Abstract
It is recommended to investigate the serology of hepatitis B virus (HBV) and vaccinate seronegative patients at the time of diagnosis in inflammatory bowel diseases (IBD). This study aimed to investigate the efficacy of HBV vaccine and factors affecting the response.
In this retrospective, observational study, HBV-seronegative IBD patients were administered 3 doses (at months 0, 1, and 6) recombinant 20 μg HbsAg. Patients’ demographics, IBD attributes, and treatment methods were investigated as the factors with potential impacts on vaccination outcomes.
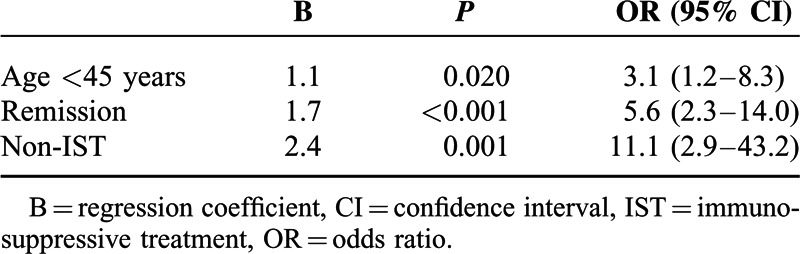
One hundred twenty-five patients with IBD were evaluated. The number of patients with Anti-HBs >10 IU/L was 71 (56.8%), and the number of patients with anti-HBs >100 IU/L was 50 (40%). Age, disease activity, Crohn disease subtype, and immunosuppressive treatment (IST) were found to have significant effects on immune response (P = 0.011, P < 0.001, P = 0.003, and P < 0.001, respectively). With multivariate analysis, age < 45 years (OR 3.1, 95% CI 1.2–8.3, P = 0.020), vaccination during remission (OR 5.6, 95% CI 2.3–14, P < 0.001), and non-IST (OR 11.1, 95% CI 2.9–43.2, P = 0.001) had favorable effects on the occurrence of adequate vaccine response.
The likelihood of achieving adequate immune response with standard HBV vaccination protocol in IBD is low. Selecting vaccination protocols with more potent immunogenicity is a better approach to achieve effective vaccine response in patients with multiple unfavorable factors.
INTRODUCTION
More than half of the patients with inflammatory bowel disease (IBD) have at least 1 risk factor for transmitting hepatitis B virus (HBV) (including endoscopic procedures, surgical interventions, and blood transfusions).1,2 Furthermore, it has been reported that HBV infection may be associated with poor prognosis in IBD patients, particularly in those receiving immunosuppressive therapy, and that this could even result in fulminant hepatic failure or death.3,4
Recently published guidelines on IBD and opportunistic infections recommended evaluating HBV serology for all IBD patients at the time of diagnosis and vaccinating seronegative patients.5,6 Relevant studies, however, demonstrate that very few IBD patients requiring HBV vaccination are actually appropriately vaccinated and have received adequate immunization.7,8 The leading reason for this is that, unfortunately, physicians dealing with IBD do not make conscious decisions on and are not informed well enough about HBV vaccination.9
The success rate in achieving protective antibody levels with standard HBV vaccination protocols in IBD is very low compared to the general population.10,11 Although several factors have been suggested to explain this, IBD itself and the increasingly popular use of immunosuppressive treatments (ISTs) are considered the major reasons.12–14
There are currently very few studies in the literature evaluating the factors that affect the outcomes of HBV vaccination in IBD. Furthermore, the algorithms to be followed in cases of failed immunizations with standard vaccination schemes have not been established. The present study aims to evaluate the factors that may affect the outcomes of standard HBV vaccinations and vaccine response in IBD.
METHODS
Patients
The study included IBD patients who received HBV vaccination and were followed up for ulcerative colitis (UC) and Crohn disease (CD) in the IBD unit of the Gastroenterology Department of Izmir Ataturk Training and Research Hospital.
