Abstract
Although discontinuation of suppressive antifungal therapy for acquired immunodeficiency syndrome (AIDS)-associated histoplasmosis is accepted for patients with immunologic recovery, there have been no published studies of this approach in clinical practice, and minimal characterization of individuals who relapse with this disease. We performed a multicenter retrospective cohort study to determine the outcome in AIDS patients following discontinuation of suppressive antifungal therapy for histoplasmosis.
Ninety-seven patients were divided into a physician-discontinued suppressive therapy group (PD) (38 patients) and a physician-continued suppressive therapy group (PC) (59 patients). The 2 groups were not statistically different at baseline, but at discontinuation of therapy and at the most recent follow-up there were significant differences in adherence to therapy, human immunodeficiency virus (HIV) RNA, and urinary Histoplasma antigen concentration. There was no relapse or death attributed to histoplasmosis in the PD group compared with 36% relapse (p < 0.0001) and 5% death (p = 0.28) in the PC group. Relapse occurred in 53% of the nonadherent patients but not in the adherent patients (p < 0.0001). Sixty-seven percent of patients with initial central nervous system (CNS) histoplasmosis relapsed compared to 15% of patients without CNS involvement (p = 0.0004), which may be accounted for by nonadherence. In addition, patients with antigenuria above 2.0 ng/mL at 1-year follow-up were 12.82 times (95% confidence interval, 2.91–55.56) more likely to relapse compared to those with antigenuria below 2.0 ng/mL.
Discontinuation of antifungal therapy was safe in adherent patients who completed at least 1 year of antifungal treatment, and had CD4 counts >150 cells/mL, HIV RNA <400 c/mL, Histoplasma antigenuria <2 ng/mL (equivalent to <4.0 units in second-generation method), and no CNS histoplasmosis.
INTRODUCTION
Histoplasma capsulatum is a dimorphic fungus that is endemic in the Ohio and Mississippi River valleys of the United States and in Central and South America, and has microfoci in the eastern United States, southern Europe, Africa, and southeastern Asia.7 Histoplasmosis is associated with significant morbidity and mortality in patients with acquired immunodeficiency syndrome (AIDS).10 However, limited experience has shown that antifungal therapy can be discontinued in selected patients with AIDS-associated histoplasmosis. In a study published in 2004,5 antifungal therapy was stopped in patients who had received at least 1 year of antifungal therapy; received 6 months of antiretroviral therapy; and had Histoplasma antigenemia and antigenuria <4.0 units, negative fungal blood cultures, and CD4 counts of at least 150 cells/μL. Of the 32 patients enrolled in the study, none relapsed. In 2007 the Infectious Diseases Society of America (IDSA) endorsed the approach outlined in the 2004 study and changed its recommendation from lifelong-maintenance antifungal therapy in the 2000 guideline8 to discontinuation of antifungal therapy if that study’s criteria were met.1,11 However, there is limited published evidence for this approach in clinical practice. Additionally, the study that informed the guideline did not include individuals who relapsed, and there is a paucity of published data on this subset of patients. Our objective in the current study was to evaluate outcome data from clinical practice during the highly active antiretroviral therapy (HAART) era and suggest additional considerations for discontinuation of antifungal therapy.
PATIENTS AND METHODS
Patients
Patients were identified by review of clinical and laboratory records and International Classification of Diseases-9th revision (ICD-9) codes from October 1995 to December 2009 at the participating institutions. Of the 97 patients we evaluated, 27 were from Indiana University Health (Indianapolis, IN), 23 were from University of Kentucky (Lexington, KY), 15 from University of Southern California (Los Angeles, CA), 14 from University of Missouri-Kansas City School of Medicine (Kansas City, MO), 12 from Emory University (Atlanta, GA), and 6 were from University of Alabama (Birmingham, AL). Eligibility for inclusion in the analysis required the availability of records and follow-up for at least 1 year.
The study was approved by the institutional review boards of each institution.
The 97 patients were divided into 2 groups: the physician-discontinued suppressive therapy (PD) group (38 patients) and the physician-continued suppressive therapy (PC) group (59 patients). This was not a randomized study. Adherence to antifungal and antiretroviral therapy was determined by the physician’s assessment and human immunodeficiency virus (HIV) RNA levels consistently below 400 c/mL. Adherence in this study referred to both antifungal and antiretroviral therapy.
Diagnosis
The diagnosis was based upon positive culture, pathology, or antigen testing accompanied by clinical findings compatible with histoplasmosis. Central nervous system (CNS) histoplasmosis was diagnosed in patients who had CNS symptoms and cerebrospinal fluid (CSF) abnormalities suggesting meningeal inflammation, or CNS imaging showing meningeal enhancement, or 1 or more cerebral masses. The laboratory basis for diagnosis of CNS histoplasmosis included positive Histoplasma antigen or antibody from CSF or positive culture or cytologic or histopathologic evidence of Histoplasma in the CSF or brain. Disseminated histoplasmosis was defined as systemic findings such as progressive weight loss, hepatosplenomegaly, extrapulmonary lymphadenopathy, mucosal or skin lesions, anemia, leukopenia, thrombocytopenia, hepatic enzyme elevation, adrenal insufficiency, or demonstration of extrapulmonary involvement by isolating the organism or visualizing it by fungal stain in extrapulmonary tissue. The clinical illness was classified as severe if the patient required management in an intensive care unit, moderate if hospitalization was required without intensive care unit admission, and mild if the illness was managed on an outpatient basis without hospitalization.
