Supplemental Digital Content is available in the text
Abstract
The primary objective of this study was to determine the association between the use of gastric acid suppressants (GAS) and the risk of developing spontaneous bacterial peritonitis (SBP) in patients with advanced liver cirrhosis (LC).
A case–control study nested within a cohort of 480,000 representatives of Taiwan National Health Insurance beneficiaries was carried out. A case was matched with 100 controls on age, gender, and index date of SBP diagnosis. GAS use was identified from the 1-year period before the index date. Conditional logistic regression analysis was used to adjust for various unbalanced covariates between users and nonusers of GAS.
A total of 947 cases of SBP were identified among the 86,418 patients with advanced LC. A significant increased risk of developing SBP was found to be associated with current (within 30 days), and recent (within 30–90 day) use of 2 different classes of GAS: proton pump inhibitors (PPIs) and histamine 2 receptor antagonists (H2RAs). The confounder adjusted rate ratio (aRR) for the current use of PPIs was 2.77 (95%CI: 1.90–4.04) and H2RAs was 2.62 (95%CI: 2.00–3.42). The risk of SBP attenuated for the recent use of PPIs (aRR: 2.20, 95%CI: 1.60–3.02) or H2RAs (aRR: 1.72, 95%CI: 1.25–2.37).
In addition, sensitivity analysis using hospitalized SBP as the primary outcome showed a similar risk for the current use of PPIs (aRR, 3.24; 95%CI: 2.08–5.05) and H2RAs (aRR 2.43; 95%CI 1.71–3.46). Furthermore, higher cumulative days of gastric acid suppression were associated with a higher risk of SBP (trend P < 0.0001).
To conclude, exposure to GAS was associated with an increased risk of SBP in patients with advanced LC. The association was more pronounced in current PPI users compared with nonusers.
INTRODUCTION
Cirrhosis and chronic liver disease were the 12th leading cause of death in the United States, causing roughly 31,000 deaths in 2009.1,2 Patients with cirrhosis have a compromised immune system. The immunocompromised state predisposes cirrhotic patients to increased risk of bacterial infection, the most common infections include spontaneous bacterial peritonitis (SBP) and urinary tract infections.3–5 Although the mechanism of developing SBP develop in cirrhotic patients is not elucidated, changes in liver such as increased intestinal permeability, altered intestinal motility, and bacterial overgrowth may play an important role to facilitate bacterial translocation.4–10 Evidence has shown patients with SBP have higher prevalence of small intestinal bacterial overgrowth (SIBO) than patients who did not have SBP.11
In addition to small intestinal dysmotility, several risk factors were found to induce SIBO. Gastric acid suppression (GAS) with proton pump inhibitors (PPIs) or histamine 2 receptor antagonists (H2RAs) was recently identified as an important risk factor for SIBO.11–13 There has been a growing safety concern regarding, the long-term use of PPIs since the introduction of PPIs. GAS with both PPIs and H2RAs raises the gastric pH and may lead to bacterial overgrowth in the alimentary tract. Recent studies found an association between GAS and increased risks of Clostridium difficile infection, community-acquired pneumonia, and nosocomial pneumonia in the susceptible individuals.14–17 Several studies18–29 investigated the association between PPI use and risk of SBP in cirrhotic inpatients, but conflicting results were found. Campbell et al showed that the use of PPI was not significant associated with SBP,20 in contrast, Goel et al24and Bajaj et al18 discovered that the association was statistically significant. Heterogeneity in patient population and exposure definition and random variation due to small sample size may be responsible for the conflicting results. Therefore, there is an urgent need for a large population-based study with various exposure definitions to determine the association. The incidence of SBP in cirrhotic patients is high, an estimated 1.5% to 3.5% of outpatients, and 10% of hospitalized cirrhotic patients may develop SBP.7 Identifying controllable risk factor of SBP along with appropriate prevention methods is therefore crucial in improving patients overall treatment outcome. In this study, we aimed to determine the potential association between the use of gastric acid suppressants, PPIs or H2RAs, and risk of SBP in cirrhotic patients by conducting a nested case–control study in a population-based health insurance claims database.
