Abstract
Central nervous system (CNS) nocardiosis is a rare disease entity caused by the filamentous bacteria Nocardia species. We present a case series of 5 patients from our hospital and a review of the cases of CNS nocardiosis reported in the literature from January 2000 to December 2011. Our results indicate that CNS nocardiosis can occur in both immunocompromised and immunocompetent individuals and can be the result of prior pulmonary infection or can exist on its own. The most common predisposing factors are corticosteroid use (54% of patients) and organ transplantation (25%). Presentation of the disease is widely variable, and available diagnostic tests are far from perfect, often leading to delayed detection and initiation of treatment. The optimal therapeutic approach is still undetermined and depends on speciation, but lower mortality and relapse rates have been reported with a combination of targeted antimicrobial treatment including trimethoprim/sulfomethoxazole (TMP-SMX) for more than 6 months and neurosurgical intervention.
INTRODUCTION
Nocardiosis is an uncommon disease caused by aerobic gram-positive bacteria in the genus Nocardia. Nocardia species have the ability to cause localized or systemic suppurative disease in humans and animals.7,41,58,61,95 Nocardiosis is primarily an opportunistic infection affecting immunocompromised patients, such as organ transplant recipients receiving pharmacologic immunosuppression, patients with low CD4 T-lymphocyte counts, and those with hematologic malignancies.41,58 However, roughly one-third of patients with nocardiosis are immunocompetent.95
The incidence of nocardiosis varies among different patient groups. In hospitalized patients, nocardiosis presents either as single-organ disease or as multifocal disease caused by dissemination of the microorganisms from a primary focus of infection. Single-organ infection most commonly manifests as lung disease (39% of cases in hospitalized patients), followed by infection of the central nervous system (CNS) (9% of cases). In this patient population disseminated disease is common and has been reported in 32% of cases.7
The genus Nocardia is composed of 13 medically important species. Nocardia asteroides, N. farcinica, N. nova and N. abscessus cause the majority of invasive infections.61,91 Patients with invasive nocardiosis are often seriously ill, and published reports on such infections are lacking. This is especially true of reports of CNS nocardiosis, which has a low prevalence. In the current review we focus on cases of CNS nocardiosis and examine the clinical manifestations, laboratory diagnosis, response to therapy, and outcome using clinical cases and the published literature.
METHODS
We identified 84 patients with nocardiosis seen during the 12 years from January 2000 to December 2011 by means of the research patient data registry at our hospital, Massachusetts General Hospital (MGH). We searched for patients using the International Classification of Disease-9th Revision (ICD-9) codes for nocardiosis. Approval of the study protocol was granted by the institutional review board of MGH.
We accessed all electronic medical records and identified 5 cases of CNS nocardiosis. We recorded data on the patients’ index hospitalization, such as demographic information, including age, sex, and race. We also recorded each patient’s underlying illnesses and intake of medications, giving emphasis to their immunologic status. In particular, we looked for the presence of malignancy, human immunodeficiency virus (HIV), transplantation status, diabetes mellitus, neutropenia, tuberculosis, dialysis, chronic obstructive lung disease, and history of cytomegalovirus (CMV) infection. Neutropenia was defined as the presence of <1500 neutrophils per μL. Regarding the patient’s drug history, we were specifically interested in immunosuppressive drugs, such as chemotherapeutic agents, calcineurin inhibitors, tumor necrosis factor-α (TNF-α) inhibitors, and corticosteroids. Patients were considered immunosuppressed if they took prednisone or a prednisone-equivalent pharmacologic agent in a dose ≥10 mg per day for at least 3 months.46
Diagnostic methods involved imaging studies, such as computerized tomography (CT) scans of the head and magnetic resonance imaging (MRI) of the brain with and without contrast, as well as cultures of blood, cerebrospinal fluid (CSF), sputum, and aspirates of cerebral abscesses. Confirmed cases of CNS nocardiosis were those with cultures positive for Nocardia species from aspiration of cerebral abscesses or CSF. Only confirmed CNS nocardiosis cases were included in this review.
We categorized each therapeutic approach as medical (including empiric and targeted antimicrobial therapy) or surgical. Empiric treatment was regarded as the administration of antibiotics in patients with a clinical presentation consistent with CNS nocardiosis, but without a positive culture. We also gathered data on the medications that were administered as prophylaxis. Finally, we recorded outcomes, taking into consideration parameters such as intensive care unit admission, relapse, complete cure, and death.
For the literature review, we reviewed cases in the English-language literature that were treated in or after January 2000 and gathered all confirmed CNS nocardiosis cases. We searched the MEDLINE database (National Library of Medicine, Bethesda, MD) using the key words terms “Nocardia,” “nocardiosis,” “CNS,” “brain,” “meningitis,” and “encephalitis.” As in our case series, only cases with proven CNS nocardiosis were included.
RESULTS
Case Series
The main characteristics of patients with confirmed CNS nocardiosis from MGH are summarized in Table 1. We identified 5 patients with confirmed nocardiosis (mean age, 59.8 yr; range, 30–83 yr). Four of the 5 patients were women. Most cases (4 of 5) presented with chronic lung disease: 1 patient had bronchiectasis, 1 had chronic obstructive pulmonary disease (COPD), 1 had bronchiectasis and idiopathic pulmonary fibrosis, and 1 had COPD and pulmonary hypertension. Other underlying conditions were autoimmune diseases, such as Hashimoto thyroiditis (n = 2) and autoimmune hepatitis (n = 1), renal insufficiency (n = 2), hematologic malignancy (Hodgkin lymphoma with bone marrow metastases treated with bone marrow transplant and complicated with graft-versus-host disease (GVHD); n = 1), and systemic amyloidosis treated with heart and stem cell transplants (n = 1). Two patients had history of CMV infection of the lungs within 6 months of being diagnosed with CNS nocardiosis. None of the patients had previous cerebral pathology, including cerebral hemorrhagic or ischemic events and head trauma; or HIV infection; neutropenia; diabetes mellitus; or tuberculosis. All 5 patients in the case series were taking corticosteroids at the time of diagnosis, 4 patients were also taking chemotherapy medications, and 2 patients were taking calcineurin inhibitors. None of the patients was taking anti-TNF-α agents.
TABLE 1.
MGH Patients With CNS Nocardiosis
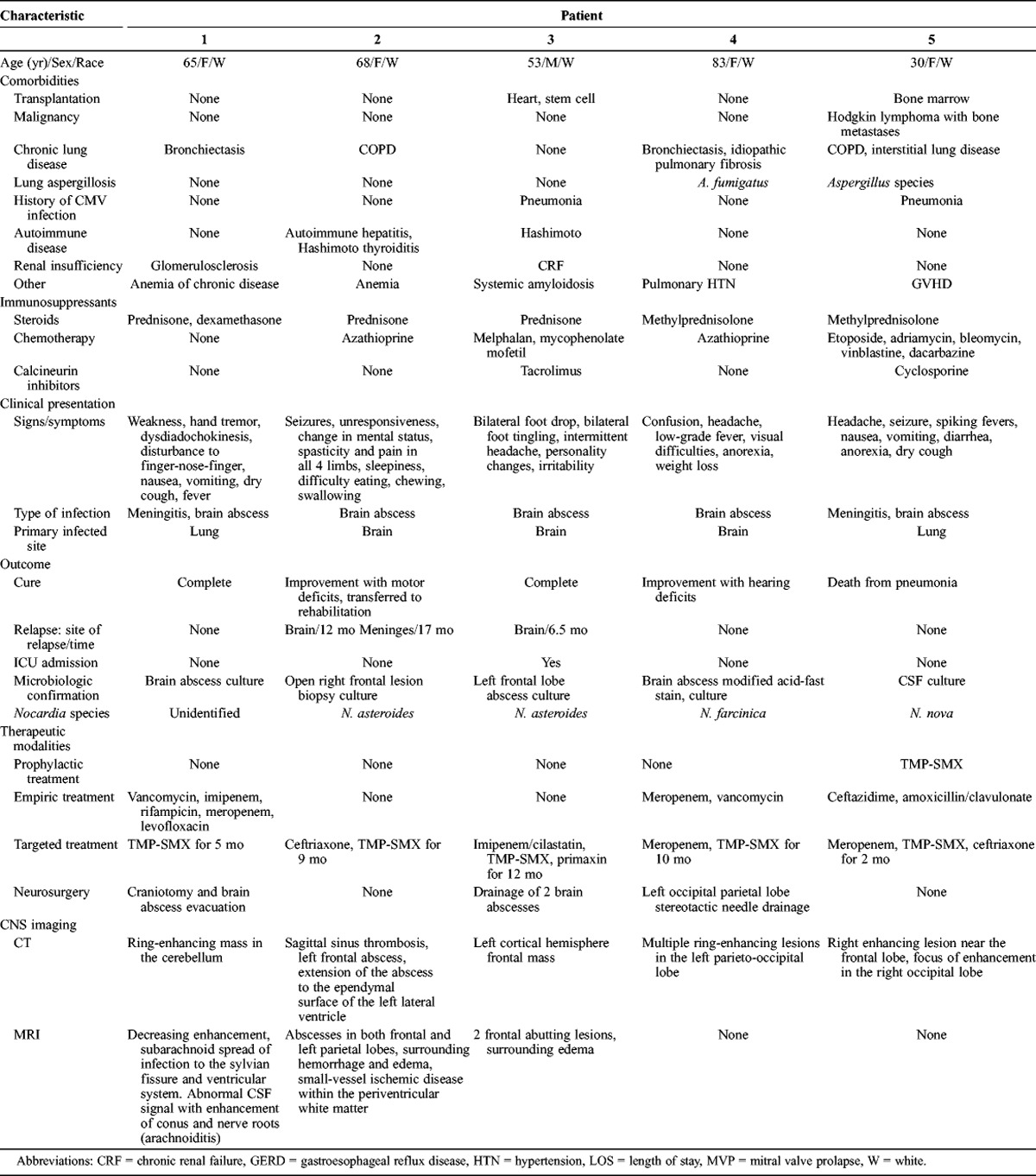
The clinical presentation in this group of patients varied. Two patients (Patients 1 and 5, Table 1) had concurrent pulmonary nocardiosis, which was the primary site of infection. These 2 patients also had systemic symptoms including fever, nausea, vomiting and anorexia, and a dry cough. Pulmonary involvement was confirmed with cultures of lung biopsy specimens, which were positive for Nocardia species. Both cases were diagnosed with meningitis and brain abscesses. There was no involvement of other organ systems. The first patient (Patient 1) presented with signs of cerebellar involvement due to the localization of the brain abscess to the cerebellum and radiologic findings suggestive of arachnoiditis. He had a negative CSF culture for Nocardia species, but high CSF protein levels (76.4 mg/dL). The second patient (Patient 5) presented with headaches and seizures, and a CT scan of the head demonstrated ring-enhancing lesions in the frontal and occipital lobes. CSF cell counts were consistent with meningitis, with the presence of red blood cells and an increased number of white blood cells with a predominance of neutrophils. CSF cultures were positive for N. nova.