Laboratory Testing
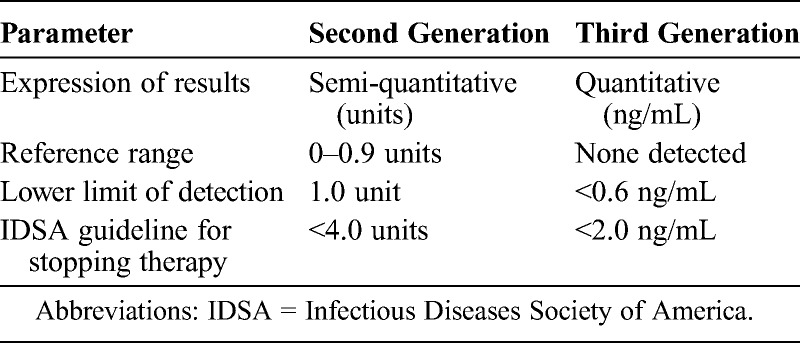
All Histoplasma antigen testing for these patients was performed at MiraVista Diagnostics. The original Histoplasma antigen enzyme immunoassay was modified as a second-generation assay in 2004 to reduce false-positive results,12 and again in 2007 as a third-generation assay to provide quantification.3 The main difference in the 2 assays was incorporation of a standard curve permitting quantification in the third-generation assay. The reference range for results considered negative was 0–0.9 units in the second-generation assay, and none detected in the third-generation assay. The IDSA guideline for stopping therapy included recommendations for both the second- and third-generation assays (2 ng/mL in the third-generation assay and 4 units in the second-generation assay)11 (Table 1). Twenty-one of 84 specimens that were tested before March 2007 that were stored at MiraVista Diagnostics at −20°C were retested in the third-generation assay. If stored specimens were not available, results in the second-generation assay were used.
TABLE 1.
Second- and Third-Generation Histoplasma Antigen Tests
Outcome
Relapse of histoplasmosis was defined as recurrence of clinical signs and symptoms compatible with histoplasmosis occurring more than 3 months after the initial episode, accompanied by positive culture, pathology, or increase in Histoplasma antigen. Death was attributed to histoplasmosis if no other cause was determined and laboratory evidence was consistent with active histoplasmosis, including positive culture, increased antigen concentration, or antigen concentration persistently higher than the upper limit of the test (≥39 ng/mL). Remission was classified based on evidence of a period of absence of clinical findings before the relapse of active histoplasmosis.
Statistical Analysis
For analysis of the relationships of variables between PD and PC patients, univariate analyses were performed using the chi-square test or the Fisher exact test for categorical variables. The Wilcoxon tests were used for continuous variables. SAS version 9.3 (SAS Institute, Cary, NC) was used for statistical analysis. To determine the variables associated with relapse, a multiple logistic regression was performed with the regression modeling the probability of relapse. Variables with a p value < 0.15 in the bivariate analysis and confounders were included in the model. The level of significance was defined as p < 0.05 (2-sided) unless otherwise specified.
RESULTS
Patients
Of 227 histoplasmosis patients, 97 met the study requirements. Eighty-six patients were excluded due to inadequate information to verify diagnosis or clinical findings or to classify outcome, and 44 were excluded because they had follow-up of less than 1 year. Histoplasmosis occurred before 2007, the year the IDSA guidelines recommended withdrawal of suppressive antifungal therapy, in 31 of 38 (81.6%) patients in the PD group and 42 of 59 (72.9%) patients in the PC group.