METHODS
Setting
We performed a cohort study with a nested case–control analysis using the Taiwan National Health Insurance Database (NHIRD). NHIRD is a database where randomized samples of patient information are captured longitudinally for more than 10 years for research purpose. The population covered by this database is demographically representative of the Taiwanese population. Data include anonymous eligibility and patient demographic characteristics (including age, sex, patient identification, and hospital and physician information), dates, hospitalizations, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, and pharmacy records (including product name, prescribed quantity, dose regimen, and route of administration) for each inpatient and outpatient visit. Since this is an electronic database study using anonymous subjects, patient consent is not required. The institutional review board at National Taiwan University has approved this study.
Study Cohort
In this study, we used a 4,800,000 representative sample that was longitudinally followed from 1999 to 2007. All patients who were 15 years or older and had an outpatient or inpatient diagnosis of liver cirrhosis in 1999 were eligible for inclusion. We adopted a new-user design by excluding any prevalent users of PPIs or H2RAs in 1999.30 We aimed to study the new cases; therefore, all patients receiving a diagnosis of SBP (ICD-9 code: 567.23) in 1999 were excluded for analysis. We followed cohort members since January 1, 2001 until the earliest occurrence of SBP, death, termination of health insurance coverage, or end of the study period (December 31, 2007).
Case Definition and Control Selection
The first episode of SBP within the follow-up period is identified by the following criteria at least 1 outpatient visit or 1 hospital admission with ICD-9-CM codes of SBP (ICD-9 code: 567.23), in addition to the procedure code for paracentasis and the prescription of compatible antibiotics. Compatible antibiotics for the treatment of SBP in this study are categorized into combination or single agent therapy based on the local treatment guideline. Combination therapy includes a cephalosporin (cefazolin, cefuroxime, ceftriaxone, cefotaxime, or cefepime) or a fluoroquinolone (ciprofloxacin or levofloxacin) in combination with an antianaerobic agent such as metronidazole. Single agent therapy includes ampicillin-sulbactam, amoxicillin-clauvanate, piperacillin-tazobactam, ticarcillin-clavulanate, flomoxef, ertapenem, meropenem, moxifloxacin, tigecycline, or a second generation of cephalosporin with coverage of anaerobes such as cefoxitin or cefmetazole. The definition of SBP was validated by a hospital record review, which showed a positive predictive value of 92%. The index date was defined as the first date of SBP diagnosis. The risk set sampling method was used to select patients for the control group; they must have comparable index dates as the patients in the experimental group. In addition, a subgroup analysis with comparable 5-year age group and sex between cases and controls was conducted.
Medication Exposure
A period of 1 year proceeding to the index date was used to determine drug exposure status. Exposure of an acid-suppressive medication is defined as any order for a prescription of PPI or H2RA. PPIs were defined as pure or compound medication containing omeprazole, esomeprazole, lansoprazole, pantoprazole, or rabeprazole. H2RAs are defined as drugs that contain cimetidine, ranitidine, or famotidine. A cumulative treatment period for ≥7 days was required for inclusion. Drug exposure periods were classified into 4 time intervals. Current use referred to a prescription of PPIs or H2RAs that covers the index date of SBP diagnosis or ends within 30 days of the index date. Recent use referred to 31 and 90 days prior to the index date. Past use indicated the use of GASs between 91 days and 1 year prior to the index date. Chronic users were patients taking PPIs or H2RAs for more than 90 days in the past year. Unexposed status was defined as no prescription or a cumulative treatment period ≤7 days in the 1 year period before the index date.
Covariates
For each individual, we collected the covariate information from cohort entry to 1 year before the year containing the index date (see appendix 1, http://links.lww.com/MD/A291). The following information was determined as potential confounders age, sex, calendar year (matching variables), indication for GAS therapy, risk factor for SBP disease, burden of comorbid conditions, indicator for frailty, and use of specific medications. The indication for PPIs or H2RAs included gastric cancer, gastrointestinal hemorrhage, gastroesophageal reflux disease or reflux esophagitis, gastrojejunal or esophageal ulcers, esophageal stricture, and esophageal varices, and functional gastrointestinal disorders. Risk factors for SBP included diabetes mellitus, chronic renal failure or hemodialysis, solid organ transplantation, presence of cancer, and malnutrition-related disorders such as cachexia, anorexia, abnormal loss of weight and alcoholism-related disease, and postgastric surgery. Liver disease related comorbidities included ascites, jaundice, portal hypertension, hepatic encephalopathy, hepatocellular carcinoma, acute and subacute necrosis of liver, and hepatorenal syndrome. The use of specific medications included NSAIDs, aspirin, systemic immunosuppressive agents and biologics, and systemic corticosteroid use. We used a combined weighted comorbidity index to quantify each individual's burden of comorbidity. This score combines the Charlson Index with Elixhauser system to offer improvements in comorbidity summarization over Charlson index alone.