Inclusion criteria: IBD patients older than 17 years who are seronegativite for antibodies against hepatitis B core protein (anti-HBc), antibodies against hepatitis surface antigen (anti-HBs), and hepatitis B surface antigen (HBsAg).
Exclusion criteria: Patients with HBV infection diagnosis or treatment history, patients vaccinated for HBV before being diagnosed with IBD, patients with chronic renal failure or hepatic cirrhosis, pregnant women, patients who did not complete the vaccination scheme, and postvaccination serologic controls were excluded.
Study Design
In this retrospective, observational study, HBV seronegative IBD patients were administered 3 doses (at months 0, 1, and 6) of recombinant 20 μg HBsAg intramuscularly (deltoid muscle region), and their anti-HBs levels 1 month after the last vaccine administration were determined.
Anti-HBs levels >10 IU/L were considered as adequate, and anti-HBs levels >100 IU/L were considered as an effective antibody response.
Patients’ demographics (age, gender), IBD attributes (disease type and duration, IBD location, type of CD, and disease activity), and treatment methods (mesalamine, steroid, thiopurine, and anti-tumor necrosis factor [TNF] agent) were evaluated as the factors with potential impact on the outcomes of HBV vaccination.
Montreal criteria were considered in determining IBD disease location and CD subtype.15 In determining IBD disease activity, CD activity index (remission <150, active ≥150) was used for CD and Mayo Clinic score (remission ≤ 2, active ≥ 3) for UC.16,17
Patients receiving prednisolone more than 20 mg/day for at least 2 weeks overlapping the vaccination period and/or patients receiving thiopurine and/or anti-TNF therapy during the vaccination were evaluated as the IST group.
Laboratory Tests
HBsAg, anti-HBc, and anti-HBs serology were determined using chemiluminescent enzyme immunoassay. Advia Centaur HBSII kits (Siemens Healthcare Diagnostics, Bayswater Victoria, Australia) were studied on an Advia Centaur XP immunoassay system (Siemens Healthcare Diagnostics, Dublin, Ireland) autoanalyzer.
Statistical Analysis