Baseline Characteristics
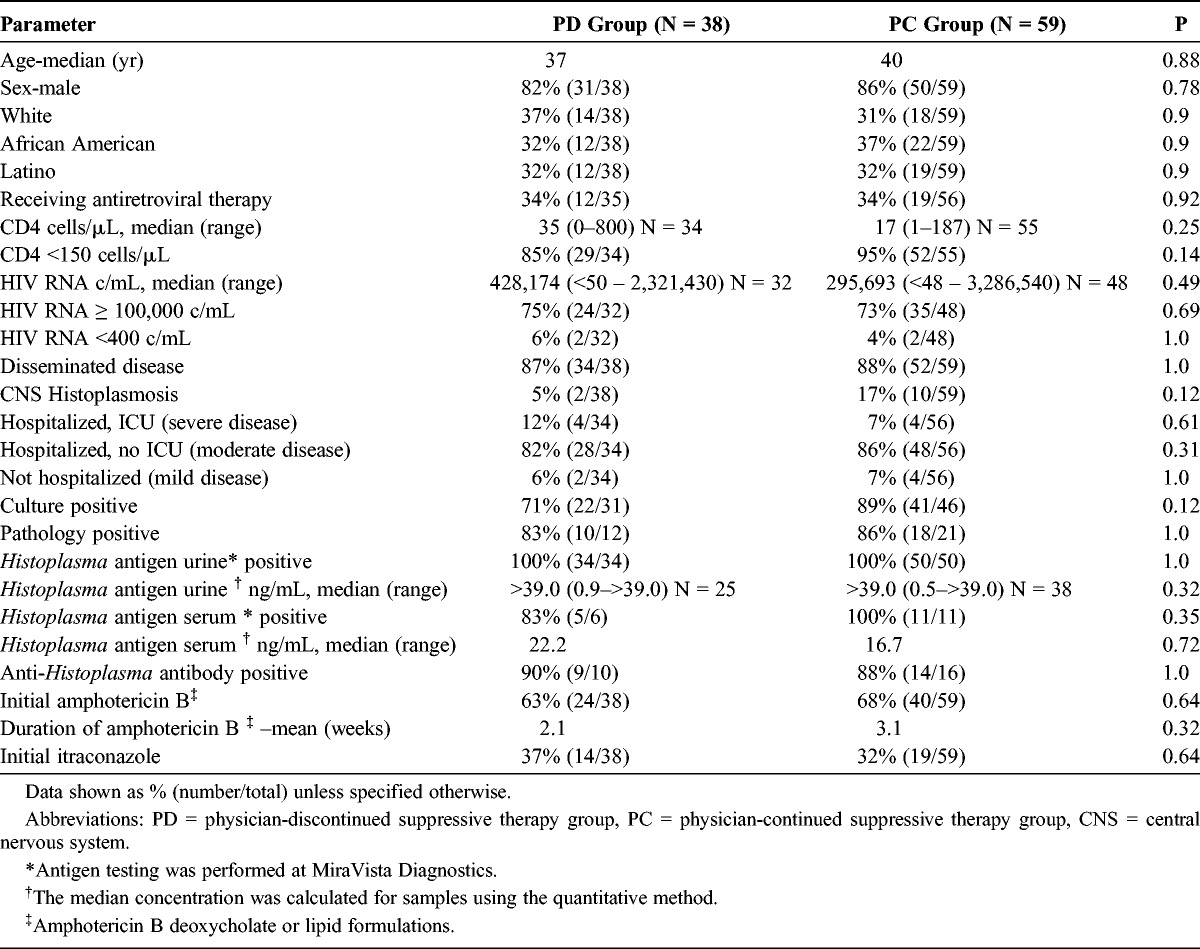
Most parameters were similar between the 2 groups (Table 2). Thirty-four percent of patients in both groups were receiving antiretroviral therapy at the initial diagnosis of histoplasmosis. Severe histoplasmosis was present in 12% of patients in the PD group and 7% in the PC group and was disseminated in 87% of the PD group compared to 88% of the PC group. The CNS was involved in 17% of the PC group and 5% of the PD group (p = 0.12). The difference in median CD4 cell count in the PD group and the PC group was not statistically significant, 35 cells/μL and 17 cells/μL, respectively (p = 0.25). Positive culture occurred in 89% of the PC group and 71% of the PD group (p = 0.12).
TABLE 2.
Characteristics at the Time of Diagnosis of Histoplasmosis
Amphotericin B was used for initial treatment in 63% of the PD and 68% of the PC group, for 2.1 and 3.1 weeks, respectively, which was followed by itraconazole. The remaining percentage of patients from each group was treated with itraconazole only. Twelve patients with CNS histoplasmosis were treated with amphotericin B deoxycolate or lipid formulation for a median duration of 7 weeks (range, 1–28 wk) followed by itraconazole. Eight patients were continued on itraconazole at the most recent follow-up; 4 patients were treated with a median duration of 10 months (range, 6–58 mo). None of them was treated with other triazoles.
Two follow-up time points were analyzed: the time of treatment discontinuation or at 1 year if treatment was not stopped, and the time of most recent follow-up.
At treatment discontinuation or at 1 year if treatment was not stopped, 87% of the PD and 39% of the PC group patients were adherent to therapy (p < 0.0001) (Table 3). Forty-one percent of the African American patients (14/34) were adherent compared to 62% of white (20/32) and 81% of Latino patients (25/31) (p < 0.0001). No relapse occurred among African American, white, or Latino patients who were adherent to therapy. The median duration of antifungal treatment at the time of discontinuation was 22 months in the PD group. Eight patients in the PC group discontinued therapy against the advice of their physicians, with a median of 9.5 months (6–44 mo) of therapy. Seventy-six percent (29/38) of the patients in the PD group had completed at least 12 months of therapy when treatment was discontinued. Nine patients completed less than 12 months of therapy: 11 months in 3, 6 months in 2, and 10, 9, 8, and 4 months in 1 patient each. The CD4 count was above 150 cells/μL in 92% of the PD and 34% in the PC group (p < 0.0001). The median HIV RNA was 50 c/mL in the PD and 5860 c/mL in the PC group (p < 0.0001). Histoplasma antigenuria was below 2.0 ng/mL in 82% of the PD and 21% of the PC group (p = 0.0005). Five patients (8%) in the PC group relapsed before receiving 1 year of treatment. No deaths were caused by histoplasmosis during the first year of therapy in either group.
TABLE 3.
