Abstract
The safety, tolerability, and efficacy data for antipsychotic drugs used in the acute phase of stroke are limited. The primary aim of this study was to examine the effectiveness and safety of typical and atypical antipsychotics on acute ischemic stroke mortality.
This observational study was conducted in a retrospective cohort of patients selected from the 2010–2011 National Health Research Institute database in Taiwan. Patients were tracked for 1 month from the time of their first hospitalization for acute ischemic stroke. A nested case–control analysis was used to estimate the odds ratio (OR) of 30-day mortality associated with antipsychotic drug, adjusted for age, gender, disease severity, and comorbidities.
The study cohort included 47,225 subjects with ischemic stroke, including 9445 mortality cases and 37,780 matched controls. After adjustment for the covariates, antipsychotics users before ischemic stroke are associated with a 73% decrease in the rate of mortality (OR 0.27; 95% CI 0.23–0.31). After ischemic stroke, the use of antipsychotics is associated with 87% decrease in the rate of mortality (OR 0.13; 95% CI 0.1–0.16). The users of conventional antipsychotics are associated with a 78% decrease in the rate of mortality (OR 0.22; 95% CI 0.18–0.26). The users of atypical antipsychotics are also associated with a 86% decrease in the rate of mortality (OR 0.14; 95% CI 0.12–0.17).
We found that 1-month mortality among acute stroke patients treated with antipsychotics is significantly lower. The benefit on lower mortality was found not only among ischemic stroke patients who had received antipsychotics previously but also among patients who start antipsychotics after their stroke.
INTRODUCTION
Diagnosis and treatment of acute stroke have advanced over the past 2 decades, but morbidity and mortality after stroke are still high. Patients who have had stroke are at significant risk for medical complications, neurological damage, and various psychiatric illnesses. Even if not always life-threatening, these complications can lead to prolonged hospitalization, delay in rehabilitation, poor functional outcomes, and increased costs of care.1–5 Acute neurological complications include brain edema, hemorrhagic transformation, recurrent stroke, seizure, and epilepsy.4,6 Psychiatric complications include depression, psychosis, confusion, and delirium.5,7,8 Many reviews have focused on medical complications and their management.2,3,8 There is a growing body of evidence to guide the management of the neurological complications1,4 but not the management of the psychiatric complications.
The incidence of delirium in the acute phase of stroke varies from 13% to 48%, depending on the study population and delirium definition.9–12 Given the longer hospitalization period and poorer prognosis, adequate treatment is important.5,7 Sedative and antipsychotic drugs are frequently used for treatment of delirium in acutely ill patients, although the evidence base for use of these medications to treat delirium is weak.13–16 The treatment recommendations for delirium after stroke are usually similar to those for delirium in patients with other diseases, because there have been no studies of delirium specifically in patients with acute stroke.4
Traditionally, haloperidol, a conventional antipsychotic, has been considered the treatment of choice.13 Because it may cause adverse extrapyramidal symptoms, haloperidol may be replaced by atypical antipsychotics such as risperidone, olanzapine, or quetiapine, which are as effective as haloperidol in controlling delirium.13,16–18 The safety, tolerability, and efficacy data for antipsychotic drugs used in the acute phase of stroke are limited. The primary aim of this study was to examine the effectiveness and effect of conventional and atypical antipsychotics on acute ischemic stroke mortality.
METHODS
Data Source
In 1995, Taiwan implemented a National Health Insurance (NHI) program that requires mandatory enrollment in the government-run, universal, single-payer insurance system and provides comprehensive benefits coverage. Currently, up to 99% of the 23 million residents of Taiwan receive medical care through the NHI program. Over 97% of the hospitals and clinics in Taiwan are contracted to provide health care services,19 which are reimbursed by the NHI Bureau, and all data related to these services are collected and input into the National Health Research Institute Database (NHIRD) by the National Health Research Institutes to provide a comprehensive record of medical care. The data consist of ambulatory care records, inpatient care records, and the registration files of the insured. The dataset includes all claims data from Taiwan's NHI program, which was implemented to pay for health care of all Taiwanese citizens.