The remaining 3 cases (Patients 2, 3, and 4, Table 1) had no other known primary focus of infection. These patients were therefore thought to have primary brain lesions. Patient 2 presented with seizures, changes in mental status, spasticity in her upper and lower limbs, hypersomnia, and dysphagia. Cerebral abscesses were found in the frontal and parietal lobes along with meningeal involvement on MRI of the brain. Patient 3 had intermittent headaches lasting for a month, personality changes, and symptoms of peripheral nerve involvement including both motor and sensory deficits. Motor deficits included foot drop, while sensory deficits included paresthesia in the feet. A brain MRI demonstrated 2 frontal lobe abscesses. Patient 4 was admitted with a low-grade fever, headaches, anorexia and weight loss, changes in mental status, and visual field deficits. A CT scan of the head revealed multiple ring-enhancing lesions in the left parietal and occipital lobes.
The predominant clinical presentation was with nonspecific generalized symptoms, which were present in 4 of 5 patients. The least common symptoms in this group of patients were bilateral foot drop and paresthesia in the feet. We note that 2 patients had concurrent lung infections with Aspergillus species confirmed with bronchoalveolar culture before a diagnosis of CNS nocardiosis was established.
The diagnosis of CNS nocardiosis was established in all 5 cases by the isolation of Nocardia species in cultures of either drained cerebral abscesses (n = 4) or CSF (n = 1). Radiologic imaging was performed in all 5 patients. CT scans of the head demonstrated the presence of ring-enhancing lesions suggestive of brain abscesses. One patient had multiple ring-enhancing lesions, while the rest had single focal lesions in different lobes of the brain. In addition to this, 1 patient had sagittal sinus thrombosis and extension of the abscess into the lateral ventricle. MRI of the brain was performed in 3 patients, yielding results consistent with the presence of abscesses in all patients. Two patients had surrounding edema on the brain MRI, and 1 patient also had hemorrhagic and ischemic changes. One patient with meningeal symptoms had a finding of arachnoiditis on MRI with abnormal CSF enhancement and extension of the infection to the ventricular system. Two patients had confirmed infection with N. asteroides, 1 with N. farcinica, and 1 with N. nova. The species of Nocardia remained unidentified in 1 patient.
Treatment included both medical and surgical interventions. Three patients received a combination of medical and surgical treatment (Patients 1, 3, and 4, Table 1). Two patients received only medical treatment with antimicrobial agents (Patients 2 and 5, Table 1). Patient 1 was treated empirically with vancomycin, imipenem, rifampicin, meropenem, and levofloxacin. When the diagnosis of CNS nocardiosis was confirmed the patient was switched to trimethoprim and sulfomethoxazole (TMP-SMX), and underwent craniotomy and abscess drainage at a later time. Patient 2 received targeted treatment only, consisting of TMP-SMX and ceftriaxone. Patient 3 was treated with imipenem and cilastatin, TMP-SMX, and primaxin as targeted treatment and then underwent craniotomy and abscess drainage. Patient 4 was treated empirically with meropenem and vancomycin and had a craniotomy and abscess drainage. The patient was switched to meropenem and TMP-SMX when the positive culture results became available. Patient 5 received empirical therapy with ceftazidime, amoxicillin and clavulanate. The patient was switched to meropenem and switched again to combination TMP-SMX and ceftriaxone for better coverage. Patient 5 had also received prophylaxis with TMP-SMX before the initiation of treatment.
It is noteworthy that there was no mortality in the group of patients treated with a combination of medical therapy and surgical intervention. Indeed 2 of them achieved complete cure. As stated above, the first patient received empiric antibiotics followed by targeted treatment and a craniotomy and abscess drainage (Patient 1, Table 1), and the other received targeted treatment followed by abscess drainage (Patient 3, Table 1). The latter suffered a recurrence of the brain abscess 6.5 months after admission. Two patients developed postinfectious neurologic deficits, specifically motor deficits and hearing impairment. Among them, 1 was treated with targeted medical therapy only (Patient 2, Table 1) and suffered 2 relapses of disease at 12 and 18 months after the initial CNS infection, which manifested as brain abscess and meningitis, respectively. This patient required rehabilitation for motor deficits following treatment at MGH. The patient who developed hearing impairment (Patient 4, Table 1) received empiric antimicrobial therapy followed by targeted therapy and drainage of the cerebral abscess. The patient was treated with antimicrobials. Treatment was monitored using radiologic imaging and was continued until there was complete resolution of lesions. One patient who had received medical treatment only (Patient 5, Table1) died of pneumonia.
Literature Review
The comorbidities of the 84 reported cases of CNS nocardiosis1–5,11,12,15,17,20–24,26,27,30–38,42,44,45,47,48,50–55,57,60,63–65,67,68,70,73–84,86,87,93,94,96–101,104,107–110,112,113 are summarized in Table 2. The mean age of the patients was 50.9 years (range, 7–77 yr). There were 56 male (67%) and 28 female (33%) patients in this group.
TABLE 2.
Comorbidities of Literature Patients With CNS Nocardiosis (n=84 Patients)
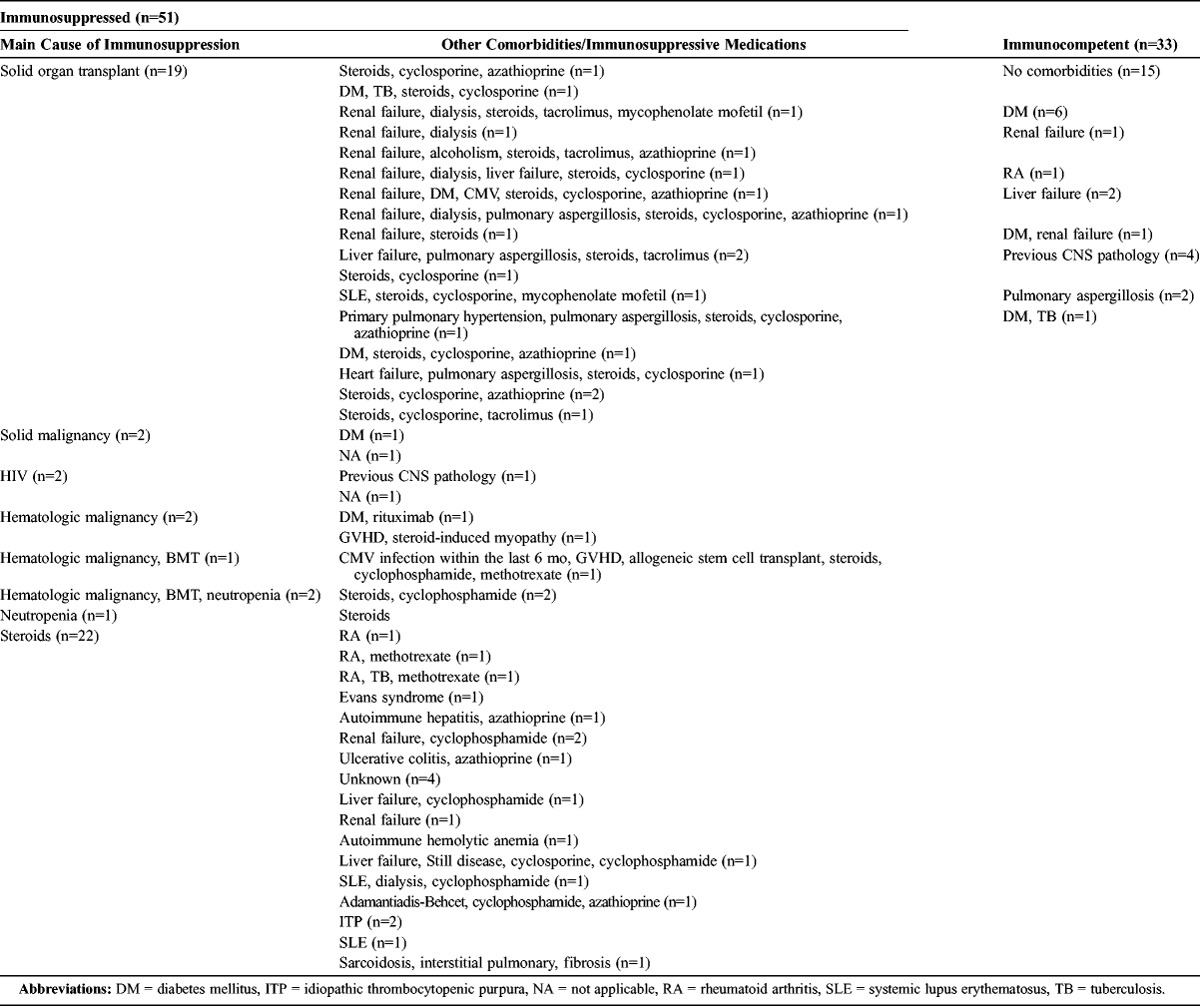
A significant proportion of patients (15/84; 18%) had no comorbid conditions.1,2,12,22,29,31,33,36,44,50,51,53,55,65,67,70,73–75,79,82,87,104,109,113 The most common comorbid condition was solid organ or bone marrow transplantation (25%). The kidney was the most commonly transplanted organ (n = 14), followed by liver (n = 3), heart (n = 2), bone marrow (n = 1), lung (n = 1), and allogeneic stem cell transplant (n = 1). One patient who had pulmonary hypertension received a dual heart and lung transplant, while another patient received liver and bone marrow transplants. The second most common comorbid condition was autoimmune disease (20%), such as rheumatoid arthritis, vasculitides, autoimmune hepatitis, systemic lupus erythematosus, autoimmune hemolytic anemia, sarcoidosis, and ulcerative colitis. A significant proportion of patients (14%) suffered from chronic renal disease, with 5 patients on dialysis. Diabetes mellitus was present in 16% of patients, and chronic liver disease was present in 11% of patients. Other comorbid conditions included malignancy (8%), with 5 cases suffering from hematologic malignancies and 2 from solid organ tumors; chronic lung disease (6%); previous CNS pathology (6%); tuberculosis (5%); neutropenia (4%); GVHD (2%); and HIV infection (2%). Two percent of patients had a history of excessive alcohol intake. We note that 7% of cases suffered from concurrent pulmonary aspergillosis, which is consistent with our findings in the MGH case series patients. Two percent of patients had a history of CMV infection within 6 months of being diagnosed with CNS nocardiosis.
