Abstract
The increasing problems in the field of osteoporotic fracture fixation results in specialized implants as well as new operation methods, for example, implant augmentation with bone cement. The aim of this study was to determine the biomechanical impact of augmentation in the treatment of osteoporotic distal femur fractures.
Seven pairs of osteoporotic fresh frozen distal femora were randomly assigned to either an augmented or nonaugmented group. In both groups, an Orthopaedic Trauma Association 33 A3 fractures was fixed using the locking compression plate distal femur and cannulated and perforated screws. In the augmented group, additionally, 1 mL of polymethylmethacrylate cement was injected through the screw. Prior to mechanical testing, bone mineral density (BMD) and local bone strength were determined. Mechanical testing was performed by cyclic axial loading (100 N to 750 N + 0.05N/cycle) using a servo-hydraulic testing machine.
As a result, the BMD as well as the axial stiffness did not significantly differ between the groups. The number of cycles to failure was significantly higher in the augmented group with the BMD as a significant covariate.
In conclusion, cement augmentation can significantly improve implant anchorage in plating of osteoporotic distal femur fractures.
INTRODUCTION
The increasing problems in the field of osteoporotic fracture fixation results in specialized implants as well as new operation methods. Therefore, in the last years, cement augmentation techniques have been used in different anatomical regions to a greater extent.1–6 Osteoporotic fractures are an increasing and unsolved problem in today’s trauma and orthopedic surgery.7,8 Melton et al9 found osteoporotic bone mineral conditions in 58% of women aged 70 to 79 years and even 84% in women over 80 years. In another investigation, they found the relative risk of death elevated for a long time after many of low or moderate energy fractures.10 These results are confirmed by the work of Morin et al11 who found an association between fractures at typical osteoporotic sites with increased mortality across all age groups.11 Especially fractures around the knee can cause a long period of nonweight bearing with known complications (eg, thrombosis, embolism, pneumonia) because of the fact that most elderly patients with nonweight bearing are bedridden.
To improve implant anchorage and to allow early mobilization, implant augmentation with bone cement was developed. This technique has been shown to increase anchorage between bone and implant and to rule out the influence of osteoporosis. Biomechanical studies could demonstrate the benefit of augmentation techniques in osteoporotic fracture fixation.1,4–6,12 Clinically, augmentation has been introduced for the treatment of osteoporotic proximal femoral fractures.13 Previously, our group has shown the potential of augmentation in osteoporotic distal femur fractures using an artificial bone model.2
As a further step, the aim of this biomechanical study was to identify the capability of cement augmentation to improve implant anchorage in osteoporotic distal femur fractures using human specimens and modified cannulated and perforated screws in an unstable fracture model fixed with an anatomical locking plate.
MATERIALS AND METHODS
Bone Samples
All experiments were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. The bone samples were provided by a local anatomical institute.
In this study, 7 pairs of fresh frozen distal femora with low bone mass were used. The mean age was 87 years (minimun 81; maximum 92); all specimens were from female donors. The proximal femur was replaced by a custom-made standardized polymethylmethacrylate (PMMA) femoral shaft of a length of 13 cm. Plate fixation was performed in a rigid manner to prevent proximal loosening or failure using 3 screws with nuts in the proximal plate holes 2, 4, and 6 (Figure 1). This setup simulates an extraarticular distal femur fracture with a gap of 1.5 cm (AO 33 A3).
FIGURE 1.
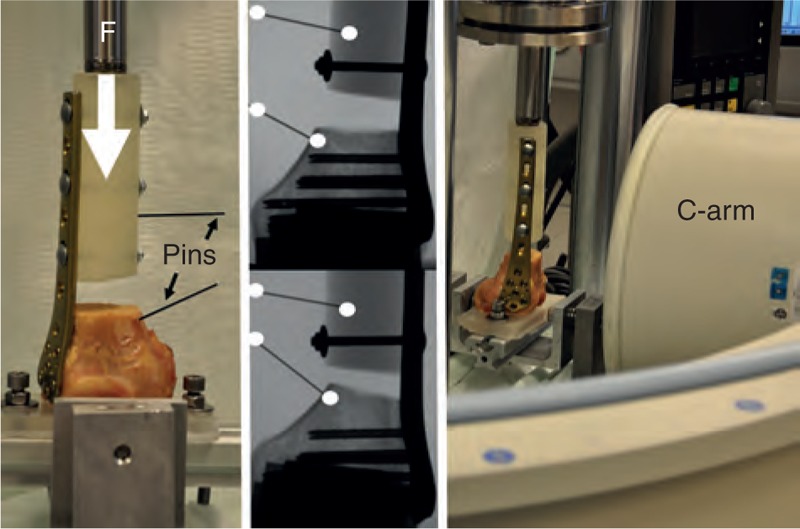
Photograph on the right is showing an overview of the test setup with the C-arm placed in anteroposterior direction. The detail image on the left shows the 2 pins (1 in the condyle and 1 in the shaft) of the specimen to allow x-ray evaluation. The x-rays in the middle showing 1 specimen before testing (top) and after failure (bottom); change in the pin angle and also in the distance pin tip and screw tip can clearly be seen.