This study was initiated after approval by the Institutional Review Board of the Buddhist Dalin Tzu Chi General Hospital, Chiayi, Taiwan. Since all identifying personal information was stripped from the secondary files before analysis, the review board waived the requirement for written informed consent from the patients involved.
Study Design
The source population consisted of all patients with ischemic stroke between 2010 and 2011 (Figure 1). Inpatients ≥18 years of age diagnosed with stroke between 2010 and 2011 and with a discharge diagnosis identified by International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) codes 433–437 were recruited for the study. All patients with ischemic stroke in this cohort were followed from cohort entry until event (mortality) or censored developed. In view of the time-varying nature of antipsychotic drugs, a nested case–control analysis within the cohort was performed.
FIGURE 1.
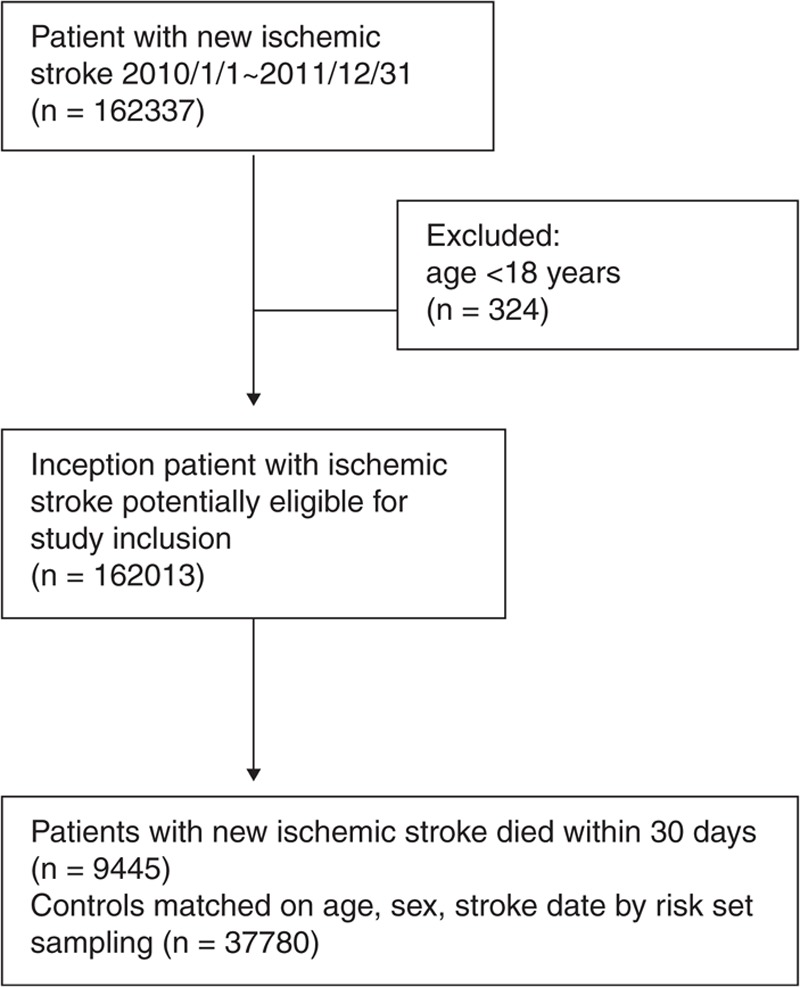
Flowchart of cohort formation.
Cases and Controls
Patients with ischemic stroke who died within 30 days after stroke were defined as cases. For each case, 4 controls who did not die within 30 days after stroke matched on the case's age (±3 years), gender, and stroke date (±3 days) were randomly selected.