The majority of patients (n = 45, 54%) received immunosuppressant pharmacologic agents before diagnosis. The most commonly used immunosuppressant agents were corticosteroids (n = 43, 51%); followed by chemotherapeutic drugs, such as cyclophosphamide, azathioprine, and methotrexate (n = 24, 29%); calcineurin inhibitors, such as cyclosporine and tacrolimus (n = 20, 24%); and anti-TNF-α agents (n = 2, 4%). Many patients received a combination of immunosuppressants (n = 32, 38%), which included corticosteroids in all 32 cases. Two patients had insufficient data regarding the intake of immunosuppressive medications. Of note, 39 patients (46%) with CNS nocardiosis were not on corticosteroids.
Fifty-four patients (64%) were considered to have primary CNS nocardiosis.2,3,17,21–23,25,31,32,35,36,42,44,45,48–54,56,63,65,67,68,74,75,77,80,82,84,87,94,96,97,99,104,107–110,113 Of these 54 patients, 43 had cerebral abscesses, 5 had meningitis, and 6 patients had both cerebral abscesses and meningitis. Among the latter 6 patients, 1 patient also had ventriculitis. Two patients who had cerebral abscesses also had spinal cord involvement. One patient had a cervical spine abscess, which was depicted in cervical MRI as a contrast-enhancing lesion at the level of C3-T1 and manifested as left-sided hemiparesis and subsequent quadriplegia. The second patient had lumbar spine abscess shown in lumbar spine MRI that manifested as back pain and leg weakness. It is worth mentioning that 2 patients with cerebral abscesses had concurrent skin involvement (subcutaneous abscess of the left leg and subcutaneous abscess of the back respectively). The second most common primary site of infection was the lung, with 27 patients (32.1%) presenting with pulmonary disease and positive cultures from specimens collected from the respiratory tract. Of note, in addition to dissemination of the primary lung infection to the CNS, 3 cases also disseminated to the skin and 2 cases to the retina. Other primary sites of infection included the epidural space (n = 2) and bone (n = 1), while in 1 patient with a cerebral abscess and testicular involvement, the primary site of infection was not clear.
The signs and symptoms of disease in the literature patients with CNS nocardiosis were variable and included focal neurologic abnormalities (51%), headache (45%), fever (40%), altered mental status (36%), seizures (28%), visual changes (21%), nausea and vomiting (21%), ataxia and falls (11%), meningism (9%), polyuria and urinary incontinence (4%), and personality changes (2%). Focal neurologic abnormalities included motor abnormalities, such as hemiparesis (n = 29) and quadriplegia (n = 1); sensory abnormalities (n = 6); and cranial nerve palsies (n = 20). Seizures were either generalized (n = 18) or focal (n = 6). Only 2 patients presented with low-grade fever.
The major species reported among the patients in the literature review were N. asteroides (35%), N. farcinica (19%), and N. cyriacigeorgica (6%). Other less common species included N. transvalensis (4%), N. brasilensis (3.6%), and N. otitidiscaviarum (2%). One patient was infected with N. paucivorans, 1 with N. veterana, 1 with N. carnea, 1 with N. exalbida, 1 with N. nova, and 1 with N. asiatica. One patient was infected 2 times; the first infection was with N. asteroides and the second was with N. transvalensis.
Regarding the laboratory diagnostic techniques, 82% of patients were diagnosed with culture of aspirates from the site of infection, 7% with polymerase chain reaction (PCR) and 16S rRNA sequencing, and 2% with acid-fast staining. Two percent of patients were diagnosed at autopsy. Among the 69 patients who were diagnosed with culture, 36 (43%) underwent aspiration of a cerebral abscess and subsequent culture of the aspirate, 26 (31%) underwent biopsy of the lesion and the biopsy specimen was cultured, and 7 (8.3%) were diagnosed with CSF culture. Of note, in 1 patient the diagnosis was confirmed with both culture of aspirate and sequencing of 16S rRNA. All literature patients underwent radiologic imaging, which consisted of either a CT scan of the head or a brain MRI with and without contrast. Most patients (78, 93%) presented with ring-enhancing lesions presumed to be cerebral abscesses. Of those, 39 patients presented with a single ring-enhancing lesion (surrounded by edema and causing mass effects in 23 cases) and 39 with multiple ring-enhancing lesions (surrounded by edema in 15 cases). Lesions were described as heterogeneous or irregular multilobulated in 12 of 78 patients. Four patients had meningeal enhancement suggestive of meningeal involvement, while 2 patients had cystic lesions on CT scan. Other imaging findings included subdural hematoma in 2 patients, ventriculitis and tethered spinal cord at L4–L5 in 1 patient, demyelination and bilateral white matter inflammation in 1 patient, sphenoid sinus hematoma in 1 patient, lumbosacral hematoma in 1 patient, postoperative hydrocephalus in 1 patient, intraventricular hemorrhage in 1 patient, and ventricular enlargement in 1 patient.
The therapeutic approaches and outcome of the literature patients with CNS nocardiosis are summarized in Table 3. Overall, 70% of patients received TMP-SMX with 15.3% mortality rate and 13.6% relapse rate (compared to 32% mortality rate and 16% relapse rate in patients who did not receive TMP-SMX). The dose of TMP-SMX was mentioned in 18 patients. Of those, 6 received 30 mg/kg per day, 6 received 15 mg/kg per day, 4 received 90 mg/kg per day, and 1 received 20 mg/kg per day. Three of those 18 patients suffered a relapse, and the doses of TMP-SMX they received were 90 mg/kg per day, 90 mg/kg per day, and 30 mg/kg per day, respectively. The duration of treatment with TMP-SMX was mentioned in 27 patients, 6 of whom suffered a relapse. Ten patients were discharged on long-term therapy with TMP-SMX for at least 6 months (0% mortality and relapse rates vs. 18.4% mortality rate and 16.3% relapse rate in patients treated with TMP-SMX for less than 6 months). Five patients were treated empirically only: 4 of them died and the fifth suffered a relapse. Fifty-four percent of all patients were treated with combination targeted antimicrobial therapy consisting of at least 2 antimicrobial agents, while 27% were treated with targeted monotherapy. TMP-SMX was most commonly combined with ceftriaxone (n = 13), imipenem (n = 10), amikacin (n = 7), linezolid (n = 5), and less frequently with doxycycline (n = 3), a quinolone (n = 3), amoxicillin (n = 1), metronidazole (n = 1), and vancomycin (n = 1). Another common combination regimen was the concurrent administration of imipenem and another drug, such as aminoglycosides (n = 7), quinolones (n = 1), and minocycline (n = 1). Ceftriaxone was combined with aminoglycosides in 3 patients, quinolones in 1 patient, amoxicillin in 1 patient, and metronidazole in 1 patient. Among patients who were treated with monotherapy, 20 received TMP-SMX, 1 patient received ceftriaxone, 1 patient received linezolid, and 1 patient received quinolones.
TABLE 3.