All statistical analyses were performed using SPSS 15.0 statistical package program. Continuous variables were expressed as mean and standard deviation or median and min–max values; categorical variables were expressed as frequencies and percentages. ROC analysis and Youden Index Method were performed to determine the optimal cut-off value of age. The chi-square test was used for categorical variables, and the Mann–Whitney test was used for continuous variables to identify the univariate impacts on immune response. Logistic regression analysis was performed to identify predictors of immune response. Risks were expressed as odds ratio (OR) with a 95% confidence interval (CI). P value of <0.05 was set for statistical significance.
Ethical Considerations
The Ethics Committee of Katip Çelebi University Faculty of Medicine, Izmir, Turkey approved this study.
RESULTS
Patient Characteristics
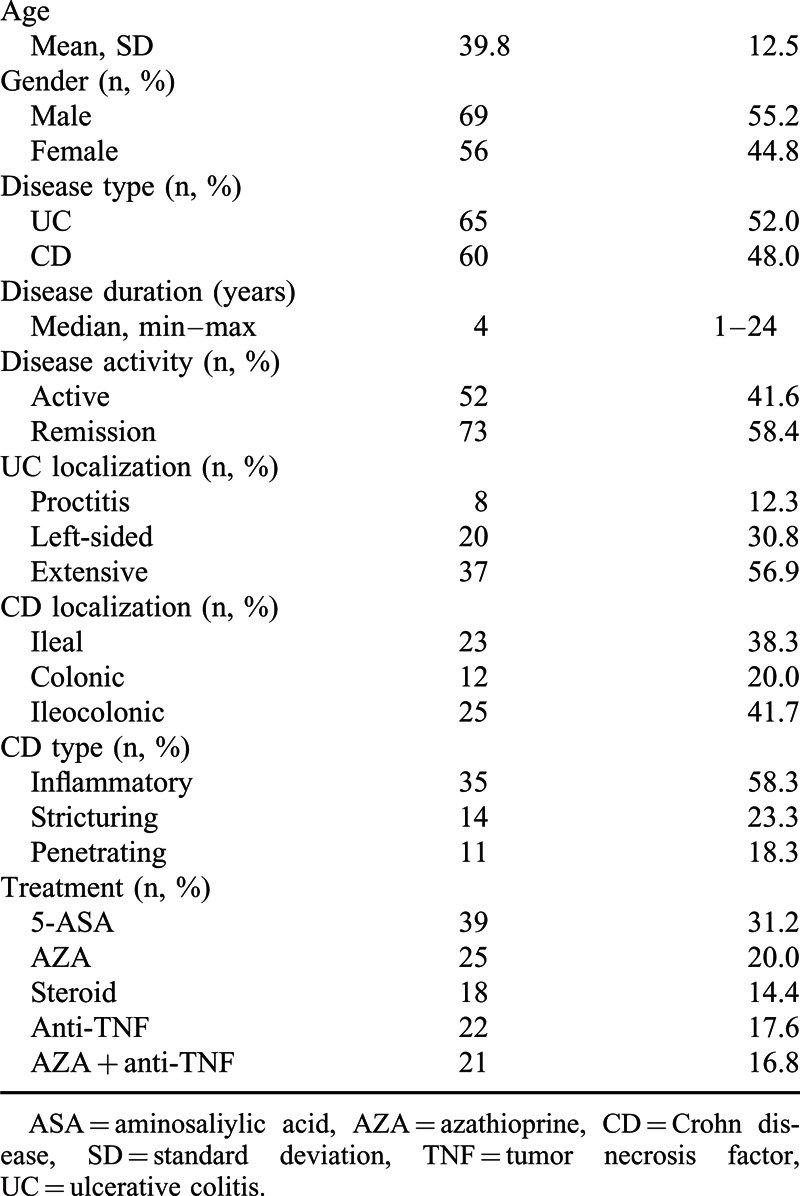
One hundred twenty-five patients with IBD were evaluated 65 (52%) with UC and 60 (48%) with CD. Their mean age was 39.8 ± 12.5 years, 69 (55.2%) of them were males, and median IBD disease duration was 4 years. Of the patients, 52 (41.6%) were in the active period and 73 (58.4%) were in the remission period. The number of patients who received immunosuppressive therapy (IST) was 86 (68.8%), and patients that did not receive immunosuppressive therapy (non-IST) were 39 (31.2%). Demographics of patients together with UC and CD localizations, CD subtypes, and treatment features are provided in Table 1.
TABLE 1.
Demographics and Clinical Variables of IBD Patients (n = 125)
Response Rates to HBV Vaccination
When HBV vaccine response was examined in the whole patient group, the number of patients with adequate immune response (anti-HBs >10 IU/L) was 71 (56.8%), and effective immune response (anti-HBs >100 IU/L) was 50 (40%). The median anti-HBs titer in patients with adequate immune response was 280 (12.8–1000) IU/L.
Factors Effecting the Response to HBV Vaccination
The univariate analysis evaluating the effect of the studied variables on HBV vaccine response demonstrated statistically significant effects of age, disease activity, CD subtype, and IST on immune response (P = 0.011, P < 0.001, P = 0.003, and P < 0.001, respectively). Gender, IBD type, disease duration, UC, and CD location had no statistically significant effect on HBV vaccine response (Tables 2 and 3).
TABLE 2.
