Abstract
Blood glucose control in patients with diabetes mellitus (DM) is reportedly influenced by the seasons, with hemoglobin A1c (HbA1c) levels decreasing in the summer or warm season and increasing in the winter or cold season. In addition, several studies have shown that sepsis is also associated with the seasons. Although both blood glucose control and sepsis can strongly affect the occurrence of severe hypoglycemia, few studies have examined the seasonal variation of severe hypoglycemia. The aim of the present study is to examine the association between severe hypoglycemia and the seasons in patients with type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), and non-diabetes mellitus (non-DM). We retrospectively reviewed all the patients with severe hypoglycemia at a national center in Japan between April 1, 2006 and March 31, 2012. A total of 57,132 consecutive cases that had visited the emergency room by ambulance were screened, and 578 eligible cases of severe hypoglycemia were enrolled in this study. The primary outcome was to assess the seasonality of severe hypoglycemia. In the T1DM group (n = 88), severe hypoglycemia occurred significantly more often in the summer than in the winter (35.2% in summer vs 18.2% in winter, P = 0.01), and the HbA1c levels were highest in the winter and lowest in the summer (9.1% [7.6%–10.1%] in winter vs 7.7% [7.1%–8.3%] in summer, P = 0.13). In the non-DM group (n = 173), severe hypoglycemia occurred significantly more often in the winter than in the summer (30.6% in winter vs 19.6% in summer, P = 0.01), and sepsis as a complication occurred significantly more often in winter than in summer (24.5% in winter vs 5.9% in summer, P = 0.02). In the T2DM group (n = 317), the occurrence of severe hypoglycemia and the HbA1c levels did not differ significantly among the seasons. The occurrence of severe hypoglycemia might be seasonal and might fluctuate with temperature changes. Patients should be treated more carefully during the season in which severe hypoglycemia is more common.
INTRODUCTION
Severe hypoglycemia is an extremely hazardous event. Recently, some studies have reported that severe hypoglycemia in patients with type 1 diabetes mellitus (T1DM) was associated with traffic accidents, QT prolongation, and sudden death.1–4 In addition, several studies have suggested that severe hypoglycemia in patients with type 2 diabetes mellitus (T2DM) was associated with an increased risk of coronary heart disease and death.5–7 In critical patients, severe hypoglycemia might be a marker of the severity of underlying disease.8–10 However, many aspects of severe hypoglycemia remain unclear.
Blood glucose control in patients with diabetes mellitus (DM) is reportedly influenced by the seasons, with hemoglobin A1c (HbA1c) levels decreasing in the summer or warm season and increasing in the winter or cold season.11–18 In addition, several reports have shown that sepsis, which is a major cause of hypoglycemia, is also associated with the seasons,19,20 and the incidence of sepsis was highest in the winter.19 Although both blood glucose control and sepsis can strongly affect the occurrence of severe hypoglycemia,21 few studies have examined the seasonal variation of severe hypoglycemia.22 Furthermore, the seasonality of severe hypoglycemia in patients with different background conditions, such as T1DM, T2DM, or non-DM, has not been previously examined. The aim of the present study is to examine the association between severe hypoglycemia and the seasons.
METHODS
Study Design and Population
We retrospectively reviewed the medical records of patients who were transported by ambulance and were diagnosed as having severe hypoglycemia at the National Center for Global Health and Medicine Hospital in Tokyo, Japan, and who were subsequently diagnosed as having severe hypoglycemia between April 1, 2006 and March 31, 2012. Severe hypoglycemia was defined as the presence of any hypoglycemic symptoms that could not be resolved by themselves and that required the medical assistance of another person after the patient had visited the emergency room by ambulance.23 The blood glucose levels were checked immediately after arrival and mainly measured using venous or arterial blood at a central laboratory (77%, 444/577), although in some cases a blood glucose meter was used (23%, 133/577). All blood glucose levels <20 mg/dL were checked at a central laboratory. We investigated the seasons during which severe hypoglycemia occurred. In addition, we assessed the association between severe hypoglycemia and the seasons in cases of severe hypoglycemia with the blood glucose levels of <45 mg/dL.5,6,10 At least 2 specialists in both diabetology and internal medicine independently reviewed all the data, including the clinical records and laboratory data. Disagreements between the reviewers were resolved by a third specialist in diabetology and internal medicine. DM was confirmed when the patient had been previously diagnosed as having DM or was taking antidiabetic medicines; the DM diagnosis was further classified into T1DM, T2DM, or other types of DM. T1DM was confirmed by a previous diagnosis or the presence of antibodies to glutamic acid decarboxylase, T2DM was confirmed by a previous diagnosis or the absence of a specific cause, and other types of DM were defined as DM with an identifiable cause other than T1DM or T2DM. Patients with cardiopulmonary arrest upon arrival and patients with other types of DM were excluded from the present study. Patients who visited the hospital by ambulance more than once were analyzed as separate cases. This study was approved by the institutional review board of the National Center for Global Health and Medicine Hospital.