Treatment and Outcome of Literature Patients
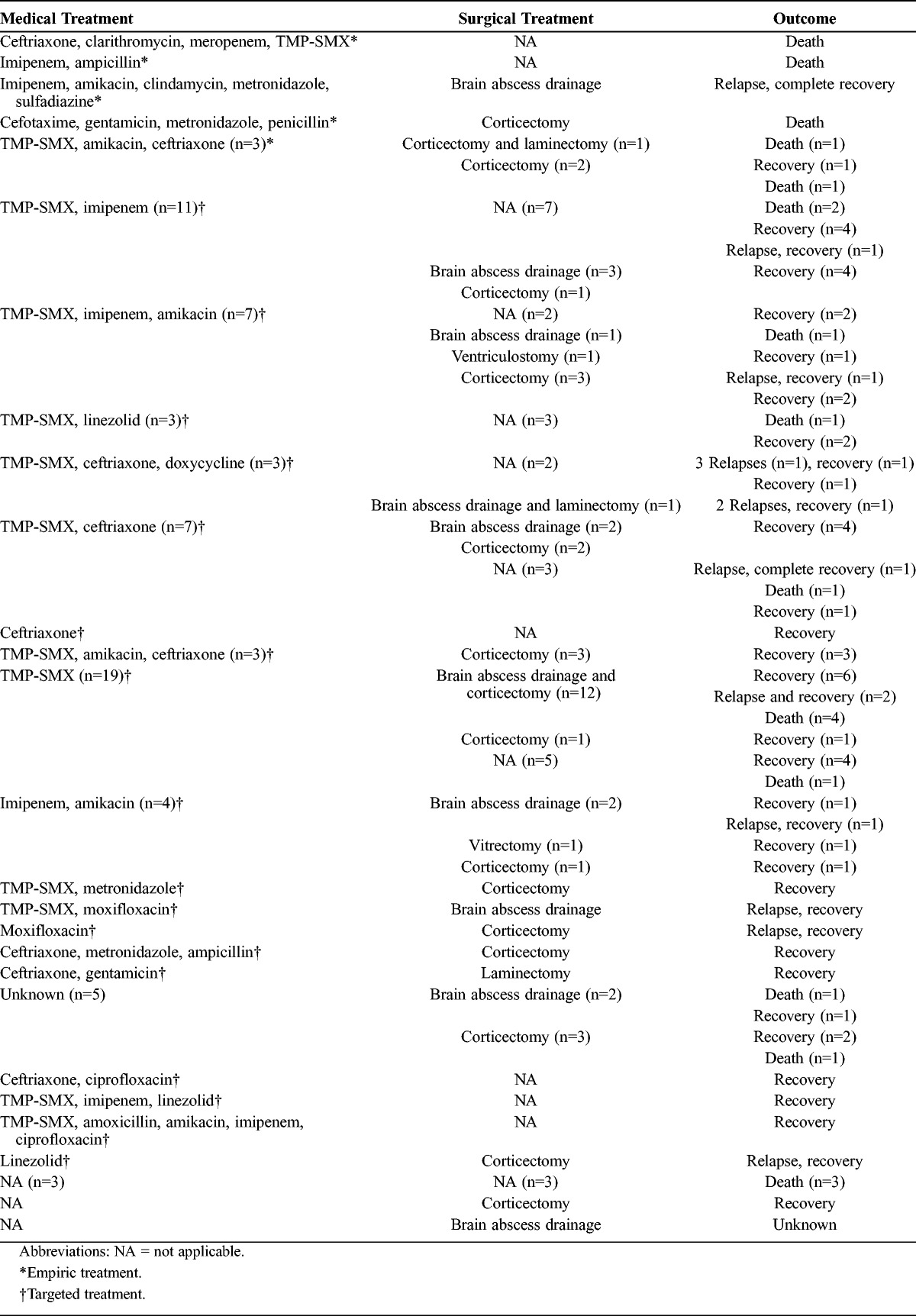
The majority of patients (74%) had a neurosurgical intervention; 26% (n = 22) had corticectomy for evacuation of a brain abscess, 17% (n = 14) had aspiration of the abscess, and 14% (n = 12) had both corticectomy and drainage of the cerebral abscess. One patient underwent ventriculostomy and another 1 vitrectomy. Also,5 patients underwent spinal neurosurgical procedures. Specifically, laminectomy was performed in 3 patients, craniotomy and laminectomy in 1 patient, and laminectomy and drainage in 1 patient. Among the 28 patients who did not have neurosurgery, 46% (n = 13) received targeted antimicrobial therapy while 2 patients received only empirical treatment.
To assess the mortality among the patients in the literature review, we divided them in 3 categories. The first category included patients who were treated with both targeted antimicrobial therapy and neurosurgery (n = 45), of whom only 3 patients died (mortality 7%, relapse rate 13%). The second category involved 23 patients who received only targeted antimicrobial therapy, of whom 5 patients died (mortality 22%, relapse rate 13%). The third category consisted of 11 patients who had only a neurosurgical procedure, of whom 4 patients died (36%). The outcome was unknown in 2 of the patients treated with neurosurgery alone; there was no relapse in the group receiving only neurosurgery. Five patients did not receive targeted antimicrobial therapy or neurosurgery, and all of them died. None of the patients who had a recurrence died.
DISCUSSION
We present 5 cases of CNS nocardiosis treated at MGH from January 2000 to December 2011, and review 84 cases reported during the same 12-year period. All patients treated at MGH were immunosuppressed, while 61% of literature patients were immunosuppressed. The most common cause for immunosuppression in both groups of patients was iatrogenic (patients taking immunosuppressive medication). Twenty-five percent of the literature cases had a history of solid organ or bone marrow transplantation. Although the clinical presentation of the disease was variable, most cases presented with generalized symptoms and had ring-enhancing lesions on their CT head or MRI brain imaging. Mortality was low in both the MGH cohort (20%) and cases in the literature (19%). Mortality was lower among patients who were managed with a combination of medical and surgical treatment (7% compared to 22% in patients treated with medical therapy alone and 36% in patients who underwent neurosurgery alone), while the relapse rate was not improved by the addition of neurosurgery to medical treatment (13%). Finally, treatment with TMP-SMX led to lower mortality and relapse rates (15.3% and 13.6% respectively, compared to 32% and 16%, respectively, in patients who did not receive TMP-SMX), and prolonged treatment for >6 months led to better outcomes than treatment for <6 months (0% mortality and relapse rates compared to 18.4% mortality rate and 16.3% relapse rate in patients treated for <6 mo).
Eighteen percent of literature patients had no comorbid conditions. Previous studies have shown that although two-thirds of patients with nocardiosis are immunosuppressed, about a third of patients with nocardiosis are immunocompetent.6,7,61 More precisely, the proportion of immunocompetent patients among all patients with CNS nocardiosis was 39% in our literature review, which correlates with the 33% reported in the past for patients with any form of nocardiosis.6,7,61 Only 2 patients in our literature review and none of our case series patients had HIV infection. In a study published in 2007 the prevalence of HIV infection among patients with pulmonary nocardiosis was 19%.69 This is much higher than our reported prevalence (2%). However, in the same study no association was found between HIV infection and dissemination to the CNS.69 One possible explanation may be that patients with advanced HIV receive prophylaxis with TMP-SMX, which might protect against dissemination to the CNS. Unfortunately, there are no studies in the literature that estimate the prevalence of HIV infection in patients with CNS nocardiosis.
Nocardia species are weakly gram-positive acid-fast filamentous branching aerobic bacilli that invade the host, grow within host cells, and cause severe disease manifesting with granulomas.91 Although the branching filaments act like fungi, they are truly bacteria. They have the enzymes catalase and superoxide dismutase, which are virulence factors that give them the ability to inactivate reactive oxygen species produced by neutrophils.61 In addition, the cell wall of Nocardia species contains a “cord factor,” a glycolipid that impedes the activation of macrophages by interferon-gamma and thus phagocytosis as well as the fusion of the phagolysosome.6,7 The host defense mechanism against Nocardia species begins with inhibition of the bacterium by the host neutrophils and macrophages,39,40 followed by a T lymphocyte-mediated response that kills the bacterium.7,29 Thus, it is reasonable that factors that decrease the number or interfere with the function of neutrophils and T lymphocytes increase susceptibility to nocardiosis. However, the fact that almost one-third of patients who get CNS nocardiosis are immunocompetent should be further investigated, and may be attributed to inherent defects of the host immune mechanisms, and particularly of cell-mediated immunity, rendering patients vulnerable to such infections.61 Studies in mice have demonstrated that mice deficient in gamma-delta T lymphocytes died after 14 days of inoculation with N. asteroides at a dose that was not lethal to the control group.56 The role of humoral immunity, however, remains undetermined. Experiments in mice have shown that antibodies are not as important as cell-mediated immunity,9,10 while experiments in rabbits support the idea that antibodies aid macrophages in phagocytosis of the bacteria.28 To our knowledge, no direct role of B lymphocytes in nocardiosis has been described thus far.90
We note that all MGH patients and approximately half of the reviewed cases4,21,22,27,30,32,35,36,38,45,47,48,52,54,60,64,67,74,77,78,80–84,86,93,97,98–101,107–110,112,113 were on corticosteroids, which are known to impair lymphocyte activation,61 at the time of their diagnosis with CNS nocardiosis. High-dose corticosteroids for long periods of time are regarded as a predisposing factor for the development of nocardiosis.106,111 However, we do not know of any published studies that investigate the association between specific doses or duration of treatment and the risk of developing the disease. All patients in our literature review who were reported as taking corticosteroids were on prednisone or a prednisone-equivalent pharmacologic agent in a dose ≥10 mg per day for at least 3 months. Calcineurin inhibitors also interfere with the activation of T lymphocytes. Forty percent (2/5) of MGH patients and 24% (20/84) of the literature patients were being treated with calcineurin inhibitors when they were diagnosed with CNS nocardiosis. The administration of TNF-α inhibitors has been linked to the development or reactivation of granulomatous diseases, such as nocardiosis or tuberculosis.103 Although none of the MGH patients was taking TNF-α inhibitors, 4% of the literature cases received TNF-α inhibitors. Eighty percent (4/5) of the MGH patients and 29% (24/84) of the literature patients received chemotherapy, which is known to decrease lymphocyte counts.