Factors Associated with Response to Hepatitis B Vaccination (Univariate Analysis)
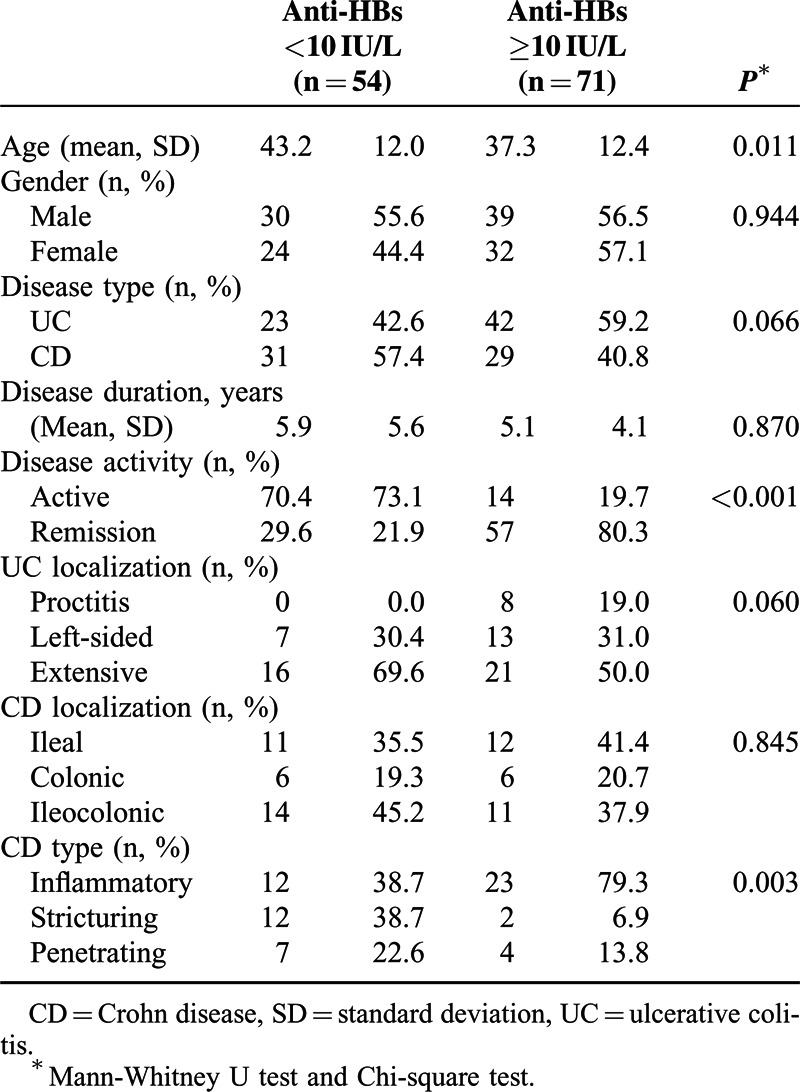
TABLE 3.
The Effect of Treatment Methods and Immunosuppressive Therapy on Immune Response
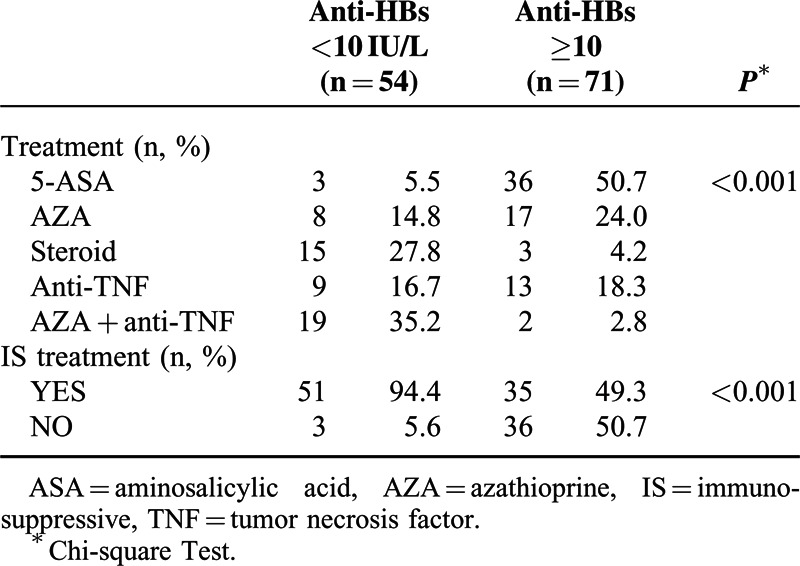
The analysis of 2 distinct anti-TNF agents (28 infliximab, 15 adalimumab) demonstrated no statistically significant difference in HBV vaccine response with different anti-TNFs (P > 0.05).
