Abstract
As robotic surgery was developed with ergonomic designs, there are expectations that the technical advantages of robotic surgery can shorten the learning curve. However, there is no comparative study, so far, to evaluate the learning curve between robotic and laparoscopic rectal cancer surgeries. Therefore, the aim of this study is to compare the learning curve of robotic low anterior resection (LAR) with laparoscopic LAR for rectal cancer.
Patients who underwent robotic or laparoscopic LAR by a single surgeon were compared retrospectively (robot n = 89 vs laparoscopy n = 89). Cumulative sum (CUSUM) was used to evaluate the learning curve. The patients were divided into phase 1 (initial learning curve period) and phase 2 (post-learning curve period). The perioperative clinicopathologic characteristics were compared by phases and surgical procedures.
According to CUSUM, the learning curve of robotic LAR was the 44th case and laparoscopic LAR was the 41st case. The learning phases were divided as follows: phase 1 (cases 1–41) versus phase 2 (cases 42–89) in the laparoscopic group, and phase 1 (cases 1–44) versus phase 2 (cases 45–89) in the robotic group. Comparison between phase 1 and phase 2 in each type of surgery showed no significant difference for the perioperative outcomes. Comparison between robotic and laparoscopic surgeries in each phase showed similar perioperative results. Pathologic outcomes were not significantly different in both procedures and phases.
The learning curve of robotic LAR for rectal cancer was similar to laparoscopic LAR, and the clinicopathologic outcomes were similar in both the procedures.
INTRODUCTION
Laparoscopic colorectal surgery has been an alternative method of open surgery with comparable clinical and oncologic outcomes.1–4 However, in terms of surgical training, it demands a more challenging process to acquire surgical competence during the learning curve. There are studies that the learning curve of laparoscopic surgery was considered as steeper than open surgery because it required to be performed in a limited pneumoperitoneum surgical space by using long nonergonomic surgical instruments.5,6
Especially, a higher level of surgical technique is necessary in laparoscopic rectal cancer surgery because of the narrow pelvic cavity that is an unfavorable circumstance to use long laparoscopic instruments. Jamali et al7 demonstrated that laparoscopic low anterior resection (LAR) required the highest level of technical challenge among laparoscopic colorectal surgeries. In this aspect, robotic surgery has advantages with the ergonomic designs of surgical instruments such as 3-dimensional view, and the 7-degrees of freedom. The elaborate manipulation of the robotic arms may aid in a precise dissection in the pelvic cavity and thus help the surgeon to perform rectal cancer surgery more easily. If these theoretical potential advantages of robotic surgery are considered, there are expectations that the advanced technologies of robotic surgery could shorten the learning curve.
So far, previous reports suggested that the learning curve of robotic surgery might be shorter than laparoscopic surgery.8–10 The learning curve of laparoscopic colorectal surgery has been from 30 to 70 cases.8,11–15 On the contrary, the learning curve of robotic surgery suggested a range of 20 to 40 cases.9,16–18 However, these studies evaluated the learning curve by a limited number of patients. Moreover, most reports of the learning curve for robotic surgery were 1-arm studies, which analyzed short-term clinical outcomes of only robotic surgery during the learning period.9,18 This means that it is uncertain whether the learning curve of robotic surgery is shorter than laparoscopic surgery in rectal cancer. Thus, this study aims to evaluate whether the learning curve of robotic rectal cancer surgery is shorter than laparoscopic rectal cancer surgery by comparing the learning curve between robotic and laparoscopic surgeries. Moreover, the perioperative clinical and pathologic outcomes were compared between both the procedures by each initial learning curve period and the post-learning curve period.
METHODS
Study Population and Patient Selection
A total of 288 patients, at the Severance Hospital, Yonsei University College of Medicine, Seoul, South Korea, from April 2006 to August 2011, were initially enrolled for this study. These patients underwent robotic or laparoscopic LAR for rectal cancer by a single surgeon (SH Baik), consecutively. The surgeon received 2 years of colorectal fellowship training (from 2004 to 2005) after 4 years of general surgery resident training. The surgeon received laparoscopic training during the colorectal fellowship period. Training for the laparoscopic surgical technique with the basic principle of colorectal cancer surgery was done by participating as an assistant surgeon and also by participating twice in an animal laboratory.
The surgeon assisted 86 cases of laparoscopic colorectal surgery during the colorectal fellowship training period prior to performing the procedures: 43 cases of laparoscopic LAR, 29 cases of laparoscopic anterior resection, 11 cases of laparoscopic right hemicolectomy, 2 cases of laparoscopic abdominoperineal resection, and 1 case of laparoscopic total proctocolectomy. On the contrary, robotic surgery was not learned during the training period because of the later adoption of robotic surgery than laparoscopic surgery. Before performing the first robotic rectal cancer surgery, the surgeon observed robotic-assisted prostatectomy that was performed by KH Rha (Department of Urology, Severance Hospital, Korea) and went to Italy to observe robotic colorectal surgeries performed by A D’Annibale (Divisione di Chirurgia Generale, Ospedale di Camposampiero, Padova, Italy), and had opportunities of dry laboratory training using the robotic surgical system. The first laparoscopic and robotic rectal cancer surgeries were performed in April and June 2006, respectively. These cases were supervised by experienced surgeons (NK Kim, KH Rha, and WJ Lee) in our institution. They proctored and gave advice on surgical tips for manipulation of the instruments and technical tips for robotic and laparoscopic surgeries during the initial learning curve period. Ten open and 6 laparoscopic rectal cancer surgeries were performed prior to the first robotic rectal cancer surgery.
In this study, there were 93 laparoscopic LAR patients and 145 robotic LAR patients using the da Vinci surgical system (Intuitive Surgical, Sunnyvale, CA). The patients who underwent synchronous operations (laparoscopy n = 4 and robot n = 14) and pelvic lymph node dissection (robot n = 1) were excluded. Therefore, the study population constituted of 89 laparoscopic and 130 robotic LAR patients. However, because this study proposed to compare the same number of consecutive patients between laparoscopic and robotic surgeries, 89 patients in the laparoscopic group and 89 patients in the robotic group were evaluated (Figure 1). During the study period, 264 cases of laparoscopic colorectal cancer surgery, except for laparoscopic LAR, were performed: 103 cases of laparoscopic anterior resection, 129 cases of laparoscopic right hemicolectomy, 28 cases of laparoscopic left hemicolectomy, 1 case of laparoscopic transverse colectomy, and 3 cases of laparoscopic subtotal colectomy. However, all robotic surgeries were performed for LAR except for only 1 case of robotic right hemicolectomy within the study period.
FIGURE 1.