Chronic lung disease was the most common comorbid condition in the MGH group of patients and its presence is a known risk factor for pulmonary nocardiosis.69 Half the literature patients with chronic lung disease had primary lung nocardiosis which subsequently disseminated to the CNS. It is possible that Nocardia species invade the respiratory tract through inhalation and this is aided by the impaired immunoprotective mechanisms in patients with lung disease. However, the patient’s immune system is able to prevent an acute lung infection by inhibiting the organism.61 The host’s response is mediated by neutrophils that do not kill the bacteria, but hinder the dissemination of the infection until activation of macrophages and T lymphocytes takes place.7,62 Thus the infection remains subclinical or is mild and self-limited. Nocardia species are able to infect cells and survive intracellularly by inhibiting the fusion of phagosomes with lysosomes.61 Nocardia species may, therefore, be carried by the macrophages across the blood brain barrier and cause CNS infection in patients who are severely immunocompromised or those with previous CNS pathology. The review of the literature discovered a low proportion of patients with COPD (6%), which highlights the fact that an association between chronic lung diseases and CNS nocardiosis has not been established thus far, possibly because investigators rarely report the concomitant presence of lung disease in patients with CNS nocardiosis.
We note that 40% (2/5) of MGH patients and 7.1% (6/84) of literature cases presented with concomitant CNS nocardiosis and pulmonary aspergillosis. The higher proportion of patients identified in our case series (40%) compared to the proportion of patients reported in the literature (7.1%) may be attributed to the limited number of studies that looked for pulmonary aspergillosis in patients infected with Nocardia species. Although concurrent pulmonary infection from Nocardia species and Aspergillus species has been described in a renal transplant patient,18 to our knowledge there is no documented association between pulmonary aspergillosis and CNS nocardiosis. Forty percent (2/5) of MGH patients and 2% (2/84) of literature patients, who were either solid (1 MGH patient and 1 literature patient) or hematologic (2 MGH patients and 1 literature patient) transplant recipients, had a history of CMV infection with pulmonary involvement. CMV infection is an established risk factor for CNS nocardiosis.89
Forty percent (2/5) of MGH patients and 6% (5/84) of literature cases had previous brain pathology, which is a risk factor for CNS nocardiosis.7,62,71 Nocardia species are known to preferentially invade and reproduce within the CNS. Specifically, experiments in mice have shown that several strains of Nocardia species possess surface receptors that recognize and bind sites on capillary endothelial cells in the CNS.8,9 The progression and clearance of the CNS infection depends on the virulence of the particular strain as well as the inoculum of Nocardia species infecting the brain.7,8 N. farcinica is more virulent than N. asteroides and leads to disseminated disease and CNS nocardiosis more frequently.25,95,102
The clinical presentation of CNS nocardiosis is variable and there are no specific signs or symptoms to guide the diagnosis. Focal neurologic abnormalities, headache, fever, and seizures were some of the most common presenting symptoms in both MGH and literature patients. Imaging reveals nonspecific radiologic features of disease, which are often misdiagnosed as tumors or abscesses. In most cases the definitive diagnosis requires invasive techniques to drain abscesses and obtain specimen cultures. Culture of biological specimens has a sensitivity of 85%–90%.85 In aerobic cultures, Nocardia species colonies have an inconsistent white, orange, yellow, or brown appearance14 and usually require 5–21 days to grow.7,25,58 These inconsistencies lead to the delay in the diagnosis, which has been associated with a higher case-fatality ratio. In our literature review, 5 patients were treated empirically due to a delay in diagnosis: 4 of the 5 died, and the remaining 1 patient who survived suffered a relapse. This highlights the necessity of alerting physicians when nocardiosis is first suspected, as specimens can be growing for 72 hours. The most accurate and dependable results are obtained using PCR, but this assay is not available in most laboratory settings.
The majority of MGH and literature patients were treated with a combination of medical therapy and neurosurgery. This combination led to a better outcome and higher survival rates: 93% survival in those treated with combination therapy versus 78% survival in patients receiving only medical management with antibiotics. Mortality was highest (36%) among patients in the literature receiving neurosurgery without targeted antimicrobial medication. There was no recurrence of CNS nocardiosis in this group of patients. This may be because patients who did not have complete resolution of their CNS nocardiosis died. The addition of neurosurgery to medical treatment does not seem to decrease the number of relapses. Some studies suggest that neurosurgery should be used in patients with CNS nocardiosis who do not respond to targeted antimicrobial therapy.59,66 However, based on the data in the current case series and literature review, a combination of targeted antimicrobial agents and neurosurgical drainage of abscesses improves the outcome in terms of both morbidity and mortality and should be considered in all patients.
TMP-SMX is the standard treatment for CNS nocardiosis due to its good penetration into the CNS. It is available in both oral and intravenous formulations. Despite the paucity of prospective randomized trials, retrospective reviews have shown higher survival rates in patients receiving regimens that include TMP-SMX.104 TMP-SMX was administered to all MGH patients in the current study and to the majority of literature patients (70% of all patients). It is likely that in the 30% of the literature cases who were not treated with TMP-SMX there was a delay in diagnosis, and patients were treated with empiric broad-spectrum antibacterial therapy. The administration of TMP-SMX led to lower mortality and relapse rates in literature patients (15.3% and 13.6%, respectively) compared to patients who did not receive the drug (32% and 16%, respectively). Only 1 of the 5 patients who received TMP-SMX in the case series died, and this patient had many comorbidities, including Hodgkin lymphoma with bone metastases, GVHD, bone marrow transplant, COPD, interstitial lung disease, and lung aspergillosis. Our findings suggest the importance of early diagnosis and administration of targeted therapy that includes TMP-SMX.
The duration of treatment with TMP-SMX also seems to influence the outcome of the disease, as patients who were treated for more than 6 months had lower mortality and relapse rates. In the literature, treatment of patients with non-CNS disease is recommended for at least 6 months, and treatment of CNS involvement is recommended for at least 12 months to prevent relapses.106 In both the case series and the literature review, no patient was treated for more than 12 months. This may be attributed to the fact that patients are often lost to follow-up after 1 year of treatment, especially when there is significant improvement of their condition. The recommended dose of TMP-SMX is 25–50 mg/kg per day.106 An association between the dose and the possibility for recurrence has not been documented, as there are not enough data in the literature regarding the therapeutic dose. In the literature review, all 3 patients who relapsed and provided information about the dose of TMP-SMX were treated adequately, and 2 of them received very high doses of the medication (90 mg/kg per day).
TMP-SMX is usually combined with imipenem or amikacin for the treatment of pulmonary and disseminated nocardiosis.14,91 Murine studies have shown that a combination of amikacin with imipenem or third-generation cephalosporin is more effective in reducing bacterial load compared to TMP-SMX.61 In the literature review only 4 patients received a combination of imipenem and amikacin without TMP-SMX; all of them recovered fully, and 1 had a relapse. However, the number of patients treated with this therapeutic scheme is too small to draw the conclusion that the combination of imipenem and amikacin improves the overall prognosis. Alternative treatments considered effective against nocardiosis are cephalosporins, amoxicillin with clavulanate, macrolides, and fluoroquinolones.13,16,92 Also, linezolid is considered an effective option against CNS nocardiosis, due to its good penetration to the CSF and its effectiveness against all species.89
While in clinical practice the initial treatment of CNS nocardiosis is empirical, targeted antimicrobial therapy should be initiated as soon as possible based on antimicrobial susceptibility testing. Speciation influences drug selection, as different species are resistant to different antibiotics. N. farcinica, N. brasilensis, and N. otitidiscaviarium have the highest rates of resistance to multiple antibiotics, including TMP-SMX, ampicillin, ceftriaxone, and imipenem, while N. asteroides is resistant to ampicillin, most fluoroquinolones, and imipenem.14,43 It is estimated, for example, that 36% of N. farcinica and 23% of N. asteroides strains are resistant to imipenem.62,71,72 Also, N. nova, N. otitidiscaviarium, and N. transvalensis are resistant to amoxicillin with clavulanate.61 On the other hand, moxiflocaxin is particularly active against N. asteroides.20,49
Finally, it is worth mentioning that the relapse rate of literature patients who were treated with targeted medical therapy was independent of the performance of neurosurgery. Specifically, 13% (11/84) of literature patients treated with the combination of medication and surgery, and an equal percentage of patients treated with medical therapy alone, recurred. The recurrence of CNS nocardiosis is multifactorial and depends on the immune status of the patient, the duration of treatment, the administration of prolonged oral antimicrobial prophylaxis after primary infection, the antimicrobial susceptibility profile of infecting nocardial strains, and, potentially, the poor penetration of drugs to the CNS.