Exposure to Antipsychotics
All prescriptions of antipsychotics, conventional, atypical, or combined use, were identified 30-day before and after the index date. Patients were grouped into the following cohorts on the basis of exposure to antipsychotic medications. The antipsychotics used in this study included typical antipsychotics: chlorpromazine, thioridazine, levomepromazine, loxapine, perphenazine, trifluoperazine, haloperidol, fluphenazine, droperidol, zuclopenthixol, and prochlorperazine; and atypical antipsychotics: amisulpride, aripiprazole, clozapine, olanzapine, paliperidone, quetiapine, risperidone, sulpiride, ziprasidone, and zotepine. Patients who took typical antipsychotic were designated conventional antipsychotic users. Patients who took atypical antipsychotics were designated atypical antipsychotic users. Patients who took conventional and atypical antipsychotics were designated combined users; the remaining patients were designated antipsychotics nonusers. Within each antipsychotics group, subjects were further divided into 3 smaller groups based on total amount of pills use (ie, low, medium, and high dose).
Comorbidities and Related Variables
We assessed patients’ comorbidities, including history of hypertension, diabetes, hyperlipidemia, atrial fibrillation, coronary artery disease, depression, dementia, schizophrenia, bipolar disorder, and post-stroke complications including pneumonia, urinary tract infection, deep vein thrombosis, acute coronary syndrome, upper gastrointestinal bleeding, seizure, and delirium. Because the NHIRD lacks information on the severity of stroke, we used intensive care unit (ICU) admission as a proxy for severity.
Statistical Analysis