Data Analysis
Characteristics of patients were presented as numbers with percentages for categorical variables and as median with interquartile range for continuous variables. We estimated the risk of SBP associated with the use of PPIs or H2RAs by constructing conditional logistic regression model for 4 types of users (current, recent, past) as compared with nonusers. Two analyses were performed. The first analysis was the crude effect estimate stratified on the 3 matching factors, age, sex, and index date. The second analysis was a regression model adjusted for the individual covariates mentioned in the previous section. A duration response analyses and subgroup analyses in high-risk patients were also carried out to further assess the robustness of our results. All statistical calculations were carried out with SAS version 9.2.
RESULTS
Study Population and Use of Medication
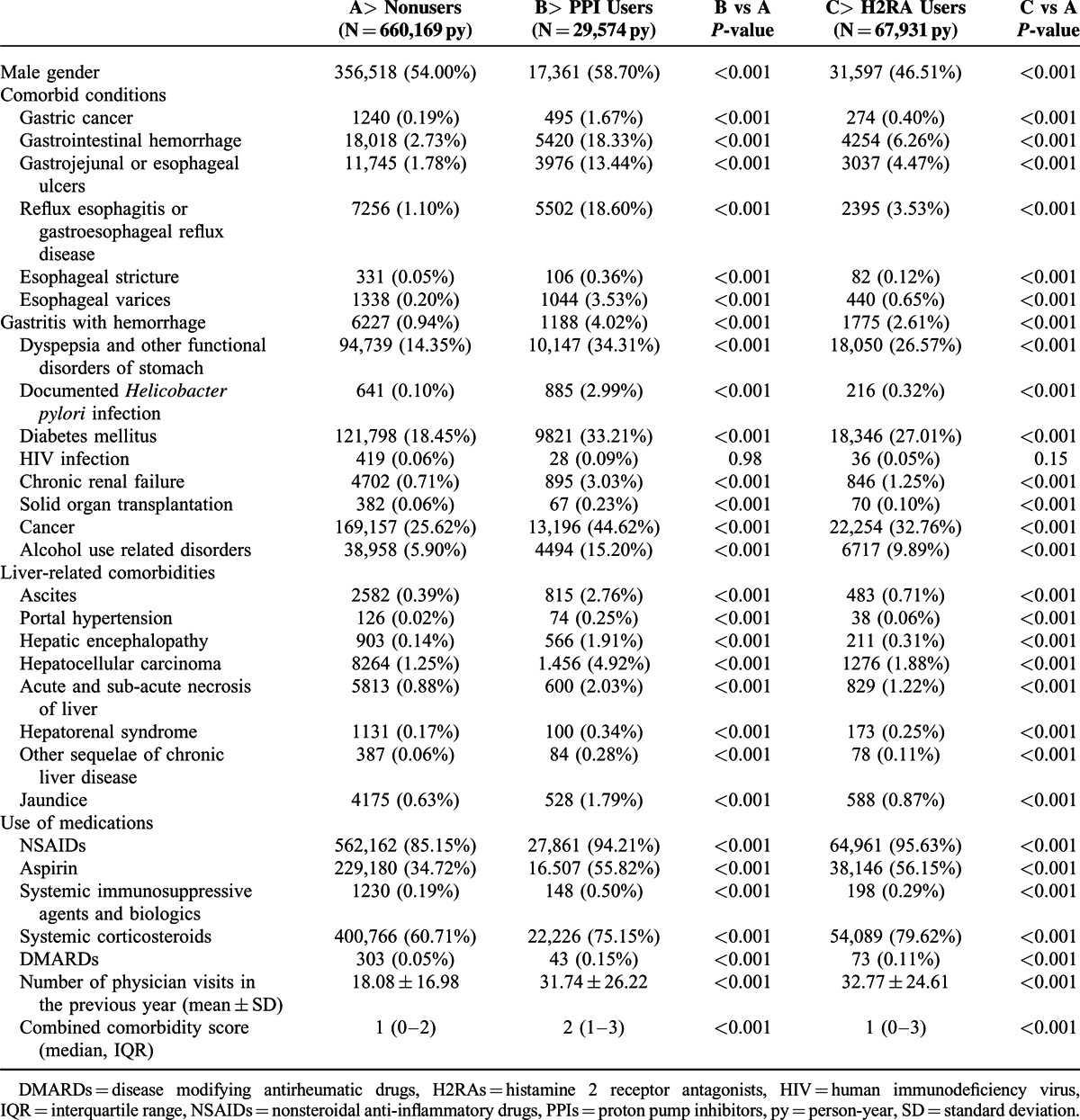
After exclusion of prevalent users of PPIs or H2RAs, prevalent cases of SBP, and children aged less than 15 year, the study cohort included 86,418 patients with LC, of which a total of 947 cases of SBP were identified. The mean age of cohort members at baseline was 43.4 ± 15.8 years. The mean length of follow-up for the cohort was 8.8 years. Overall the crude incidence of SBP among this cohort of cirrhotic patients is 124.8/100,000 person-years. The characteristics among PPI users, H2RA users, and nonusers are shown in Table 