Abstract
To investigate the clinical features of Rhupus syndrome, we retrospectively reviewed the medical records of 56 patients with Rhupus who were hospitalized at the Peking Union Medical College Hospital, Beijing, China, between January 2000 and March 2013. We analyzed the clinical manifestations of Rhupus syndrome and compared these with a control group of 160 randomly selected systemic lupus erythematosus (SLE) patients without coexisting rheumatoid arthritis (RA). In our center, 1.30% (56/4301) of hospitalized SLE patients had Rhupus syndrome. The median disease duration was 8.0 years and 83.9% had RA onset. All Rhupus patients showed radiological erosion in the joints. Compared with the control group, Rhupus patients had a longer disease duration, higher prevalence of anticyclic citrullinated peptide antibody and rheumatoid factor, higher incidence of symmetrical polyarthritis with more joint deformities and rheumatic nodules, and increased erythrocyte sediment rate and c-reactive protein levels (P < 0.005). In addition, a lower SLE disease activity index and incidences of malar rash, hemolytic anemia, renal and neurological involvement (P < 0.005), and hypocomplementemia (P < 0.05) was observed in the Rhupus group.
Rhupus syndrome is rare in SLE patients. Most Rhupus patients had RA onset and a distinctive clinical profile characterized by more severe RA-associated and mild SLE-associated damage. Specific autoantibodies and imaging findings could be helpful for making accurate Rhupus diagnoses.
INTRODUCTION
In 1971, Peter Schur1 coined the term “rhupus” to describe patients who satisfy the criteria for both systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). However, this definition did not establish whether this coexistence is a distinct clinical entity or is only the coincident presence of both conditions in the same patient. Rhupus syndrome is a rare condition and compared with SLE has minor visceral organ involvement.2 With increased numbers of emerging Rhupus syndrome case reports, to date, the cumulative number of globally reported Rhupus cases is about 140.3 To improve the understanding of this rare disorder, we analyzed data from 56 patients hospitalized with Rhupus syndrome in our center to investigate the clinical features of Rhupus syndrome, and compared them with 160 randomly selected SLE patients without RA hospitalized for the same period. To our knowledge, this is the largest Rhupus syndrome patient cohort in the literature.
PATIENTS AND METHOD
Patients
We retrospectively reviewed the clinical charts of 4301 patients who were diagnosed with SLE and admitted to the Peking Union Medical College Hospital (PUMCH), Beijing, China, between January 2000 and March 2013. Of these 4301 patients, 56 were diagnosed with SLE having RA (Rhupus syndrome group) over the study period. As a control group, we randomly selected 160 SLE patients without RA who were hospitalized in our center for the same period. PUMCH is a university-based hospital and the referral center of complicated patients nationwide. The diagnosis of SLE was based on the SLE classification criteria revised by the American College of Rheumatology in 1997.4 The diagnosis of RA was based on the RA classification criteria revised by the American Rheumatism Association in 1987.5 The disease activity of SLE was evaluated with the systemic lupus erythematosus disease activity index (SLEDAI).6 The local institutional review board approved the study. Because the study was based on a review of medical records that had been obtained for clinical purposes, the requirement for written informed consent was waived.
Methods
Medical records were retrospectively reviewed and the collected clinical data included demographic data, disease duration, disease signs and symptoms, joint involvement, visceral organ disorders, laboratory and imaging findings, SLEDAI, and treatments.
Statistical Analysis
Statistical analysis was carried out using SPSS 16.0 for Microsoft Windows. Numerical and categorical data were expressed as mean ± standard deviation (range) and percentage, respectively. Means were compared between the Rhupus group and the control group using the Mann–Whitney U test. Comparisons of various categorical clinical manifestations between the two groups were performed with Pearson’s chi-square or Fisher’s exact test (when expected frequencies were <5). All probabilities were two-sided, with P values <0.05 considered statistically significant.