Implants
The plate used in this study was the locking compression plate for the distal femur (LCP DF; Synthes GmbH, Solothurn, Switzerland) made of titanium alloy. We used left and right plates (according to the anatomical side) shortened to 6 proximal holes. Distal fixation was performed for both groups identically with the corresponding cannulated and perforated 5-mm self-tapping locking screws (Synthes GmbH). The screws comprise four 1.1-mm radial perforations at a distance of 10 and 15 mm from the screw tip (Figure 2A).
FIGURE 2.
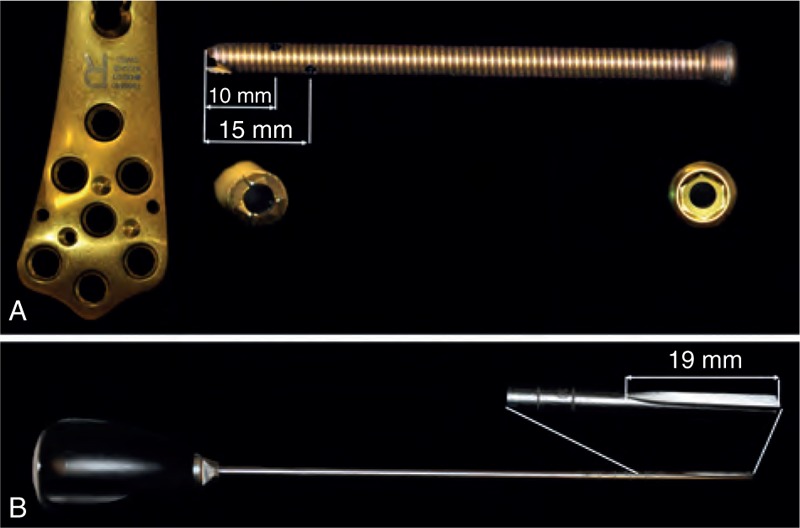
(A) Implants used in this study: LCP and cannulated, perforated screws. (B) DensiProbe, which was used to determine the local bone strength by measuring the peak torque necessary to destroy the local trabecular structure.
Bone Quality Assessment
Bone mineral density (BMD) was determined in the distal femoral condyles using peripheral quantitative computed tomography (pQCT) (XtremeCT, Scanco Medical AG, Bassersdorf, Switzerland).
Additionally, we used a DensiProbe (AO Foundation)14 to determine the local bone strength at the position of the distal screws by measuring the breakaway torque of the trabecular bone. A custom-made probe with a blade tip diameter of 3.8 and 19 mm blade length was used (Figure 2B). After temporarily fixing the plate to the specimen using forceps, a custom-made drill sleeve was fixed to the first drill hole and the distance from the plate to the medial cortex was measured using a custom-made caliper (Figure 3A). Thereby, the drill depth and the depth for the DensiProbe measurement was calculated (Figure 3B). The hole was predrilled and the probe was driven in by hammer stokes to the determined depth (Figure 3C and D). Bone strength was measured 10 mm behind the screw tip at a length of 19 mm. A 10 Nm torque measuring device (Mecmesin; Brütsch/Rüegger AG, Zürich, Switzerland) was attached to the probe and the handle was turned 90° clockwise. Maximum torque was recorded for all 7 distal screws. This was performed for all 7 distal screw holes.
FIGURE 3.
