Abstract
Digital ischemia associated with cancer (DIAC) is increasing in frequency and recent reports have suggested the concept of paraneoplastic manifestation. The aims of this study were to characterize the clinical presentation of DIAC and identify clinical features that could lead physicians to diagnose underlying cancer.
From January 2004 to December 2011, 100 patients were hospitalized in the Department of Internal Medicine at Rouen University Hospital, France for a first episode of DI. Fifteen (15%) exhibited symptomatic or asymptomatic cancer during the year preceding or following vascular episode and constituted the DIAC group. Other patients without cancer made up the digital ischemia (DI) group.
Median time between diagnosis of cancer and episode of digital necrosis was 2 months [0.25–9]. Diagnosis of DI and concomitant cancer was made in 7 of the 15 patients, while DI preceded the malignant disorder in 2 cases and followed it in 6 cases. Histological types were adenocarcinoma for 7 (46.7%), squamous cell carcinoma for 4 (26.7%), and lymphoid neoplasia for 3 patients (20%). Six patients (40%) had extensive cancer. Three patients were lost to follow-up and 5 patients died <1 year after diagnosis of cancer. Cancer treatment improved vascular symptoms in 6 patients (40%). Patients with DIAC, compared to patients with DI, were significantly older (56 years [33–79] vs 46 [17–83] P =0.005), and had significantly lower hemoglobin and hematocrit levels (12.7 g/dl vs 13.9 g/dl; P =0.003 and 38% vs 42%; P =0.003, respectively). Patients with DIAC had a higher platelet rate (420 vs 300 G/L P =0.01), and 6 patients with DIAC (40%) had thrombocytosis. There was no difference between groups either in C-reactive protein level (12 mg/L vs 5 mg/L; P =0.08) or regarding cardiovascular risk factors, presence of autoimmunity, or monoclonal protein.
This retrospective study suggests that DIAC may be more prevalent than previously reported. Outcomes of the 2 diseases were not strictly chronologically parallel. However, in the majority of cases, treatment of the tumor resolved vascular involvement. Our findings suggest that age >50 years and thrombocytosis should alert physicians to consider a possible occult malignancy when digital necrosis occurs.
INTRODUCTION
Acral vascular syndromes mainly including digital ischemia (DI), Raynaud’s phenomenon, or erythermalgia are frequent causes of consultation or hospitalization in internal medicine and dermatology (Table 1). Incidence of DI is 2 cases per 100,000 persons per year.1,2 Diagnosis is mainly based on clinical symptoms, including pain, permanent digital blanching or cyanosis, and desquamation or ulceration of the fingers. Etiological investigations lead to separate acral vascular syndromes in idiopathic or secondary form, and according to the acute or progressive onset of the acral syndrome, different etiologies can be advanced. Main causes identified are smoking, autoimmune connective tissue diseases (especially systemic sclerosis), vasculitis, and local injuries that is hammer syndrome.3 Cancer is also known as a possible etiology of acral vascular syndrome (Figures 1 and 2) with specific clinical features (eg, trophic ulcers),4–9 leading to a prevalence of paraneoplastic acral vascular syndromes ranging from 2.2%–8% of cases.8,10–12 Surprisingly, data in the literature regarding digital ischemia associated with cancer (DIAC) contain essentially case reports and analysis of the neoplasm implicated. Occurrence of DI may coincide, follow or antedate diagnosis of cancer, or herald its recurrence. Mechanisms of this phenomenon are not clear. Vasospasm, overproduction of vasoconstrictor factors, intimal proliferation, intraluminal thrombosis, vasculitis, immune complex, or drug toxicity are the main hypothetic factors leading to DI. Awareness that cancer can cause this nonmetastatic symptom is important for early diagnosis and treatment of an occult neoplasm and management of DI. Thus, better knowledge of DIAC might be useful for physicians.
TABLE 1.
Main Acral Vascular Syndromes According to Their Pathomechanisms
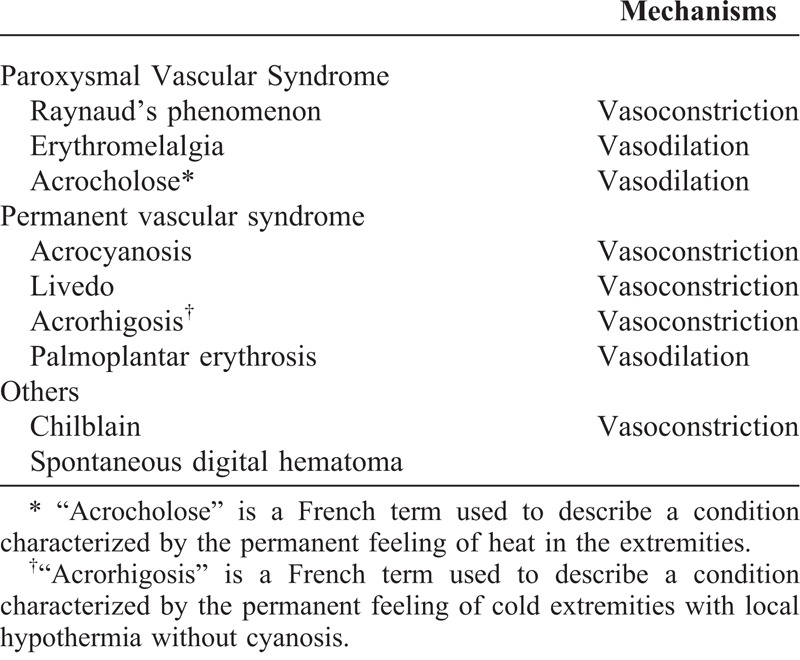
FIGURE 1.
Digital ischemia in a patient with lung adenocarcinoma.