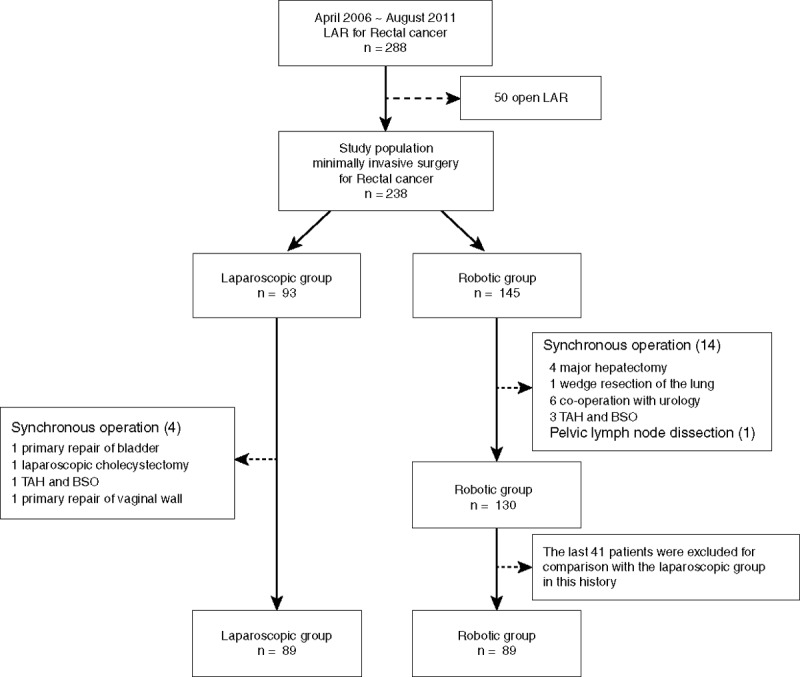
Flow chart of patient selection. TAH-BSO = total abdominal hysterectomy with bilateral salpingo-oopherectomy.
The patients were explained about the detailed characteristics of the robotic and the laparoscopic procedures before surgery. The final choice between robotic and laparoscopic surgeries was decided by the patient’s preference with an informed consent.
This study was approved by the Institutional Review Board of Severance Hospital (IRB No. 4-2013-0467).
Data Collection and Evaluation Parameters
Perioperative clinical outcomes were retrospectively evaluated using the Yonsei Colorectal Cancer Database and electronic medical charts. The database was established by the prospective data collection.
The total operation time was defined as the time from the first skin incision to the final closure of the abdominal wall. The surgeon console time and docking time were evaluated to analyze more detailed processes of robotic surgery. The surgeon console time was defined as the actual time that a surgeon purely performed at the robotic console. Docking time was the time from moving robotic instruments in the surgical field to setting robotic arms into the port sites.
The American Society of Anesthesiologists (ASA) Classification was used for categorizing the preoperative status of the patients. The location of the tumor was divided by the distance from the anal verge: low (<5 cm), mid (5.1–10 cm), and upper rectum (10.1–15 cm). Conversion was defined as the unintended extension of a laparotomy (>4 cm) to complete the operation. Postoperative complications that occurred within 30 postoperative days were stratified by the Clavien–Dindo classification.19 Major postoperative complications were defined as a complication with a grade of III or IV.
Pathologic outcomes were evaluated by the tumor nodes metastasis (TNM) stage.20 Involvement of the circumferential resection margin (CRM) was defined when a primary tumor was present within 1 mm from the mesorectal fascia.21 Local recurrence was defined as the relapse of a tumor from the primary site by radiological or histological confirmation.
The learning curve period was evaluated using statistical methods. All patients of each of the robotic and the laparoscopic group were divided into 2 phases: phase 1 of the initial learning curve period and phase 2 of the post-learning curve period. Clinicopathologic outcomes were compared between each robotic LAR and laparoscopic LAR group, and subgroup comparisons were performed according to the groups (robotic LAR vs laparoscopic LAR) and learning curve phases (phase 1 vs phase 2).
Surgical Techniques
Robotic LAR was performed using the hybrid technique in all cases of this study. The hybrid technique is composed of 2 steps and is a combination of laparoscopic and robotic procedures. The ligation of inferior mesenteric vessels and the mobilization of both left colon and splenic flexure were performed by conventional laparoscopic techniques. Then, rectal dissection throughout the mesorectal plane was performed by the robotic system in accordance with total mesorectal excision (TME) or tumor-specific mesorectal excision principles.22,23 Intracorporeal anastomosis with the double stapling method was applied to all cases. The specimen was extracted through the minilaparotomy (3–4 cm incision) by extension of the left lower da Vinci trocar site. Further details of the surgical techniques are explained in our previous reports.22,23
Laparoscopic LAR was performed in the same manner as described above. However, all procedures were carried out using laparoscopic instruments.
Statistical Analyses
Continuous variables were analyzed by the independent two-sample t test. Categorical variables were performed by the χ2 test or Fisher exact test. The SPSS program (Statistical Product and Service Solution 20 for Windows; SPSS Inc, Chicago, IL) was used for statistical analyses. The moving average method and cumulative sum (CUSUM) were used to evaluate the learning curve in terms of operation times. P values of <0.05 were considered statistically significant.
In this study, we analyzed the operation times by using the moving average method. This method was created by an average of subsets, which were modified by adding new data to the subsets and then shifting forward all data sets. It was useful to apprehend the flow of overall data and detect cumulative changes from average values. After the patients were arranged chronologically, the total operation time, the surgeon console time, and the docking time were evaluated by this method. In this study, a moving average order of 20 was used.
CUSUM is a statistical technique, which is useful to quantitatively assess the learning curve. It is a statistical method of sequential analysis tests, which was initially used for quality control in the industrial process.24 In the aspect of surgical performance, CUSUM is a useful method to detect persistent changes between the individual data and the mean value. It allows investigators to visualize indiscernible data trends and judge whether the variations of performance is acceptable. Because it shows the sequential difference between the individual data and the mean value, the learning curve can be intuitively represented while detecting consistent changes.25–27 The CUSUM of the first case was calculated from the difference between the operation time and the mean of all cases. Then, the CUSUM of the second case was calculated by the difference between the operation time of the second case and the mean value added with the CUSUM of the first case. This process was continued until the final case. In this study, CUSUM was a crucial method to determine the learning curve period.