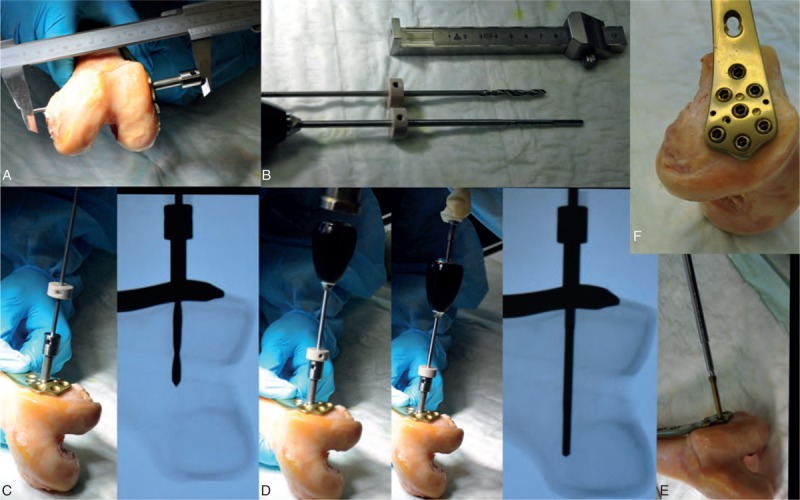
Process of instrumentation. (A) Measuring condyle width to calculate the screw length. (B) Drill bit and the DensiProbe adjusted for the measured screw length with stop rings. (C) Drilling the hole to the determined depth and x-ray control. (D) Hammering in the DensiProbe and measuring the local bone strength by turning the probe 90° and x-ray control. (E) Inserting the screw. (F) Final result before augmentation.
Instrumentation and Augmentation
We performed a pairwise comparison, hence, left and right specimens of a pair were randomly assigned to group I (nonaugmented) or group II (augmented). For both groups, the instrumentation followed the principles of the LCP surgical technique of the manufacturer. Prior testing plane x-rays excluded bony lesions.
After local bone strength was determined, a 2.5-mm guide wire was inserted into the drill hole. Appropriate cannulated and perforated screws (2–5 mm distance to the medial cortex was assured) were introduced over the K-wire and fixed using a 4 Nm torque limiter (Figure 3E and F). Screws were placed monocortical to prevent any cement leakage to the medial side. Another concern about bicortical screw placement were clinical experiences. Patients with bicortical screws in the distal femur can suffer from significant medial knee pain. Therefore, in both groups, as long as possible, monocortical screws were chosen.
After instrumentation and x-ray control in the augmented group, 1 mL of PMMA-based bone cement (Traumacem V + ; Synthes GmbH, Oberdorf, Switzerland) was introduced to each of the distal screws using the corresponding syringe kit and an 8-gauge vertebroplasty needle with side-opening cannula (Synthes GmbH, Oberdorf, Switzerland). The cement was mixed following the manufacturer’s instructions; the cannula was prefilled and then inserted into the screw to the full depth. During injection, the cannula was slowly rotated and withdrawn. After implant placement and cement injection, cement distribution was controlled using an image intensifier (Figure 4). Before biomechanical testing, specimens were stored at room temperature for 6 hours to allow cement curing.
FIGURE 4.
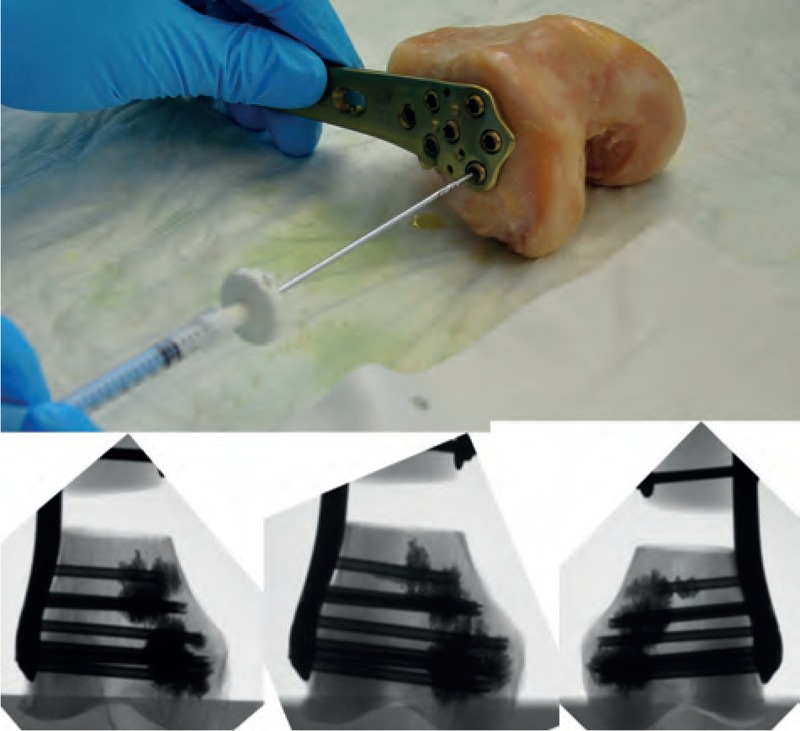
Augmentation process with the side-opening cannula (top); 1 mL cement was injected per screw. The x-rays on the bottom show 3 example results after augmentation.