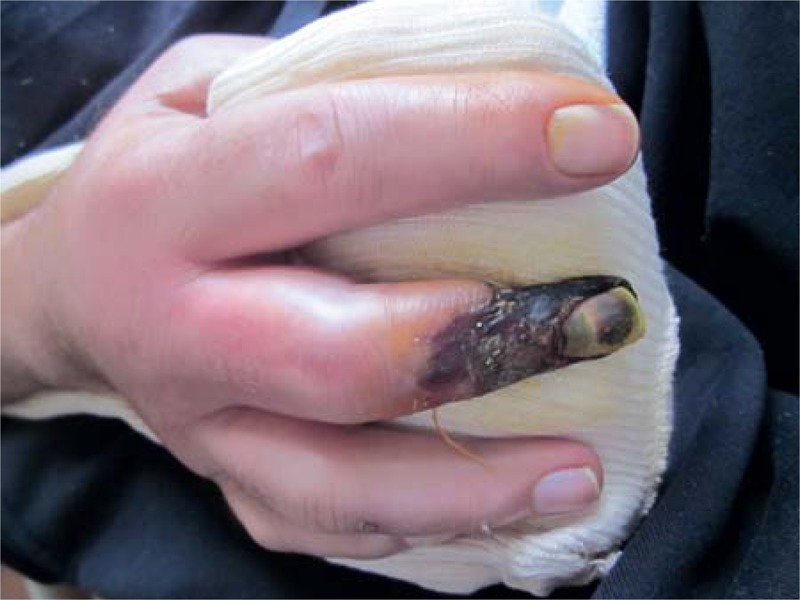
FIGURE 2.
Digital ischemia in patient with sclerodermia and breast adenocarcinoma.
Therefore, the aim of our study was to characterize the clinical presentation of DIAC and to identify clinical particularities that could lead to the diagnosis of underlying cancer in patients presenting DI. We report here the experience of the Department of Internal Medicine at Rouen University Hospital, France.
MATERIAL AND METHODS
Patients
In this retrospective cohort study, we consecutively included patients hospitalized for DI in our Department of Internal Medicine between January 2004 and December 2011. Inclusion criterion was a first episode of DI diagnosed because of painful and permanent digital blanching, cyanosis, or ulceration of the fingers. Exclusion criteria were past DI, traumatic DI. Patients were classified in 2 subgroups, which were analyzed and compared separately according to diagnosis of cancer. Neoplasia was systematically screened depending on clinical or biological abnormalities. Usually, all patients underwent a thoracic and abdominal computed tomography-scanner (CT scan) in addition to routine biological samples. Thus, patients with cancer diagnosed the year before or after occurrence of DI constituted the DIAC group, whereas other patients without cancer made up the DI group. Both groups were evaluated using the same schedule. Clinical assessment included age, sex, main cardiovascular predisposing factors (hypertension, diabetes, dyslipidemia, and tobacco), presence of Raynaud’s phenomenon preceding DI episode, and treatment with oral antiplatelet agents, vasodilator agents such as calcium channel blocker, or buflomedil. Initial clinical evaluation was performed by vascular physicians. Biological data reviewed were complete blood count, C-reactive protein (CRP), blood protein electrophoresis, and immunologic markers [antinuclear antibodies (ANA), antineutrophil cytoplasmic antibodies (ANCA)]. Thrombogenic factors, that is, cryoglobulin and antiphospholipid (anticardiolipin and/or antibeta2glycoprotein antibodies, IgG or IgM, presence of lupus anticoagulant) were also collected. Results of nailfold capillary microscopy, Doppler ultrasound examination of the upper limbs, and CT scan or angiography were recorded when performed. Main vascular therapies such as vasodilators, hypervolemic hemodilution, prostacyclin analogs and sometimes surgical treatment (thoracic sympathectomy, amputation) were also collected. Diagnosis of scleroderma was based on international classification from Leroy et al13 while the diagnosis of thromboangiitis obliterans was made according to the Olin’s criteria.14
Treatment Efficacy
Response to treatment was analyzed according to clinical criteria: complete healing, ulceration, gangrene, amputation or relapse. Regarding cancer, we also assessed disease free survival and absence of recurrence. Cure was defined by the designated oncologist.
Group Comparison
The following parameters were compared in the DI and DIAC groups: mean age and sex; predisposing cardiovascular factors, Raynaud’s phenomenon, and treatment preceding DI; laboratory features (hemoglobin rate, hematocrit, platelet rate, CRP, immunologic markers, cryoglobulin, and monoclonal protein), and results of nailfold capillary microscopy, Doppler ultrasound examination of the upper limbs, CT scan, or angiography.
Statistical Analysis
For group comparison involving binary data, we used chi square test or Fisher exact test, depending on expected cell count (more or <5, respectively). Comparisons involving continuous data were performed using Mann Whitney test. Significance level was set at P =0.05. Statistical analysis was conducted using StatView version 5.0.
Ethical Review
Patients were informed that some medical data could be used in further medical publication with the strict respect of their anonymous. An ethical review committee is not necessary for this retrospective and exclusively observational study.
RESULTS
Population
One hundred and thirty six files were reviewed, but only 100 were analyzed because 36 patients had previous manifestations of DI. (Figure 3).
FIGURE 3.
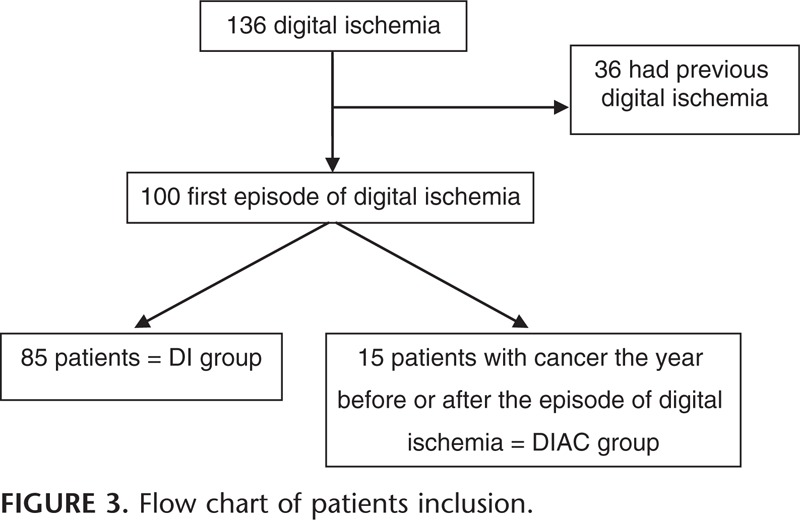
Flow chart of patients inclusion.