RESULTS
Demographic Features
Fifty-six patients (9 male and 47 female) were diagnosed with Rhupus syndrome at PUMCH between January 2000 and March 2013. The proportion of Rhupus syndrome patients among SLE patients hospitalized during the same period was 1.30% (56/4301). The diagnosis of Rhupus syndrome was made at ages ranging from 22 years to 86 years (median age 45.5 y), and disease onset of Rhupus occurred between ages 13 and 78 (median onset age 30.5 y). The disease duration of the 56 Rhupus patients was between 1 year and 40 years (median 8.0 y). Among these, 47 patients (83.9%) were diagnosed with RA at the onset of disease and then developed SLE between 1 year and 40 years later (median 7.8 y). Four patients (7.1%) had a diagnosis of SLE prior to the RA diagnosis with an interval of 16.5 years between diagnoses (range 8–29 y). Five patients (8.9%) were diagnosed with RA and SLE concomitantly. In the control group, 20 male and 140 female SLE patients without RA coexistence were diagnosed between the ages of 10 years and 69 years (median age 33.0 y), and their disease duration was between 0.06 year and 28 years (median 2.7 y). There was no statistically significant difference between Rhupus syndrome and the control group in terms of age and sex (P > 0.05). Rhupus syndrome patients had significantly longer disease durations than the control group (P < 0.001).
Clinical Manifestations
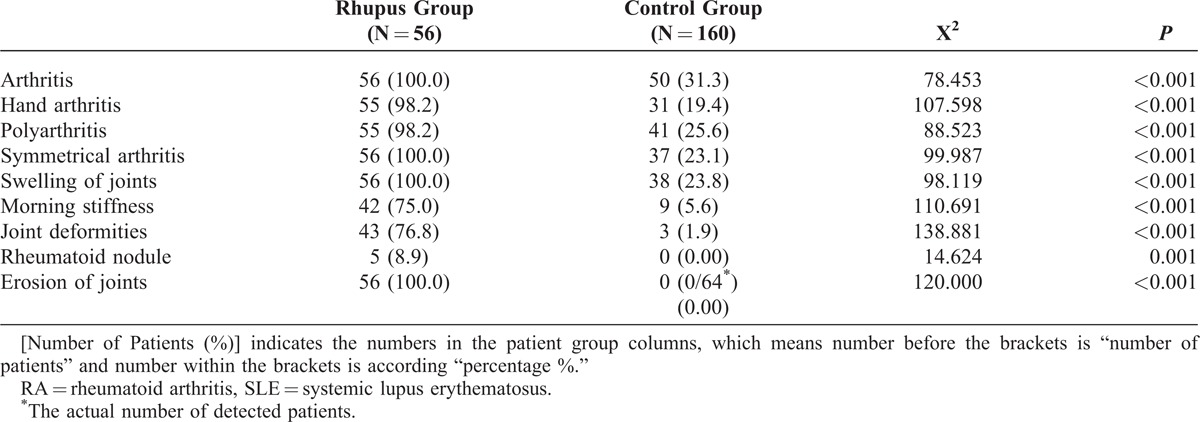
Each Rhupus syndrome patient had symmetrical arthritis, joint swelling, and radiological abnormalities [51 patients had erosion of the joints in posterior–anterior radiographs of the hands, while 5 patients showed joint surface destruction in magnetic resonance imaging (MRI) of the hands]. In the control group, 50 patients (31.3%) had arthritis without erosion as assessed by plain radiographs, and among these, 3 patients (1.9%) had Jaccoud’s arthropathy in the hands. The incidences of hand arthritis, polyarthritis, symmetrical arthritis, joint swelling, morning stiffness, joint deformities (P < 0.001), and rheumatoid nodules (P < 0.005) in Rhupus syndrome patients were significantly higher than the control group (Table 1).
TABLE 1.