Conclusions
CNS nocardiosis occurs in both immunocompromised and immunocompetent individuals. There is a higher incidence of CNS nocardiosis in immunocompromised patients, most commonly patients on corticosteroids and transplant patients, and patients with previous brain pathology. Delay in the diagnosis of the disease may be caused by its nonspecific and variable clinical presentation, nonspecific radiologic findings, and variable bacterial culture morphology. CNS nocardiosis can lead to death even with targeted antimicrobial therapy and neurosurgical drainage of abscesses. Antimicrobial agents used as the mainstay of treatment include TMP-SMX, and the combination of imipenem and amikacin. The results of the current study indicate that the most effective treatment is targeted antimicrobial therapy based on the antimicrobial susceptibility profile of the infecting nocardial strain in combination with neurosurgical drainage of abscesses.
Overall, administration of a therapeutic scheme that includes TMP-SMX for more than 6 months is probably associated with lower mortality and relapse rates. The current study is limited by the number of patients involved; we were not able to reach any definitive conclusions about the most effective therapeutic approach. However, due to the rarity of the disease, this limitation may be impossible to circumvent.
Abbreviations
- CMV
cytomegalovirus
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- CSF
cerebrospinal fluid
- CT
computed tomography
- GVHD
graft-versus-host disease
- HIV
human immunodeficiency virus
- MGH
Massachusetts General Hospital
- MRI
magnetic resonance imaging
- PCR
polymerase chain reaction
- TMP/SMX
trimethoprim/sulfomethoxazole
- TNF-α
tumor necrosis factor-α
Footnotes
Financial support and conflicts of interest: The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Alp E, Yildiz O, Aygen B, Sumerkan B, Sari I, Koc K, Couble A, Laurent F, Boiron P, Doganay M. Disseminated nocardiosis due to unusual species: two case reports. Scand J Infect Dis. 2006;38:545–548. [DOI] [PubMed] [Google Scholar]
- 2.Al Soub H, Almaslamani M, Al Khuwaiter J, El Deeb Y, Khatab MA. Primary Nocardia meningitis in a patient without a predisposing condition: case report and review of the literature. Scand J Infect Dis. 2007;39:737–741. [DOI] [PubMed] [Google Scholar]
- 3.Arends JE, Stemerding AM, Vorst SP, de Neeling AJ, Weersink AJ. First report of a brain abscess caused by Nocardia veterana. J Clin Microbiol. 2011;49:4364–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azap A, Arslan H, Ergin F, Karakayali H, Haberal M. Disseminated Nocardia asteroides and coinfection with Trichophyton rubrum in a renal transplant recipient. Transpl Infect Dis. 2002;4:223–225. [DOI] [PubMed] [Google Scholar]
- 5.Barnaud G, Deschamps C, Manceron V, Mortier E, Laurent F, Bert F, Boiron P, Vinceneux P, Branger C. Brain abscess caused by Nocardia cyriacigeorgica in a patient with human immunodeficiency virus infection. J Clin Microbiol. 2005;43:4895–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaman BL, Black CM, Doughty F, Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985;47:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7:213–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaman BL, Beaman L, Kjelstrom JA, Ogata SA. Bacteria and neurodegeneration. In: Calne D, ed. Neurodegenerative Diseases. Orlando, FL: WB Saunders; 1994:319–338. [Google Scholar]
- 9.Beaman BL, Boiron P, Beaman L, Brownell GH, Schaal K, Gombert ME. Nocardia and nocardiosis. J Med Vet Mycol. 1992;30:317–331. [PubMed] [Google Scholar]
- 10.Beaman BL, Gershwin ME, Ahmed A, Scates SM, Deem R. Response of CBA/N x DBA2/F1 mice to Nocardia asteroides. Infect Immun. 1982;35:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belhocine W, Purgus R, Almasalma M, Moal V, Edouard S, Berland Y, Raoult D, Fournier PE. Nocardia carnea infection in a kidney transplant recipient. Transplant Proc. 2010;42:4359–4360. [DOI] [PubMed] [Google Scholar]
- 12.Borm W, Gleixner M. Nocardia brain abscess misinterpreted as cerebral infarction. J Clin Neurosci. 2003;10:130–132. [DOI] [PubMed] [Google Scholar]
- 13.Brown JM, McNeil MM. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, eds. Manual of Clinical Microbiology. 8th ed Washington, DC: ASM Press; 2003:502–531. [Google Scholar]
- 14.Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ., Jr Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budzik JM, Hosseini M, Mackinnon AC, Jr, Taxy JB. Disseminated Nocardia farcinica: literature review and fatal outcome in an immunocompetent patient. Surg Infect (Larchmt). 2012;13:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgert SJ. Nocardiosis: a clinical review. Infect Dis Clin Pract. 1999;8:27–32. [Google Scholar]
- 17.Carradice D, Szer J. Cerebral nocardiosis after allogeneic bone marrow transplantation. Intern Med J. 2004;34:698–699. [DOI] [PubMed] [Google Scholar]
- 18.Carter JM, Green WR, Callender CO, Peters B. Pulmonary cavitation with Nocardia and Aspergillus in a renal transplant patient. J Natl Med Assoc. 1990;82:527–528,530-531. [PMC free article] [PubMed] [Google Scholar]
- 19.Cercenado E, Marin M, Sanchez-Martinez M, Cuevas O, Martinez-Alarcon J, Bouza E. In vitro activities of tigecycline and eight other antimicrobials against different Nocardia species identified by molecular methods. Antimicrob Agents Chemother. 2007;51:1102–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakrabarti P, Nandi SS, Todi SK. Nocardia brain abscess in a diabetic patient. Indian J Pathol Microbiol. 2008;51:151–153. [DOI] [PubMed] [Google Scholar]
- 21.Charfeddine K, Kharrat M, Yaich S, Abdelmalek R, Hakim H, Bahloul H, Jarraya F, Hammami A, Hachicha J. Systemic nocardiosis with multiple brain abscesses in a renal transplant recipient: successfully treated with antibiotics alone. Saudi J Kidney Dis Transpl. 2002;13:498–500. [PubMed] [Google Scholar]
- 22.Chow E, Moore T, Deville J, Nielsen K. Nocardia asteroides brain abscesses and meningitis in an immunocompromized 10-year-old child. Scand J Infect Dis. 2005;37:511–513. [DOI] [PubMed] [Google Scholar]
- 23.Chung TT, Lin JC, Hsieh CT, Chen GJ, Ju DT. Nocardia farcinica brain abscess in an immunocompetent patient treated with antibiotics and two surgical techniques. J Clin Neurosci. 2009;16:1675–1677. [DOI] [PubMed] [Google Scholar]
- 24.Cianfoni A, Calandrelli R, De Bonis P, Pompucci A, Lauriola L, Colosimo C. Nocardia brain abscess mimicking high-grade necrotic tumor on perfusion MRI. J Clin Neurosci. 2010;17:1080–1082. [DOI] [PubMed] [Google Scholar]
- 25.Conville PS, Witebsky FG. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, eds. Manual of Clinical Microbiology. 9th ed Washington, DC: ASM Press; 2007:515–542. [Google Scholar]
- 26.Cortese I, Nath A. Case 11: a young woman with ring-enhancing brain lesions. MedGenMed. 2006;8:3. [PMC free article] [PubMed] [Google Scholar]
- 27.Dahan K, El Kabbaj D, Venditto M, Pastural M, Delahousse M. Intracranial Nocardia recurrence during fluorinated quinolones therapy. Transpl Infect Dis. 2006;8:161–165. [DOI] [PubMed] [Google Scholar]
- 28.Davis-Scibienski C, Beaman BL. Interaction of alveolar macrophages with Nocardia asteroides: immunological enhancement of phagocytosis, phagosome-lysosome fusion, and microbicidal activity. Infect Immun. 1980;30:578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deem RL, Doughty FA, Beaman BL. Immunologically specific direct T lymphocyte-mediated killing of Nocardia asteroides. J Immunol. 1983;130:2401–2406. [PubMed] [Google Scholar]
- 30.Dehghani M, Davarpanah MA. Epididymo-orchitis and central nervous system nocardiosis in a bone marrow transplant recipient for acute lymphoblastic leukemia. Exp Clin Transplant. 2009;7:264–266. [PubMed] [Google Scholar]
- 31.Dias M, Nagarathna S, Mahadevan A, Chandramouli BA, Chandramuki A. Nocardial brain abscess in an immunocompetent host. Indian J Med Microbiol. 2008;26:274–277. [DOI] [PubMed] [Google Scholar]
- 32.Duran E, Lopez L, Martinez A, Comunas F, Boiron P, Rubio MC. Primary brain abscess with Nocardia otitidiscaviarum in an intravenous drug abuser. J Med Microbiol. 2001;50:101–103. [DOI] [PubMed] [Google Scholar]