General Characteristics of Patients
One hundred patients were finally analyzed. Median age at onset of DI was 47.5 years [17–83]. Sex ratio was 1.7 (63 men and 37 women). Seventy-seven patients (77%) were smokers (51 active), 7 (7%) were diabetics, 12 (12%) had hypertension, and 13 (13%) had dyslipidemia. Twelve patients (12%) had past symptomatic atherosclerosis (stroke, myocardial infarction, peripheral arterial disease). Mean number of cardiovascular risk factors was 1 and only 10 patients (10%) had no cardiovascular risk factor. Thirty-nine patients (39%) had Raynaud’s phenomenon preceding DI. Bilateral symptoms were involved in 19 cases (19%). Positive autoimmunity was found in 31 patients (31%), mostly ANA (n = 29) and antiproteinase-3-ANCA (n = 2). Thrombogenic factors were found in 7 patients (7%), including cryoglobulin in 3 cases (1 type I, 1 type II and 1 type III), and paraprotein in 6 patients (3 IgG Kappa, 1 IgG Lambda, 2 IgM Kappa). None of our patients had significant antiphospholipid antibodies or lupus anticoagulant.
Median hemoglobin rate was 13.75 g/dl [8.3–18], median hematocrit was 41% [23–52], median platelet rate was 305 G/L [140–947], and median value of CRP was 5 mg/L [5–80]. Regarding morphological data, capillaroscopic findings were abnormal for 18 patients (18%), including 10 with systemic sclerosis presenting architectural disorganization, megacapillaries, hemorrhages, and avascular areas. The other 8 patients had unspecific microangiopathy, mostly extravasations and dilatations of the venous segment. Upper limb arterial Doppler and angiography were performed in 50 and 13 cases respectively. Abnormalities were detected in two thirds of patients who underwent ultrasound examination and in all patients who experienced angiography. Arterial thrombosis (n = 31), digital arteritis (n = 7), decrease in fingertip blood flow (n = 4), vasospasm (n = 1), and thoracic outlet syndrome (n = 1) were the most frequent abnormalities detected.
Prevalence of Cancer Related DI
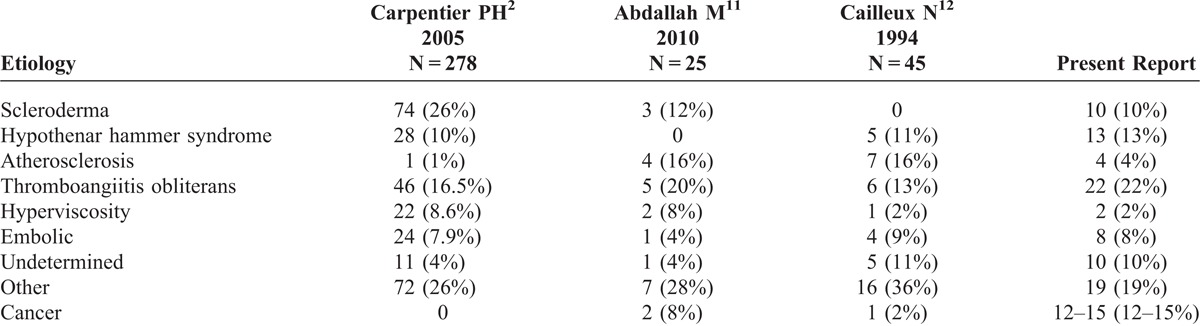
Systemic sclerosis (10%), hypothenar hammer syndrome (13%), thromboangiitis obliterans (22%), and atherosclerosis (4%) were the most frequently related etiologies in the 100 patients hospitalized (Table 2). No etiology was found in 10 cases (10%). Fifteen patients (15%) were diagnosed with cancer during the year preceding or following occurrence of DI. Of these fifteen patients, only 3 were known to also have scleroderma. Therefore prevalence of DI associated exclusively with cancer was 12%.
TABLE 2.
Etiology of Digital Ischemia in Our Study and in the Literature
With Cancer
General Characteristics
The 15 patients were 7 women and 8 men (sex ratio: 1.14), with a median age of 56 years [33–79]. Many patients had 1 or more cardiovascular comorbidities: arterial hypertension (n = 3, 20%), diabetes (n = 2, 13.3%), smoking (n = 10, 66.7%), dyslipidemia (n = 1, 6.7%), and atherosclerosis (n = 4, 26.7%). Moreover, several patients were on antiplatelet therapy (n = 7, 46.7%). Three patients had scleroderma. One patient was on chemotherapy with carboplatin or gemcitabine.
Clinical Manifestations Related to DI
DI was mostly unilateral (n = 13, 86.7%) and toes were never affected. Raynaud’s phenomenon preceded DI in about half of cases (7 patients) and was frequently bilateral (n = 6).
Laboratory Tests and Other Investigations
Median hemoglobin and hematocrit rate were 12.7 g/dl [9.9–15.3] and 38% [31–45] respectively. Median platelet rate was 420 G/L [140–947] and 6 patients (40%) had thrombocytosis (platelet rate higher than 450 G/L), in this subgroup median platelet rate was 601.5 G/L [548–947], whereas median CRP was 12 mg/L [5–63]. Antinuclear antibodies were found in 7 patients (46.7%), median titer was 1:1280 [1:640–1:1280], mainly speckled pattern without specificity. Two patients exhibited cryoglobulinemia, 1 type I IgM Kappa and 1 type III (Table 3).
TABLE 3.