1. Compared with nonusers, users of GAS are associated with higher burden of gastrointestinal diseases, diabetes mellitus, chronic renal disease, chronic pulmonary disease, immunocompromised disease, and chronic liver disease related complications. Patients who were prescribed with nonsteroidal antiinflammatory agents or steroid were also more likely to be prescribed with GAS.
TABLE 1.
Characteristics Between Users and Nonusers of Gastric Acid Suppressants
Multivariate Analysis
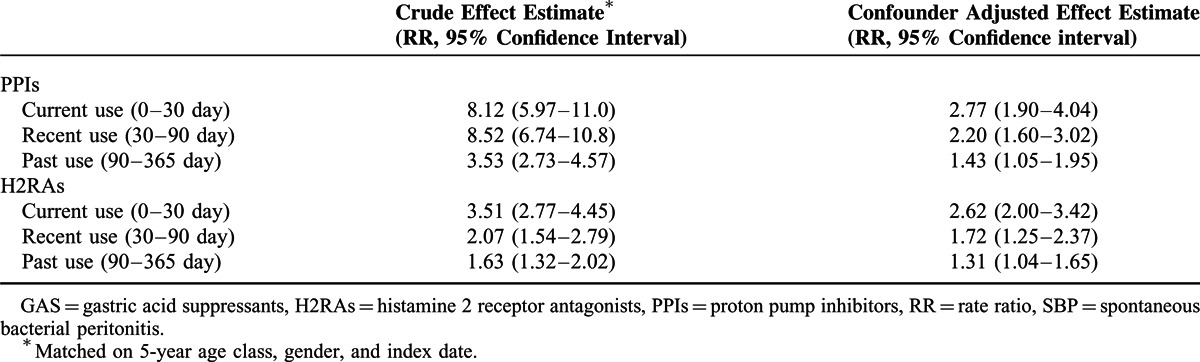
Table 2 shows the crude and adjusted effect of current, recent, past, and chronic users of PPIs or H2RAs and incident SBP. The crude RR associating current drug use with SBP was 8.12 (95% confidence interval: 5.97–11.04) for PPIs and 3.51 (95%CI 2.77–4.45) for H2RAs as compared with nonusers. Adjustment for potential confounders attenuated the RR to 2.77 (95%CI 1.90–4.04) for PPIs and 2.62 (95%CI 2.00–3.42) for H2RAs. The effect size decreases with time since exposure to GAS. Recent use of PPIs was associated with lower risk for SBP (adjusted RR 2.20, 95% CI 1.60–3.02) as compared with the current use of PPIs. Similar trend was found in users of H2RAs. Recent use of H2RAs was associated with an attenuated risk for SBP (adjusted RR 1.72, 95%CI: 1.25–2.37). Past use of either PPIs or H2RAs was also associated with increased risk of SBP (past users of PPIs: 1.43, 95% CI 1.05–1.95 and H2RAs: 1.31, 95% CI 1.21–1.73).
TABLE 2.