RESULTS
Patient Characteristics
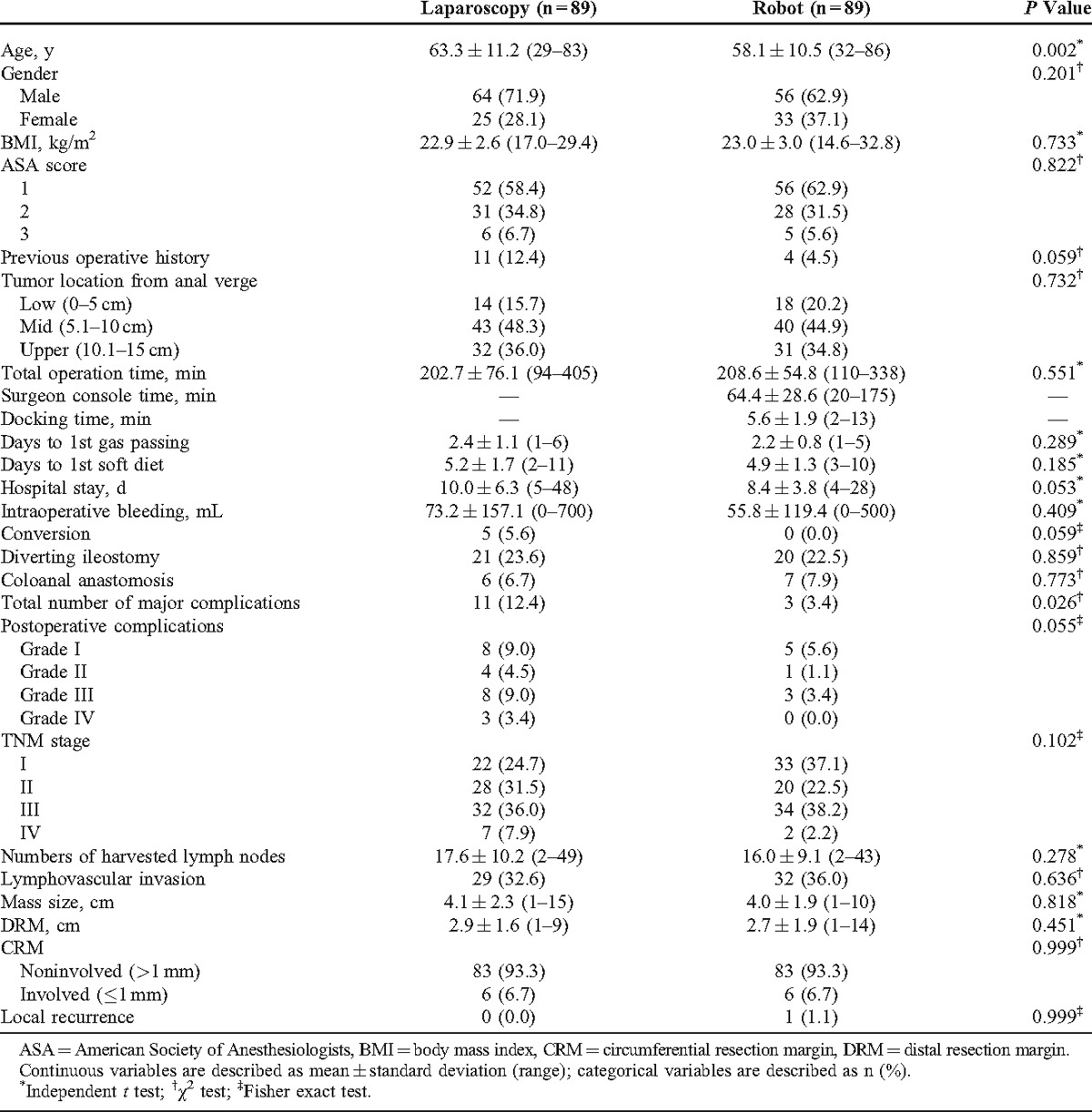
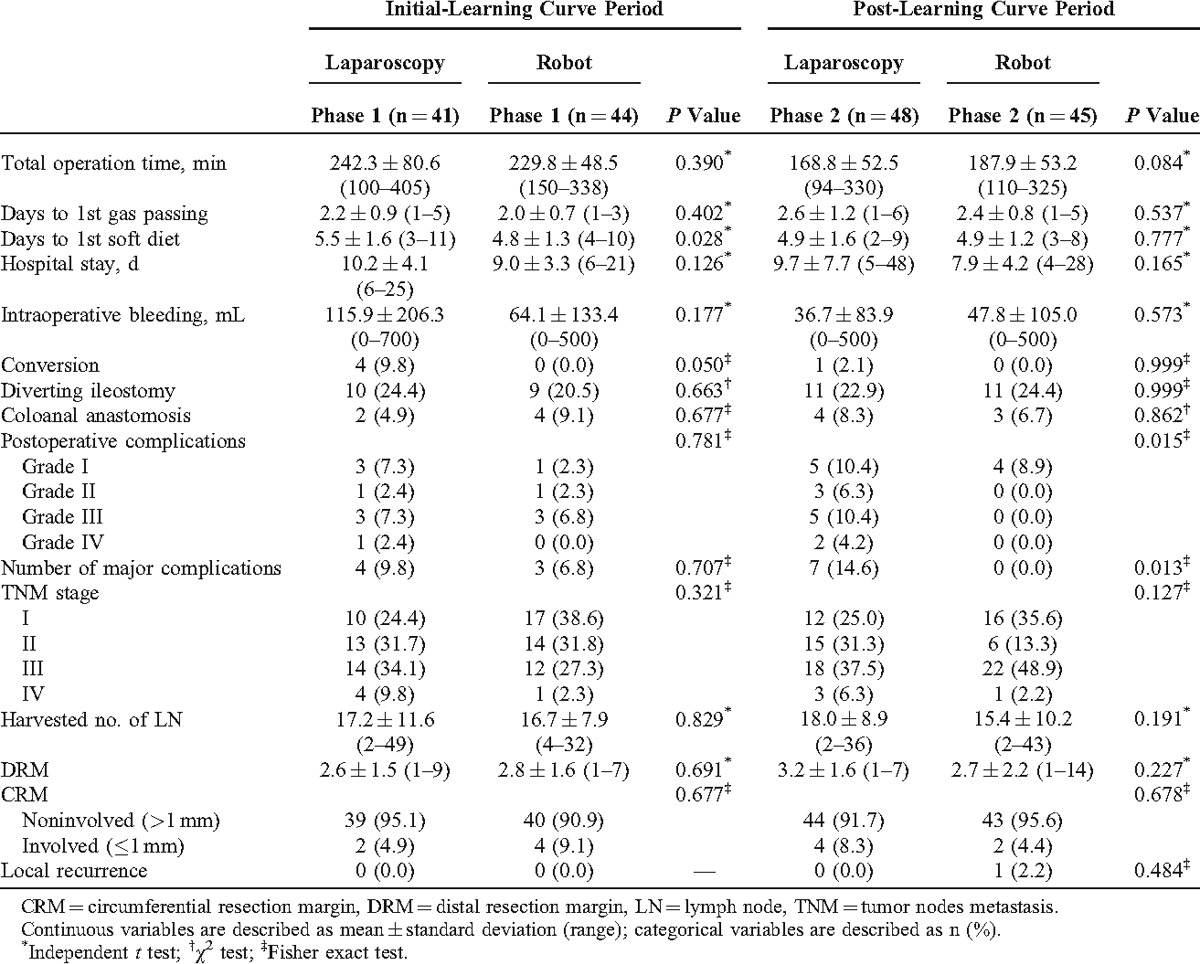
The mean age of the robotic group was younger than the laparoscopic group (robotic group; 58.1 ± 10.5 [mean ± standard deviation] years vs laparoscopic group; 63.3 ± 11.2 years) (P = 0.002). The total number of major complications for laparoscopic surgery was higher than robotic surgery (robotic group; 3.4% vs laparoscopic group; 12.4%) (P = 0.026). The total operation time was not significantly different between the robotic and the laparoscopic surgeries (robotic group; 208.6 ± 54.8 minutes [range, 110–338 minutes] vs laparoscopic group; 202.7 ± 76.1 minutes [range, 94–405 minutes]) (P = 0.551). Other parameters showed no significant difference between the robotic and the laparoscopic groups (Table 1).
TABLE 1.