Laboratory and Other Investigation Findings in 14 Patients With DIAC
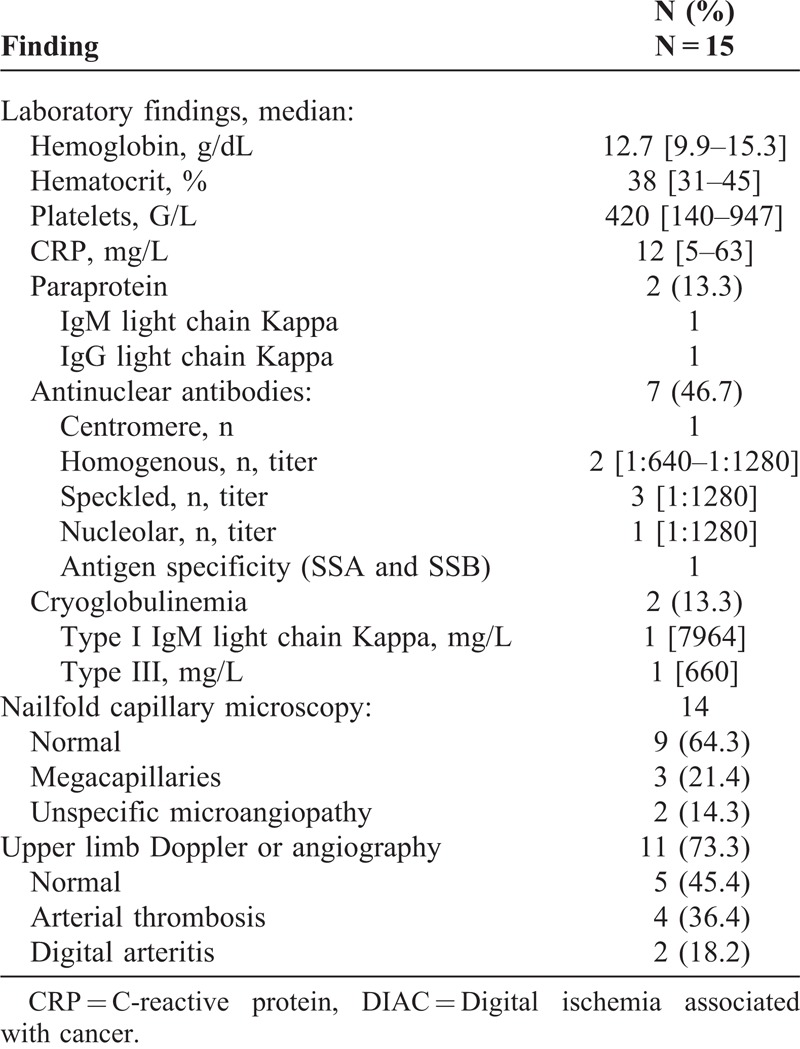
Nailfold capillary microscopy was performed in 14 of the 15 patients and was often normal except in 5 patients (35.7%), 3 of whom had known scleroderma with architectural disorganization and megacapillaries. Upper limb arterial Doppler or upper limb angiography was performed in 11 of the 15 patients and showed abnormalities in about half of all cases when performed (56.6%), which were mostly arterial thrombosis (n = 4), or digital arteritis (n = 2). None of our patients had histological investigation.
Associated Malignancies
Histological types of cancer were: adenocarcinoma for 7 patients (46.7%), including 2 lungs, 2 breasts, 1 colon, 1 stomach, and in 1 case prostate; squamous cell carcinoma for 4 patients (26.7%), including 2 cervices, 1 lung, and 1 larynx; melanoma for 1 patient (6.7%); and malignant lymphoid neoplasia for 3 patients (20%). Six patients (40%) had extensive cancer; 4 of them with metastasis and the other 2 with neoplastic ascites.
In the DI group, only 1 patient (1.2%) was diagnosed with cancer more than 1 year preceding DI: a 41-year old woman with nonmetastatic breast adenocarcinoma. Diagnosis was made 84 months prior to first episode of DI. Cancer was cured on occurrence of DI, with diagnosis of thromboangiitis obliterans.
Timing of Onset of Cancer
Median time between discovery of cancer and episode of digital necrosis was 2 months [0.25–9]. Diagnosis of DI and concomitant cancer was made in 7 patients (46.7%), whereas DI preceded the malignant disorder in 2 cases and followed it in 6 cases. Seven patients (46.7%) had systemic symptoms of cancer including fever (n = 2), sweating (n = 2), asthenia (n = 4), weight loss (n = 4), and symptoms associated with tumor mass (n = 3).
Therapy and Follow-up
All patients were given vasodilators including calcium channel blocker or buflomedil. Hypervolemic hemodilution therapy was systematically performed to decrease hematocrit level <35%. Prostacyclin analog therapy was administered in 7 patients. Four patients (26.7%) had amputation of the finger, 2 (13.3%) before diagnosis of cancer and 2 (13.3%) after diagnosis because of insufficient response to chemotherapy. Moderate improvement was reported in only 6 patients (40%). Median length of follow-up in the 15 patients with DIAC was 28 months [4–118]. Three patients (20%) were lost to follow-up and 5 (33.3%) died >1 year after diagnosis of cancer. Associated cancer treatment improved vascular symptoms in 6 patients (40%). Five patients (33.3%) had no recurrence of DI after cure of cancer; 1 patient (6.7%) had no recurrence and is still followed for multiple myeloma. Only 1 patient (6.7%) had other DI relapses during follow-up despite cure of cancer but presence of scleroderma.
Comparing DI With and Without Cancer
Patients with DIAC were significantly older than patients in the DI group (56 years [33–79] vs 46 [17–83] P = 0.005). There was no difference between groups regarding sex ratio, smoking, diabetes, and other cardiovascular risk factors. Nevertheless, patients in the DI group, were more frequently treated by oral antiplatelet agents (P = 0.01), although they no longer had atherosclerosis (P = 0.15).
Previous Raynaud’s phenomenon was not more frequent in patients with DIAC but was more often unilateral without significant difference with the DI group (Table 4).
TABLE 4.