Seasons and Temperatures
According to the data from the Japan Meteorological Office, the mean (lowest–highest) temperatures for each month between 1981 and 2012 in Tokyo, Japan, were as follows: 6.0°C (2.4°C–9.8°C) in January, 6.5°C (2.9°C–10.4°C) in February, 9.3°C (5.5°C–13.2°C) in March, 14.6°C (10.7°C–18.8°C) in April, 18.9°C (15.4°C–22.8°C) in May, 22.1°C (19.1°C–25.5°C) in June, 25.9°C (23.1°C–29.5°C) in July, 27.5°C (24.6°C–31.4°C) in August, 23.9°C (21.2°C–27.3°C) in September, 18.5°C (15.5°C–21.9°C) in October, 13.3°C (9.9°C–16.9°C) in November, and 8.6°C (5.0°C–12.3°C) in December. The period from July to September, which was the warmest 3 months of the year, was defined as summer. The period from January to March was defined as winter, the period from April to June was defined as spring, and the period from October to December was defined as autumn. The mean temperatures of these seasons over the 30 years from 1981 to 2010 were 7.3°C in winter, 18.5°C in spring, 25.7°C in summer, and 13.5°C in autumn. The study period was just 6 years, and each season was evenly included. We also researched the association between severe hypoglycemia and temperature. The periods during which severe hypoglycemia occurred were also classified into 3 groups according to mean monthly temperature: less than 10°C (T<10), 10°C to 20°C (T10–20), and >20°C (T>20); each group included 4 months of the year.
HbA1c, Sepsis, and Other Measurements
The HbA1c levels were measured at the nearest time within 3 months of arrival, and most of the data were measured within 1 month of arrival (94.9%, 169/178). Sepsis was defined as systemic inflammatory response syndrome in response to infection. Systemic inflammatory response syndrome was regarded as the presence of ≥2 of the following criteria: body temperature >38°C or <36°C, heart rate >90 beats/min, respiratory rate >20 breaths/min, and white blood cell >12,000/mm3. The serum creatinine and potassium levels were measured upon arrival. The estimated glomerular filtration rate (GFR) was calculated using the following formula, as recommended by the Japanese Society of Nephrology: estimated GFR (mL/min/1.73 m2) = 194 × Cre−1.094 × Age−0.287 (×0.739, if the patient was female).24
Statistical Methods
Patients were categorized into the T1DM, T2DM, and non-DM groups. Data were presented as the number (%) and median with the lower and upper ends of the interquartile range (IQR). Continuous variables were compared using the Wilcoxon rank sum test or the Kruskal–Wallis tests. Categorical variables were compared using chi-square tests or Fisher exact tests. We analyzed the differences in the frequency of severe hypoglycemia between winter and summer and between the T<10 and T>20 group. P values <0.05 according to a 2-sided test were considered statistically significant for all the tests. All the analyses were performed using Stata software, version 11.1 (Stata Corp, College Station, TX).