Effect of GAS Therapy on the Risk of Incident SBP in Patients With Advanced Liver Cirrhosis
Sensitivity Analysis
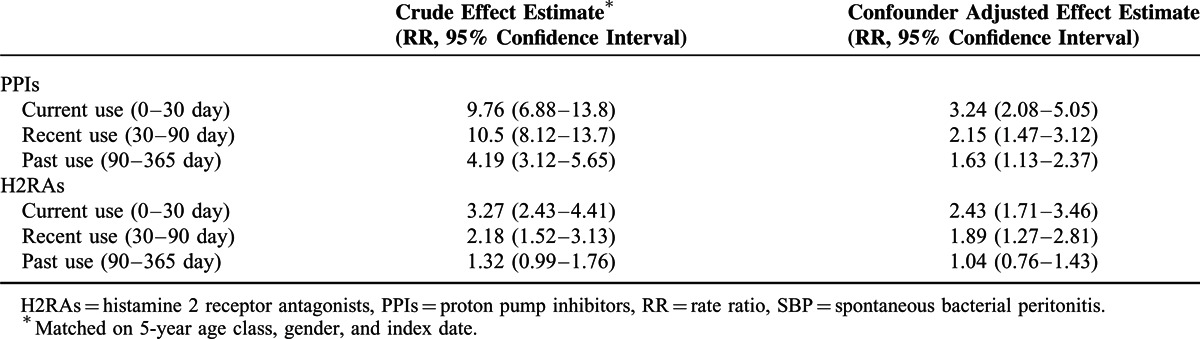
To verify the robustness of our findings, we conducted a sensitivity analysis by using a more strictly defined outcome, hospitalized SBP. Hospitalized SBP referred to hospitalized patients with a primary or secondary diagnosis of SBP. The results were in line with our primary analysis. The use of PPIs or H2RAs in previous 1 year was associated with increased risk of SBP. Results of sensitivity analysis by different drug exposure periods were summarized in Table 3.
TABLE 3.
Results of Sensitivity Analysis Using Hospitalized SBP as the Primary Endpoint
Duration-Response Analysis
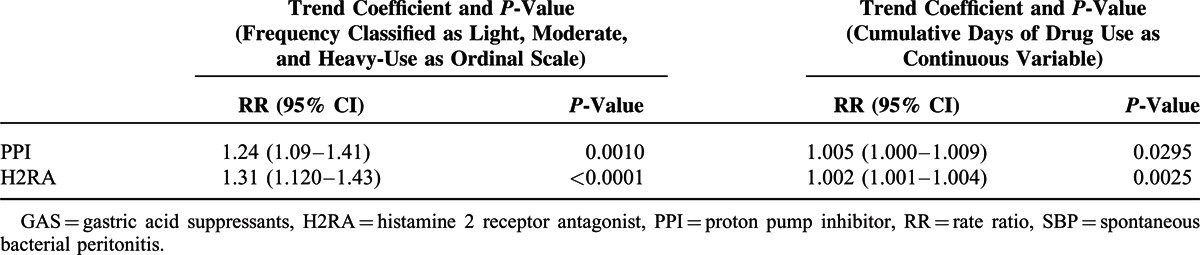
Multivariate analysis showed the risk of SBP increased from light (<7 cumulative days) to moderate (7–30 cumulative days) to heavy-use (> 30 cumulative days) categories for both PPI (trend P = 0.001) and H2RA (trend P < 0.0010). The incremental risk of SBP was 1.005 (1.000–1.009) for each day use of PPI and 1.002 (1.001–1.004) for each day use of H2RAs (Table 4).
TABLE 4.