Characteristics at the Time of Discontinuation of Suppressive Therapy or at 1 Year in Those Who Did Not Stop Suppressive Therapy, and at Most Recent Follow-Up
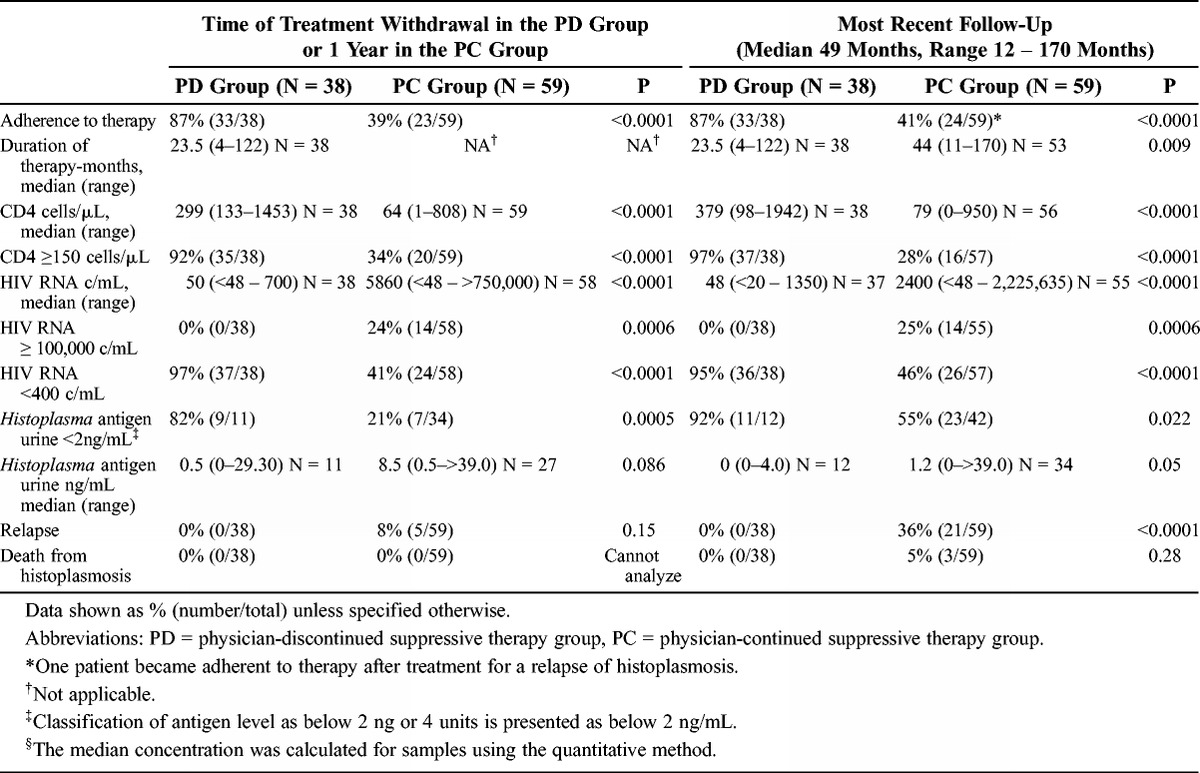
At the most recent follow-up visit, the median duration of follow-up from the initial diagnosis was 64.5 months in the PD and 54.4 months in the PC group (p = 0.028). The median duration of treatment was 23.5 months in the PD and 44 months in the PC group (p = 0.009). One patient in the PC group subsequently became adherent to therapy, and the physician discontinued suppressive treatment. For the last follow-up analysis, that patient was analyzed in the PC group. The CD4 count was above 150 cells/mL in 97% of the PD and 28% of the PC group (p < 0.0001). The HIV RNA was below 400 c/mL in 95% of the PD and 46% of the PC group (p ≤ 0.0001). Histoplasma antigenuria was below 2.0 ng/mL in 92% of the PD and 55% of the PC group (p = 0.022). None of the patients in the PD group had relapsed compared to 21 of 59 (36%) in the PC group (p < 0.0001), and none of the patients in the PD group died from histoplasmosis compared to 3 of 59 (5%) in the PC group (p = 0.28). Death was also more common in nonadherent patients, 3 of 37 (8%) compared to none of 60 adherent patients (p = 0.08).