- 33.Durmaz R, Atasoy MA, Durmaz G, Adapinar B, Arslantas A, Aydinli A, Tel E. Multiple nocardial abscesses of cerebrum, cerebellum and spinal cord, causing quadriplegia. Clin Neurol Neurosurg. 2001;103:59–62. [DOI] [PubMed] [Google Scholar]
- 34.Eisenblatter M, Disko U, Stoltenburg-Didinger G, Scherubl H, Schaal KP, Roth A, Ignatius R, Zeitz M, Hahn H, Wagner J. Isolation of Nocardia paucivorans from the cerebrospinal fluid of a patient with relapse of cerebral nocardiosis. J Clin Microbiol. 2002;40:3532–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elmaci I, Senday D, Silav G, Ekenel F, Balak N, Ayan E, Akinci M, Isik N, Yazici S. Nocardial cerebral abscess associated with mycetoma, pneumonia, and membranoproliferative glomerulonephritis. J Clin Microbiol. 2007;45:2072–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Everett CM, Dhillon H, Samarasinghe D, Berry L, Warwick S, Turner B. A case of cerebral nocardiosis following brief immunosuppression. Eur J Neurol. 2006;13:431–432. [DOI] [PubMed] [Google Scholar]
- 37.Fellows GA, Kalsi PS, Martin AJ. Nocardia farcinica brain abscess in a patient without immunocompromise. Br J Neurosurg. 2007;21:301–303. [DOI] [PubMed] [Google Scholar]
- 38.Fihman V, Bercot B, Mateo J, Losser MR, Raskine L, Riahi J, Loirat P, Pors MJ. First successful treatment of Nocardia farcinica brain abscess with moxifloxacin. J Infect. 2006;52:99–102. [DOI] [PubMed] [Google Scholar]
- 39.Filice GA, Beaman BL, Krick JA, Remington JS. Effects of human neutrophils and monocytes on Nocardia asteroides: failure of killing despite occurrence of the oxidative metabolic burst. J Infect Dis. 1980;142:432–438. [DOI] [PubMed] [Google Scholar]
- 40.Filice GA. Inhibition of Nocardia asteroides by neutrophils. J Infect Dis. 1983;151:47–56. [DOI] [PubMed] [Google Scholar]
- 41.Filice GA. Nocardiosis in persons with human immunodeficiency virus infection, transplant recipients, and large, geographically defined populations. J Lab Clin Med. 2005;145:156–162. [DOI] [PubMed] [Google Scholar]
- 42.Ghalib MB, Hassan LS. Prolonged unconsciousness in a patient with End-stage Renal Disease. Saudi J Kidney Dis Transpl. 2006;17:273–277. [PubMed] [Google Scholar]
- 43.Glupczynski Y, Berhin C, Janssens M, Wauters G. Determination of antimicrobial susceptibility patterns of Nocardia spp. from clinical specimens by Etest. Clin Microbiol Infect. 2006;12:905–912. [DOI] [PubMed] [Google Scholar]
- 44.Green JS, Abeles SR, Uslan DZ, Mehta SR. Persistent neutrophilic meningitis in an immunocompetent patient after basilar skull fracture: case report. BMC Infect Dis. 2011;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta S, Faughnan ME, Prud’homme GJ, Hwang DM, Munoz DG, Kopplin P. Sarcoidosis complicated by cirrhosis and hepatopulmonary syndrome. Can Respir J. 2008;15:124–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hahn BH, McDermott RP, Jacobs SB, Pletscher LS, Beale MG. Immunosuppressive effects of low doses of glucocorticoids: effects on autologous and allogeneic mixed leukocyte reactions. J Immunol. 1980;124:2812–2817. [PubMed] [Google Scholar]
- 47.Hashimoto M, Johkura K, Ichikawa T, Shinonaga M. Brain abscess caused by Nocardia nova. J Clin Neurosci. 2008;15:87–89. [DOI] [PubMed] [Google Scholar]
- 48.Hemmersbach-Miller M, Martel AC, Benitez AB, Sosa AO. Brain abscess due to Nocardia otitidiscaviarum: report of a case and review. Scand J Infect Dis. 2004;36:381–384. [DOI] [PubMed] [Google Scholar]
- 49.Hoogkamp-Korstanje JA, Roelofs-Willemse J. Comparative in vitro activity of moxifloxacin against gram-positive clinical isolates. J Antimicrob Chemother. 2000;45:31–39. [DOI] [PubMed] [Google Scholar]
- 50.Iannotti CA, Hall GS, Procop GW, Tuohy MJ, Staugaitis SM, Weil RJ. Solitary Nocardia farcinica brain abscess in an immunocompetent adult mimicking metastatic brain tumor: rapid diagnosis by pyrosequencing and successful treatment. Surg Neurol. 2009;72:74–79. [DOI] [PubMed] [Google Scholar]
- 51.Joung MK, Kong DS, Song JH, Peck KR. Concurrent nocardia related brain abscess and semi-invasive pulmonary aspergillosis in an immunocompetent patient. J Korean Neurosurg Soc. 2011;49:305–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Justiniano M, Glorioso S, Dold S, Espinoza LR. Nocardia brain abscesses in a male patient with SLE: successful outcome despite delay in diagnosis. Clin Rheumatol. 2007;26:1020–1022. [DOI] [PubMed] [Google Scholar]
- 53.Kandasamy J, Iqbal HJ, Cooke RP, Eldridge PR. Primary Nocardia farcinica brain abscess with secondary meningitis and ventriculitis in an immunocompetent patient, successfully treated with moxifloxacin. Acta Neurochir (Wien). 2008;150:505–506. [DOI] [PubMed] [Google Scholar]
- 54.Kaswan KK, Vanikar AV, Feroz A, Patel HV, Gumber M, Trivedi HL. Nocardia infection in a renal transplant recipient. Saudi J Kidney Dis Transpl. 2011;22:1203–1204. [PubMed] [Google Scholar]
- 55.Khan SH, Sanche SE, Robinson CA, Pirouzmand F. N. paucivorans infection presenting as a brain abscess. Can J Neurol Sci. 2006;33:426–427. [DOI] [PubMed] [Google Scholar]
- 56.King DP, Hyde DM, Jackson KA, Novosad DM, Ellis TN, Putney L, Stovall MY, Van Winkle LS, Beaman BL, Ferrick DA. Cutting edge: protective response to pulmonary injury requires gamma delta T lymphocytes. J Immunol. 1999;162:5033–5036. [PubMed] [Google Scholar]
- 57.Kundranda MN, Spiro TP, Muslimani A, Gopalakrishna KV, Melaragno MJ, Daw HA. Cerebral nocardiosis in a patient with NHL treated with rituximab. Am J Hematol. 2007;82:1033–1034. [DOI] [PubMed] [Google Scholar]
- 58.Lederman ER, Crum NF. A case series and focused review of nocardiosis: clinical and microbiologic aspects. Medicine (Baltimore). 2004;83:300–313. [DOI] [PubMed] [Google Scholar]
- 59.Lee GY, Daniel RT, Brophy BP, Reilly PL. Surgical treatment of nocardial brain abscesses. Neurosurgery. 2002;51:668–671. [PubMed] [Google Scholar]
- 60.Leis JA, Bunce PE, Lee TC, Gold WL. Brain and lung lesions in an immunocompromised man. CMAJ. 2011;183:573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891–903. [DOI] [PubMed] [Google Scholar]
- 62.Lerner PI. Nocardia species. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 4th ed New York: Churchill Livingstone; 1995:2273–2280. [Google Scholar]
- 63.Lin YJ, Yang KY, Ho JT, Lee TC, Wang HC, Su FW. Nocardial brain abscess. J Clin Neurosci. 2010;17:250–253. [DOI] [PubMed] [Google Scholar]
- 64.Lopez FA, Johnson F, Novosad DM, Beaman BL, Holodniy M. Successful management of disseminated Nocardia transvalensis infection in a heart transplant recipient after development of sulfonamide resistance: case report and review. J Heart Lung Transplant. 2003;22:492–497. [DOI] [PubMed] [Google Scholar]
- 65.Malincarne L, Marroni M, Farina C, Camanni G, Valente M, Belfiori B, Fiorucci S, Floridi P, Cardaccia A, Stagni G. Primary brain abscess with Nocardia farcinica in an immunocompetent patient. Clin Neurol Neurosurg. 2002;104:132–135. [DOI] [PubMed] [Google Scholar]
- 66.Mamelak AN, Obana WG, Flaherty JF, Rosenblum ML. Nocardial brain abscess: treatment strategies and factors influencing outcome. Neurosurgery. 1994;35:622–631. [DOI] [PubMed] [Google Scholar]
- 67.Marchandin H, Eden A, Jean-Pierre H, Reynes J, Jumas-Bilak E, Boiron P, Laurent F. Molecular diagnosis of culture-negative cerebral nocardiosis due to Nocardia abscessus. Diagn Microbiol Infect Dis. 2006;55:237–240. [DOI] [PubMed] [Google Scholar]
- 68.Marlowe M, Ali-Ahmad D, Cherrick I, Higgins MJ, Kiska DL, Domachowske JB. Central nervous system nocardiosis in an immunocompetent child. Pediatr Infect Dis J. 2000;19:661–662. [DOI] [PubMed] [Google Scholar]
- 69.Martinez Tomas R, Menendez Villanueva R, Reyes Calzada S, Reyes Calzada S, Santos Durantez M, Valles Tarazona JM, Modesto Alapont M, Gobernado Serrano M. Pulmonary nocardiosis: risk factors and outcomes. Respirology. 2007;12:394–400. [DOI] [PubMed] [Google Scholar]
- 70.Mascarenhas NB, Lam D, Lynch GR, Fisher RE. PET imaging of cerebral and pulmonary Nocardia infection. Clin Nucl Med. 2006;31:131–133. [DOI] [PubMed] [Google Scholar]