Comparison of Joint Manifestations Between Rhupus Syndrome (Rhupus Group) and SLE Patients Without RA (Control Group) [Number of Patients (%)]
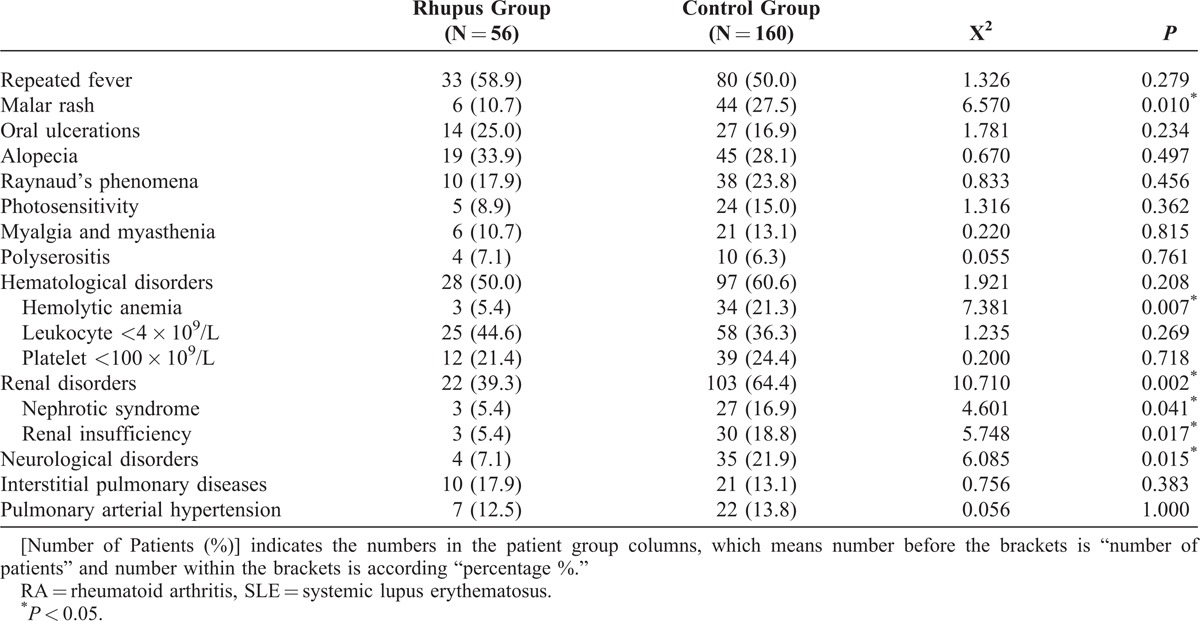
Comparisons of extra-articular manifestations showed lower incidences of malar rash, renal disorder (including nephrotic syndrome and renal insufficiency), and neurological disorders in patients with Rhupus syndrome as compared with the control group (Table 2). Rhupus patients had lower disease activity (SLEDAI scores) (8.43 ± 5.37) when compared with SLE patients without coexisting RA (11.46 ± 5.96) (P = 0.001).
TABLE 2.