RESULTS
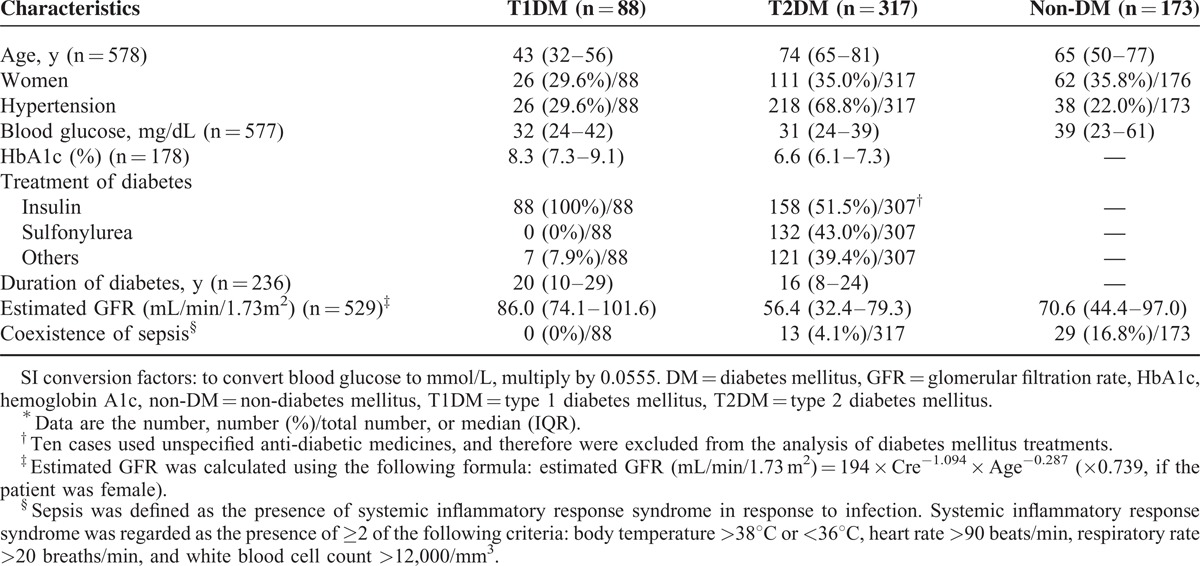
A total of 57,132 consecutive cases that had visited the emergency room by ambulance were screened, and 593 cases had severe hypoglycemia. Among these cases, 15 patients who had other types of DM were excluded; consequently, 578 cases were enrolled in the present study. The percentages of patients with re-occurrence of severe hypoglycemia were 14.2% in the T1DM group and 8.8% in the T2DM group. In the T1DM (n = 88), T2DM (n = 317), and non-DM (n = 173) groups, the median (IQR) ages were 43 (32–56) years, 74 (65–81) years, and 65 (50–77) years, the percentages of women were 29.6%, 35.0%, and 35.8%, and the blood glucose levels were 32 (24–42) mg/dL, 31 (24–39) mg/dL, and 39 (23–61) mg/dL, respectively (Table 1). All patients with T1DM received insulin treatment and >90% of the patients with T2DM received insulin or sulfonyl urea.
TABLE 1.
Characteristics Upon Arrival∗
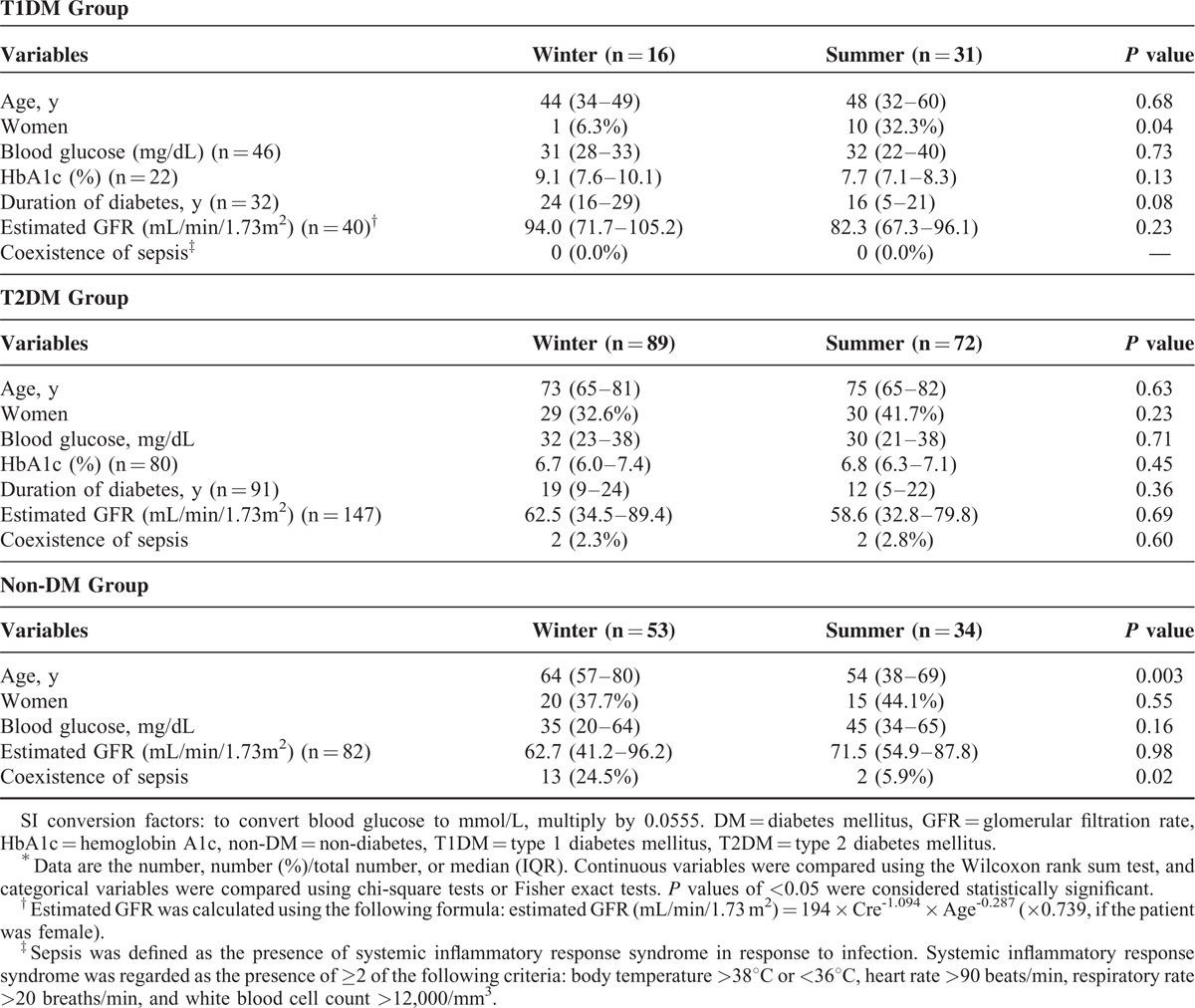
The seasonal variations in the occurrence of severe hypoglycemia for the T1DM, T2DM, and non-DM groups are shown in Figure 1, and the clinical profiles in winter and summer for these groups are presented in Table 2. In the T1DM group, severe hypoglycemia occurred significantly more often in summer than in winter (35.2% in summer vs 18.2% in winter, P = 0.01) (Figure 1A), and the median (IQR) HbA1c levels were 9.1% (7.6%–10.1%) in winter, 8.4% (8.3%–8.7%) in spring, 7.7% (7.1%–8.3%) in summer, and 9.0% (7.3%–10.0%) in autumn. Although the HbA1c levels in the T1DM group did not differ significantly among the seasons (P = 0.13), the HbA1c levels were highest in winter and lowest in summer. Although the sex ratios in the T1DM group were significantly different between winter and summer, sepsis was not observed. Age, blood glucose levels, duration of DM, and estimated GFR in the T1DM group did not significantly differ between these seasons. In the T2DM group, the occurrence of severe hypoglycemia was not significantly different between