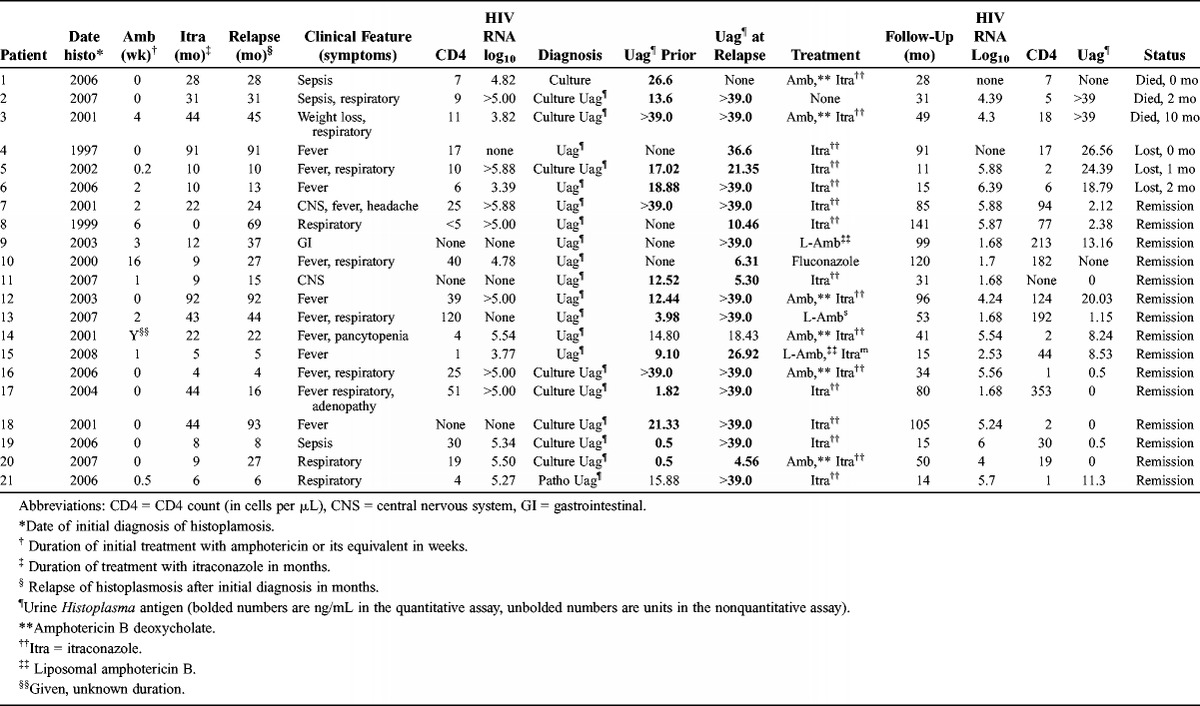
Clinical and laboratory findings, treatment, and outcome in the relapse patients are summarized in Table 4. The median duration of treatment at the time of relapse was 18.6 months. Respiratory illness was the most common manifestation, occurring in 48% of cases. A sepsis syndrome was observed in 14% and CNS disease in 10% of cases. Histoplasma was isolated from blood or other body fluids from 9 of the 21 relapse patients (42.8%). High or increasing concentrations of antigen were detected in 20 patients (95%). Antigenuria increased at least 2.0 ng/mL in 12 of 16 (75%) relapse patients in whom a prior concentration was available, compared to none of 21 patients who did not relapse (p < 0.0001). In 3 relapse patients, antigen ≥39.0 ng/mL in the current and prior specimens precluded detection of an increase at relapse. Antigenuria declined from 12.5 to 5.3 ng/mL in 1 patient who relapsed with meningitis, but the antigen concentration was ≥39.0 ng/mL in the CSF. Three patients died (14%) from histoplasmosis, 3 were lost to follow-up, and 15 (71%) achieved clinical remission.
TABLE 4.
Clinical and Laboratory Findings and Outcome in Patients Who Relapsed
Analysis of Relapses
Most baseline characteristics were similar (Table 5). The higher relapse rate in African American patients, 52% (11/21), and lower rate in Latino patients, 14% (3/21) (p = 0.06), was attributable to differences in adherence to therapy. CNS histoplasmosis was more common in the patients who relapsed (38%) than in those who did not relapse (5%) (p = 0.0004). Similarly, 8 of 12 (67%) patients with CNS histoplasmosis relapsed compared to 13 of 85 (15%) patients without CNS involvement (p = 0.0004).
TABLE 5.
Characteristics in Patients Who Did or Did Not Relapse
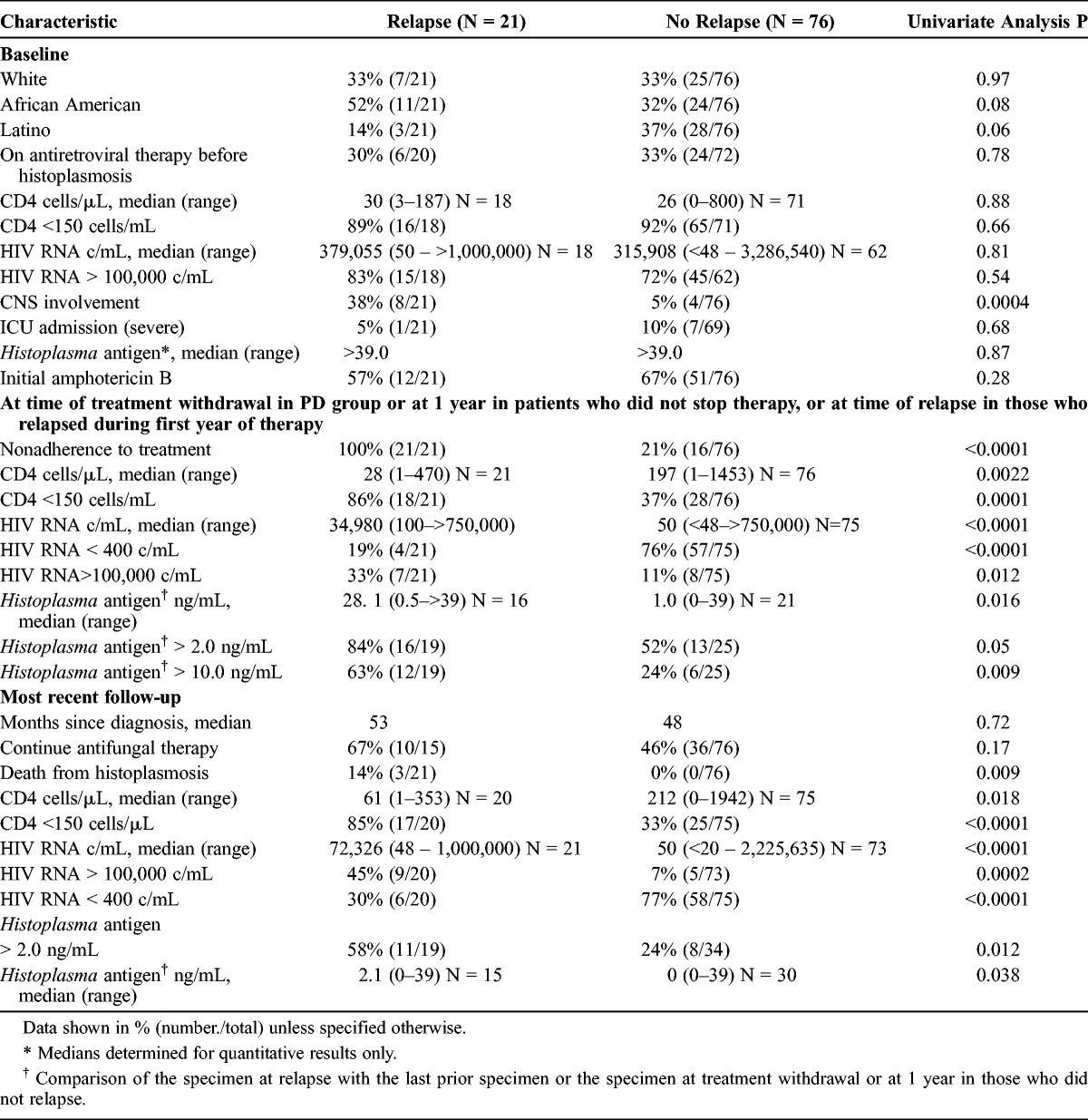