Comparison of Extra-Articular Manifestations Between Rhupus Syndrome (Rhupus Group) and SLE Patients Without RA (Control Group) [Number of Patients (%)]
Laboratory Findings
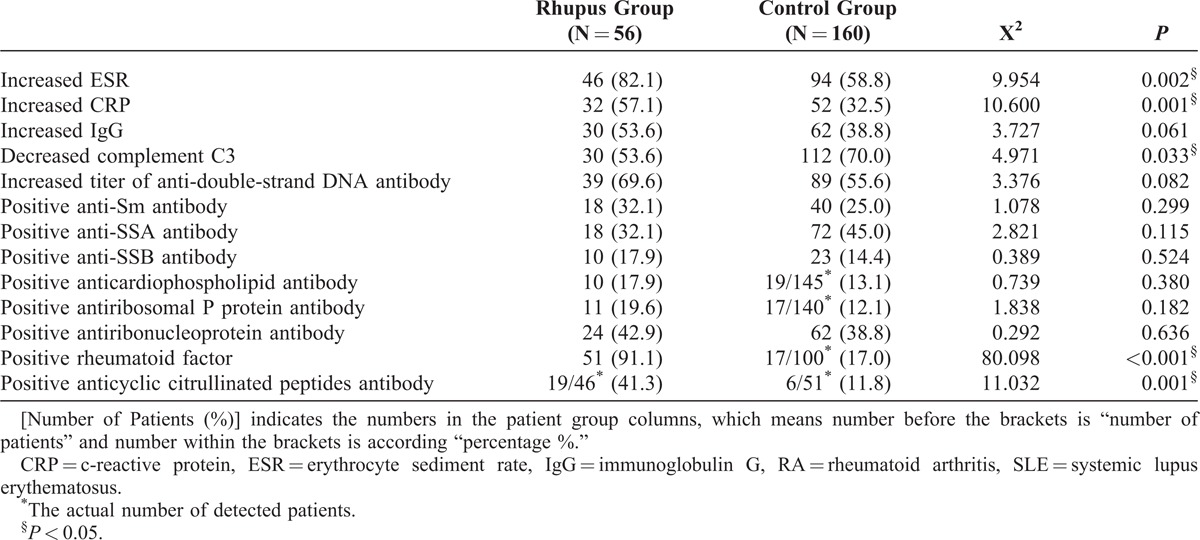
In both groups, all patients had positive antinuclear antibody results. Rheumatoid factor (RF) and anticyclic citrullinated peptide (CCP) antibody were significantly more prevalent in the Rhupus group than in the control group. The incidences of increased erythrocyte sediment rate (ESR) and c-reactive protein (CRP) were also significantly higher in Rhupus patients (P < 0.005) while the frequency of hypocomplementemia was lower (P < 0.05) (Table 3).
TABLE 3.
Comparison of Laboratory Findings Between Rhupus Syndrome Patients (Rhupus Group) and SLE Patients Without RA (Control Group) [Number of Patients (%)]
Treatments
Upon diagnosis, every patient with Rhupus syndrome was treated with systemic corticosteroids combined with 1–3 disease-modifying antirheumatic drugs (DMARDs) (e.g., methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine). Cyclophosphamide, mycophenolate mofetil, or cyclosporin A was used to treat Rhupus patients with visceral organ involvement. There were fewer cases treated with intravenous pulse methylprednisolone therapy (1 g/day for 3 consecutive days) in the Rhupus syndrome group (12 vs 78, P < 0.001). The corticosteroid dosage (equivalent to prednisone, mg/kg/d) at the beginning of the disease course was lower in the Rhupus group compared with the control group (P < 0.001), but there was no statistically significant difference in the maximum dosage (P = 0.087) found between the two groups.
DISCUSSION
The term “Rhupus syndrome” is used to describe the coexistence of SLE and RA, wherein patients have symmetrical erosive arthritis and characteristic manifestations of SLE.7 The definition of Rhupus syndrome remains controversial, as the immunopathological process of SLE is considered to be the exact opposite of RA. Abnormal activation of T helper type 2 cell (Th2) cytokines plays a central role in SLE while T helper type 1 cells (Th1) participate in RA.8 Thus, the overlap of SLE and RA has a very low incidence (0.01%–0.2%) in patients with arthritis,9 and the incidence is <2% in patients with connective tissue diseases.2,9,10 In our study, the incidence of Rhupus syndrome in SLE patients was 1.30%. The increasing application of ultrasonic imaging and MRI in joint examinations enabled early detection of bone erosion, which may have led to the increased incidence of Rhupus syndrome in SLE patients that was recently reported.11
We found that most Rhupus syndrome patients were diagnosed with onset of RA (83.9%), some with onset of SLE, and the rest were diagnosed with SLE and RA concurrently. These findings are consistent with the previous reports.11 Rhupus patients with onset of RA typically developed SLE within 7.8 years (median time), and Rhupus patients with onset of SLE developed RA within 16.5 years (median time). This result is similar to earlier studies that documented a slowly progressive disease course for Rhupus syndrome.9–11
Arthralgia and arthritis are the most common symptom of SLE, and they occur very early in the disease course. About 90% of patients diagnosed with SLE have arthralgia or arthritis during their course and 34% of patients have arthritis at disease onset.12 Some rheumatologists have classified Rhupus syndrome as a subset of SLE with severe arthritis.7,12 SLE shows three types of articular involvement: intermittent nonerosive polyarthritis usually found in the hands, wrists, and knees; nonerosive deforming arthritis referred to as Jaccoud’s joint; and arthritis with joint deformities and specific erosion, that is, Rhupus syndrome.13 Most patients with SLE have transient, migratory, and reversible arthritis without erosion.14 A few SLE patients have severe deformities in hands or feet, which was termed as Jaccoud’s arthropathy with subluxation seen in plain radiolographs.14 Jaccoud’s joint usually involves