winter and summer (28.1% in winter vs 22.7% in summer, P = 0.12) (Figure 1B). Among the patients with T1DM receiving insulin, the occurrence of severe hypoglycemia also did not differ significantly between winter and summer (30.4% in winter vs 23.4% in summer, P = 0.16). The median HbA1c levels in the T2DM group were 6.7% (6.0%–7.4%) in winter, 6.5% (6.1%–7.6%) in spring, 6.8% (6.3%–7.1%) in summer, and 6.4% (5.9%–7.0%) in autumn. There were no significant differences between these values (P = 0.19). In addition, there were no significant differences between the clinical profiles, such as age, sex, blood glucose levels, duration of DM, and estimated GFR, between winter and summer periods in the T2DM group. Although patients with sepsis were observed in the T2DM group, the occurrence of severe hypoglycemia without sepsis did not differ significantly between winter and summer (28.6% in winter vs 23.0% in summer, P = 0.11). In the non-DM group, the highest frequency of severe hypoglycemia was observed in winter and the lowest was observed in summer; these differences were significant (30.6% in winter vs 19.6% in summer, P = 0.01) (Figure 1C). In addition to age, the coexistence of sepsis in the non-DM group differed significantly between winter and summer (24.5% in winter vs 5.9% in summer, P = 0.02). Because of the multicollinearity between sepsis and age,25 further analyses were conducted in terms of sepsis. The occurrence of severe hypoglycemia in non-DM patients without sepsis was not significantly different between winter and summer (27.8% in winter vs 22.2% in summer, P = 0.27). When the cases of severe hypoglycemia were limited to those with the blood glucose levels of <45 mg/dL, similar seasonal variations in the occurrence of severe hypoglycemia were observed in all groups (37.3% in summer vs 17.9% in winter in the T1DM group [P = 0.01], 22.9% in summer vs 28.2% in winter in the T2DM group [P = 0.10], and 16.3% in summer vs 31.5% in winter in the non-DM group [P = 0.01]).
FIGURE 1.