At the time of treatment discontinuation or at 1 year, the findings associated with relapse included nonadherence to therapy, CNS involvement, higher urinary Histoplasma antigen concentration, HIV RNA, and CD4 cell count. All relapse patients were nonadherent to therapy, compared to 16 of 76 (21%) patients who did not relapse (p < 0.0001). Twenty-one of 37 (57%) nonadherent patients relapsed compared to none of the 60 adherent patients (p < 0.0001). Eight of 12 patients with CNS disease relapsed, and all 8 were nonadherent to therapy. The viral load data for 5 of 8 CNS patients were available at the time of relapse, median of 100,000 c/mL (range, 60,600–750,000 c/mL).
Relapse patients had a higher median antigenuria concentration than those who did not relapse, 28.1 ng/mL compared with 1.0 ng/mL, respectively (p = 0.016). Antigenuria was >10 ng/mL in 63% of the relapse patients compared with 24% of the nonrelapse patients (p = 0.009), and relapse occurred in 12 of the 18 (67%) patients with antigenuria >10 ng/mL compared with 7 of the 19 (37%) patients with lower concentrations (p = 0.07).
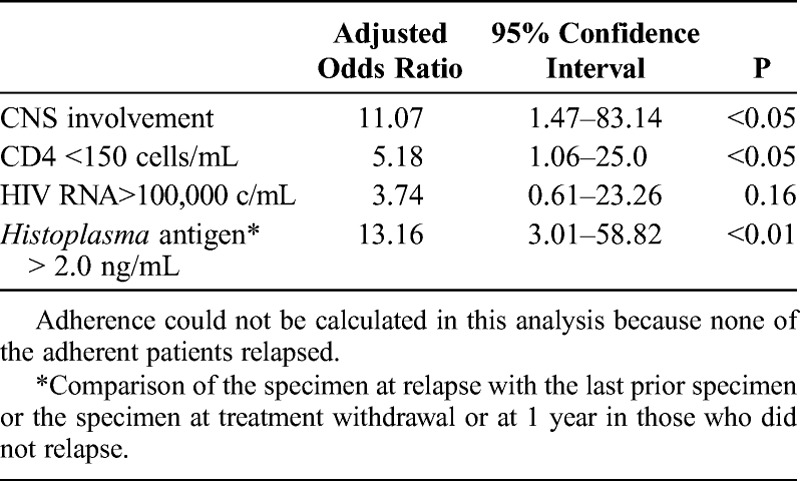
In a multivariate analysis, patients with CNS disease were more likely to relapse compared to those without CNS disease (odds ratio [OR], 11.07; 95% confidence interval [CI], 1.47–83.14) (Table 6). All of the patients with CNS disease also were nonadherent with treatment for both antiretroviral therapy and antifungal drugs. Those with antigenuria ≥2.0 ng/mL at 1-year follow-up were 13.16 (95% CI, 3.01–58.82) times more likely to relapse compared to those with antigenuria <2.0 ng/mL, and those patients with a CD4 count <150 cells/μL were 5.18 (95% CI, 1.06–25.0) times more likely to relapse compared to their counterparts. The OR for adherence could not be calculated because none of the adherent patients relapsed. However, HIV RNA ≥100,000 copies/mL at 1 year or withdrawal of therapy was not a statistically significant risk factor for relapse (OR, 3.74; 95% CI, 0.61–23.26).
