3 ). Well-designed trial investigating this procedural aspect are surely needed.
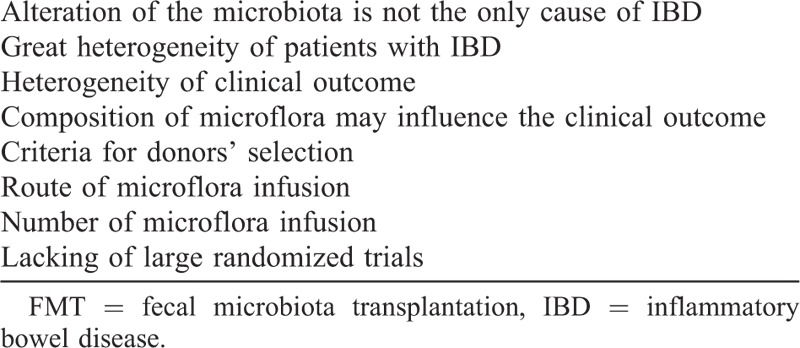
In conclusion, the application of FMT for the management of IBD may be an intriguing and suggestive therapeutic option, but data are actually uncertain, because several clinical, pathophysiological, and methodological issues have to be elucidated (Table 4). Current experiences consist only of occasional reports, whereas large and methodologically sound studies are lacking. Specifically, well-designed controlled trials are necessary to evaluate safety and to develop optimal protocols for the use of FMT in IBD. The issue of the homogeneity of the target patients with IBD (for disease phenotype, disease activity, and treatment), together with a clear definition of the outcomes variables (symptom resolution and mucosal healing) and the safety profile, will need to be addressed in future studies.
TABLE 3 (Continued).
FMT for the Management of IBD
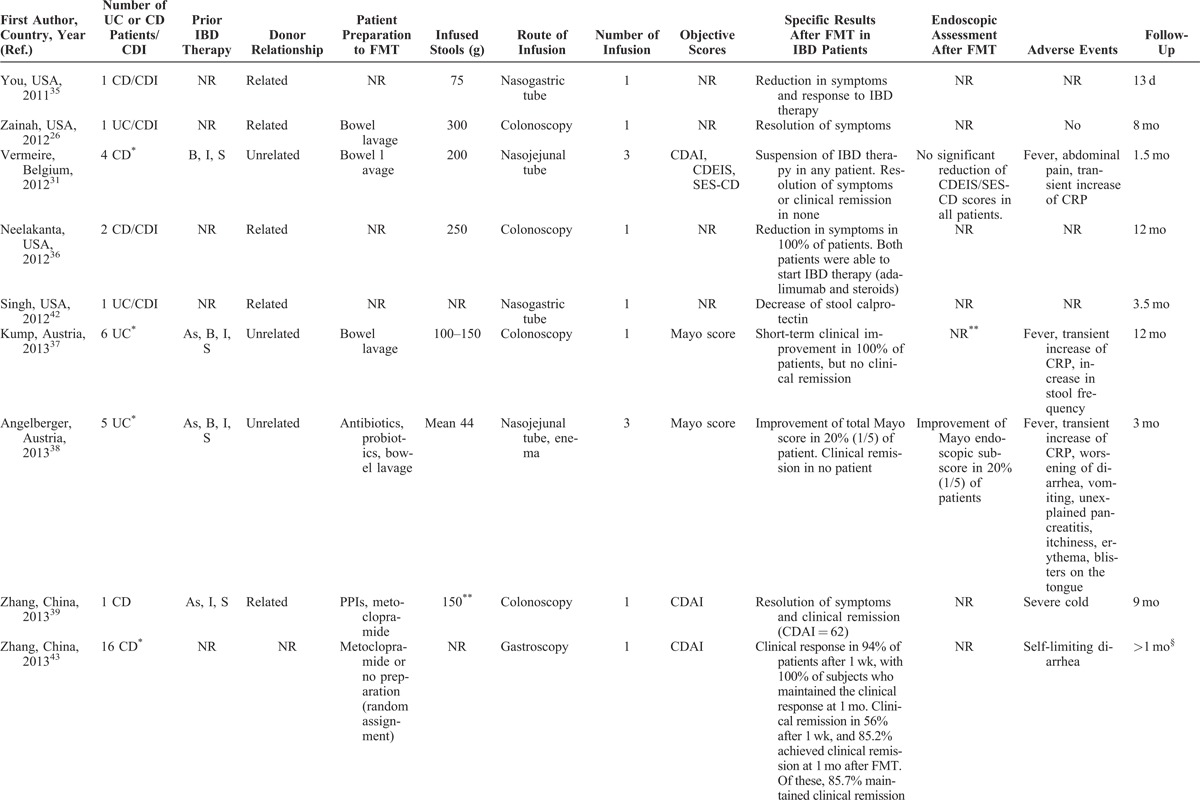
TABLE 4.