Biomechanical Testing
We used a slightly modified test setup, which was introduced earlier by our group.15,16 The specimens were physiologically loaded. Force was applied via a ball and socket joint proximally. The distal femur was placed in a preshaped mold, which was fixed to a seesaw able to tilt medial and lateral (Figure 1). Testing was performed using a servo-hydraulic testing machine (MTS 858 Mini Bionix II; MTS, Eden Prairie, MN) equipped with a 4 kN load cell. Cyclic sinusoidal axial loading was performed at 2 Hz until failure of the construct. Starting at a 750 N maximum compression force, the load was monotonically increased at 0.05 N/cycle.17 The load valley was maintained at 100 N throughout the test.
Data Acquisition and Evaluation
Time, axial load, and axial displacement were recorded from the test system transducers at a frequency of 64 Hz. Axial stiffness was calculated from the mean load–displacement curve between cycles 20 and 39.
Before testing, two 1.25-mm K-wires were attached to the femoral shaft component and the femoral condyle in mediolateral direction to serve as landmarks for x-ray data evaluation with regard to varus collapse by change of angle between the wires (Figure 1). Fluoroscopic imaging was performed in anteroposterior direction using an image intensifier (Siemens Arcadis Varic; Siemens Medical Solutions AG, Munich, Germany) every 250 cycles with the specimen in a preloaded condition (100 N). Varus collapse was determined from the radiographs by means of a custom-made software routine (Matlab 7.9 R2009b, Image processing Toolbox; The MathWorks GmbH, Ismaning, Germany). The number of cycles to 4° varus collapse with respect to the initial x-ray was identified for all specimens.
After assuring normal distribution of the test data (Shapiro-Wilk test), paired t tests were carried out to identify differences between study groups with regard to BMD, stiffness, and cycles to failure. Additionally, a cox-regression was performed for the cycles to failure. Pearson correlation coefficient R was calculated for BMD, maximum torque, and cycles to failure. The software package SPSS 18.0 (SPSS Inc, Chicago, IL) was used for all statistical evaluations. Level of significance was α = 0.05.
RESULTS
Bone Quality
The mean BMD determined by pQCT was 153.3 mgHA/cm3 (standard deviation [SD] 48) in the augmented group and 157.3 mgHA/cm3 (SD 37) in the nonaugmented group; this difference was not significant (P = 0.7, power = 0.04). The mean maximum torque measured using the DensiProbe tool was 0.407 Nm (SD 0.26) for the augmented and 0.407 Nm (SD 0.23) for the nonaugmented group (P = 0.99, power = 0.02). The Pearson correlation between BMD and DensiProbe values (mean value of all 7 screw holes) was significant (R = 0.84, P < 0.01).
Axial Stiffness
The mean axial stiffness was 385.5 N/mm (SD 78) for the augmented specimens and 366.7 (SD 58) for the nonaugmented. This difference was not significant (P = 0.446, power = 0.1).
Cycles to Failure
Mean number of cycles to failure was 23,483 (SD 5715) in the augmented group and 17,643 (SD 5483) in the nonaugmented group (Figure 5). This difference was significant (P = 0.011, power = 0.67). Figure 6 shows the cumulative survival from a cox-regression analysis, the difference between the 2 groups was found to be significant (P = 0.007) with the BMD as a significant covariate (P = 0.014).
FIGURE 5.