SPSS (version 15, SPSS Inc, Chicago, IL) was used for data analysis. Crude and adjusted odds ratios (ORs) of mortality cases associated with antipsychotics use with 95% confidence intervals (CIs) were estimated by multivariate logistic regression to account for the matching of cases and controls. The ORs were adjusted for patients’ characteristics (age, gender, and comorbidities), admission to an ICU, length of stay and current use, dose, and past use of antipsychotics. Comorbidities included hypertension, diabetes, hyperlipidemia, atrial fibrillation, coronary artery disease, depression, dementia, schizophrenia, bipolar disorder, and post-stroke complications including pneumonia, urinary tract infection, deep vein thrombosis, acute coronary syndrome, upper gastrointestinal bleeding, seizure, and delirium. A 2-sided P value (P < 0.05) was used to determine statistical significance.
RESULTS
The study cohort included 47,225 subjects with ischemic stroke. Table 1 summarizes the characteristics of 9445 cases of mortality and 37,780 matched controls. The mortality cases and their matched controls were around 77 years of age and 60.8% were men. The mortality cases had more admitted to ICU and longer length of stay. The mortality cases had higher incidence of complications. Table 2 compares these characteristics among the mortality cases according to the use of antipsychotics, showing that antipsychotics users were more likely to have dementia and depression, but similar with respect to other comorbidities. Table 3 compares these characteristics among the matched controls according to the use of antipsychotics.
TABLE 1.
Characteristics of Mortality Cases and Their Matched Controls Selected From a Cohort of Patients With Ischemic Stroke
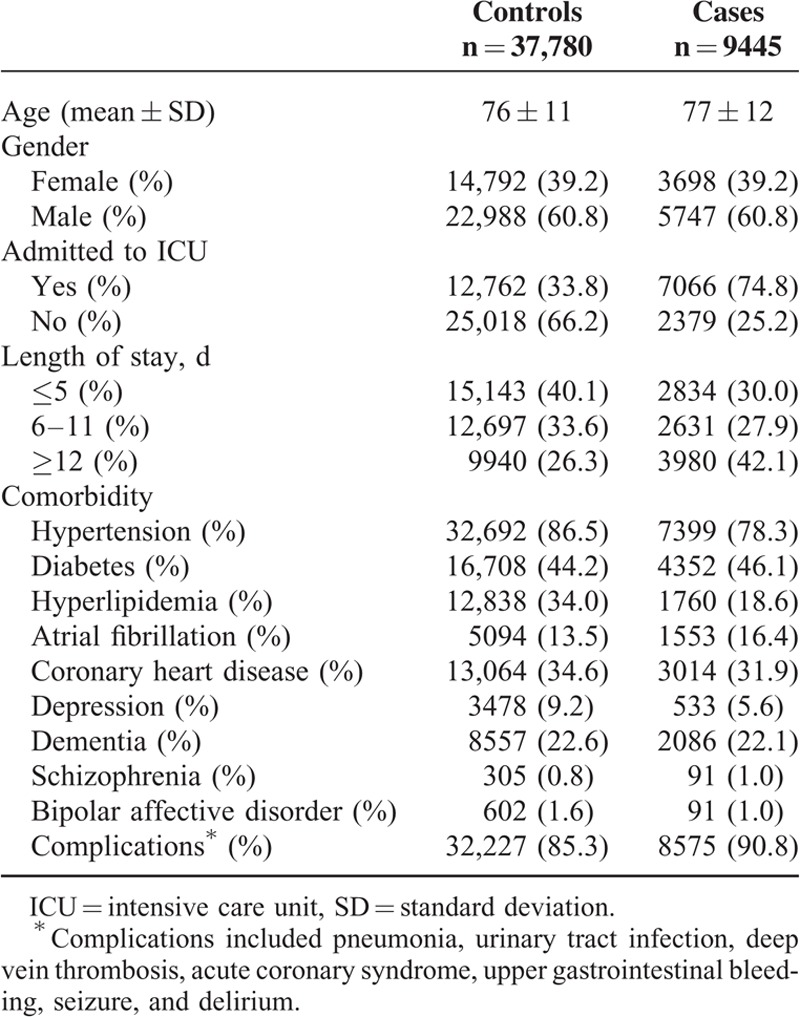
TABLE 2.
Characteristics of Mortality Cases Selected From Cohort of Patients With Ischemic Stroke, According to Current Use of Antipsychotic Medications After Stroke (n = 9445)
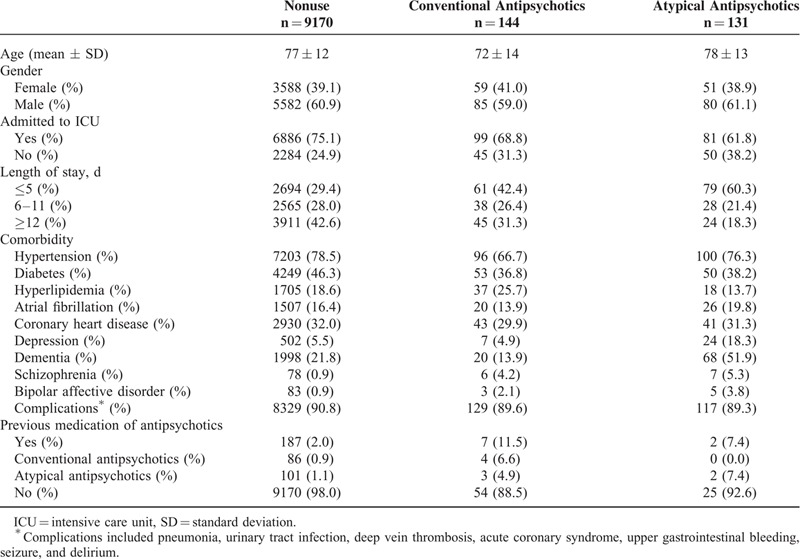
TABLE 3.