Overall Characteristics Between Laparoscopic and Robotic Surgeries
Learning Curve Period: Phase 1 Versus Phase 2
The learning curve period of this study was determined as the 41st case of laparoscopic surgery and the 44th case of the robotic surgery by the following analysis.
According to the moving average method, the total operation time of laparoscopic surgery tended to decrease with cumulative number of cases (Figure 2A). In contrast, robotic surgery showed a different pattern from laparoscopic surgery: a concave-shaped graph with 2 peak points, as the 21st case of the first peak (total operation time, 233.4 minutes) and the 67th case of the second peak (209.3 minutes), respectively. The surgeon console time showed similar graph patterns with the total operation time of robotic surgery: the 19th case of the first peak (75.5 minutes) and the 63rd case of the second peak (63.9 minutes). Docking time was 5.6 ± 1.9 minutes (range 2–13), and it was relatively consistent, as shown in Figure 2B.
FIGURE 2.
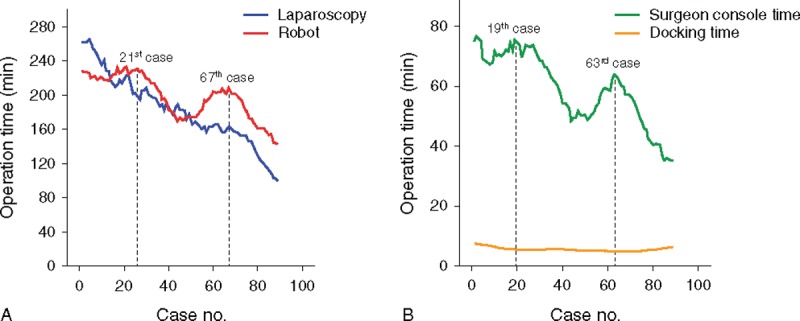
Moving average method for the operation time. (A) Total operation times of laparoscopic (n = 89) and robotic surgery (n = 89); the first peak point of robotic surgery is the 21st case, the second point, the 67th case. (B) SCT and docking time of robotic surgery; the first peak point of SCT is the 19th case, and the second peak point, the 63rd case. SCT = surgeon console time.
According to CUSUM of Figure 3, the peak point of the total operation time was the 41st case of laparoscopic surgery (CUSUM, 1626.9 minutes) and the 45th case of robotic surgery (944.6 minutes). The surgeon console time was shown at the 44th case of the peak point (464.1 minutes). The CUSUM of robotic surgery between the total operation time and the surgeon console time also represented a similar pattern. The CUSUM of the docking time showed the peak point at the 21st case (36.6 minutes). Figure 4 shows the CUSUM of robotic surgery (n = 130, robotic cases, while 89 laparoscopic cases that were performed). The peak point of the total operation time was not changed.
FIGURE 3.
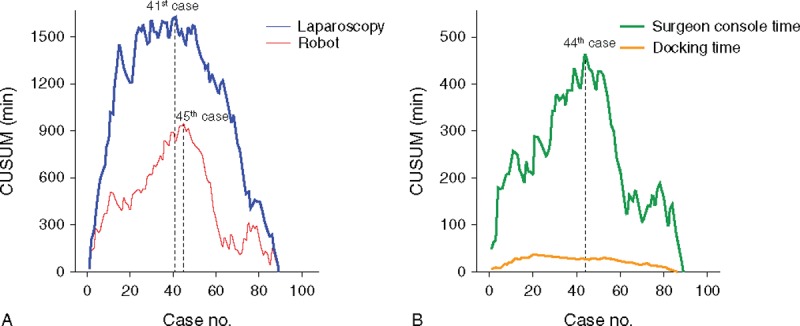
CUSUM for the operation time. (A) CUSUM for the total operation time of laparoscopy and robot; the peak point of CUSUM in laparoscopic surgery was the 41st case, and in robotic surgery, the 45th case. (B) CUSUM graph of SCT and docking time in robotic surgery; The peak point of the CUSUM was the 44th case of SCT, and the 21st case of docking time. CUSUM = cumulative sum, SCT = surgeon console time.
FIGURE 4.
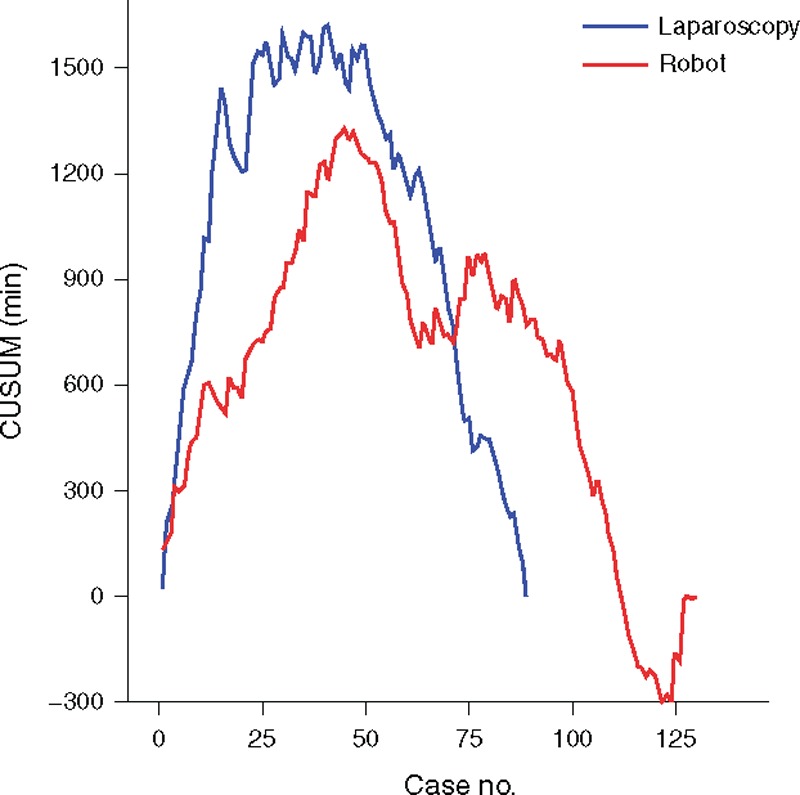
CUSUM comparison for the total operation time between robotic surgery (n = 130) and laparoscopic surgery (n = 89); the peak points of CUSUM in both procedures did not change with the original results using the same number of robotic and laparoscopic cases (n = 89). CUSUM = cumulative sum.
According to these results, the total operation time of laparoscopic LAR and the surgeon console time of robotic LAR were used to divide the learning curve into the initial learning period (phase 1) and the post-learning curve period (phase 2) from the CUSUM method. Finally, the phases were defined as follows: phase 1 (cases 1–41) versus phase 2 (cases 42–89) in the laparoscopic LAR group, and phase 1 (cases 1–44) versus phase 2 (cases 45–89) in the robotic LAR group. In robotic surgery, the surgeon console time was used to assess the learning curve instead of the total operation time because it reflects the actual operative times to perform in the robotic console. The reason why we selected CUSUM as the decisive method to determine the learning curves was that CUSUM was adequate to visualize the sequential changes of the operation time with discernable points. Although the moving average method was useful to apprehend the overall trend of the operation times, it was not appropriate to find a critical point of the learning curve. Thus, in this present study, the learning curve of laparoscopic LAR was the 41st case and robotic LAR was the 44th case in terms of operation time by CUSUM.