In UC, disease extension inversely effected the response but did not reach a statistical significance (P = 0.06).
ROC analysis revealed a positive correlation between age and immune response (AUC = 0.633, P = 0.011). Cut-off analysis for age ≥45 with Youden index showed 74.6% sensitivity, 48.1% specificity, and 63.2% accuracy.
With the post hoc analysis of CD subtypes affecting HBV vaccine response, immune response to vaccination in inflammatory type CD was significantly greater compared to stricturing CD (P = 0.001). Other paired comparisons among CD subtypes did not demonstrate any significant immune response differences (P > 0.05).
With the post hoc analysis of treatment subgroups, no significant differences in vaccine immune response were found between “Steroid versus azathioprine (AZA) + anti-TNF combination” and “AZA monotherapy versus anti-TNF monotherapy” (P > 0.05). With all other paired comparisons, treatment methods caused significant differences in immune response (P < 0.05).
The negative effect of IST on adequate immune response was also observed. Anti-HBs was >100 IU/L in 24.4% of the patients receiving IST and in 74.4% of the non-IST patients (P < 0.001) (Figure 1).
FIGURE 1.
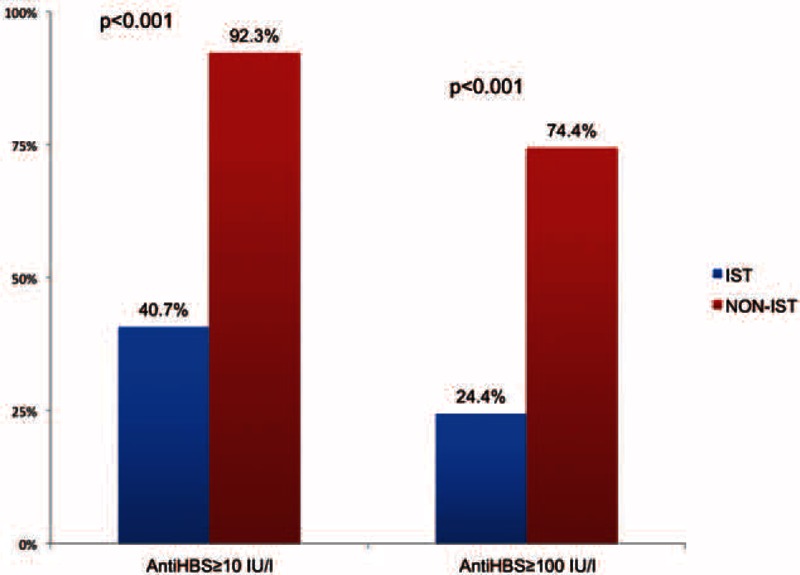
The effect of immunosuppressive therapy on adequate and effective immune response.
With the logistic regression analysis of the variables found to be effective in achieving immune response to HBV vaccine with the univariate analysis, age, disease activity, and IST were significantly involved in the model. In terms of achieving adequate immune response, age < 45 years (OR 3.1, 95% CI 1.2–8.3, P = 0.020), vaccination during remission (OR 5.6, 95% CI 2.3–14, P < 0.001), and non-IST IBD treatment (OR 11.1, 95% CI 2.9–43.2, P = 0.001) had favorable effects (Table 4). Although a significant difference was found in the immune response between CD subtypes with univariate analysis, CD subtypes were not studied as a part of the multivariate analysis considering that it would not represent an accurate effect for all patients.
TABLE 4.
Multivariate Analysis of Factors Affecting Immune Response (Logistic Regression)
DISCUSSION
We evaluated the factors that may affect the outcomes of standard HBV vaccinations and the immune response resulting from vaccination in IBD patients. The proportion of patients achieving adequate immune response (anti-HBs >10 IU/L) was 56.8% and effective immune response (anti-HBs >100 IU/L) was 40%. Immune response rates obtained with HBV vaccination in IBD are known to be lower compared with healthy populations, but different results were reported from a few studies on this subject (Altunöz 76%, Gisbert 41%, and Cossio-Gil 50%).14,18,19 These different results may be explained by the different characteristics of the patient groups across studies with regards to factors that are influential on immune response including immunosuppressive therapy (IST) and disease activity.