Characteristics of Matched Controls Selected From Cohort of Patients With Ischemic Stroke, According to Current Use of Antipsychotic Medications After Stroke (n = 37,780)
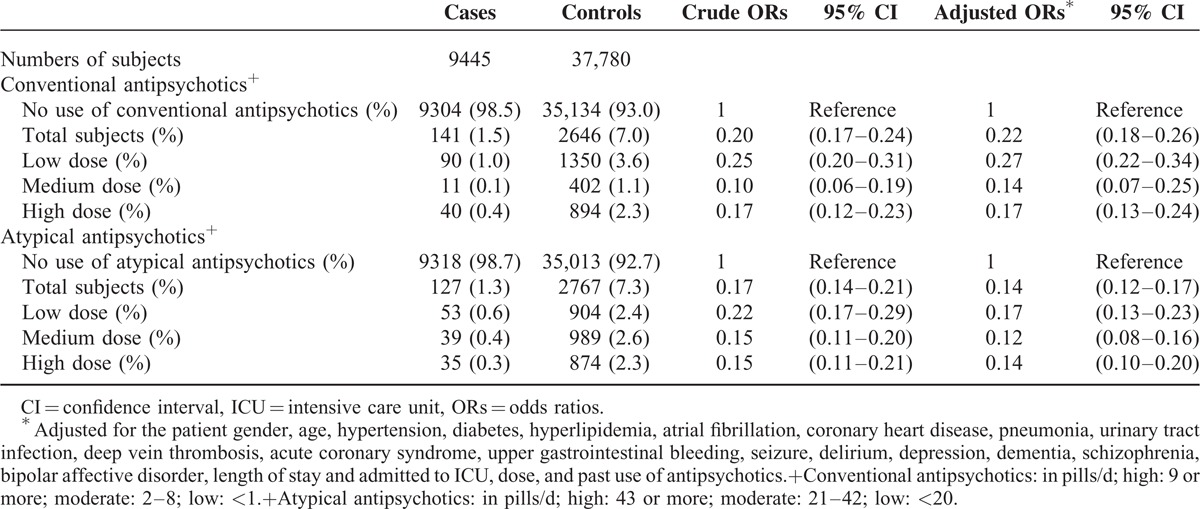
Table 4 shows that, after adjustment for differences in the covariates, antipsychotics users before ischemic stroke are associated with a 73% decrease in the rate of mortality (OR 0.27; 95% CI 0.23–0.31). After ischemic stroke, the use of antipsychotics is associated with 87% decrease in the rate of mortality (OR 0.13; 95% CI 0.1–0.16). The cumulative dose did not affect the benefit of antipsychotics use on ischemic stroke. The risk for mortality among users of conventional and atypical antipsychotics is shown in Table 5. The users of conventional antipsychotics are associated with a 78% decrease in the rate of mortality (OR 0.22; 95% CI 0.18–0.26). The users of atypical antipsychotics are also associated with a 86% decrease in the rate of mortality (OR 0.14; 95% CI 0.12–0.17).
TABLE 4.
Crude and Adjusted Rate Ratios of Mortality Associated With Current Use, Dose, and Past Use of Antipsychotics
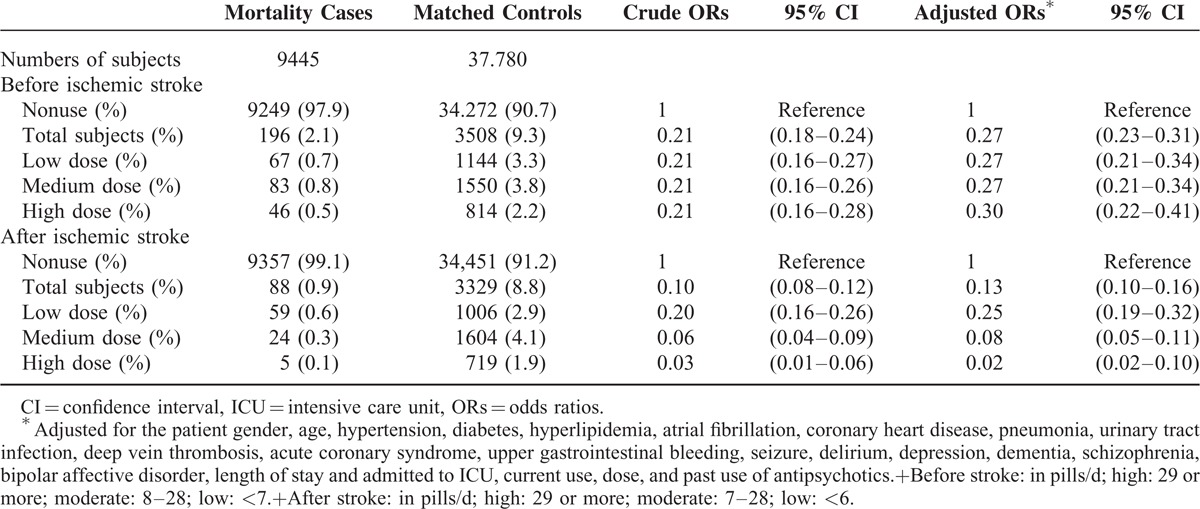
TABLE 5.