General Characteristics and Comparison Between DI Group and DIAC Group
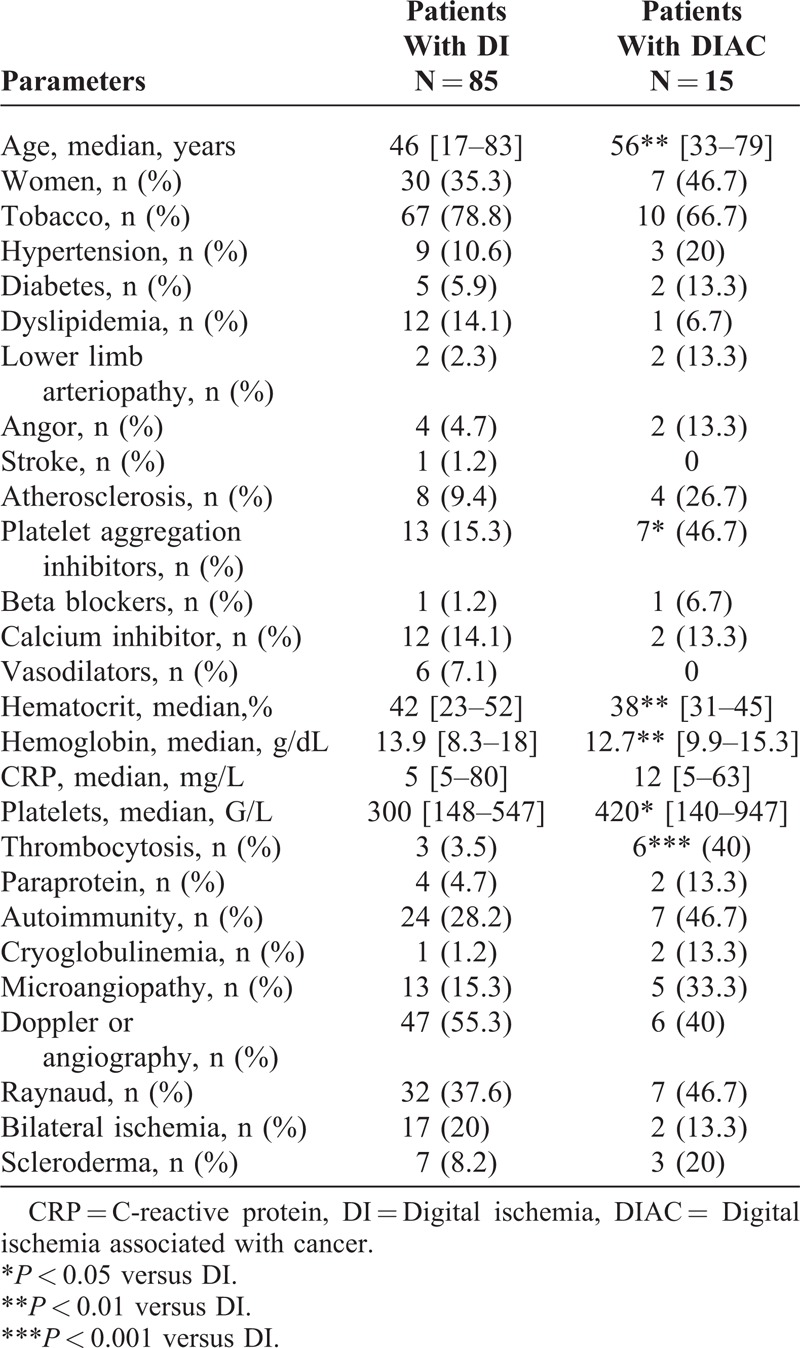
There was no difference regarding presence of immunologic markers, cryoglobulin or monoclonal protein. However, patients with DIAC had significantly lower hemoglobin and hematocrit levels than patients with DI (12.7 g/dl vs 13.9 g/dl; P = 0.003 and 38% vs 42%; P = 0.003 respectively). They also had a higher platelet rate (420 G/L vs 300 G/L P = 0.01). Moreover, 6 patients with DIAC (40%) had thrombocytosis compared to 3 patients (3.5%) in the DI group (P < 0.001). Nevertheless, CRP level was not significantly different (12 mg/L vs. 5 mg/L; P = 0.08).
We failed to find any statistically significant difference between groups regarding nailfold capillary microscopy, upper limb arterial Doppler or upper limb angiography.
DISCUSSION
The major findings of the present study are that 15% of patients exhibited symptomatic or asymptomatic cancer during the year preceding or following vascular episode mostly associated with thrombocytosis. DI associated with cancer is uncommon but increasingly frequent, leading to the concept of paraneoplastic manifestation. To our knowledge, this current study is 1 of the largest retrospective series of patients which assesses the association between DI and cancer. Since the first described case of DI associated with cancer,15 other studies have reported a prevalence of cancer ranging between 2% and 8% (Table 2).11,12 Nevertheless, populations were smaller and our report included only first episode of DI from tertiary center recruitment in vascular pathology, where prevalence of multiple comorbidities is higher. Moreover, differences in prevalence could be explained by the interval retained in our definition of DI related to cancer. Indeed, some authors included in their definition an interval ranging from 1 month to 2 years.16 Similarly, studies on the relationship between cancer and other rheumatic diseases suggest that paraneoplastic syndromes may either precede by 2 years, appear concurrently with, or follow diagnosis of cancer.17 However, despite differences in inclusion criteria between the studies, we found an identical time course of occurrence of DI compared to other studies. Thus, DI occurred before cancer in 13.3% of cases and the majority of patients developed DI and cancer simultaneously. As described by others, the clinical course of DI was closely linked to evolution of the tumor.17 Indeed, 6 patients had considerable symptomatic improvement after treatment of cancer, whereas 5 died >1 year after diagnosis of cancer. These results are consistent with those published by Poszepczynska-Guigné et al, which showed that definitive regression occurred with tumor removal in 50% of cases and that the other half stabilized or worsened.18
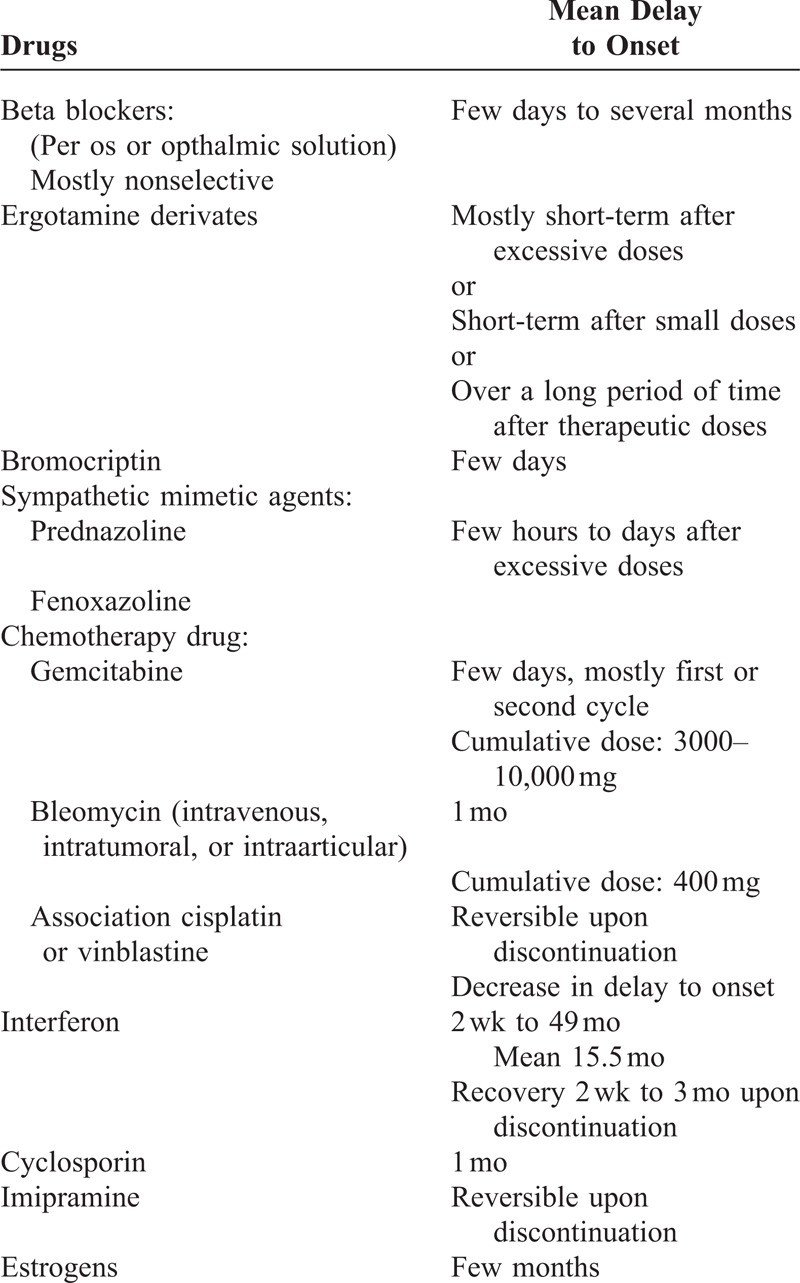
Moreover, some patients have other risk factors for developing DI. Thus, of the 15 patients with DIAC, 3 were active smokers, 7 had already been smokers and 3 had systemic sclerosis. Finally, only 4 patients (26.7%) did not have scleroderma or had not been exposed to tobacco, 1 of whom had undergone chemotherapy with carboplatin and gemcitabine, known to induce vascular damage.19–22 This result is slightly different from that of Chow et al in which approximately two thirds of patients had no predisposing factor for DI.16 Others drugs have been reported to induce acral vascular syndrome (Table 5).23–27 However, variable delay to onset and unknown prevalence make imputability difficult to assess. Several studies have demonstrated an increased frequency of cancer in patients with systemic sclerosis.28,29 Launay et al showed that delay between onset of systemic sclerosis and diagnosis of cancer was <12 months in 61.4% of cases.30 Anticancer therapy did not improve systemic sclerosis in any of their patients. In our study, diagnosis of both diseases was made simultaneously in our 3 cases and anticancer therapy significantly reduced sclerosis for 1 patient. Paraneoplastic scleroderma and sclerodermiform syndrome has already been described.31 The very short delay between onset of systemic sclerosis and cancer is suggestive of a pathophysiological link.