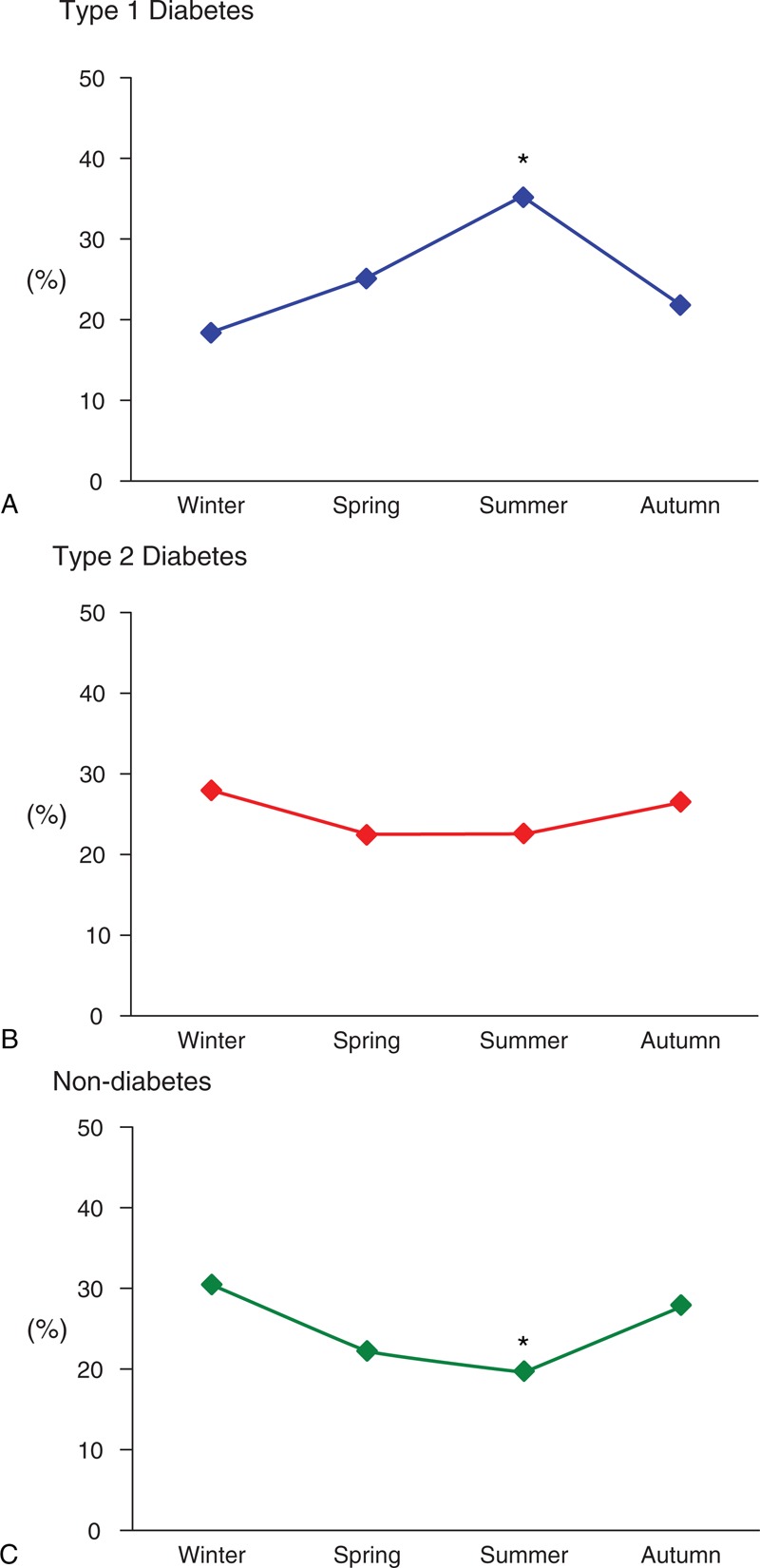
Seasonal variations in severe hypoglycemia. Type 1 diabetes mellitus (Panel A), type 2 diabetes mellitus (Panel B), and non-diabetes mellitus (Panel C). The occurrence rates of severe hypoglycemia in summer and winter were compared using chi-square tests. P values of <0.05 were considered statistically significant. ∗Summer results indicate statistical significance.
TABLE 2.