Crude and Adjusted Rate Ratios of Mortality Associated With Conventional Antipsychotics and Atypical Antipsychotics
DISCUSSION
Using a large population-based cohort of 47,225 subjects with ischemic stroke, we found that both conventional and atypical antipsychotic drug users had lower 1-month mortality. The benefit on lower mortality was found not only among ischemic stroke patients who had received antipsychotics previously but also among patients who start antipsychotics after their stroke.
Atypical antipsychotics are used in the United States for the treatment of schizophrenia.20,21 Because of their superior efficacy, safety, and tolerability relative to conventional agents, atypical antipsychotics have become the mainstay of treatment in various neurological and psychiatric disorders.22 However, concerns have been raised that atypical antipsychotics may increase the risk of adverse events, including death and stroke.23–26 In contrast with these reports, studies from large observational administrative database studies suggest that atypical antipsychotics compared with conventional antipsychotics do not increase the risk of stroke.27–29 To date, the association between the use of atypical antipsychotics and the risk of stroke remains controversial. A recent investigation of the 90-day mortality rate after recent stroke among schizophrenia patients by Kang et al30 found lower mortality among patients who received antipsychotic medication before the stroke. Another study by Prior et al31 found that preadmission use of antipsychotics was associated with a higher risk of severe stroke, a longer duration of hospital stay, and a higher post-stroke mortality. The findings of this study suggest that, among past antipsychotics users, antipsychotics do not increase the risk of mortality for ischemic stroke. Racial and ethnic disparities in response to antipsychotics should be taken into consideration.
Many deleterious mechanisms of ischemic stroke have been proposed. In the ischemic brain, massive release of dopamine can amplify the neuronal damage caused by excitotoxicity and energy deprivation.32,33 Serotonin is also shown to modulate the postsynaptic effects of glutamate and lead to reduction of blood flow during cerebral ischemia.34 Atypical antipsychotics have been shown to have neuroprotective properties against cerebral ischemia in animal studies.35,36 The antidopaminergic and antiserotonergic effects of atypical antipsychotics could theoretically be beneficial by augmenting blood flow during ischemic stroke.34 Our study found that patients with acute ischemic stroke who received atypical antipsychotics had lower mortality, consistent with findings in animal models. Therefore, we propose that the observed lower mortality may be due to the neuroprotective effect of atypical antipsychotics.
In this study, we also showed that it is safe to treat patients in the acute phase after ischemic stroke with conventional antipsychotics, such as haloperidol. Haloperidol may interfere with recovery after stroke and therefore should be avoided if possible.37 In the practice guideline by the American Psychiatric Association for the treatment of delirium, haloperidol is the first choice,38 despite its anticholinergic side effects, a risk factor for delirium.39
Our study had several NHIRD database-related limitations. First, the diagnoses of stroke and any other comorbid conditions were dependent on ICD codes from the NHIRD database. The NHI Bureau of Taiwan, however, has made every effort to verify the accuracy of diagnosis by conducting random chart reviews and patient interviews. The accuracy of the NHIRD in recording ischemic stroke diagnoses is up to 95%.40 Second, an additional limitation of the NHIRD database was its lack of information on stroke severity. We used ICU admission and length of stay as proxies for severity. Third, the limitations of this study are those of observational studies, with the potential for uncontrolled confounding by prescription indication being the most troublesome. It is not possible to completely exclude prescription indication bias. Among both cases and controls, antipsychotics users were more likely to have dementia and depression. Nonetheless, given the magnitude and statistical significance of the observed effects in this study, these limitations were unlikely to have affected our results. In fact, the strengths of our study are that it was a nationwide population-based study, with near complete follow-up information on the whole study population (99%), and that the dataset was routinely monitored for diagnostic accuracy by the NHI Bureau of Taiwan.