TABLE 5.
Main Drugs Inducing Acral Vascular Syndromes
Regarding cancer disease, DI was essentially associated with adenocarcinoma (lung, breast and digestive) in 46.7% of cases. Hematological malignancies were the second most frequent type of cancer. Raimondo et al reported gastrointestinal cancer as the most common malignancy, followed by lung, hematologic, and gynecologic neoplasia.32 In addition, 40% of our patients with DI had metastatic localizations. This result is similar to that of the French cohort of 68 patients in which metastatic dissemination was present in 41%.18
To date, no study has investigated the clinical or biological profiles of patients with DI associated with cancer. Firstly, we observe that patients with DIAC were older than patients without cancer.11,33,34 This finding confirms that paraneoplastic syndrome usually occurs in older patients.17 Secondly, history of Raynaud’s phenomenon was present in 46.7% of patients before onset of DI. This result is consistent with data suggesting that spastic Raynaud’s phenomenon could precede development of arterial obstruction33 and that its prevalence in acute digital gangrene associated with cancer could reach 53% of cases.18 However, we failed to find any association between bilateral or unilateral Raynaud’s phenomenon and DIAC. In the same way, bilateral ischemia was not more frequent in patients with DIAC compared to patients without cancer, while most published data described bilateral and symmetric lesions.16 In our study, only 2 patients had bilateral ischemia suggesting that local mechanisms are probably also implicated. Indeed, similar to Trousseau’s syndrome, multiple mechanisms have been suggested to explain digital ischemic symptoms in association with malignancy. First, vasospasm, attributed to sympathetic hyperactivity caused by metastatic tumor invasion of sympathetic nerves or by a vasoconstrictive substance produced by the tumor cells, has been suggested as the main mechanism of ischemia.15,18 In our study, advanced tumors were found in nearly half of patients with poor outcome in 33.3% of cases, suggesting high potential for aggression. Second, thromboembolic mechanism by fragments of the tumor has also been described in histological analysis which showed fibrinoid necrosis of the vessels, intimal proliferation and heterogeneous inflammatory cell infiltrates or tissue necrosis with venous and/or arterial thrombosis.35,36 Hypothesis of an immunologic mechanism is supported in literature by several arguments. First, arteritis may be related to tumor antigen-antibody complexes with subsequent complement activation in contact with the arterial wall.37 Secondly, positive but nonspecific findings included elevated antinuclear antibodies or abnormal cryoglobulin may be explained by a deficiency in suppressor T cell function and proliferation of monoclonal B lymphoid cells.38 Cryoglobulins is another important immune mechanism leading to increased blood viscosity, complement activation and leukocytoclastic vasculitis or intravascular deposition of cryoproteins causing obliteration of the microvascular space.39 In our study, 46.7% of patients with DIAC had positive antinuclear antibodies and 13.3% had cryoglobulinemia (2 patients) supporting the hypothesis described above. In previous reports, antinuclear antibodies were found in 24% of cases, whereas cryoglobulinemia was detected in 13% of patients.18
Regarding, the frequency of positive antinuclear antibodies in DIAC, we thought that Nailfold capillary microscopy could add some informative data. Unfortunately, capillaroscopy was often normal except in 5 patients and no additional information was given by its result.