Clinical Profiles in Winter and Summer in the T1DM, T2DM, and non-DM Groups∗
Severe hypoglycemia in the T1DM group occurred significantly more often in the T>20 group than in the T<10 group (45.4% in T>20 vs 27.3% in T<10, P = 0.01). The HbA1c levels in the T<10, T10–20, and T>20 groups were 9.9% (8.0%–10.0%), 8.3% (6.8%–9.0%), and 8.0% (7.3%–8.4%), respectively, and the HbA1c levels were significantly different between the T>20 and the T<10 groups (P = 0.04). In the T2DM group, the occurrence of severe hypoglycemia did not differ significantly between the T<10 and the T>20 groups (37.2% in the T<10 group vs 30.6% in the T>20 group, P = 0.07). The HbA1c levels in the T<10, T10–20, and T>20 groups were 6.6% (5.9%–7.2%), 6.6% (5.9%–7.7%), and 6.7% (6.2%–7.1%), respectively, and the HbA1c levels between the T>20 and T<10 groups were not significantly different (P = 0.28). In the non-DM group, severe hypoglycemia occurred more frequently in the T<10 group than in the T>20 group (37.0% in T<10 vs 26.0% in T>20, P = 0.02). The rate of sepsis complications in the non-DM group differed significantly between the T<10 and the T>20 groups (23.4% in T<10 vs 6.7% in T>20, P = 0.03). In the non-DM patients without sepsis, the occurrence of severe hypoglycemia did not differ significantly between the T<10 and T>20 groups (34.0% in T<10 vs 29.2% in T>20, P = 0.37).
DISCUSSION
This study is, to the best of our knowledge, the first study to reveal a seasonal variation in the frequency of severe hypoglycemia in patients with T1DM, T2DM, and non-DM. Severe hypoglycemia in patients with T1DM occurred more frequently in summer or during periods of warm temperatures than in winter or during periods of cold temperatures. Conversely, severe hypoglycemia in non-DM patients occurred more frequently in winter or during periods of cold temperatures than in summer or during periods of warm temperatures. Meanwhile, severe hypoglycemia in patients with T2DM was not significantly associated with the seasons. Hashimoto et al22 have reported that the patients with severe hypoglycemia required significantly more frequent emergency admissions during the cold season than during the warm season. However, the study has not taken into account the different types of DM, and the patients were all hospitalized and of 60 years of age or older. Therefore, these factors may have led to different results than those obtained in our study.
The HbA1c levels in T1DM patients with severe hypoglycemia were lowest in summer and highest in winter and were significantly lower during periods of warm temperatures than during periods of cold temperatures. These results regarding the seasonal variation of HbA1c levels were consistent with those of previous studies.11,12 Nordfeldt et al11 suggested that HbA1c levels in patients with T1DM were significantly lower in spring and summer than in autumn and winter. Moreover, Mianowska et al12 reported that the lowest HbA1c levels in patients with T1DM were observed in summer and the highest were observed in winter, with differences consistently exceeding 0.44%. One of the major reasons for this difference might be that the physical activity levels were higher in summer than in winter,26–28 and high physical activity levels could improve insulin resistance and strengthen insulin action. Herbst et al29 revealed that the HbA1c levels were lower in T1DM patients with greater levels of physical activity. Meanwhile, the increased HbA1c levels in patients with T1DM in winter, in comparison with those in summer, may be caused not only by lower levels of physical activity but also by lower frequency of hospital visits and higher body weight fluctuation in winter, shifts in working time, and insulin adjustment from summer to winter. In addition, plasma cortisol and tissue sensitivity to glucocorticoids are higher in winter, which could contribute to increased body fat and insulin resistance.30 These data support the finding that the HbA1c levels in this study population with T1DM were lower in summer than in winter. As a result, low blood glucose levels might lead to frequent hypoglycemia and severe hypoglycemia.31
In the patients with T2DM, both the occurrence of severe hypoglycemia and the HbA1c levels did not differ significantly among the seasons. In addition, no association between the occurrence of severe hypoglycemia and the environmental temperature was seen. Some studies suggested that the HbA1c levels in patients with T2DM fluctuated and decreased in summer, compared with in winter.16–18 However, Dasgupta et al32 reported that there were no significant differences in the HbA1c levels among seasons despite a lower number of daily steps in autumn and winter than in spring and summer. Because the daily fluctuation of blood glucose levels in patients with T2DM is much smaller than that in patients with T1DM, the minimal change in HbA1c levels in the patients with T2DM might not have much influence on the occurrence of severe hypoglycemia. In addition, the main anti-diabetic treatment for patients with T1DM is insulin and severe hypoglycemic events may be frequent when their HbA1c levels are low. However, the dosage of anti-diabetic medicines for type 2 diabetic patients with relatively low HbA1c levels may be lowered, or the treatment changed to other remedies with decreased risk of hypoglycemia. Although the difference was not statistically significant, severe hypoglycemia was more frequent in winter and during periods of cold temperature than in summer and during periods of warm temperature. A loss of appetite caused by acute infections may have resulted in an increased occurrence of severe hypoglycemia during the cold season.22 A larger number of T2DM patients with severe hypoglycemia should be analyzed to confirm these conclusions.