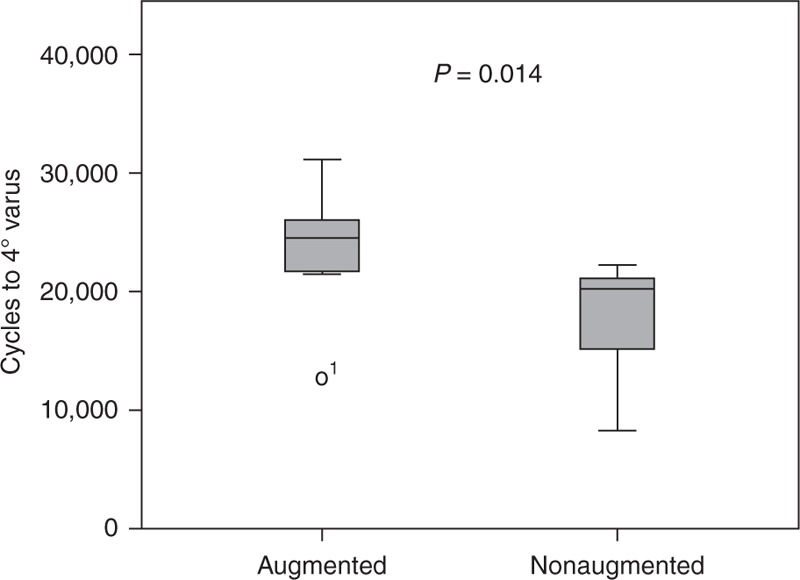
Box-plot diagram showing the number of cycles until failure for the augmented and nonaugmented group.
FIGURE 6.
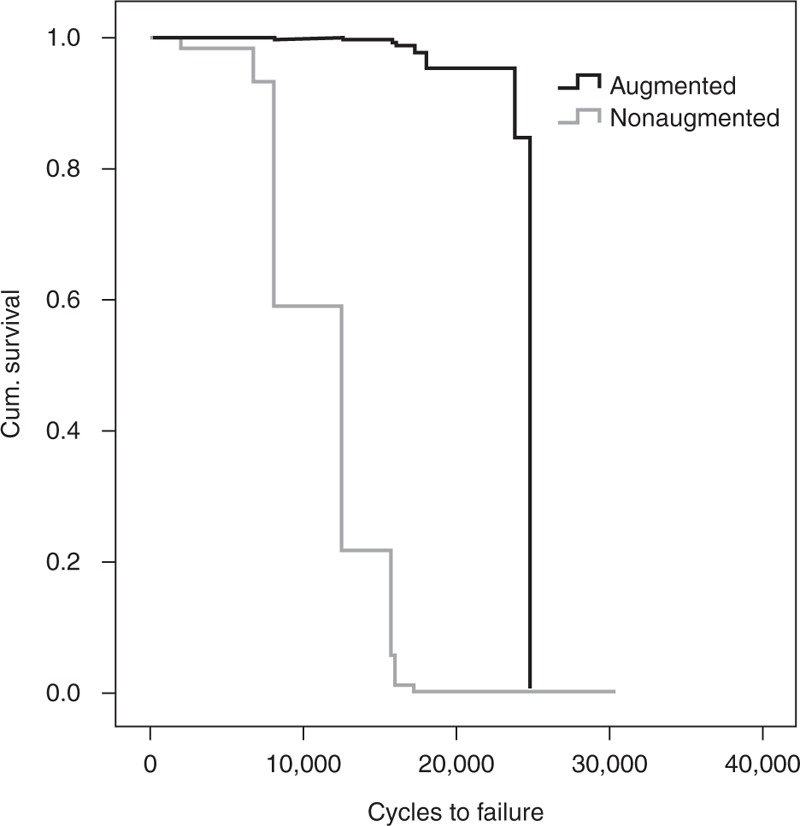
Graph showing a survival data of the augmented and the nonaugmented group, the difference is significant (P = 0.007).
In case of augmentation, no significant correlation of the number of cycles to failure and BMD (R = 0.726, P = 0.065) or DensiProbe values (R = 0.534, P = 0.217) was detected. In contrast, for the nonaugmented specimens, a significant correlation could be found for the number of cycles to failure compared to the DensiProbe values (R = 0.781; P = 0.038). No significant correlation was found for the number of cycles to failure and BMD (R = 0.718, P = 0.069).
Failure Mode
The failure mode significantly differs between both the groups, whereas screw cutout was the mode of failure in all the nonaugmented specimens, and the screw and plate breakage occurred in all augmented specimens (Figure 7).
FIGURE 7.