Relationship Between Length of GAS Prescription and Risk of SBP in Patients With Advanced Liver Disease
Subgroup Analyses
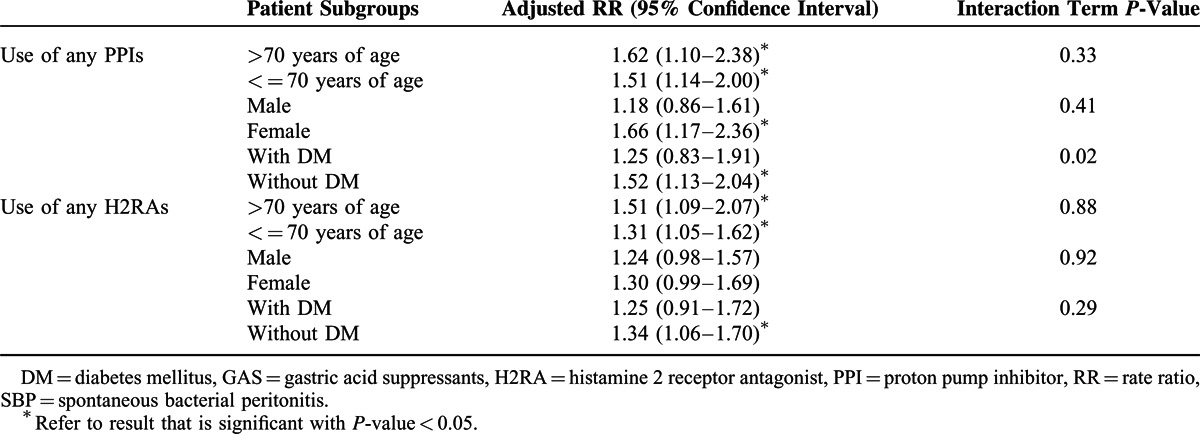
To investigate whether there is a differential risk of SBP among different populations of GAS users (>7 cumulative days), we performed predefined subgroup analyses (Table 5). We found that the risk of SBP varies among different subgroups, and the interaction term reached statistical significance (P-value < 0.05) for only the diabetes mellitus (DM) subgroup that was using PPIs. The risk increase of SBP in PPI users (compared with nonusers) was more substantial in subjects without DM than in subjects with DM (1.52 vs 1.25). In addition, we found that female users of PPIs or H2RAs tend to have a higher risk increase of SBP, but the interaction term was not significant.
TABLE 5.
Risk of Incident SBP Associated with Use of GAS in Different Patient Groups
DISCUSSION
The current study involving 86,418 cirrhotic patients found a significant association between SBP and use of GAS. Cirrhotic patients receiving a PPI have an overall 2.8 times greater risk of developing SBP within 30 days compared with those not receiving this medication. Cirrhotic patients taking H2RA demonstrated an attenuated but significant risk of developing SBP compared with nonusers. The current use of GASs was associated with highest risk, while recent and past use was also associated with a nonnegligible risk. In addition, the associations between the use of GAS and SBP in cirrhotic patients remain statistical significant across subgroups regardless of differences in age and sex. Our findings are consistent with 2 recent meta-analyses evaluating GAS and the risk of SBP. A meta-analysis consists of 4 observational studies with 772 patients were conducted by Trikudanathan et al.19 An increased risk of SBP among PPI users (OR 2.77, 95% CI: 1.82–4.23) was found. Another meta-analysis, which includes 8 observational studies and 3815 cirrhotic patients, was conducted by Deshpande et al. The results indicated that both PPI and H2RA therapies are associated with increased risk of SBP, with a summary OR of 3.15 (95% CI: 2.09–4.74) for PPI and 1.71 (95% CI: 2.09–4.74) for H2RA.23 Moreover, 1 observational study using a propensity score matched cohort of 402 paired cirrhotic patients with ascites noted that PPI use was the independent risk factor for SBP (hazard ratio, 1.396, 95% CI: 1.057–1.843).26 Combing our findings and previous studies,19,23,26 we tend to conclude GAS therapy, especially PPI are associated with increased risk of SBP in cirrhotic patients.
Potential mechanisms for gastric acid suppression to increase the risk of SBP were proposed in several studies. In vitro studies showed PPIs or H2RAs have direct cytotoxicity to inflammatory cells such as lymphocytes, neutrophils, or natural killer cells. In vivo study indicated that use of PPIs may lead to compromise upper gastrointestinal tract barrier function, and was able to induce a significant transmucosal leak in the upper GI tract.31 Based on the analysis of blood samples before and after PPI treatment in 10 healthy subjects, Zedtwitz-Liebenstein et al32 found that PPI can impair production of reactive oxygen intermediates by neutrophils, and cause an increase of intracellular Ca2+ concentrations in resting neutrophils that was associated with reduced bactericidal activity. All of these effects may cause the change of the natural gut microbial ecology,33 and subsequently lead to increased bacterial colonization in the gastrointestinal tract. Overall, acid-suppressive therapy may predispose to bacterial overgrowth within gastrointestinal tract and translocation across the impaired epithelial barrier.11 Therefore, it may contribute to the development of SBP.