In the non-DM patients, the occurrence of severe hypoglycemia was significantly higher in winter and during periods of cold temperatures than in summer and during the periods of warm temperatures. The patients without DM had a broad range of causes of severe hypoglycemia, and sepsis was one of the major causes.4,21,33 The rate of sepsis complications in this study was also significantly higher in winter than in summer, and this finding was consistent with that of a previous report.19 The occurrence of severe hypoglycemia in non-DM patients without sepsis was not significantly different between winter and summer. Although unknown variables might exist, an increase in the incidence of sepsis might increase the risk of severe hypoglycemia in non-DM patients.
Our study had several limitations. First, this research was limited to a specific geographical area and the data were reviewed retrospectively. In addition, missing data might have influenced the results and the statistical analyses. Thus, further large-scale prospective studies without missing data are needed to confirm the present results. However, the occurrence of severe hypoglycemia is extremely rare, and we examined around 600 cases of severe hypoglycemia that were classified into T1DM, T2DM, and non-DM. We believe that our study provides extremely important information about severe hypoglycemia. Second, some unknown variables might exist. Dietary habits, duration of sunshine, and the amount of rainfall might also be associated with the seasons. Although these factors should be considered, many previous reports support our data, and we are confident of the accuracy of the results in the present study. Third, the causality between severe hypoglycemia and sepsis remains unknown. Therefore, further research is required. However, sepsis is a major cause of severe hypoglycemia, and we believe the association between the seasonality of severe hypoglycemia in non-DM patients and the incidence of sepsis to be an important finding.
In conclusion, this study suggested that severe hypoglycemia in patients with T1DM or without DM might be associated with the seasons and that the incidence of severe hypoglycemia in these patients may fluctuate with temperature changes, whereas severe hypoglycemia in patients with T2DM might not be associated with the seasons or environmental temperatures. Severe hypoglycemia is an extremely hazardous event, and we should treat patients more carefully during the season in which severe hypoglycemia can be expected to occur frequently. We believe that the results of this study will contribute greatly to the prevention of severe hypoglycemia and that an understanding of the seasonality of severe hypoglycemia could improve healthcare planning.
Footnotes
Abbreviations: GFR = glomerular filtration rate, HbA1c = hemoglobin A1c, IQR = interquartile range, Non-DM = non-diabetes mellitus, T<10 = less than 10°C, T>20 = more than 20°C, T10–20 = 10°C–20°C, T1DM = type 1 diabetes mellitus, T2DM = type 2 diabetes mellitus.
Ethics approval was obtained from the institutional review board of the National Center for Global Health and Medicine in Tokyo, Japan.
TT conceived the study. TT, MKa, and MN designed the protocol. TT, RY-H, HK, MKi, RH, and AK contributed to the data collection and preparation. TT, RY-H, MKi, HN, and MN analyzed all the data. TT, RY-H, MKa, and MN wrote the report. All the authors contributed to the interpretation of the results and approved the final version.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Tattersall RB, Gill GV. Unexplained deaths of type 1 diabetic patients. Diabet Med 1991; 8:49–58. [DOI] [PubMed] [Google Scholar]
- 2.Rossing P, Breum L, Major-Pedersen A, et al. Prolonged QTc interval predicts mortality in patients with Type 1 diabetes mellitus. Diabet Med 2001; 18:199–205. [DOI] [PubMed] [Google Scholar]
- 3.Gruden G, Giunti S, Barutta F, et al. QTc interval prolongation is independently associated with severe hypoglycemic attacks in type 1 diabetes from the EURODIAB IDDM complications study. Diabetes Care 2012; 35:125–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsujimoto T, Yamamoto-Honda R, Kajio H, et al. Vital signs, QT prolongation, and newly diagnosed cardiovascular disease during severe hypoglycemia in type 1 and type 2 diabetic patients. Diabetes Care 2014; 37:217–225. [DOI] [PubMed] [Google Scholar]
- 5.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363:1410–1418. [DOI] [PubMed] [Google Scholar]