Finally, our finding in patients with DIAC, with a platelet rate significantly higher than patients without cancer, supports the hypothesis that state of hypercoagulability is the other main mechanism involved in paraneoplastic ischemia. More interestingly, 6 patients with DIAC (40%) had thrombocytosis, which is an indirect parameter of hypercoagulability.40,41 Otherwise, evidence suggests that cancer cells may induce a state of hypercoagulability leading to increased risk of thrombosis.33,42,43
This study has some limitations, the major 1 is our recruitment which is probably different from other centers. The extensive investigations performed within 1 year after the occurrence of DI could explain the high frequency of cancer as compared to those previously published. However, in literature, frequencies of cancer are probably more related to the definition used to consider DI related to cancer rather than the type of recruitment center. Therefore, according to classifications, patients with DI receiving a chemotherapy for a cancer, may be include in the cancer group or in the toxic group because it is difficult to confirm which mechanism is the most involved in the occurrence of DI (cancer or drugs?). This is the same problematic with tobacco and cancer leading to a great variation in the rate of DIAC. The second limitation is the retrospective nature of the study, however, medical records of DI are currently include in a register since 2008 which may lead to a reduced bias of memory and of analysis.
CONCLUSION
There is increasing evidence that tumors may be associated with DI. The pathogenesis of this association are partially known. Cancer appears as an ischemia-triggering factor, since the outcomes of the 2 diseases were not strictly chronologically parallel. However, in the majority of cases, treatment of the tumor resolved vascular involvement leading to the concept of paraneoplastic syndrome. Specific signs including age >50 years and thrombocytosis associated with DI should alert physicians to considering possible occult malignancies.
ACKNOWLEDGEMENTS
We are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her help in editing the manuscript and to Estelle Houivet, Biometry Department Rouen University Hospital, for her help in supervising statistical analysis.
We are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for writing assistance and review of the manuscript in English.
BIBLIOGRAPHY
UNCITED REFERENCES
Footnotes
Abbreviations: ANA = antinuclear antibodies, ANCA = antineutrophil cytoplasmic antibodies, CRP = C-reactive protein, CT scan = computed tomography-scanner, DI = digital ischemia, DIAC = digital ischemia associated with cancer.
The authors have no funding or conflicts of interest to disclose.
References
- 1.Pentti J, Salenius JP, Kuukasjärvi P, et al. Outcome of surgical treatment in acute upper limb ischaemia. Ann Chir Gynaecol. 1995; 84:25–28. [PubMed] [Google Scholar]
- 2.Carpentier PH, Guilmot JL, Hatron PY, et al. [Digital ischemia, digital necrosis]. J Mal Vasc. 2005; 30:4S29–4S37. [PubMed] [Google Scholar]
- 3.Gayraud M. Raynaud’s phenomenon. Joint Bone Spine. 2007; 74:e1–e8. [DOI] [PubMed] [Google Scholar]
- 4.Decross AJ, Sahasrabudhe DM. Paraneoplastic Raynaud’s phenomenon. The American Journal of Medicine. 1992; 92:571–572. [DOI] [PubMed] [Google Scholar]
- 5.Schildmann EK, Davies AN. Paraneoplastic Raynaud’s phenomenon—good palliation after a multidisciplinary approach. J Pain Symptom Manage. 2010; 39:779–783. [DOI] [PubMed] [Google Scholar]
- 6.Sahan C, Ucer T, Aksakal E. A case of hepatocellular carcinoma who admitted with Raynaud’s phenomenon. Rheumatol Int. 2006; 27:87–89. [DOI] [PubMed] [Google Scholar]
- 7.Nomura T, Kawasaki T, Tanabe T, et al. Ectopic cystic thymoma associated with Raynaud’s phenomenon. Ann Thorac Cardiovasc Surg. 2007; 13:118–121. [PubMed] [Google Scholar]
- 8.Auboire L, Landy S, Perrot J-Y, et al. [A negative first-line work-up of Raynaud’s phenomenon: and what if it were cancer?] J Mal Vasc. 2010; 35:35–37. [DOI] [PubMed] [Google Scholar]
- 9.Allen D, Robinson D, Mittoo S. Paraneoplastic Raynaud’s phenomenon in a breast cancer survivor. Rheumatol Int. 2010; 30:789–792. [DOI] [PubMed] [Google Scholar]
- 10.Vayssairat M, Fiessinger JN, Housset E. [Digital necroses of the upper limb. 86 cases]. Nouv Presse Med. 1977; 6:931–934. [PubMed] [Google Scholar]
- 11.Abdallah M, Hamzaoui S, Larbi T, et al. Profil étiologique des nécroses digitales des membres supérieurs : analyse de 25 observations. J Mal Vasc. 2010; 35:12–16. [DOI] [PubMed] [Google Scholar]
- 12.Cailleux N, Levesque H, Gilbert P, et al. [Digital necrosis of the arm excluding scleroderma. Retrospective study of 45 cases]. J Mal Vasc. 1994; 19:22–26. [PubMed] [Google Scholar]
- 13.LeRoy EC, Medsger TA. Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001; 28:1573–1576. [PubMed] [Google Scholar]