Comparison of Patient Characteristics According to the Learning Curve Phase
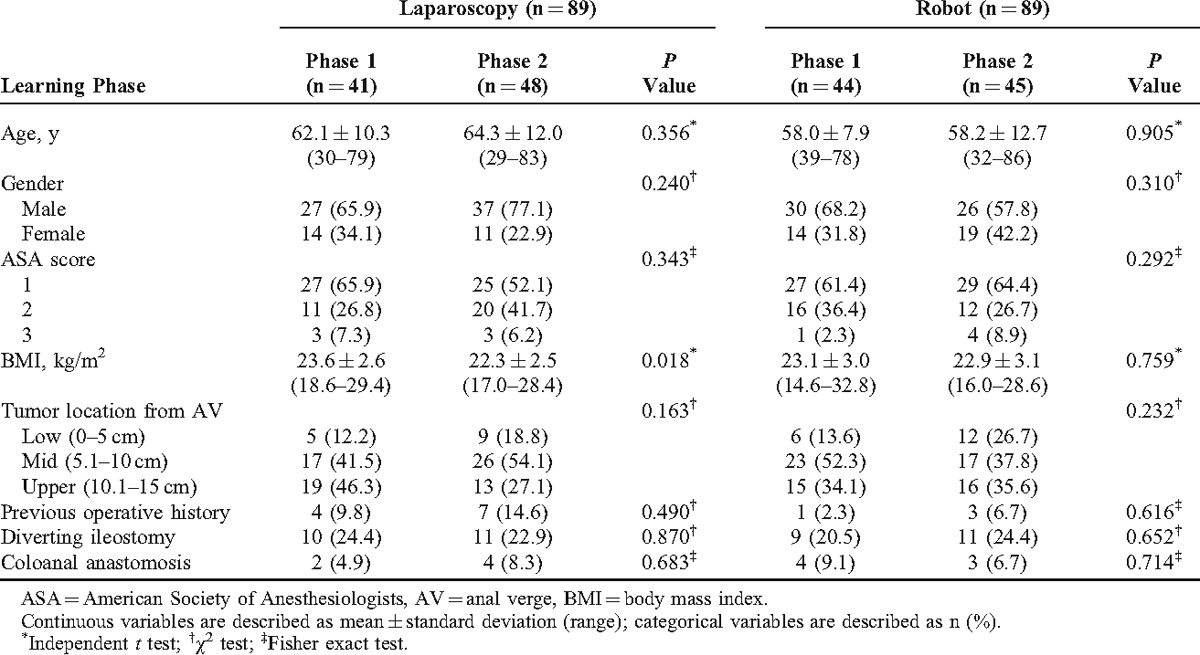
Comparison of patient characteristics between the learning curve phases showed that all parameters had no significant differences except for the BMI of laparoscopic surgery. The BMI of laparoscopic surgery was 23.6 ± 2.6 kg/m2 of phase 1 and 22.3 ± 2.5 kg/m2 of phase 2 (P = 0.018). Age, sex, ASA score, and tumor locations showed no significant differences between robotic and laparoscopic LAR. Moreover, previous operative history, incidence of diverting ileostomy, and coloanal anastomosis were not significantly different between phase 1 and phase 2 in both the groups (Table 2).
TABLE 2.
Comparison of Patient Characteristics According to Learning Curve Phases: Phase 1, the Initial Learning Curve Period; Phase 2, the Post-Learning Curve Period
Comparison of Clinicopathologic Outcomes According to the Learning Curve Phase (Robotic Group: Phase 1 vs Phase 2; Laparoscopic Group: Phase 1 vs Phase 2)
Short-Term Outcomes
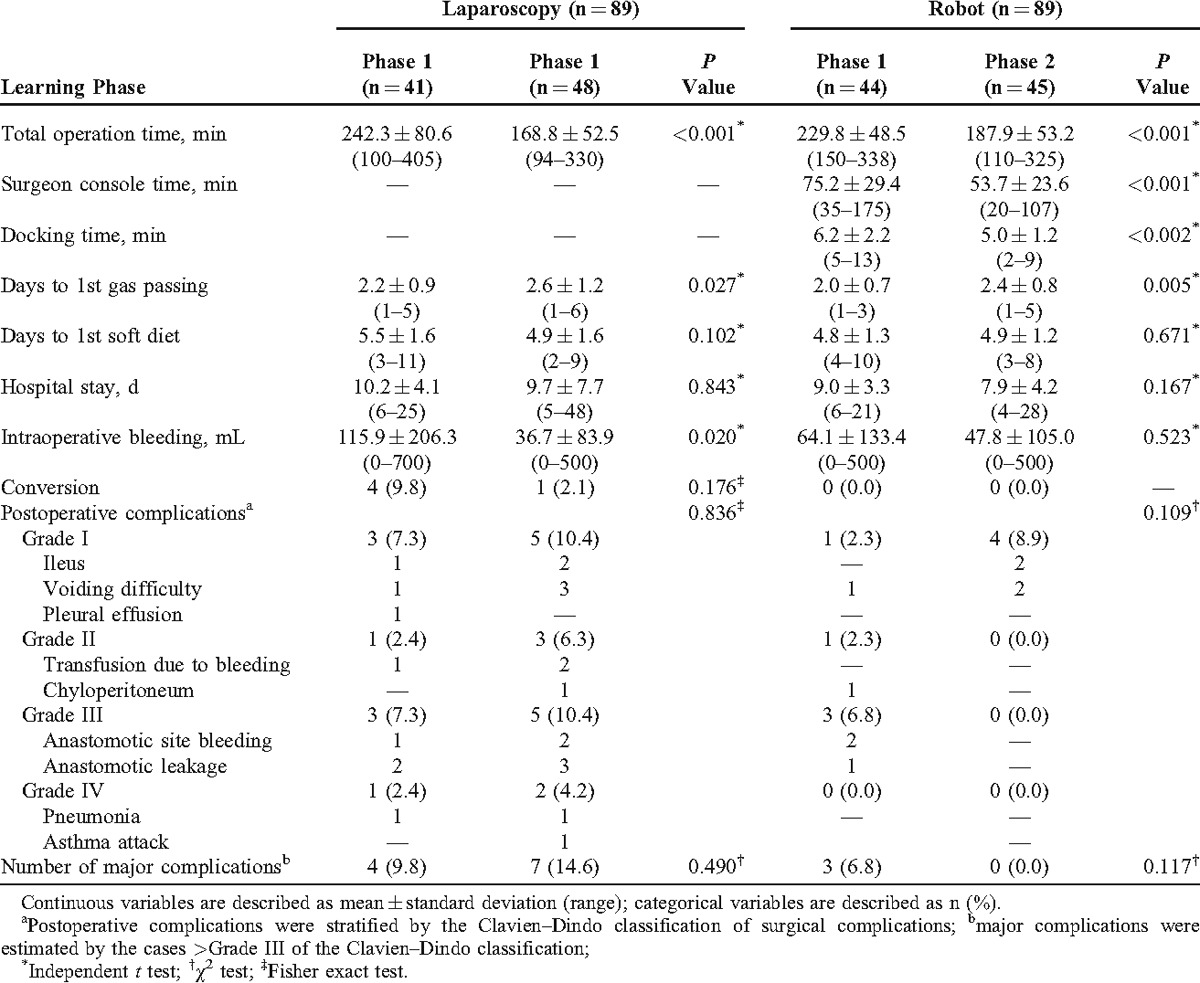
The total operation time of phase 2 was shorter than phase 1 in both laparoscopic and robotic groups. The surgeon console time and docking time during phase 2 were also shorter than phase 1 in the robotic group. However, days to 1st gas passing of phase 2 were longer than phase 1 in both the groups (P = 0.027 and 0.005, respectively). The intraoperative bleeding amounts of phase 2 were less than phase 1 in the laparoscopic group (phase 1: 115.9 ± 206.3 mL vs phase 2: 36.7 ± 83.9 mL; P = 0.020). However, the robotic group showed no significant difference of intraoperative bleeding amounts between the phases (P = 0.523). Days to 1st soft diet and hospital stay showed no significant difference in both the groups. Conversion in the laparoscopic group was 4 cases (9.8%) of phase 1 and 1 case (2.1%) of phase 2 (P = 0.176). However, there were no conversions in all phases of robotic surgery.