Our study has several major strengths. First, this study is based on a true population-based database with full coverage of hospital admissions and prescriptions for PPI or H2RA. It represents the real world situation, and makes our results more generalizable to other settings. Second, the incidence of SBP in North America and most European countries is less than 5/100,000 person-years; therefore, a larger sample size of more than 10 million may be required to reach adequate statistical power. In contrast, the incidence of SBP is around 122/100,000 person-years in this sample, so larger number of case patients and controls can be identified using this database. The large sample size allowed adjustments for all the possible risk factors, and different subgroup analyses. Third, by using the longitudinal database of NHIRD, we can define the interval between the receipt of PPI or H2RA therapy and occurrence of SBP. Thus, allowing duration response analysis.
However, this study has several limitations. First, our claim database lacked the microbiological data to confirm SBP cases, and outcome misclassification is possible. However, we felt that our definition of SBP is rather stringent. SBP cases need to have ICD-9-CM codes of SBP plus both the procedure code for paracentasis and the ICD-9-CM codes for prescription of compatible antibiotics. Furthermore, our case definition was validated by a hospital record review, which showed a positive predictive value of 92%. Second, as our study is an observational study, the possibility of residual confounding due to uncontrolled significant cofounders cannot be totally excluded. Third, protopathic bias may play a role in the observed higher risk of SBP in patients taking GASs. Protopathic bias arises when early gastrointestinal symptoms of SBP are the cause of GAS prescription. However, protopathic bias cannot totally explain the observed strong association that persisted for more than 30 days before the SBP diagnosis. A sensitivity analysis that excluded the most recent users (ie, within 7 days of index date) also did not change the effect estimate significantly. Finally, variations in physicians’ practice may also play a role in the observed results.
In conclusion, our study suggests that the use of PPIs or H2RAs is associated with increased risk for SBP, especially in new initiators within 30 days. As a substantial body of literature supports that gastric acid environment is important for human host defense to SBP infection, further validation of this association in multiple populations is warranted. Before such studies are available, gastric acid suppressants should be prescribed cautiously to patients with risk of developing SBP.
Acknowledgments
The authors thank the staff of the Core Labs, Department of Medical Research, National Taiwan University Hospital for technical support. Also like to thank Shih-Hao Lee and Yi-Hsian Lin for their help with statistical analysis.
Footnotes
Abbreviations: GAS = gastric acid suppression, H2RA = histamine 2 receptor antagonist, PPI = proton pump inhibitor, RR = rate ratio, SBP = spontaneous bacterial peritonitis.
All authors participated in the study conception, design, and statistical analysis planning.
STROBE
This study is supported by Taiwan National Science Foundation Grant NSC101-3114-Y-002-003, NSC 100-2314-B-002-138-MY3, and NSC102-2314-B-002-131-MY3; and National Taiwan University Hospital Yunlin Branch Research Grant, NTUHYL101.N014.
No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep 2013; 61:1–117. [PubMed] [Google Scholar]
- 2.Xu J, Kochanek KD, Murphy SL, et al. Deaths: final data for 2007. Natl Vital Stat Rep 2010; 58:1–19. [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. Hepatol 2010; 53:397–417. [DOI] [PubMed] [Google Scholar]
- 4.Runyon BA. Management of adult patients with ascites caused by cirrhosis. Hepatology (Baltimore, MD) 1998; 27:264–272. [DOI] [PubMed] [Google Scholar]
- 5.van Vlerken LG, Huisman EJ, van Hoek B, et al. Bacterial infections in cirrhosis: role of proton pump inhibitors and intestinal permeability. Eur J Clin Invest 2012; 42:760–767. [DOI] [PubMed] [Google Scholar]
- 6.Evans LT, Kim WR, Poterucha JJ, et al. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology (Baltimore, MD) 2003; 37:897–901. [DOI] [PubMed] [Google Scholar]
- 7.Jamil S, Ahmed S, Memon A, et al. Factors predicting the recurrence of spontaneous bacterial peritonitis in patients with cirrhosis. J Coll Phys Surg Pak 2011; 21:407–410. [PubMed] [Google Scholar]
- 8.Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology (Baltimore, MD) 1984; 4:1209–1211. [DOI] [PubMed] [Google Scholar]