- 7.Goto A, Arah OA, Goto M, et al. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ 2013; 347:f4533. [DOI] [PubMed] [Google Scholar]
- 8.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 2007; 35:2262–2267. [DOI] [PubMed] [Google Scholar]
- 9.Hermanides J, Bosman RJ, Vriesendorp TM, et al. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med 2010; 38:1430–1434. [DOI] [PubMed] [Google Scholar]
- 10.The NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367:1108–1118. [DOI] [PubMed] [Google Scholar]
- 11.Nordfeldt S, Ludvigsson J. Seasonal variation of HbA1c in intensive treatment of children with type 1 diabetes. J Pediatr Endocrinol Metab 2000; 13:529–535. [DOI] [PubMed] [Google Scholar]
- 12.Mianowska B, Fendler W, Szadkowska A, et al. HbA(1c) levels in schoolchildren with type 1 diabetes are seasonally variable and dependent on weather conditions. Diabetologia 2011; 54:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins T, Saw S, Sikaris K, et al. Seasonal variation in hemoglobin A1c: is it the same in both hemispheres? J Diabetes Sci Technol 2009; 3:668–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakura H, Tanaka Y, Iwamoto Y. Seasonal fluctuations of glycated hemoglobin levels in Japanese diabetic patients. Diabetes Res Clin Pract 2010; 88:65–70. [DOI] [PubMed] [Google Scholar]
- 15.Liang WW. Seasonal changes in preprandial glucose, A1C, and blood pressure in diabetic patients. Diabetes Care 2007; 30:2501–2502. [DOI] [PubMed] [Google Scholar]
- 16.Ishii H, Suzuki H, Baba T, et al. Seasonal variation of glycemic control in type 2 diabetic patients. Diabetes Care 2001; 24:1503. [DOI] [PubMed] [Google Scholar]
- 17.Sohmiya M, Kanazawa I, Kato Y. Seasonal changes in body composition and blood HbA1c levels without weight change in male patients with type 2 diabetes treated with insulin. Diabetes Care 2004; 27:1238–1239. [DOI] [PubMed] [Google Scholar]
- 18.Gikas A, Sotiropoulos A, Pastromas V, et al. Seasonal variation in fasting glucose and HbA1c in patients with type 2 diabetes. Prim Care Diabetes 2009; 3:111–114. [DOI] [PubMed] [Google Scholar]
- 19.Danai PA, Sinha S, Moss M, et al. Seasonal variation in the epidemiology of sepsis. Crit Care Med 2007; 35:410–415. [DOI] [PubMed] [Google Scholar]
- 20.Hodgin KE, Moss M. The epidemiology of sepsis. Curr Pharm Des 2008; 14:1833–1839. [DOI] [PubMed] [Google Scholar]
- 21.Malouf R, Brust JC. Hypoglycemia: causes, neurological manifestations, and outcome. Ann Neurol 1985; 17:421–430. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto T, Morita A, Hashimoto Y, et al. Seasonal variation of severe hypoglycemia in hospitalized patients 60 years of age or older presenting to an emergency center hospital between 2004 and 2010. Intern Med 2013; 52:2721–2726. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes A. Standards of medical care in diabetes-2012. Diabetes Care 2012; 35 Suppl 1:S11–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53:982–992. [DOI] [PubMed] [Google Scholar]
- 25.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 2006; 34:15–21. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Olendzki BC, Li W, et al. Seasonal variation in food intake, physical activity, and body weight in a predominantly overweight population. Eur J Clin Nutr 2006; 60:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merrill RM, Shields EC, White GL, Jr, et al. Climate conditions and physical activity in the United States. Am J Health Behav 2005; 29:371–381. [DOI] [PubMed] [Google Scholar]
- 28.Dannenberg AL, Keller JB, Wilson PW, et al. Leisure time physical activity in the Framingham Offspring Study. Description, seasonal variation, and risk factor correlates. Am J Epidemiol 1989; 129:76–88. [DOI] [PubMed] [Google Scholar]
- 29.Herbst A, Bachran R, Kapellen T, et al. Effects of regular physical activity on control of glycemia in pediatric patients with type 1 diabetes mellitus. Arch Pediatr Adolesc Med 2006; 160:573–577. [DOI] [PubMed] [Google Scholar]
- 30.Walker BR, Best R, Noon JP, et al. Seasonal variation in glucocorticoid activity in healthy men. J Clin Endocrinol Metab 1997; 82:4015–4019. [DOI] [PubMed] [Google Scholar]
- 31.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977–986. [DOI] [PubMed] [Google Scholar]
- 32.Dasgupta K, Joseph L, Pilote L, et al. Daily steps are low year-round and dip lower in fall/winter: findings from a longitudinal diabetes cohort. Cardiovasc Diabetol 2010; 9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2009; 94:709–728. [DOI] [PubMed] [Google Scholar]