Postoperative complications stratified by the grades showed no significant difference between phase 1 and phase 2 in both laparoscopic and robotic LAR (P = 0.836 and 0.109, respectively). Major complications, including grades III and IV, also did not show any statistical differences between the phases in both groups (P = 0.490 and 0.117, respectively). However, the complications in phase 2 of the robotic group were only grade I although there was no statistical significance in comparison between phase 1 and phase 2 of the robotic group (Table 3).
TABLE 3.
Short-term Outcomes and Postoperative Complications According to the Learning Curve Phases
Pathologic Outcomes
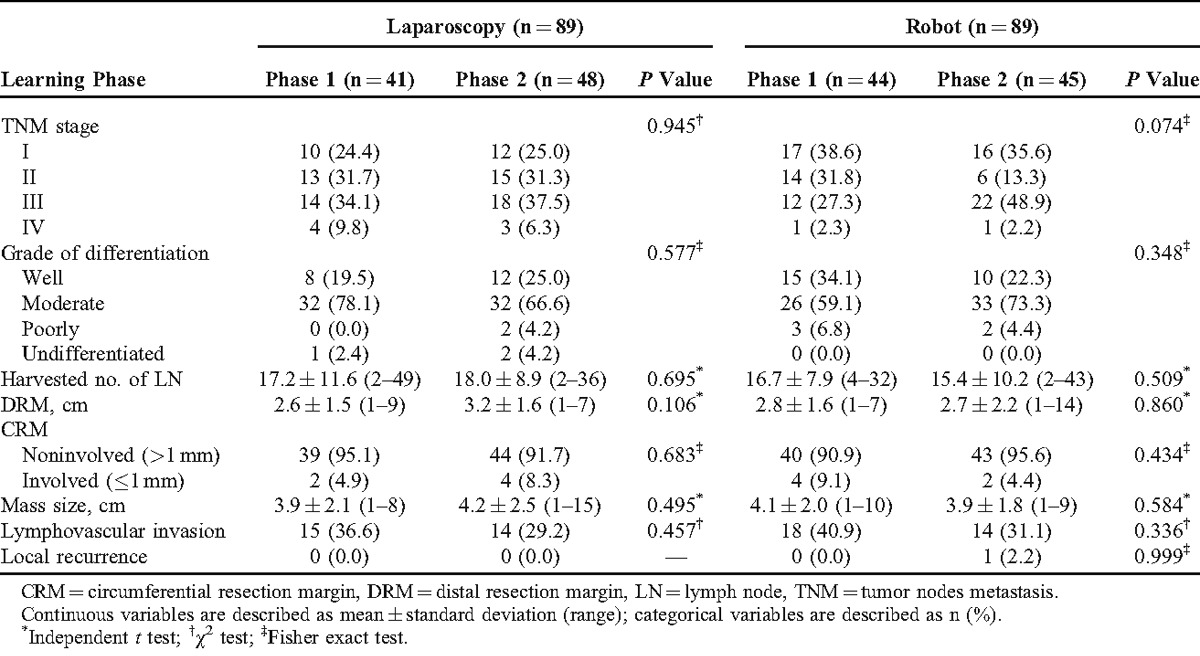
There were no significant differences in all parameters with respect to pathologic outcomes according to the phases of laparoscopic and robotic groups. Postoperative pathologic stage and histologic grade distribution were similar in both phases of laparoscopic and robotic groups. The mean number of harvested lymph nodes was more than 12 in both the groups. This is regarded as the number to achieve oncologic safety and was not significantly different between the phases of both robotic and laparoscopic groups (P = 0.509 and 0.695, respectively).
Distal resection margin and CRM involvements showed no significant differences between the phases in both the groups. Mass size, lymphovascular invasion, and local recurrence had no significant difference between the phases in both groups (Table 4).
TABLE 4.
Pathologic Outcomes Between Laparoscopic and Robotic Surgeries According to the Learning Curve Phases
Comparison of Clinicopathologic Outcomes in Phase 1 and Phase 2 Between Groups (Phase 1: Robotic vs Laparoscopic Group; Phase 2: Robotic vs Laparoscopic Group)
In phase 1, days to 1st soft diet of robotic surgery was shorter than laparoscopic surgery (laparoscopy: 5.5 ± 1.6 days vs robot: 4.8 ± 1.3 days) (P = 0.028). However, in phase 2, there was no significant difference of days to 1st soft diet between the groups (P = 0.777). Conversion occurred more frequently in phase 1 of the laparoscopic group than the robotic group (P = 0.050). Meanwhile, there was no statistical difference of conversion rate between the groups of phase 2 (P = 0.999).
Laparoscopic surgery had more postoperative major complications (14.6% vs 0.0%, P = 0.013) and total postoperative complications (31.3% vs 8.9%, P = 0.015) than robotic surgery in phase 2. However, there was no significant difference of major complications and total postoperative complications in phase 1 (P = 0.707 and 0.781, respectively).
TNM stage, the number of harvested lymph nodes, distal resection margin, CRM involvement, and local recurrence rate were not significantly different between the laparoscopic and robotic groups in phase 1 and phase 2. Local recurrence occurred in 1 patient (TNM stage, T2N0M0) of phase 2 in the robotic group. In this patient, the disease-free survival period was 49.5 ± 21.7 months (range 1–80 months). Median follow-up period of all enrolled cases was 54.0 ± 19.7 months (range 1–80 months) (Table 5).
TABLE 5.