tendinitis, but not erosion, synovitis, or outstanding joint swelling or tenderness. In our study group, 31.3% patients in the control group (SLE without coexisting RA) had articular involvement (1.9% with Jaccoud’s joint and 29.4% with intermittent nonerosive arthritis), but arthritis in Rhupus patients was more severe. We speculate that when persistent symmetrical multiple swelling and tenderness is present, especially in the hands, and is accompanied by long-standing morning stiffness, the patients should be considered to have Rhupus syndrome. Some rheumatologists suggest that rheumatoid nodules in SLE patients should be a risk factor for Rhupus syndrome.15 In our study, only 5 Rhupus syndrome patients had rheumatoid nodules. This result differs from that reported in the literature and may be associated with ethnic differences of the patients in this study.
Rhupus patients have lower incidences of malar rash, hemolytic anemia, and renal and neurological involvement compared with the control group. Rhupus patients rarely have severe renal disorders such as nephrotic syndrome and renal insufficiency. The SLEDAI scores, initial corticosteroid dosages, and the usage ratio of methylprednisolone pulse therapy are lower in the Rhupus patients than in the control group. Previous studies also showed that Rhupus patients have mild SLE activity and lower incidence of visceral organ involvement compared with SLE patients without RA.2 The immunoglobulin M-type of RF could compete with complement for binding to circulating immunocompounds, which, in turn, helps to protect the body from complement activation. As mild activity of SLE and better prognosis is presented in Rhupus patients, lower amounts of corticosteroids and immunosuppressive agents were given to treat Rhupus syndrome.11
Whether Rhupus is a distinct entity with overlapping RA and SLE or is a subset of SLE is a subject of debate, as some patients with Rhupus have specific antibodies of SLE. In the present study, we demonstrated that Rhupus patients showed a prevalence of anti-double-stranded DNA and anti-Sm antibodies that was similar to those of lupus patients without RA coexistence. However, Rhupus patients display a clinical and serological profile that differs significantly from SLE with more “robust” features of RA such as severe, erosive, and deforming arthropathy as well as a significantly high prevalence of RF and anti-CCP antibodies, while having mild SLE disease activity and much lower rates of visceral organ involvement. Our findings support the contention that Rhupus is an overlap of RA and SLE and not merely a specific subset of SLE.14,16
The serum CRP level is usually normal or slightly increased in most patients with active SLE, and a highly elevated CRP level is almost always associated with infections. CRP levels were previously found to be significantly higher in SLE patients with erosive arthritis compared with nonerosive arthritis patients,7 which is in agreement with our results. As such, it would be reasonable to speculate that erosive arthritis occurs in SLE patients with obviously increased CRP.
The anti-CCP antibody has high sensitivity and specificity for the diagnosis of RA and is significantly associated with radiological joint erosion.17,18 Some SLE patients also have anti-CCP antibody, which is reflected in a report by Zhao et al.19 that showed 13.8% anti-CCP antibody positivity in 138 Chinese SLE patients. Other studies indicated that the presence of anti-CCP antibody in SLE patients increased the risk of developing erosive/deforming arthritis by 18–28-fold.17,20 Tani et al.11 also found an association of RF positivity and joint erosion in SLE patients.11 In our study, the percentage of patients showing positive results for RF and anti-CCP antibody was significantly higher in the Rhupus group than in the control group. Since RF and anti-CCP antibody positivity is associated with erosive arthritis, SLE patients with such indicators should be aggressively treated to control joint inflammation.