- 14.Olin JW. Thromboangiitis obliterans (Buerger’s disease). N Engl J Med. 2000; 343:864–869. [DOI] [PubMed] [Google Scholar]
- 15.Hawley PR, Johnston AW, Rankin JT. Association between digital ischaemia. Br Med J. 1967; 3:208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow SF, McKenna CH. Ovarian cancer and gangrene of the digits: case report and review of the literature. Mayo Clin Proc. 1996; 71:253–258. [DOI] [PubMed] [Google Scholar]
- 17.Naschitz JE, Rosner I, Rozenbaum M, et al. Rheumatic syndromes: clues to occult neoplasia. Semin Arthritis Rheum. 1999; 29:43–55. [DOI] [PubMed] [Google Scholar]
- 18.Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002; 47:47–52. [DOI] [PubMed] [Google Scholar]
- 19.Dasanu CA. Gemcitabine: vascular toxicity and prothrombotic potential. Expert Opin Drug Saf. 2008; 7:703–716. [DOI] [PubMed] [Google Scholar]
- 20.Staff S, Lagerstedt E, Seppänen J, et al. Acute digital ischemia complicating gemcitabine and carboplatin combination chemotherapy for ovarian cancer. Acta Obstet Gynecol Scand. 2011; 90:1296–1297. [DOI] [PubMed] [Google Scholar]
- 21.Holstein A, Bätge R, Egberts E-H. Gemcitabine induced digital ischaemia and necrosis. Eur J Cancer Care (Engl). 2010; 19:408–409. [DOI] [PubMed] [Google Scholar]
- 22.Clowse MEB, Wigley FM. Digital necrosis related to carboplatin and gemcitabine therapy in systemic sclerosis. J Rheumatol. 2003; 30:1341–1343. [PubMed] [Google Scholar]
- 23.Cacoub P, De Lacroix I, Tazi Z, et al. [Drug-induced iatrogenic arterial diseases]. Rev Med Interne. 1995; 16:827–832. [DOI] [PubMed] [Google Scholar]
- 24.Garcia GD, Goff JM, Hadro NC, et al. Chronic ergot toxicity: A rare cause of lower extremity ischemia. J Vasc Surg. 2000; 31:1245–1247. [DOI] [PubMed] [Google Scholar]
- 25.Mekinian A, Kyndt X, Girard-Buttaz I, et al. [Digital ischemia related to bromocriptin]. Rev Med Interne. 2008; 29:805–807. [DOI] [PubMed] [Google Scholar]
- 26.Sharma AK, Sunil S, Rustom R, et al. Cyclosporin A-related Raynaud’s phenomenon in a renal transplant recipient. Transpl Int. 2002; 15:517–518. [DOI] [PubMed] [Google Scholar]
- 27.Schapira D, Nahir AM, Hadad N. Interferon-induced Raynaud’s syndrome. Semin Arthritis Rheum. 2002; 32:157–162. [DOI] [PubMed] [Google Scholar]
- 28.Wooten MM. Systemic Sclerosis and Malignancy: A Review of the Literature. Journal January 2008. 2008; 101:59–62. [DOI] [PubMed] [Google Scholar]
- 29.Derk CT, Rasheed M, Artlett CM, et al. A cohort study of cancer incidence in systemic sclerosis. J Rheumatol. 2006; 33:1113–1116. [PubMed] [Google Scholar]
- 30.Launay D, Le Berre R, Hatron P-Y, et al. Association between systemic sclerosis and breast cancer: eight new cases and review of the literature. Clin Rheumatol. 2004; 23:516–522. [DOI] [PubMed] [Google Scholar]
- 31.Hounkpati A, Marie I, Paillotin D, et al. [Paraneoplastic sclerodermiform syndrome]. Rev Mal Respir. 2010; 27:251–256. [DOI] [PubMed] [Google Scholar]
- 32.Raimondo L. Digital Ischemia in Patients with Solid Tumors: a Case Report and Review of the Literature. Journal of Cancer Therapy. 2011; 02:281–284. [Google Scholar]
- 33.Taylor LM, Hauty MG, Edwards JM, et al. Digital ischemia as a manifestation of malignancy. Ann Surg. 1987; 206:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer. 2005; 5:655–662. [DOI] [PubMed] [Google Scholar]
- 35.Paolini MV, Jankilevich G, Graziano C, et al. Fulminant digital necrosis in a patient with prostate adenocarcinoma. Allergol Immunopathol (Madr). 2010; 38:48–50. [DOI] [PubMed] [Google Scholar]
- 36.Kuhnert C, Groza M, Fohrer C, et al. [Digital arteritis preceding the diagnosis of a malignant B cell lymphoma]. Rev Med Interne. 2008; 29:666–668. [DOI] [PubMed] [Google Scholar]
- 37.Andrasch RH, Bardana EJ, Porter JM, et al. Digital ischemia and gangrene preceding renal neoplasm. An association with sarcomatoid adenocarcinoma of the kidney. Arch Intern Med. 1976; 136:486–488. [PubMed] [Google Scholar]
- 38.Abu-Shakra M, Buskila D, Ehrenfeld M, et al. Cancer and autoimmunity: autoimmune and rheumatic features in patients with malignancies. Ann Rheum Dis. 2001; 60:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferri C, Mannini L, Bartoli V, et al. Blood viscosity and filtration abnormalities in mixed cryoglobulinemia patients. Clin Exp Rheumatol. 1990; 8:271–281. [PubMed] [Google Scholar]
- 40.Randi ML, Stocco F, Rossi C, et al. Thrombosis and hemorrhage in thrombocytosis: evaluation of a large cohort of patients (357 cases). J Med. 1991; 22:213–223. [PubMed] [Google Scholar]
- 41.Levin J, Conley C. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964; 114:497–500. [DOI] [PubMed] [Google Scholar]
- 42.Vayssairat M, Fiessinger JN, Bordet F, et al. [Relationship between digital necrosis of the upper limbs and malignant conditions] (author’s transl). Nouv Presse Med. 1978; 7:1279–1282. [PubMed] [Google Scholar]
- 43.Sack GH, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977; 56:1–37. [PubMed] [Google Scholar]