Comparison of the Learning Curve Phases Between Laparoscopic and Robotic Surgeries
DISCUSSION
According to CUSUM, the learning curve of laparoscopic surgery was the 41st case and that of robotic surgery was the 44th case of the surgeon console time in this study. Although there were expectations that the technical advantages of the robotic system could shorten the learning curve of robotic colorectal surgery, the results of this study did not prove this assumption. The learning curve of robotic LAR was similar to laparoscopic LAR. Moreover, this study showed that the learning curve of robotic surgery was relatively longer than previous studies.15–17,28
To the best of our knowledge, the learning curve of laparoscopic colorectal surgery was reported as 30 to 70 cases.10,12–14 The results of our study for laparoscopic surgery corresponds with the previous reports. However, the learning curve of robotic colorectal surgery is uncertain so far because of the late adoption in 2001.29 A few previous studies reported that the learning curve of robotic colorectal surgery is from 15 to 35 cases.9,16,18 Bokhari et al16 suggested that the learning curve of robotic colorectal surgery was achieved at 15 to 25 cases with 3 learning phases after analyzing 50 patients by using CUSUM. Jiménez-Rodriguez et al18 also reported that the estimated learning curve in the 43 patients with robotic rectal surgery was 21 to 23 cases with 3 phases. The latest studies in 2013 by Sng et al9 reported multiphasic learning curve of robotic rectal surgery from 197 patients. They suggested that the initial learning curve period was 35 cases by the analysis of CUSUM.
In this present study, the learning curve of robotic surgery was estimated as the 44th case with a bimodal distribution from the analysis of CUSUM (Figure 3). The 44th case is longer than the results of the previous studies.9,16,18 The reason of this different results can be interpreted by discrepancy of number of enrolled patients in each study. Previous reports, which enrolled <50 patients represented the learning curve as 20 to 25 cases.16,18 Interestingly, as shown in Figure 2 of this study, the total operation time of robotic surgery showed a 2-peak-shaped concave graph by the moving average method. If the total sample sizes are limited to 50 cases, there is the risk of making a hasty conclusion that the learning curve of robotic surgery is the 21st case because of the first highest turning point. This aspect can explain why the previous study reported a relatively shorter learning curve as 15 to 35 cases compared with the present study.9,16,18 In terms of the small sample size, it is noticeable that an increased number of enrolled patients can lead to different findings and obtain a more reliable conclusion about the learning curve definition.
In this study, the most important finding is that the learning curve of robotic LAR was similar to laparoscopic LAR. Although the advanced technologies of the robotic system might give a positive effect to shorten the learning curve period of robotic LAR, some drawbacks of robotic technology also can affect the learning curve. At the initial training period of robotic surgery, the surgeon needs more knowledge about the robotic system and intensive practice to manipulate the complicated robotic instruments compared to the operation of conventional simple laparoscopic instruments. The absence of tactile sense during surgery can be an obstacle to manipulate soft tissues in a narrow pelvic cavity during the initial learning period. These limitations of the robotic system might countervail the advantages of the robotic system. Moreover, robotic surgery is basically the same as laparoscopic surgery because both procedures are performed in the pneumoperitoneum using long surgical instruments. For these reasons, the learning curve period according to CUSUM might be similar between both procedures in this study.
It can be assumed that perioperative clinical and pathologic outcomes during the learning curve phase may be compromised because of the surgeon’s poor surgical skills during the learning curve. However, short-term clinical and pathologic outcomes of robotic surgery were similar between phase 1 and phase 2. These results were also similar to laparoscopic LAR. These aspects can support the feasibility of the learning curve period in both laparoscopic and robotic surgeries.
The comparison of short-term outcomes in both surgeries by phases showed that robotic LAR had faster 1st soft diet and lower conversion rate than laparoscopic LAR during phase 1 (Table 5). Postoperative complications were lower in robotic LAR during phase 2 (P = 0.015). The technological advantages of robotic surgery can affect lower conversion rates and faster recovery during the initial learning period.30 However, this assumption is not appropriate to consider this result as a final conclusion because the ages of the robotic group were younger than the laparoscopic group in this study. Moreover, the retrospective nonrandomized study design of this study has inevitable selection bias, and this is a major limitation of this study.
There are several limitations that generalize the findings of this study. The enrolled patients were not randomized to either the robotic or laparoscopic groups and the consecutive patients did not have the same anatomical features, which affects the technical difficulty during the operation. In this study, more male patients underwent laparoscopic surgery than robotic surgery although the difference did not reach statistical significance (laparoscopy [71.9%] vs robot [62.9%]; P = 0.201). The surgical technique is usually technically demanding in male patients because they have a relatively narrow pelvis than female patients. This can be a potential selection bias in this study.
The most important issue is that validation using the data of other surgeons is necessary to obtain a general conclusion because this study was evaluated with consecutive cases by a single surgeon. There may be different results compared to this study and among surgeons if the data of other surgeons are evaluated. However, these limitations can affect the specific learning curve case, but relatively not affect the results in which the learning curve case was similar between robotic and laparoscopic LAR. For additional explanation, the one surgeon’s inherited ability for the surgical technique may equally influence both the robotic and laparoscopic procedures. Because of this reason, the question about the difference of learning curves between both procedures may be similar to the conclusion of this study although the specific learning curve case can be longer or shorter according to each surgeon’s inherited ability for their surgical technique. This is the main concept of this study. This study focused on evaluating whether the learning curve of robotic LAR is different or similar compared to laparoscopic LAR.
Previous surgical experience of open or laparoscopic surgeries prior to the first robotic surgery can influence the learning curve of robotic surgery. In this study, 10 open and 6 laparoscopic LARs were performed. However, manipulation of the instrumental tips and controlling the surgical field are different between robotic and laparoscopic surgeries. The essence of rectal cancer surgery is to keep the principle of TME, which requires a more sophisticated manipulation of surgical instruments. Thus, prior surgical experience before robotic surgery may not significantly influence the learning curve of robotic surgery.
In this study, the last 41 cases were excluded from the 130 total cases of robotic surgery. The main reason of exclusion was to compare the learning curve and clinicopathologic outcomes more objectively by using the same number of consecutive patients between the robotic and laparoscopic groups. The reason why a comparison of the same number of consecutive patients between groups is objective is that the learning curve of surgical procedure depends on number of consecutive cases and does not depend on the period. Thus, we matched the same number of cases between laparoscopic (n = 89) and robotic surgeries (n = 89). However, if data should be compared based on the same period, 89 cases of the laparoscopic group should be compared to the 130 cases of robotic surgery. However, in the robotic group, the last 41 cases that were excluded could not change the conclusion of learning curve of robotic surgery (44th case) because there were already 89 cases. Moreover, the results shown in Figure 4 support this point.
Another limitation of this study is that other robotic or laparoscopic procedures other than LAR was performed during the learning curve period. During the learning curve period of laparoscopic surgery, 43 cases of laparoscopic anterior resection, 40 cases of laparoscopic right hemicolectomy, 10 cases of laparoscopic left hemicolectomy, and 1 case of laparoscopic subtotal colectomy were performed. There were no robotic cases of such surgeries mentioned above during the learning curve period of robotic surgery. This surgical experience can be biased to compare the learning curve between robotic and laparoscopic LAR because it may shorten the learning curve of laparoscopic surgery. However, these surgical experiences might only be a tiny influence because these surgical procedures only involved resection of the colon. In this study, the evaluated surgical procedure was LAR for rectal cancer, which is a technically demanding procedure.