Very few data are available concerning Rhupus syndrome treatment, and the data that do exist are based on a few case studies and small series.11 Generally, treatment regimens including low-to-moderate dosages of corticosteroids with multiple DMARDs (e.g., methotrexate and leflunomide) could be used in Rhupus patients with prominent joint involvement to prevent the progression of erosive arthritis. However, Pipili et al.13 found that some Rhupus syndrome patients showed inadequate responses to DMARDs.13 Both mycophenolate mofetil21 and cyclosporin A22 were reported to be effective in treating Rhupus syndrome,21,22 while tumor necrosis factor inhibitors showed little effect on Rhupus or SLE, and may even lead to disease aggravation, despite reports of their success in RA treatments,3 while rituximab and abatacept appear to be more promising in Rhupus treatments.23,24 In a prospective, open study, effects of rituximab were seen not only for joint manifestations, with significant reductions in DAS28, but also for other lupus manifestations, such as improved SLEDAIs, for Rhupus patients who showed an inadequate response to corticosteroids in low and medium doses or nonbiological DMARD monotherapy or combination therapy.24 The prognosis of Rhupus syndrome often depends on the severity of vital organ involvement, but is typically better than SLE and worse than RA.
CONCLUSION
Rhupus syndrome is a special overlap syndrome of RA and SLE that is characteristically manifested by more RA-associated and less SLE-associated damage. Specific antibody profiles and radiological imaging could assist in making diagnoses of Rhupus syndrome. The treatment and prognosis of Rhupus syndrome is different than that for RA or SLE. The proper recognition and prompt early diagnosis of Rhupus syndrome is important for choosing suitable therapies and improving patient prognosis.
Footnotes
Abbreviations: CCP = cyclic citrullinated peptide, CRP = c-reactive protein, DMARDs = disease-modifying antirheumatic drugs, ESR = erythrocyte sediment rate, RA = rheumatoid arthritis, RF = rheumatoid factor, SLE = systemic lupus erythematosus, SLEDAI = SLE disease activity index.
JL and HW contributed equally to this article.
All authors made substantial contributions to the conception and design of this study. Wu Honghua and Huang Xinxiang acquired the data. Li Jing performed the data analysis and interpretation and wrote the manuscript. Zheng Wenjie provided critical revisions of the manuscript. Zhao Yan, Zeng Xiaofeng, and Liu Wanli also critically reviewed the manuscript and provided valuable input. All authors read and approved the final manuscript.
This study was supported by the Chinese National High Technology Research and Development Program, Ministry of Science and Technology, under Grants 2012AA02A513 and 2014AA020527, and the Chinese Medical Association, under Grant 12040670367.
The authors declare no conflicts of interest.