CONCLUSION
We found that 1-month mortality among acute stroke patients treated with antipsychotics is significantly lower. The benefit on lower mortality was found not only among ischemic stroke patients who had received antipsychotics previously but also among patients who start antipsychotics after their stroke. Well-designed studies focusing on antipsychotics use in acute stroke patients are needed to define optimal care.
Acknowledgments
This study is based in part on data from the NHIRD provided by the Bureau of National Health Insurance and Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
Footnotes
Abbreviations: ICD-9 = International Classification of Diseases-Ninth Revision, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Johnston KC, Li JY, Lyden PD, et al. Medical and neurological complications of ischemic stroke experience from the RANTTAS trial. Stroke 1998; 29:447–453. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol 2010; 9:105–118. [DOI] [PubMed] [Google Scholar]
- 3.Tong X, Kuklina EV, Gillespie C, et al. Medical complications among hospitalizations for ischemic stroke in the United States from 1998 to 2007. Stroke 2010; 41:980–986. [DOI] [PubMed] [Google Scholar]
- 4.Balami JS, Chen R-L, Grunwald IQ, et al. Neurological complications of acute ischaemic stroke. Lancet Neurol 2011; 10:357–371. [DOI] [PubMed] [Google Scholar]
- 5.McManus J, Pathansali R, Stewart R, et al. Delirium post-stroke. Age Ageing 2007; 36:613–618. [DOI] [PubMed] [Google Scholar]
- 6.Weimar C, Roth MP, Zillessen G, et al. Complications following acute ischemic stroke. Eur Neurol 2002; 48:133–140. [DOI] [PubMed] [Google Scholar]
- 7.Robinson RG. Poststroke depression: prevalence, diagnosis, treatment, and disease progression. Biol Psychiatry 2003; 54:376–387. [DOI] [PubMed] [Google Scholar]
- 8.Langhorne P, Stott D, Robertson L, et al. Medical complications after stroke a multicenter study. Stroke 2000; 31:1223–1229. [DOI] [PubMed] [Google Scholar]
- 9.Gustafson Y, Olsson T, Eriksson S, et al. Acute confusional states (delirium) in stroke patients. Cerebrovas Dis 1991; 1:257–264. [Google Scholar]
- 10.Caeiro L, Ferro JM, Albuquerque R, et al. Delirium in the first days of acute stroke. J Neurol 2004; 251:171–178. [DOI] [PubMed] [Google Scholar]
- 11.Sheng AZ, Shen Q, Cordato D, et al. Delirium within three days of stroke in a cohort of elderly patients. J Am Geriatr Soc 2006; 54:1192–1198. [DOI] [PubMed] [Google Scholar]
- 12.Henon H, Lebert F, Durieu I, et al. Confusional state in stroke relation to preexisting dementia, patient characteristics, and outcome. Stroke 1999; 30:773–779. [DOI] [PubMed] [Google Scholar]
- 13.Lonergan E, Britton A, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev 2007; CD005594. [DOI] [PubMed] [Google Scholar]
- 14.Girard TD, Pandharipande PP, Carson SS, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the mind randomized, placebo-controlled trial. Crit Care Med 2010; 38:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seitz DP, Gill SS, Zyl LTv. Antipsychotics in the treatment of delirium: a systematic review. J Clin Psychiatry 2007; 68:11–21. [DOI] [PubMed] [Google Scholar]
- 16.Rea RS, Battistone S, Fong JJ, et al. Atypical antipsychotics versus haloperidol for treatment of delirium in acutely ill patients. Pharmacotherapy 2007; 27:588–594. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics 2002; 43:171–174. [DOI] [PubMed] [Google Scholar]
- 18.Boettger S, Breitbart W. Atypical antipsychotics in the management of delirium: a review of the empirical literature. Palliat Support Care 2005; 3:227–237. [DOI] [PubMed] [Google Scholar]