- 71.McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McNeil MM, Brown JM, Hutwagner LC, Schiff TA. Evaluation of therapy for Nocardia asteroides complex infections. Infect Dis Clin Pract. 1995;4:287–292. [Google Scholar]
- 73.Menku A, Kurtsoy A, Tucer B, Yildiz O, Akdemir H. Nocardia brain abscess mimicking brain tumour in immunocompetent patients: report of two cases and review of the literature. Acta Neurochir (Wien). 2004;146:411–414. [DOI] [PubMed] [Google Scholar]
- 74.Mete B, Yemisen M, Demirel AE, Ozaras R, Mert A, Ozturk R, Tabak F. A case of nocardiasis complicated with meningitis in a patient with immune thrombocytopenic purpura. Blood Coagul Fibrinolysis. 2010;21:185–187. [DOI] [PubMed] [Google Scholar]
- 75.Mongkolrattanothai K, Ramakrishnan S, Zagardo M, Gray B. Ventriculitis and choroid plexitis caused by multidrug-resistant Nocardia pseudobrasiliensis. Pediatr Infect Dis J. 2008;27:666–668. [DOI] [PubMed] [Google Scholar]
- 76.Montoya JP, Carpenter JL, Holmes GP, Hurley DL, Winn R. Disseminated Nocardia transvalensis infection with osteomyelitis and multiple brain abscesses. Scand J Infect Dis. 2003;35:189–196. [DOI] [PubMed] [Google Scholar]
- 77.Moon JH, Cho WS, Kang HS, Kim JE. Nocardia brain abscess in a liver transplant recipient. J Korean Neurosurg Soc. 2011;50:396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mooraki A, Jenabi A, Bastani B. Resolution of pulmonary and cerebral nocardiosis in renal transplant patient despite continued immunosuppression: a case report. Transplant Proc. 2003;35:2694–2695. [DOI] [PubMed] [Google Scholar]
- 79.Murray RJ, Himmelreich U, Gomes L, Ingham NJ, Sorrell TC. Cerebral nocardiosis characterized by magnetic resonance spectroscopy in vivo. Clin Infect Dis. 2002;34:849–852. [DOI] [PubMed] [Google Scholar]
- 80.Naqi R, Ahsan H, Azeemuddin M. Cerebral nocardiosis. J Pak Med Assoc. 2011;61:697–699. [PubMed] [Google Scholar]
- 81.Ng EW, Zimmer-Galler IE, Green WR. Endogenous Nocardia asteroides endophthalmitis. Arch Ophthalmol. 2002;120:210–213. [PubMed] [Google Scholar]
- 82.O’Neill E, Fitzpatrick F, Smyth E. Nocardial brain abscess: an unusual cause of acute confusion. Ir Med J. 2005;98:116. [PubMed] [Google Scholar]
- 83.Ono M, Kobayashi Y, Shibata T, Maruyama D, Kim SW, Watanabe T, Mikami Y, Tobinai K. Nocardia exalbida brain abscess in a patient with follicular lymphoma. Int J Hematol. 2008;88:95–100. [DOI] [PubMed] [Google Scholar]
- 84.Ozturk S, Tufan F, Alisir S, Gorcin S, Guven D, Cagatay A, Turkmen A. A case of isolated Nocardia asteroides brain abscess in a kidney transplant recipient. Transplant Proc. 2006;38:3121–3124. [DOI] [PubMed] [Google Scholar]
- 85.Palmer DL, Harvey RL, Wheeler JK. Diagnostic and therapeutic considerations in Nocardia asteroides infection. Medicine (Baltimore). 1974;53:391–401. [DOI] [PubMed] [Google Scholar]
- 86.Pamuk GE, Pamuk ON, Tabak F, Mert A, Ozturk R, Aktuglu Y. Systemic Nocardia infection in a patient with Behcet’s disease. Rheumatology (Oxford). 2001;40:597–599. [DOI] [PubMed] [Google Scholar]
- 87.Patil A, Cherian A, Iype T, Sandeep P. Nocardial brain abscess in an immunocompetent individual. Neurol India. 2011;59:779–782. [DOI] [PubMed] [Google Scholar]
- 88.Patil NP, Nadkarni NJ, Sharma NR. Nocardiosis: clinical and pathological aspects. In: Poblet Martinez E, ed. Histopathology—Reviews and Recent Advances. 2012; Chapter 5 [e-book; http://dx.doi.org/10.5772/2991].
- 89.Peleg AY, Husain S, Qureshi ZA, Silveira FP, Sarumi M, Shutt KA, Kwak EJ, Paterson DL. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis. 2007;44:1307–1314. [DOI] [PubMed] [Google Scholar]
- 90.Rico G, Ochoa R, Oliva A, Gonzalez-Mendoza A, Walker SM, Ortiz-Ortiz L. Enhanced resistance to Nocardia brasiliensis infection in mice depleted of antigen-specific B cells. J Immunol. 1982;129:1688–1693. [PubMed] [Google Scholar]
- 91.Saubolle MA, Sussland D. Nocardiosis: review of clinical and laboratory experience. J Clin Microbiol. 2003;41:4497–4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saubolle MA. Aerobic actinomycetes. Clin Lab Medicine. 2002;2:1201–1220. [Google Scholar]
- 93.Shin JH, Lee HK. Nocardial brain abscess in a renal transplant recipient. Clin Imaging. 2003;27:321–324. [DOI] [PubMed] [Google Scholar]
- 94.Sonesson A, Oqvist B, Hagstam P, Bjorkman-Burtscher IM, Miorner H, Petersson AC. An immunosuppressed patient with systemic vasculitis suffering from cerebral abscesses due to Nocardia farcinica identified by 16S rRNA gene universal PCR. Nephrol Dial Transplant. 2004;19:2896–2900. [DOI] [PubMed] [Google Scholar]
- 95.Sorrel T, Mitchell DH, Iredell JR, Chen SCA, Nocardia species. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed Philadelphia: Churchill Livingstone/Elsevier; 2010:3199–3207. [Google Scholar]
- 96.Soto-Hernandez JL, Moreno-Andrade T, Gongora-Rivera F, Ramirez-Crescencio MA. Nocardia abscess during treatment of brain toxoplasmosis in a patient with AIDS, utility of proton MR spectroscopy and diffusion-weighted imaging in diagnosis. Clin Neurol Neurosurg. 2006;108:493–498. [DOI] [PubMed] [Google Scholar]
- 97.Srinivas KV, Freigoun OS, Rabie A, Want MA. Cerebral nocardiosis in a renal transplant recipient: a case report. Saudi J Kidney Dis Transpl. 2000;11:583–586. [PubMed] [Google Scholar]
- 98.Sud S, Buxi T, Anand I, Rohatgi A. Nocardiosis of the brain and lungs. Indian J Radiol Imaging. 2008;18:218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tada Y, Fukuoka M, Mitamura M, Koarada S, Suematsu R, Inoue H. H, Ohta A, Nagasawa K. Nocardiosis in adult-onset Still’s disease and vasculitis syndrome. Am J Med Sci. 2008;336:77–80. [DOI] [PubMed] [Google Scholar]
- 100.Tamm M, Chhajed P, Malouf M, Glanville A. Cavitary opacity following lung transplantation. Respiration. 2001;68:428–431. [DOI] [PubMed] [Google Scholar]
- 101.Vossmerbaeumer U, Spandau UH, Kleinhuber K, Zeller S, Chatzikonstantinou A, Ruggiero S, Birck R. Arrest of endogenous ocular nocardiosis under linezolid therapy. Acta Ophthalmol. 2000;88:381–383. [DOI] [PubMed] [Google Scholar]
- 102.Wallace RJ, Jr, Brown BA, Tsukamura M, Brown JM, Onvi GO. Clinical and laboratory features of Nocardia nova. J Clin Microbiol. 1991;29:2407–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–1265. [DOI] [PubMed] [Google Scholar]
- 104.West KR, Mason RC, Sun M. Nocardia spinal epidural abscess 14-year follow-up. Orthopedics. 2012;35:128–131. [DOI] [PubMed] [Google Scholar]
- 105.Wilson JP, Turner HR, Kirchner KA, Chapman SW. Nocardial infections in renal transplant recipients. Medicine (Baltimore). 1989;68:38–57. [DOI] [PubMed] [Google Scholar]
- 106.Wilson JW. Nocardiosis: update and clinical overview. Mayo Clin Proc. 2012;87:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xi L, Lu C, Zeng F, Chen W, Wu S, Mikami Y. Subcutaneous and brain abscesses caused by Nocardia farcinica in China. Chin Med J (Engl). 2000;113:862-864. [PubMed] [Google Scholar]
- 108.Yamada SM, Nakai E, Toyonaga S, Nakabayashi H, Park KC, Shimizu K. A rapidly enlarging nocardial brain abscess mimicking malignant glioma. J Nippon Med Sch. 2005;72:308–311. [DOI] [PubMed] [Google Scholar]
- 109.Yasuda N, Ohmori S, Usui T. A case of Evans’ syndrome complicated with multiple nocardial abscesses: a long-term survivor under corticosteroid therapy. Int J Hematol. 2001;74:233–234. [DOI] [PubMed] [Google Scholar]
- 110.Yorke RF, Rouah E. Nocardiosis with brain abscess due to an unusual species, Nocardia transvalensis. Arch Pathol Lab Med. 2003;127:224–226. [DOI] [PubMed] [Google Scholar]
- 111.Young LS, Rubin RH. Mycobacterial and nocardial diseases in the compromised host. In: Rubin RH, Young LS, eds. A Clinical Approach to Infection in the Compromised Host. 4th ed New York: Kluwer Academic; 2002:257–261. [Google Scholar]
- 112.Young WF. Syringomyelia presenting as a delayed complication of treatment for nocardia brain abscess. Spinal Cord. 2000;38:265–269. [DOI] [PubMed] [Google Scholar]
- 113.Zakaria A, Elwatidy S, Elgamal E. Nocardia brain abscess: severe CNS infection that needs aggressive management; case report. Acta Neurochir (Wien). 2008;150:1097–1101. [DOI] [PubMed] [Google Scholar]