- 9.Sheer TA, Runyon BA. Spontaneous bacterial peritonitis. Digest Dis (Basel, Switzerland) 2005; 23:39–46. [DOI] [PubMed] [Google Scholar]
- 10.Such J, Frances R, Munoz C, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology (Baltimore, MD) 2002; 36:135–141. [DOI] [PubMed] [Google Scholar]
- 11.Chang CS, Chen GH, Lien HC, et al. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology (Baltimore, MD) 1998; 28:1187–1190. [DOI] [PubMed] [Google Scholar]
- 12.Bauer TM, Steinbruckner B, Brinkmann FE, et al. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol 2001; 96:2962–2967. [DOI] [PubMed] [Google Scholar]
- 13.Parkman HP, Urbain JL, Knight LC, et al. Effect of gastric acid suppressants on human gastric motility. Gut 1998; 42:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deshpande A, Pant C, Pasupuleti V, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol 2012; 10:225–233. [DOI] [PubMed] [Google Scholar]
- 15.Jump RL, Pultz MJ, Donskey CJ. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob Agents Chemother 2007; 51:2883–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laheij RJ, Sturkenboom MC, Hassing RJ, et al. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004; 292:1955–1960. [DOI] [PubMed] [Google Scholar]
- 17.Prod’hom G, Leuenberger P, Koerfer J, et al. Nosocomial pneumonia in mechanically ventilated patients receiving antacid, ranitidine, or sucralfate as prophylaxis for stress ulcer. A randomized controlled trial. Ann Intern Med 1994; 120:653–662. [DOI] [PubMed] [Google Scholar]
- 18.Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009; 104:1130–1134. [DOI] [PubMed] [Google Scholar]
- 19.Trikudanathan G, Israel J, Cappa J, et al. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients – a systematic review and meta-analysis. Int J Clin Pract 2011; 65:674–678. [DOI] [PubMed] [Google Scholar]
- 20.Campbell MS, Obstein K, Reddy KR, et al. Association between proton pump inhibitor use and spontaneous bacterial peritonitis. Digest Dis Sci 2008; 53:394–398. [DOI] [PubMed] [Google Scholar]
- 21.Choi EJ, Lee HJ, Kim KO, et al. Association between acid suppressive therapy and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Scand J Gastroenterol 2011; 46:616–620. [DOI] [PubMed] [Google Scholar]
- 22.de Vos M, De Vroey B, Garcia BG, et al. Role of proton pump inhibitors in the occurrence and the prognosis of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Liver Int 2013; 33:1316–1323. [DOI] [PubMed] [Google Scholar]
- 23.Deshpande A, Pasupuleti V, Thota P, et al. Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. J Gastroenterol Hepatol 2013; 28:235–242. [DOI] [PubMed] [Google Scholar]
- 24.Goel GA, Deshpande A, Lopez R, et al. Increased rate of spontaneous bacterial peritonitis among cirrhotic patients receiving pharmacologic acid suppression. Clin Gastroenterol Hepatol 2012; 10:422–427. [DOI] [PubMed] [Google Scholar]
- 25.Mandorfer M, Bota S, Schwabl P, et al. Proton pump inhibitor intake neither predisposes to spontaneous bacterial peritonitis or other infections nor increases mortality in patients with cirrhosis and ascites. PloS One 2014; 9:e110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Min YW, Lim KS, Min BH, et al. Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: a propensity score matched cohort study. Aliment Pharmacol Ther 2014; 40:695–704. [DOI] [PubMed] [Google Scholar]
- 27.Miura K, Tanaka A, Yamamoto T, et al. Proton pump inhibitor use is associated with spontaneous bacterial peritonitis in patients with liver cirrhosis. Intern Med (Tokyo, Japan) 2014; 53:1037–1042. [DOI] [PubMed] [Google Scholar]
- 28.Ratelle M, Perreault S, Villeneuve JP, et al. Association between proton pump inhibitor use and spontaneous bacterial peritonitis in cirrhotic patients with ascites. Can J Gastroenterol Hepatol 2014; 28:330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terg R, Casciato P, Garbe C, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol 2014. [DOI] [PubMed] [Google Scholar]
- 30.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003; 158:915–920. [DOI] [PubMed] [Google Scholar]
- 31.Mullin JM, Valenzano MC, Whitby M, et al. Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther 2008; 28:1317–1325. [DOI] [PubMed] [Google Scholar]
- 32.Zedtwitz-Liebenstein K, Wenisch C, Patruta S, et al. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Criti Care Med 2002; 30:1118–1122. [DOI] [PubMed] [Google Scholar]
- 33.Fried M, Siegrist H, Frei R, et al. Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut 1994; 35:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