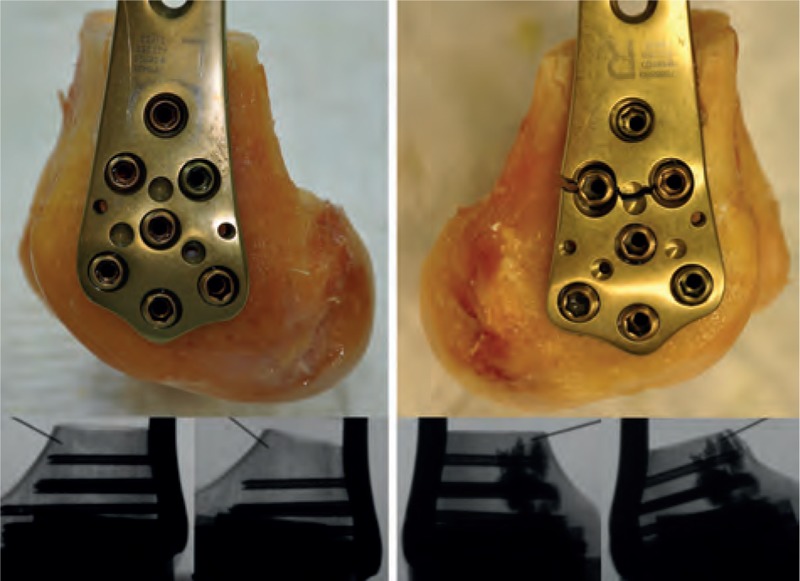
Construct failure because of the cutout in the nonaugmented specimens (left) with a photo after failure and x-rays before test (left) and after failure (right); all augmented specimen failed by implant breakage (right), x-rays showing screws after failure (right) still in the same position compared to prior testing (left).
DISCUSSION
Osteoporotic fractures of the distal femur with or without any kind of arthroplasty are an underestimated problem that will become more pronounced in the near future. The present study investigates the biomechanical advantages of cement augmentation in osteoporotic distal femur fractures using cannulated and perforated locking screws. Screw augmentation with 1-mL bone cement per screw significantly increased the number of cycles until construct failure. Additionally, the failure mode changed from screw cutout in the nonaugmented to screw and plate breakage in the augmented specimen. These results were confirmed by the DensiProbe and BMD measurements. We found no significant correlation for the augmented specimens and can conclude that screw augmentation in the distal femur can rule out the influence of osteoporosis in this biomechanical setup.
In 2 feasibility studies, our group adapted the procedure of implant augmentation to the distal femur and explored its potential in artificial osteoporotic bone models. Furthermore, in contrast to the present study, standard (not cannulated, not perforated) locking screws were used and this is why cement injection was required prior to screw placement. With these studies, our group could show that in the case of osteoporosis, implant augmentation can significantly increase mechanical stability and thus has the potential to reduce implant failure in an artificial osteoporotic model.2 These results can be confirmed by the present study also for the human cadaveric specimen with the use of cannulated and perforated screws.
Biomechanical studies should be as near as possible to physiological conditions. Therefore, Paller et al18 developed a 6 degree-of-freedom unconstrained setup for the femur to allow physiologic loading and also physiologic failing. One big disadvantage is the increasing instability of the setup, which only allows static loading. They applied axial compression of 3 mm/min until catastrophic failure. In our opinion, cyclic loading is more important than 6 degrees-of-freedom. It is possible to create clinical failure modes only with cyclic loading. Paller et al18 found the varus angulation and medial translation to be the main failure mechanism. This is also the clinical reported mode of failure. Therefore, our setup allowed these motions and we observed the varus angulation as mode of failure by x-rays.
Implant augmentation has been evaluated for several anatomical regions in the past years. Klos et al4 found significant higher stiffness values, decreased neutral zone values, and significant higher number of cycles to failure because of augmentation of the calcaneal screw in hindfoot arthrodesis nailing. Stoffel et al12 investigated cement augmentation of dynamic hip screws and found significant improvement of the cutout strength in the augmented group.12
Sermon et al6 investigated the potential of augmentation in the treatment of proximal femoral fractures using the proximal femoral nail antirotational with the helical blade. In the artificial bone model, augmentation could significantly increase the number of cycles until failure.6 In a second study, Sermon et al5 were able to confirm the findings in human cadaveric specimens.
Fliri et al3 investigated the application of bone cement in a prophylactic manner. They injected 8 to 14 mL of cement in V-shape to the proximal femur and found a significant higher energy absorption until failure in the augmented specimen.