In this study, the effects of age, disease activity, CD subtype, and IST on immune response following HBV vaccination were significant. Gender, IBD type, disease duration, UC, and CD locations had no significant effect on vaccine response. It is possible to observe the effect of age on immune response when examining the outcomes of HBV vaccination in both in healthy populations and IBD groups.12,20 Population-based studies generally indicate <40 years age group as the age group with better vaccine responses, while in an IBD study, the average age was 41 years in the group with vaccine response.21,22 In our series, the average age of the group with vaccine response was 37.3 years, and the cut-off value was 45 years for the age limit that is a determinant for vaccine response. Data from studies on IBD and other immune-mediated inflammatory disorders suggest that IST had a general unfavorable effect on immune response obtained with vaccination.14,18,23
Initiating vaccination during the active period affected the immune response to vaccination. During the period in which the disease was active, vaccination may not result in adequate immune response due to increased inflammatory cytokines and disturbances in cellular and humoral immunity. In a study by Altunöz et al,14 vaccine response was significantly lower in IBD patients during the active phase.
The subgroup analysis of vaccine response according to treatment demonstrated that the poorest immune response was in the group treated with AZA and anti-TNF combination and in the group receiving steroid treatment in our study. There is not adequate data on the effect of combination treatments with AZA and anti-TNFs on immune response. On the other hand, earlier studies demonstrated negative effects of steroids on T lymphocyte functions and immunoglobulin production.24,25 Although it has been described that steroid use in more than 1 vaccination session was associated with poor response to HBV vaccination.12
Two diseases do not seem to differ with respect to immune response to HBV vaccine both in our study and other studies.14,19,21 IBD disease location had no significant effect on immune response to HBV in our study. There was no relationship between disease location and immune response in CD, but there was a significant difference between CD subtypes. The best response to HBV vaccine was in the inflammatory type CD group, and the worst was in stricturing type disease. There are not any studies reporting a significant difference in vaccine response between CD subtypes. This may be explained by the fact that patients with stricturing disease have a higher mean age and disease duration. On the other hand, transforming growth factor-β is known to have a significant involvement in the pathogenesis of stricturing CD.26,27 In addition, there are several studies demonstrating a better immunological response to HBV vaccination if the vaccine is combined with inhibition of TGF-β.28,29
All patients should be screened and vaccinated if seronegative for HBV during the initial diagnosis of IBD regardless of whether they have or not risk factors such as being a healthcare worker, using intravenous drugs, having HIV. European Crohn's and Colitis Organisation guidelines recommend administrations of accelerated double-dose at 0, 1, 2 months followed by revaccination (0, 1, and 2 months) at a double dose if there is not an adequate response in IBD. However, even the double-dose schedule that seems more effective than the standard one has little impact on seroconversion, thus the need for a more effective vaccination should be underlined.18 More efficient HBV vaccination alternatives such as intradermal (ID) route rather than intramuscular (IM) route in patients with renal disease are investigated and much higher efficacy of ID than IM in dialysis patients occurred despite a lower vaccine dose administrated by ID route.30 However, there are not related studies with IBD patients.
The serological response should be assessed 1 to 2 months after the last dose. Levels of anti-HBs at least 100 IU/L are advisable to achieve adequate seroprotection particularly if anti-TNF treatment is planned because most of the patients will lose seroprotection after vaccination (18% per patient-year).31 The response rate to HBV vaccine can potentially be significantly lower among patients who are being treated with IS so the vaccination should preferably administered at least 14 days prior to IS treatment.14 On the other hand, HBV vaccination can be effectively administered even after IS has been already initiated.32 The frequency of monitoring is not well decided, but controls for anti-HBs yearly or every 2 years are reasonable especially in countries with intermediate and high endemicity.5
Finally, we can emphasize that there are few limitations in our study. Obesity, smoking, and nutritional factors such as low albumin levels were not evaluated, which might be cofactors for unsuccessful vaccine response.