TABLE 6.
Factors Associated With Relapse by Multiple Logistic Regression Analysis
Long-term follow-up (median, 49 mo; range, 12–170 mo) information was available for 18 of the 21 relapse patients and all 76 nonrelapse patients (see Table 5). Two-thirds of the relapse patients continued to be prescribed antifungal therapy at the last follow-up compared to 46% of the nonrelapse patients (p = 0.17). Death caused by histoplasmosis was more common in the relapse patients than in nonrelapse patients, 14% and 0%, respectively (p = 0.009). HIV RNA was higher in the relapse patients than in those who did not relapse, 72,326 and 50 c/mL, respectively (p < 0.0001), and the CD4 count was lower in the relapse than nonrelapse patients, 61 and 212 cells/μL, respectively (p = 0.018). Histoplasma antigenuria ≥2.0 ng/mL was more common in the relapse than nonrelapse patients, 58% and 24%, respectively (p = 0.012), and median level was higher in the relapse than nonrelapse patients, 2.1 ng/mL and 0 ng/mL, respectively (p = 0.038).
DISCUSSION
To our knowledge, this is the first analysis of factors associated with the relapse of histoplasmosis in HIV-infected patients in the HAART era. The primary objective of this study was to determine the outcome of physician-directed discontinuation of chronic suppressive therapy for histoplasmosis in patients with AIDS. There were no relapses or deaths caused by histoplasmosis in the PD group, supporting the safety of discontinuation of therapy in many patients. The IDSA guideline recommends that patients complete at least 1 year of antifungal and 6 months of antiretroviral therapy, and have CD4 count >150 cells/mL, negative fungal blood cultures, and antigen concentrations in urine and serum <2.0 ng/mL before discontinuing antifungal treatment.11 Only 76% of PD patients had received at least 1 year of antifungal treatment, but none relapsed, suggesting that 12 months of therapy may not be required in all cases if immune function improves in response to antiviral therapy. One such patient recovered with only antiretroviral therapy, without antifungal therapy. Ninety-two percent of the PD patients had CD4 counts ≥150 cells/mL. Fungal blood cultures appear to be unnecessary in the decision to stop therapy. Urinary antigen levels at discontinuation of therapy were measured in only 29% of patients and were <2.0 ng/mL in 92%.
The PD and PC groups differed in most parameters at discontinuation of therapy or at 1 year, the most important of which was adherence to therapy. Eighty-seven percent of the PD group was adherent compared to 39% of the PC group (p < 0.0001). CD4 counts were lower and HIV RNA and Histoplasma antigen levels were higher in the PC group (p < 0.0001). All relapses occurred in patients who were nonadherent to therapy. Relapse occurred in 53% of the nonadherent but in none of the adherent patients (p < 0.0001). Eight percent of nonadherent patients died compared to none of the adherent patients (p = 0.08). All of the deaths followed relapse of histoplasmosis in the PC group. Comparing laboratory parameters in both groups, there was better viral suppression, higher CD4 cell counts, and lower antigen concentrations in the PD group.
Relapse was more common in patients with CNS histoplasmosis. Sixty-seven percent of patients with CNS histoplasmosis relapsed compared to 15% of those without CNS involvement (p = 0.0004). All 8 CNS cases that relapsed were nonadherent to therapy, while 4 CNS cases that did not relapse were adherent to therapy. Relapse in CNS histoplasmosis may be explained by nonadherence and poor CNS penetration by itraconazole.2 Relapse occurred in none that were adherent compared to 42% of those that were nonadherent.
Monitoring antigenuria concentration appeared to aid in the management of these patients.9 Relapse occurred in 67% of patients with antigenuria >10 ng/mL compared to 27% of those with lower concentrations (p = 0.009). Elevated antigen level supported the diagnosis of relapse in 95% of cases. Median concentration at the time of relapse had increased by 20.1 ng/mL. One patient, who relapsed with CNS histoplasmosis, exhibited a decline in antigenuria but had a concentration >39 ng/mL in the CSF. Rising antigen concentrations even in the absence of symptoms raises concern about relapse.