- 19.Chiang T-L. Taiwan's 1995 health care reform. Health Policy 1997; 39:225–239. [DOI] [PubMed] [Google Scholar]
- 20.Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ 2000; 321:1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005; 353:2335–2341. [DOI] [PubMed] [Google Scholar]
- 22.Maixner SM, Mellow AM, Tandon R. The efficacy, safety, and tolerability of antipsychotics in the elderly. J Clin Psychiatry 1998; 60:29–41. [PubMed] [Google Scholar]
- 23.Douglas IJ, Smeeth L. Exposure to antipsychotics and risk of stroke: self controlled case series study. BMJ 2008; 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacchetti E, Trifirò G, Caputi A, et al. Risk of stroke with typical and atypical anti-psychotics: a retrospective cohort study including unexposed subjects. J Psychopharmacol 2008; 22:39–46. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann N, Lanctôt KL. Do atypical antipsychotics cause stroke? CNS Drugs 2005; 19:91–103. [DOI] [PubMed] [Google Scholar]
- 26.Wooltorton E. Risperidone (risperdal): Increased rate of cerebrovascular events in dementia trials. Can Med Assoc J 2002; 167:1269–1270. [PMC free article] [PubMed] [Google Scholar]
- 27.Herrmann N, Mamdani M, Lanctôt KL. Atypical antipsychotics and risk of cerebrovascular accidents. Am J Psychiatry 2004; 161:1113–1115. [DOI] [PubMed] [Google Scholar]
- 28.Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry 2005; 66:1090–1096. [DOI] [PubMed] [Google Scholar]
- 29.Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005; 330:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang J-H, Xirasagar S, Lin H-C. Lower mortality among stroke patients with schizophrenia: a nationwide population-based study. Psychosom Med 2011; 73:106–111. [DOI] [PubMed] [Google Scholar]
- 31.Prior A, Laursen TM, Larsen KK, et al. Post-stroke mortality, stroke severity, and preadmission antipsychotic medicine use—a population-based cohort study. PloS One 2014; 9:e84103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan FH, Saha M, Chakrabarti S. Dopamine induced protein damage in mitochondrial-synaptosomal fraction of rat brain. Brain Res 2001; 895:245–249. [DOI] [PubMed] [Google Scholar]
- 33.Surmeier DJ, Bargas J, Hemmings HC, Jr, et al. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron 1995; 14:385–397. [DOI] [PubMed] [Google Scholar]
- 34.Globus MYT, Busto R, Dietrich WD, et al. Effect of ischemia on the in vivo release of striatal dopamine, glutamate, and gamma-aminobutyric acid studied by intracerebral microdialysis. J Neurochem 1988; 51:1455–1464. [DOI] [PubMed] [Google Scholar]
- 35.Yulug B, Yildiz A, Güzel O, et al. Risperidone attenuates brain damage after focal cerebral ischemia in vivo. Brain Res Bull 2006; 69:656–659. [DOI] [PubMed] [Google Scholar]
- 36.Yulug B, Yildiz A, Hüdaoglu O, et al. Olanzapine attenuates brain damage after focal cerebral ischemia in vivo. Brain Res Bull 2006; 71:296–300. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein LB. Rehabilitation and recovery after stroke. Curr Treatment Options Neurol 2000; 2:319–328. [DOI] [PubMed] [Google Scholar]
- 38.Practice guideline for the treatment of patients with delirium. American psychiatric association. Am J Psychiatry 1999; 156:1–20. [PubMed] [Google Scholar]
- 39.Han L, McCusker J, Cole M, et al. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med 2001; 161:1099–1105. [DOI] [PubMed] [Google Scholar]
- 40.Cheng CL, Kao YHY, Lin SJ, et al. Validation of the National Health Insurance Research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011; 20:236–242. [DOI] [PubMed] [Google Scholar]