In conclusion, the success rate in achieving adequate immune response with standard HBV vaccination protocols in IBD is very low. Older age, disease flare, and IS treatment seem to play a pivotal role in vaccine response. Effective immune response (anti-HBs >100 IU/L) should be targeted in IBD by vaccines with more potent immunogenicity.
Footnotes
Abbreviations: AZA = azathioprine, CD = Crohn disease, CI = confidence interval, HBV = hepatitis B virus, IBD = inflammatory bowel diseases, ID = intradermal, IST = immunosuppressive treatment, OR = odds ratio, TNF = tumor necrosis factor, UC = Ulcerative colitis.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Hou JK, Velayos F, Terrault N, et al. Viral hepatitis and inflammatory bowel disease. Inflamm Bowel Dis 2010; 16:925–932. [DOI] [PubMed] [Google Scholar]
- 2.Tolentino YF, Fogaca HS, Zaltman C, et al. Hepatitis B virus prevalence and transmission risk factors in inflammatory bowel disease patients at Clementino Fraga Filho university hospital. World J Gastroenterol 2008; 14:3201–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gisbert JP, Chaparro M, Esteve M. Review article: prevention and management of hepatitis B and C infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2011; 33:619–633. [DOI] [PubMed] [Google Scholar]
- 4.Sansone S, Guarino M, Castiglione F, et al. Hepatitis B and C virus reactivation in immunosuppressed patients with inflammatory bowel disease. World J Gastroenterol 2014; 20:3516–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014; 8:443–468. [DOI] [PubMed] [Google Scholar]
- 6.Wasan SK, Baker SE, Skolnik PR, et al. A practical guide to vaccinating the inflammatory bowel disease patient. Am J Gastroenterol 2010; 105:1231–1238. [DOI] [PubMed] [Google Scholar]
- 7.Wilckens V, Kannengiesser K, Hoxhold K, et al. The immunization status of patients with IBD is alarmingly poor before the introduction of specific guidelines. Scand J Gastroenterol 2011; 46:855–861. [DOI] [PubMed] [Google Scholar]
- 8.Loras C, Saro C, Gonzalez-Huix F, et al. Prevalence and factors related to hepatitis B and C in inflammatory bowel disease patients in Spain: a nationwide, multicenter study. Am J Gastroenterol 2009; 104:57–63. [DOI] [PubMed] [Google Scholar]
- 9.Wasan SK, Coukos JA, Farraye FA. Vaccinating the inflammatory bowel disease patient: deficiencies in gastroenterologists knowledge. Inflamm Bowel Dis 2011; 17:2536–2540. [DOI] [PubMed] [Google Scholar]
- 10.Carrera E, Manzano R, Garrido E. Efficacy of the vaccination in inflammatory bowel disease. World J Gastroenterol 2013; 19:1349–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magro F, Abreu C. Immunisations in Crohn's disease: who? why? what? when? Best Pract Res Clin Gastroenterol 2014; 28:485–496. [DOI] [PubMed] [Google Scholar]
- 12.Vida Pérez L, Gómez Camacho F, García Sánchez V, et al. Adequate rate of response to hepatitis B virus vaccination in patients with inflammatory bowel disease. Med Clin (Barc) 2009; 132:331–335. [DOI] [PubMed] [Google Scholar]
- 13.Sempere L, Almenta I, Barrenengoa J, et al. Factors predicting response to hepatitis B vaccination in patients with inflammatory bowel disease. Vaccine 2013; 31:3065–3071. [DOI] [PubMed] [Google Scholar]
- 14.Altunöz ME, Senateş E, Yeşil A, et al. Patients with inflammatory bowel disease have a lower response rate to HBV vaccination compared to controls. Dig Dis Sci 2012; 57:1039–1044. [DOI] [PubMed] [Google Scholar]