References
- 1.Schur PH. Systemic lupus erythematosus. In: Beeson PB, McDermott W, eds. Cecil-loeb Textbook of Medicine. 13th ed Philadelphia, PA: WB Saunders; 1971:821. [Google Scholar]
- 2.Simon JA, Granados J, Cabiedes J, et al. Clinical and immunogenetic characterization of Mexican patients with ’rhupus. Lupus. 2002;11:287–292. [DOI] [PubMed] [Google Scholar]
- 3.Iaccarino L, Gatto M, Bettio S, et al. Overlap connective tissue disease syndromes. Autoimmun Rev. 2013;12:363–373. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 5.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. [DOI] [PubMed] [Google Scholar]
- 6.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–640. [DOI] [PubMed] [Google Scholar]
- 7.Amezcua-Guerra LM, Springall R, Marquez-Velasco R, et al. Presence of antibodies against cyclic citrullinated peptides in patients with ’rhupus’: a cross-sectional study. Arthritis Res Ther. 2006;8:R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayakawa S, Komine-Aizawa S, Osaka S, et al. Rembrandt’s Maria Bockenolle has a butterfly rash and digital deformities: overlapping syndrome of rheumatoid arthritis and systemic lupus erythematosus. Med Hypotheses. 2007;68:906–909. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez A, Quintana G, Matteson EL, et al. Lupus arthropathy: historical evolution from deforming arthritis to rhupus. Clin Rheumatol. 2004;23:523–526. [DOI] [PubMed] [Google Scholar]
- 10.Prete M, Racanelli V, Digiglio L, et al. Extra-articular manifestations of rheumatoid arthritis: An update. Autoimmun Rev. 2011;11:123–131. [DOI] [PubMed] [Google Scholar]
- 11.Tani C, D’Aniello D, Delle Sedie A, et al. Rhupus syndrome: assessment of its prevalence and its clinical and instrumental characteristics in a prospective cohort of 103 SLE patients. Autoimmun Rev. 2013;12:537–541. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez A, Quintana G, Rondon F, et al. Lupus arthropathy: a case series of patients with rhupus. Clin Rheumatol. 2006;25:164–167. [DOI] [PubMed] [Google Scholar]
- 13.Pipili C, Sfritzeri A, Cholongitas E. Deforming arthropathy in systemic lupus erythematosus. Eur J Intern Med. 2008;19:482–487. [DOI] [PubMed] [Google Scholar]
- 14.van Vugt RM, Derksen RH, Kater L, et al. Deforming arthropathy or lupus and rhupus hands in systemic lupus erythematosus. Ann Rheum Dis. 1998;57:540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter Cohen M, Steiner G, Smolen JS, et al. Erosive arthritis in systemic lupus erythematosus: analysis of a distinct clinical and serological subset. Br J Rheumatol. 1998;37:421–424. [DOI] [PubMed] [Google Scholar]
- 16.Damian-Abrego GN, Cabiedes J, Cabral AR. Anti-citrullinated peptide antibodies in lupus patients with or without deforming arthropathy. Lupus. 2008;17:300–304. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med. 2007;146:797–808. [DOI] [PubMed] [Google Scholar]
- 18.Im CH, Kang EH, Ryu HJ, et al. Anti-cyclic citrullinated peptide antibody is associated with radiographic erosion in rheumatoid arthritis independently of shared epitope status. Rheumatol Int. 2009;29:251–256. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Li J, Li XX, et al. What can we learn from the presence of anti-cyclic citrullinated peptide antibodies in systemic lupus erythematosus? Joint Bone Spine. 2009;76:501–507. [DOI] [PubMed] [Google Scholar]
- 20.Chan MT, Owen P, Dunphy J, et al. Associations of erosive arthritis with anti-cyclic citrullinated peptide antibodies and MHC Class II alleles in systemic lupus erythematosus. J Rheumatol. 2008;35:77–83. [PubMed] [Google Scholar]
- 21.Benavente EP, Paira SO. Rhupus: report of 4 cases. Reumatol Clin. 2011;7:333–335. [DOI] [PubMed] [Google Scholar]
- 22.Seo SR, Lee SJ, Park DJ, et al. Successful treatment using cyclosporine in a patient with rhupus complicated by aplastic anemia: a case report and review of the literature. Clin Exp Rheumatol. 2011;29:708–711. [PubMed] [Google Scholar]
- 23.Pinto LF, Velasquez CJ, Prieto C, et al. Rituximab induces a rapid and sustained remission in Colombian patients with severe and refractory systemic lupus erythematosus. Lupus. 2011;20:1219–1226. [DOI] [PubMed] [Google Scholar]
- 24.Andrade-Ortega L, Irazoque-Palazuelos F, Munoz-Lopez S, et al. Efficacy and tolerability of rituximab in patients with rhupus. Reumatol Clin. 2013;9:201–205. [DOI] [PubMed] [Google Scholar]