Our group investigated the advantages of augmenting lag screws. Using low volumes of bone cement, we could significantly decrease relaxation and maximum compression force until failure.1 Additionally, our group could demonstrate a considerable potential of implant augmentation in osteoporotic distal femur fractures in an artificial osteoporotic distal femur model. Augmentation significantly decreased displacement after 45,000 cycles by 3.4 times compared to nonaugmented specimens.2
Although above-mentioned studies prove biomechanical benefits of implant augmentation in various fields, there are still some concerns about this technique such as curing of PMMA-based bone cements generates a temperature increase, which may cause thermally induced necrosis. Boner et al19 and Fliri et al20 investigated the local temperature increase during augmentation of hip screws using 3 and 6 mL of cement. Both studies concluded that augmentation of hip screws with up to 6 mL of PMMA cement does not cause thermal necrosis.
The removal of augmented implants is another aspect that should be considered. Waits et al21 found comparable maximum torque during removal of cemented and noncemented standard pedicle screws. In contrast, the maximum torque for removal of augmented cannulated and perforated screws was 12 times higher.
Another open question is the identification of patients indicated for this technique. In the second feasibility study, we have not found a biomechanical benefit of augmentation in nonosteoporotic bone samples.22 Thus, only osteoporotic patients benefit from additional augmentation, and nonosteoporotic will be put at an additional risk of the above-mentioned side effects and possible complications. Furthermore in this work, we have shown that measuring the local bone strength is a promising option to predict osteoporosis and the need for an additional cement application.
However, this study also has limitations: first of all, the small number of samples. Because of the limited availability of osteoporotic human bones and ethical reasons, we try to keep the number of specimens as low as possible. The differences we found between the groups (number of cycles to failure and especially the mode of failure) differed significantly; therefore, only 7 pairs were tested. Additionally, this is an in vitro biomechanical study, which tries to get to physiologic conditions as near as possible. Therefore, we used an as much unconstrained setup as possible for cyclic loading (proximally—ball and socket joint; distally—individual mold and a seesaw table to tilt medial and lateral). The distal tilt table was constrained to 15° medial and lateral and therefore allowed varus movement. This setup allowed cyclic axial loading closed to physiologic conditions and was able to create clinical failure modes.
CONCLUSION
Cement augmentation of a distal femur locking compression plate using cannulated and perforated screws is an easy to perform procedure, which can significantly increase the number of cycles until failure and thus carries potential to prevent complications in osteoporotic patients. Additionally, the local bone strength measured by DensiProbe significantly correlates with failure and BMD and thus, in our opinion is an appropriate device for decision making with regard to cement augmentation in porotic bone.
ACKNOWLEDGMENT
The authors would like to thank the Synthes GmbH (Solothurn, Switzerland) for providing the implant materials (plates, screws, and bone cement).
Footnotes
Abbreviations: AO/OTA = Orthopaedic Trauma Association, BMD = bone mineral density, LCP = locking compression plate, LCP DF = locking compression plate distal femur, PMMA = polymethylmethacrylate, pQCT = peripheral quantitative computed tomography.
The authors have no conflicts of interest to disclose.
This investigation was performed with the assistance of the AO Foundation via the AOTRAUMA Network (Grant No. AR2008_01).