- 15.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19:5A–36A. [DOI] [PubMed] [Google Scholar]
- 16.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976; 70:439–444. [PubMed] [Google Scholar]
- 17.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 18.Gisbert JP, Menchén L, García-Sánchez V, et al. Comparison of the effectiveness of two protocols for vaccination (standard and double dosage) against hepatitis B virus in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2012; 35:1379–1385. [DOI] [PubMed] [Google Scholar]
- 19.Cossio-Gil Y, Martínez-Gómez X, Campins-Martí M, et al. Immunogenicity of hepatitis B vaccine in patients with inflammatory bowel disease and the benefits of revaccination. J Gastroenterol Hepatol 2014. [DOI] [PubMed] [Google Scholar]
- 20.Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006; 55:1–33.quiz CE1-4. [PubMed] [Google Scholar]
- 21.Gisbert JP, Villagrasa JR, Rodríguez-Nogueiras A, et al. Efficacy of hepatitis B vaccination and revaccination and factors impacting on response in patients with inflammatory bowel disease. Am J Gastroenterol 2012; 107:1460–1466. [DOI] [PubMed] [Google Scholar]
- 22.WHO Publication. Hepatitis B vaccines: WHO position paper-recommendations. Vaccine 2010; 28:589–590. [DOI] [PubMed] [Google Scholar]
- 23.Rahier JF, Moutschen M, Van Gompel A, et al. Vaccinations in patients with immune-mediated inflammatory diseases. Rheumatology (Oxford) 2010; 49:1815–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashwell JD, Vacchio MS, Galon J. Do glucocorticoids participate in thymocyte development? Immunol Today 2000; 21:644–646. [DOI] [PubMed] [Google Scholar]
- 25.Fedor ME, Rubinstein A. Effects of long-term low-dose corticosteroid therapy on humoral immunity. Ann Allergy Asthma Immunol 2006; 97:113–116. [DOI] [PubMed] [Google Scholar]
- 26.Burke JP, Mulsow JJ, O’Keane C, et al. Fibrogenesis in Crohn's disease. Am J Gastroenterol 2007; 102:439–448. [DOI] [PubMed] [Google Scholar]
- 27.Feagins LA. Role of transforming growth factor-(in inflammatory bowel disease and colitis-associated colon cancer. Inflamm Bowel Dis 2010; 16:1963–1968. [DOI] [PubMed] [Google Scholar]
- 28.Zou Q, Zhong Y, Su H, et al. Enhancement of humoral and cellular responses to HBsAg DNA vaccination by immunization with praziquantel through inhibition TGF-beta/Smad2, 3 signaling. Vaccine 2010; 28:2032–2038. [DOI] [PubMed] [Google Scholar]
- 29.Du X, Chen X, Zhao B, et al. Astragalus polysaccharides enhance the humoral and cellular immune responses of hepatitis B surface antigen vaccination through inhibiting the expression of transforming growth factor (and the frequency of regulatory T cells. FEMS Immunol Med Microbiol 2011; 63:228–235. [DOI] [PubMed] [Google Scholar]
- 30.Fabrizi F, Dixit V, Magnini M, et al. Meta-analysis: intradermal vs. intramuscular vaccination against hepatitis B virus in patients with chronic kidney disease. Aliment Pharmacol Ther 2006; 24:497–506. [DOI] [PubMed] [Google Scholar]
- 31.Gisbert JP, Villagrasa JR, Rodríguez-Nogueiras A, et al. Kinetics of anti-hepatitis B surface antigen titers after hepatitis B vaccination in patients with inflammatory bowel disease. Inflamm Bowel Dis 2013; 19:554–558. [DOI] [PubMed] [Google Scholar]
- 32.Dotan I, Werner L, Vigodman S, et al. Normal response to vaccines in inflammatory bowel disease patients treated with thiopurines. Inflamm Bowel Dis 2012; 18:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]