Principal Key Concerns for FMT in IBD
At our Department, we have successfully adopted the FMT procedure to give the best therapeutic option for patients with recurrent C difficile infection. Despite the many difficulties, first of all, concerning the coordination of a medical staff with different skills (including gastroenterology, microbiology, and infectious disease expertise), our program has evolved since to become a routine clinical practice for this kind of patients. The realization of our project initially found many obstacles at the institutional level and has made a lot of effort before going out of bounds by a simple research project and becoming a procedure of good clinical practice. We think that considerable and greater further efforts are needed before to make FMT a treatment as effective in IBD.
Footnotes
Abbreviations: CD = Crohn disease, FMT = fecal microbiota transplantation, IBD = inflammatory bowel disease, UC = ulcerative colitis.
GC had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.md-journal.com).
References
- 1.Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88–96. [DOI] [PubMed] [Google Scholar]
- 2.Eiseman B, Silen W, Bascom GS, et al. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854–859. [PubMed] [Google Scholar]
- 3.Schwan A, Sjolin S, Trottestam U, et al. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983;2:845. [DOI] [PubMed] [Google Scholar]
- 4.Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932–1940. [DOI] [PubMed] [Google Scholar]
- 5.Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591–1597. [DOI] [PubMed] [Google Scholar]
- 6.Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142:490–496. [DOI] [PubMed] [Google Scholar]
- 7.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. [DOI] [PubMed] [Google Scholar]
- 8.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503–516. [DOI] [PubMed] [Google Scholar]
- 11.Halme L, Paavola-Sakki P, Turunen U, et al. Family and twin studies in inflammatory bowel disease. World J Gastroenterol. 2006;12:3668–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62:1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DT. Curbing our enthusiasm for fecal transplantation in ulcerative colitis. Am J Gastroenterol. 2013;108:1631–1633. [DOI] [PubMed] [Google Scholar]
- 15.Manichanh C, Borruel N, Casellas F, et al. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. [DOI] [PubMed] [Google Scholar]
- 16.Vermeire S, Rutgeerts P. IBD in 2012: pathogenesis and management of IBD-thinking outside the box. Nat Rev Gastroenterol Hepatol. 2013;10:67–69. [DOI] [PubMed] [Google Scholar]
- 17.Whelan K, Quigley EM. Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29:184–189. [DOI] [PubMed] [Google Scholar]
- 18.Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: patients are ready, are we? Inflamm Bowel Dis. 2012;18:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennet JD, Brinkman M. Treatment of ulcerative-colitis by implantation of normal colonic flora. Lancet. 1989;1:164. [DOI] [PubMed] [Google Scholar]
- 20.Damman CJ, Miller SI, Surawicz CM, et al. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1452–1459. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 22.Chambers D, Rodgers M, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies. J Clin Epidemiol. 2009;62:1253–1260. [DOI] [PubMed] [Google Scholar]
- 23.Grehan MJ, Borody TJ, Leis SM, et al. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastroenterol. 2010;44:551–561. [DOI] [PubMed] [Google Scholar]
- 24.Borody TJ, Warren EF, Leis S, et al. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42–47. [DOI] [PubMed] [Google Scholar]
- 25.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infec Dis. 2003;36:580–585. [DOI] [PubMed] [Google Scholar]
- 26.Zainah H, Silverman A. Fecal bacteriotherapy: a case report in an immunosuppressed patient with ulcerative colitis and recurrent Clostridium difficile infection. Case Rep Infect Dis. 2012;2012:810943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borody TJ, George L, Andrews P, et al. Bowel-flora alteration—a potential cure for inflammatory bowel-disease and irritable bowel syndrome. Med J Aust. 1989;150:604. [DOI] [PubMed] [Google Scholar]
- 28.Borody T, Leis S, McGrath K, et al. Treatment of chronic constipation and colitis using human probiotic infusions. In: Probiotics, Prebiotics and New Foods Conference; September 2001; Universita Urbaniana, Rome, Italy; 2–4. [Google Scholar]
- 29.Borody T, Campbell J, Torres M, et al. Reversal of idiopathic thrombocytopenic purpura [ITP] with fecal microbiota transplantation (FMT). Am J Gastroenterol. 2011;106:S352. [Google Scholar]
- 30.Borody T, Torres M, Campbell J, et al. Reversal of inflammatory bowel disease (IBD) with recurrent faecal microbiota transplants (FMT). Am J Gastroenterol. 2011;106:S366. [Google Scholar]
- 31.Vermeire S, Joossens M, Verbeke K, et al. Pilot study on the safety and efficacy of faecal microbiota transplantation in refractory Crohn’s disease. Gastroenterology. 2012;142:S360. [Google Scholar]
- 32.Borody TJ, Wettstein AR, Leis S, et al. Clostridium difficile complicating inflammatory bowel disease: pre- and post-treatment findings. Gastroenterology. 2008;134:A361. [Google Scholar]