References
- 1.Wahnert D, Hofmann-Fliri L, Schwieger K, et al. Cement augmentation of lag screws: an investigation on biomechanical advantages. Arch Orthop Trauma Surg. 2013;133:373–379. [DOI] [PubMed] [Google Scholar]
- 2.Wahnert D, Lange JH, Schulze M, et al. The potential of implant augmentation in the treatment of osteoporotic distal femur fractures: a biomechanical study. Injury. 2013;44:808–812. [DOI] [PubMed] [Google Scholar]
- 3.Fliri L, Sermon A, Wahnert D, et al. Limited V-shaped cement augmentation of the proximal femur to prevent secondary hip fractures. J Biomater Appl. 2013;28:136–143. [DOI] [PubMed] [Google Scholar]
- 4.Klos K, Wahnert D, Gueorguiev B, et al. Development of a technique for cement augmentation of nailed tibiotalocalcaneal arthrodesis constructs. Clin Biomech (Bristol, Avon). 2010;25:576–581. [DOI] [PubMed] [Google Scholar]
- 5.Sermon A, Boner V, Boger A, et al. Potential of polymethylmethacrylate cement-augmented helical proximal femoral nail antirotation blades to improve implant stability: a biomechanical investigation in human cadaveric femoral heads. J Trauma Acute Care Surg. 2012;72:E54–E59. [DOI] [PubMed] [Google Scholar]
- 6.Sermon A, Boner V, Schwieger K, et al. Biomechanical evaluation of bone-cement augmented proximal femoral nail antirotation blades in a polyurethane foam model with low density. Clin Biomech (Bristol, Avon). 2012;27:71–76. [DOI] [PubMed] [Google Scholar]
- 7.Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury. 2006;37:691–697. [DOI] [PubMed] [Google Scholar]
- 8.Raschke MJ, Stange R, Kosters C. Treatment of periprosthetic and peri-implant fractures: modern plate osteosynthesis procedures. Chirurg. 2012;83:749–759. [DOI] [PubMed] [Google Scholar]
- 9.Melton LJ, 3rd, Chrischilles EA, Cooper C, et al. Perspective. How many women have osteoporosis? J Bone Miner Res. 1992;7:1005–1010. [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ, 3rd, Achenbach SJ, Atkinson EJ, et al. Long-term mortality following fractures at different skeletal sites: a population-based cohort study. Osteoporos Int. 2013;24:1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morin S, Lix LM, Azimaee M, et al. Mortality rates after incident non-traumatic fractures in older men and women. Osteoporos Int. 2011;22:2439–2448. [DOI] [PubMed] [Google Scholar]
- 12.Stoffel KK, Leys T, Damen N, et al. A new technique for cement augmentation of the sliding hip screw in proximal femur fractures. Clin Biomech (Bristol, Avon). 2008;23:45–51. [DOI] [PubMed] [Google Scholar]
- 13.Kammerlander C, Gebhard F, Meier C, et al. Standardised cement augmentation of the PFNA using a perforated blade: A new technique and preliminary clinical results. A prospective multicentre trial. Injury. 2011;42:1484–1490. [DOI] [PubMed] [Google Scholar]
- 14.Suhm N, Hengg C, Schwyn R, et al. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127:469–474. [DOI] [PubMed] [Google Scholar]
- 15.Wähnert D, Hoffmeier K, Fröber R, et al. Distal femur fractures of the elderly: different treatment options in a biomechanical comparison. Injury. 2011;42:655–659. [DOI] [PubMed] [Google Scholar]
- 16.Wähnert D, Hoffmeier KL, von Oldenburg G, et al. Internal fixation of type-C distal femoral fractures in osteoporotic bone. J Bone Joint Surg Am. 2010;92:1442–1452. [DOI] [PubMed] [Google Scholar]
- 17.Windolf M, Muths R, Braunstein V, et al. Quantification of cancellous bone-compaction due to DHS blade insertion and influence upon cut-out resistance. Clin Biomech (Bristol, Avon). 2009;24:53–58. [DOI] [PubMed] [Google Scholar]
- 18.Paller DJ, Frenzen SW, Bartlett CS, 3rd, et al. A three-dimensional comparison of intramedullary nail constructs for osteopenic supracondylar femur fractures. J Ortho Trauma. 2013;27:93–99. [DOI] [PubMed] [Google Scholar]
- 19.Boner V, Kuhn P, Mendel T, et al. Temperature evaluation during PMMA screw augmentation in osteoporotic bone–an in vitro study about the risk of thermal necrosis in human femoral heads. J Biomed Mater Res B Appl Biomater. 2009;90:842–848. [DOI] [PubMed] [Google Scholar]
- 20.Fliri L, Lenz M, Boger A, et al. Ex vivo evaluation of the polymerization temperatures during cement augmentation of proximal femoral nail antirotation blades. J Trauma Acute Care Surg. 2012;72:1098–1101. [DOI] [PubMed] [Google Scholar]
- 21.Waits C, Burton D, McIff T. Cement augmentation of pedicle screw fixation using novel cannulated cement insertion device. Spine (Phila Pa 1976). 2009;34:E478–E483. [DOI] [PubMed] [Google Scholar]
- 22.Wahnert D, Lange JH, Schulze M, et al. A laboratory investigation to assess the influence of cement augmentation of screw and plate fixation in a simulation of distal femoral fracture of osteoporotic and non-osteoporotic bone. Bone Joint J. 2013;95-B:1406–1409. [DOI] [PubMed] [Google Scholar]