There could have been a crossover effect of learning techniques that could have influenced the learning curve because the surgeon performed both laparoscopic and robotic surgeries during the study period. However, we think that there was rare crossover effects of learning techniques between both the procedures. The main difference between robotic and laparoscopic surgeries is rectal dissection with TME. The manipulation of instruments while controlling the surgical team are differentiated during rectal dissection in both the procedures. These characteristics were reflected in the differently shaped graphs between laparoscopic and robotic surgeries as shown in Figure 2. Because surgeon console time reflects actual robotic procedures, similar patterns between total operation time and surgeon console time can demonstrate that robotic procedures had independent factors from the laparoscopic procedures.
This study is the first comparative study of the learning curve between laparoscopic and robotic surgery for colorectal cancer with a relative large number of cases. The CUSUM was the statistical method, which can detect the perceptible changes of the learning process among indistinguishable operative data. The results of this study could be more reliable in terms of the large number of cases and precise comparative CUSUM analysis compared to previous studies. Thus, the results of this study could give us some clue to develop a new module for proper surgical training.
In conclusion, this study demonstrated that the learning curve of robotic LAR for rectal cancer was similar to laparoscopic LAR although there are many advanced technologies of the robotic system. Moreover, short-term outcomes compared in the learning phases were not significantly different between laparoscopic and robotic LAR except days to 1st soft diet, conversion rates, and postoperative complications. Future advanced studies are necessary to clarify the present conclusion.
ACKNOWLEDGMENT
The authors would like to thank Mi Sun Park (professional proofreader) for the English revision of this manuscript. She has given permission to be named.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BMI = body mass index, CRM = circumferential resection margin, CUSUM = cumulative sum, LAR = low anterior resection, TME = total mesorectal excision, TNM = tumor nodes metastasis.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Ng SS, Lee JF, Yiu RY, et al. Long-term oncologic outcomes of laparoscopic versus open surgery for rectal cancer: a pooled analysis of 3 randomized controlled trials. Ann Surg. 2014;259:139–147. [DOI] [PubMed] [Google Scholar]
- 2.Jayne DG, Thorpe HC, Copeland J, et al. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–1645. [DOI] [PubMed] [Google Scholar]
- 3.Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100:75–82. [DOI] [PubMed] [Google Scholar]
- 4.Group TCOoSTS. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. [DOI] [PubMed] [Google Scholar]
- 5.Miskovic D, Ni M, Wyles SM, et al. Learning curve and case selection in laparoscopic colorectal surgery: systematic review and international multicenter analysis of 4852 cases. Dis Colon Rectum. 2012;55:1300–1310. [DOI] [PubMed] [Google Scholar]
- 6.Park IJ, Choi GS, Lim KH, et al. Multidimensional analysis of the learning curve for laparoscopic resection in rectal cancer. J Gastrointest Surg. 2009;13:275–281. [DOI] [PubMed] [Google Scholar]
- 7.Jamali FR, Soweid AM, Dimassi H, et al. Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch Surg. 2008;143:762–767. [DOI] [PubMed] [Google Scholar]
- 8.Son GM, Kim JG, Lee JC, et al. Multidimensional analysis of the learning curve for laparoscopic rectal cancer surgery. J Laparoendosc Adv Surg Tech A. 2010;20:609–617. [DOI] [PubMed] [Google Scholar]
- 9.Sng KK, Hara M, Shin JW, et al. The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc. 2013;27:3297–3307. [DOI] [PubMed] [Google Scholar]
- 10.Bege T, Lelong B, Esterni B, et al. The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution’s experience. Ann Surg. 2010;251:249–253. [DOI] [PubMed] [Google Scholar]
- 11.Schlachta CM, Mamazza J, Seshadri PA, et al. Defining a learning curve for laparoscopic colorectal resections. Dis Colon Rectum. 2001;44:217–222. [DOI] [PubMed] [Google Scholar]
- 12.Kayano H, Okuda J, Tanaka K, et al. Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc. 2011;25:2972–2979. [DOI] [PubMed] [Google Scholar]
- 13.Akiyoshi T, Kuroyanagi H, Ueno M, et al. Learning curve for standardized laparoscopic surgery for colorectal cancer under supervision: a single-center experience. Surg Endosc. 2011;25:1409–1414. [DOI] [PubMed] [Google Scholar]
- 14.Ito M, Sugito M, Kobayashi A, et al. Influence of learning curve on short-term results after laparoscopic resection for rectal cancer. Surg Endosc. 2009;23:403–408. [DOI] [PubMed] [Google Scholar]
- 15.Wishner JD, Baker JW, Jr., Hoffman GC, et al. Laparoscopic-assisted colectomy. The learning curve. Surg Endosc. 1995;9:1179–1183. [DOI] [PubMed] [Google Scholar]
- 16.Bokhari MB, Patel CB, Ramos-Valadez DI, et al. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc. 2011;25:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akmal Y, Baek JH, McKenzie S, et al. Robot-assisted total mesorectal excision: is there a learning curve? Surg Endosc. 2012;26:2471–2476. [DOI] [PubMed] [Google Scholar]
- 18.Jimenez-Rodriguez RM, Diaz-Pavon JM, de la Portilla de Juan F, et al. Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis. 2013;28:815–821. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edge S, Byrs SR, Compton CC, et al. , editors. AJCC Cancer Staging Manual. 7th ed New York, NY: Springer; 2010. [Google Scholar]
- 21.Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. [DOI] [PubMed] [Google Scholar]
- 22.Baik SH, Ko YT, Kang CM, et al. Robotic tumor-specific mesorectal excision of rectal cancer: short-term outcome of a pilot randomized trial. Surg Endosc. 2008;22:1601–1608. [DOI] [PubMed] [Google Scholar]
- 23.Baik SH, Lee WJ, Rha KH, et al. Robotic total mesorectal excision for rectal cancer using four robotic arms. Surg Endosc. 2008;22:792–797. [DOI] [PubMed] [Google Scholar]
- 24.Yap CH, Colson ME, Watters DA. Cumulative sum techniques for surgeons: a brief review. ANZ J Surg. 2007;77:583–586. [DOI] [PubMed] [Google Scholar]
- 25.Van Rij AM, McDonald JR, Pettigrew RA, et al. Cusum as an aid to early assessment of the surgical trainee. Br J Surg. 1995;82:1500–1503. [DOI] [PubMed] [Google Scholar]
- 26.Biau DJ, Resche-Rigon M, Godiris-Petit G, et al. Quality control of surgical and interventional procedures: a review of the CUSUM. Qual Saf Health Care. 2007;16:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohl H. The cusum plot: its utility in the analysis of clinical data. N Engl J Med. 1977;296:1044–1045. [DOI] [PubMed] [Google Scholar]
- 28.D’Annibale A, Pernazza G, Monsellato I, et al. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc. 2013;27:1887–1895. [DOI] [PubMed] [Google Scholar]
- 29.Ballantyne GH, Merola P, Weber A, Wasielewski A. Robotic solutions to the pitfalls of laparoscopic colectomy. Osp Ital Chir. 2001;7:405–412. [Google Scholar]
- 30.Baik SH, Kwon HY, Kim JS, et al. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol. 2009;16:1480–1487. [DOI] [PubMed] [Google Scholar]