- 33.Wettstein A, Borody TJ, Leis SM, et al. Fecal Bacteriotherapy—An Effective Treatment for Relapsing Symptomatic Clostridium difficile Infection. Paris, France: United European Gastroenterology Federation; 2007. [Google Scholar]
- 34.Mellow M, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection—results and follow-up. Am J Gastroenterol. 2010;105:S135. [PubMed] [Google Scholar]
- 35.You D, Johnson M, Duplessis C, et al. Successful use of fecal bacteriotherapy in severe Crohn’s colitis and refractory Clostridium difficile infection. Am J Gastroenterol. 2011;106:S315. [DOI] [PubMed] [Google Scholar]
- 36.Neelakanta A, Moudgal V, Upadhyay N, et al. Successful treatment of refractory Clostridium difficile infection (CDI) with intestinal microbiota transplant (IMT) in two patients with inflammatory bowel disease (IBD) and its effects on IBD. Gastroenterology. 2012;142:S395. [Google Scholar]
- 37.Kump PK, Gröchenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155–2165. [DOI] [PubMed] [Google Scholar]
- 38.Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1620–1630. [DOI] [PubMed] [Google Scholar]
- 39.Zhang FM, Wang HG, Wang M, et al. Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn’s disease. World J Gastroenterol. 2013;19:7213–7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quera R, Espinoza R, Estay C, et al. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J Crohn’s Colitis. 2014;8:252–253. [DOI] [PubMed] [Google Scholar]
- 41.Borody TJ, Wettstein A, Nowak A, et al. Fecal microbiota transplantation (FMT) eradicates clostridium difficile infection (CDI) in inflammatory bowel disease (IBD). UEG J. 2013;1suppl 1:A57. [Google Scholar]
- 42.Singh N, Suskind D, Wahbeh G. Fecal bacteriotherapy in a 6 year old patient with ulcerative colitis and Clostridium difficile. Inflamm Bowel Dis. 2012;18:S69. [Google Scholar]
- 43.Zhang F, Wang H, Wang M, et al. Standard fecal microbiota transplantation through mid-gut is an effective therapy of refractory Crohn’s disease. J Gastroenterol Hepatol. 2013;28:29. [DOI] [PubMed] [Google Scholar]
- 44.De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11:1036–1038. [DOI] [PubMed] [Google Scholar]
- 45.Fischer M, Rex DK, Cook GK. Fecal microbiota transplantation for recurrent Clostridium difficile in patients with prolonged immunosuppression. UEG J. 2013;1suppl 1:A380. [Google Scholar]
- 46.Landy J, Al-Hassi HO, Mann ER, et al. A prospective controlled pilot study of fecal microbiota transplantation for chronic refractory pouchitis. Gastroenterology. 2013;144(5 suppl 1):S897. [Google Scholar]
- 47.Youssef MA, Gavin M. Fecal microbiota transplant: a case report in an immunosuppressed patient with Crohn’s disease and recurrent Clostridium difficile infection. Gastroenterology. 2013;144(5 suppl 1):S626. [Google Scholar]
- 48.Gordon H, Harbord M. A patient with severe Crohn’s colitis responds to faecal microbiota transplantation. J Crohns Colitis. 2014;8:256–257. [DOI] [PubMed] [Google Scholar]
- 49.Wang M, Wang H, Zhang F. Standard fecal microbiota transplantation through mid-gut is effective therapy for refractory ulcerative colitis. J Gastroenterol Hepatol. 2013;28:590.23527756 [Google Scholar]
- 50.Kunde S, Pham A, Bonczyk S, et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597–601. [DOI] [PubMed] [Google Scholar]
- 51.Vandenplas Y, Veereman G, van der Werff Ten Bosch J, et al. Fecal microbial transplantation in a one-year-old girl with early onset colitis—caution advised. J Pediatr Gastroenterol N utr. January 2, 2014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 52.Russell GH, Kaplan JL, Youngster I, et al. Fecal transplant for recurrent Clostridium difficile infection in children with and without inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;58:588–592. [DOI] [PubMed] [Google Scholar]
- 53.Smits LP, Bouter KE, de Vos WM, et al. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946–953. [DOI] [PubMed] [Google Scholar]
- 54.Kassam Z, Lee CH, Yuan Y, et al. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–508. [DOI] [PubMed] [Google Scholar]
- 55.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal bacteriotherapy. Clin Gastroenterol Hepatol. 2011;9:1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection. A systematic review. J Clin Gastroenterol. 2014;48:693–702. [DOI] [PubMed] [Google Scholar]
- 57.Willing BP, Dicksved J, Halfvarson J, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–1854. [DOI] [PubMed] [Google Scholar]
- 58.Hansen J, Gulati A, Sartor RB. The role of mucosal immunity and host genetics in defining intestinal commensal bacteria. Curr Opin Gastroenterol. 2010;26:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerich ME, McGovern DP. Towards personalized care in IBD. Nat Rev Gastroenterol Hepatol. 2013;11:287–299. [DOI] [PubMed] [Google Scholar]
- 60.Shan M, Gentile M, Yeiser JR, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joonssens M, Huys G, Cnockaert M, et al. Dysbiosis of the fecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut. 2011;60:631–637. [DOI] [PubMed] [Google Scholar]
- 62.Hedin CR, Stagg AJ, Whelan K, et al. Family studies in Crohn’s disease: new horizons in understanding disease pathogenesis, risk and prevention. Gut. 2012;61:311–318. [DOI] [PubMed] [Google Scholar]