Several limitations of this study require consideration. First, Histoplasma antigen, fungal culture, HIV RNA, and CD4 cell counts were not obtained with consistency or at regular intervals. Second, follow-up information obtained by medical record review may not accurately describe the clinical status of the patient. Third, determination of adherence based on physicians’ observations and HIV RNA may not be as accurate as other measures of adherence.4 Fourth, although univariate and multivariate analysis showed that there was a significantly higher relapse rate in CNS histoplasmosis, the difference might all be contributed by nonadherence. Adherence could not be calculated as a factor in multivariate analysis because none of the adherent patients relapsed. Finally, antigen testing in serum was performed in too few patients to assess its role in making decisions about stopping treatment. However, other studies have shown more rapid clearance of antigenemia than antigenuria, suggesting that monitoring either or even both may be useful for assessing the outcome of therapy6 and for diagnosing relapse.9
In conclusion, although 24% patients stopped therapy before completing a 12-month course and did not relapse, the recommendation to treat for at least 12 months seems prudent. Similarly, although antigen concentration at discontinuation of therapy was determined in only 29% of patients, relapse was 13.18 times more likely in patients with antigenuria ≥2.0 ng/mL. Other guideline criteria that should be retained include antiretroviral treatment for at least 6 months and CD4 cell count of at least 150 cells/mL. In the current study we identified 3 other criteria that should be considered in decisions to stop treatment. Nonadherence to therapy was the main predictor for relapse. HIV RNA <400 c/mL, a marker for adherence, also should be a criterion for stopping treatment. Relapse was 11.07 times more likely in patients with CNS histoplasmosis, which may be related to nonadherence. Fungal blood culture may not be necessary at the end of therapy for adherent patients. Finally, additional therapy and continued monitoring for relapse is recommended in patients with rising HIV RNA, rising antigen levels, declining CD4 counts <150 cells/μL, or in patients who have been nonadherent to therapy.
Abbreviations
- AIDS
acquired immunodeficiency syndrome
- CI
confidence interval
- CNS
central nervous system
- CSF
cerebrospinal fluid
- HAART
highly active antiretroviral therapy
- HIV
human immunodeficiency virus
- IDSA
Infectious Diseases Society of America
- OR
odds ratio
- PC
physician-continued suppressive therapy group
- PD
physician-discontinued suppressive therapy group
Footnotes
Conflicts of interest: The following authors have had relationships with the companies listed: AMA: Gilead Sciences; JWB: Merck, Pfizer, Mayne Pharma, Astellas; RNG: Pfizer, Bavarian Nordic, Pazvax, Virapharma, GlaxoSmithKline; DMB: Gilead, Viral Ed; MR: MiraVista Diagnostics; LJW: MiraVista Diagnostics and MiraBella Technologies.
REFERENCES
- 1.Anonymous. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. AIDSinfo. 2013:16. [Google Scholar]
- 2.Black KE, Baden LR. Fungal infections of the CNS: treatment strategies for the immunocompromised patient. CNS Drugs. 2007;21:293–318. [DOI] [PubMed] [Google Scholar]
- 3.Connolly PA, Durkin MM, Lemonte AM, Hackett EJ, Wheat LJ. Detection of Histoplasma antigen by a quantitative enzyme immunoassay. Clin Vaccine Immunol. 2007;14:1587–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farley J, Hines S, Musk A, Ferrus S, Tepper V. Assessment of adherence to antiviral therapy in HIV-infected children using the Medication Event Monitoring System, pharmacy refill, provider assessment, caregiver self-report, and appointment keeping. J Acquir Immune Defic Syndr. 2003;33:211–218. [DOI] [PubMed] [Google Scholar]
- 5.Goldman M, Zackin R, Fichtenbaum CJ, Skiest DJ, Koletar SL, Hafner R, Wheat LJ, Nyangweso PM, Yiannoutsos CT, Schnizlein-Bick CT, Owens S, Aberg JAAIDS Clinical Trials Group A5038 Study Group. Safety of discontinuation of maintenance therapy for disseminated histoplasmosis after immunologic response to antiretroviral therapy. Clin Infect Dis. 2004;38:1485–1489. [DOI] [PubMed] [Google Scholar]
- 6.Hage CA, Kirsch EJ, Stump TE, Kauffman CA, Goldman M, Connolly P, Johnson PC, Wheat LJ, Baddley JW. Histoplasma antigen clearance during treatment of histoplasmosis in patients with AIDS determined by a quantitative antigen enzyme immunoassay. Clin Vaccine Immunol. 2011;18:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kauffman CA. Histoplasmosis. Clin Chest Med. 2009;30:217–225. [DOI] [PubMed] [Google Scholar]
- 8.Wheat J, Sarosi G, McKinsey D, Hamill R, Bradsher R, Johnson P, Loyd J, Kauffman C. Practice guidelines for the management of patients with histoplasmosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:688–95. [DOI] [PubMed] [Google Scholar]
- 9.Wheat LJ, Connolly-Stringfield P, Blair R, Connolly K, Garringer T, Katz BP. Histoplasmosis relapse in patients with AIDS: detection using Histoplasma capsulatum variety capsulatum antigen levels. Ann Intern Med. 1991;115:936–941. [DOI] [PubMed] [Google Scholar]
- 10.Wheat LJ, Connolly-Stringfield PA, Baker RL, Curfman MF, Eads ME, Israel KS, Norris SA, Webb DH, Zeckel ML. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore). 1990;69:361–374. [DOI] [PubMed] [Google Scholar]
- 11.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, Kauffman CAInfectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–825. [DOI] [PubMed] [Google Scholar]
- 12.Wheat LJ, Witt J, III, Durkin M, Connolly P. Reduction in false antigenemia in the second generation Histoplasma antigen assay. Med Mycol. 2007;45:169–171. [DOI] [PubMed] [Google Scholar]


