Abstract
According to the Barcelona Clinic Liver Cancer (BCLC) guidelines, transarterial chemoembolization (TACE) is recommended for BCLC stage B hepatocellular carcinoma (HCC). However, an investigation of the use of resection for BCLC stage B is needed. Therefore, we compared the efficacy and safety of hepatic resection (HR) with that of TACE in treating intermediate HCC.
We retrospectively enrolled 923 patients with BCLC stage B HCC who underwent TACE (490 cases) or HR (433 cases). The baseline characteristics, postoperative recoveries, and long-term overall survival rates of the patients in these 2 groups were compared. Subgroup analyses and comparisons were also performed between the 2 groups.
The baseline demographic and tumor characteristics, in-hospital mortality rate, and 30-day mortality rate were comparable between the 2 groups. However, the patients in the resection group suffered from more serious complications compared with those in the TACE group (11.1% vs 4.7%, respectively, P < 0.01) as well as longer hospital stays (P < 0.05). The resection patients had significantly better overall survival rates than the TACE patients (P < 0.01). In the TACE group, patients with Lipiodol retention showed much higher 1-, 3-, and 5-year overall survival rates than those in the noncompact Lipiodol retention group (P < 0.01). Subgroup analyses revealed that patients with 1 to 3 tumor targets showed much better 1-, 3-, and 5-year overall survival rates in the resection group (P < 0.01), but no difference was observed for the patients with >3 targets.
Our clinical analysis suggests that patients with BCLC stage B HCC should be recommended for resection when 1 to 3 targets are present, whereas TACE should be recommended when >3 targets are present.
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 80% to 90% of primary liver cancers; it is the fifth most common cancer worldwide for men and the seventh for women.1 The prognosis for HCC patients is determined by tumor status, liver function reserve, general health, and treatment efficacy.2 More than 80% of HCC patients present with cirrhosis; only 10% to 15% of these patients have potentially resectable tumors.3 Liver transplantation (LT), surgical resection, and radiofrequency ablation (RFA) are the 3 potential radical treatments for early-stage HCC patients.4 LT results in the widest possible resection margin, removes the underlying cirrhotic liver, and restores hepatic function; thus, it should be considered as the most effective treatment for these patients. However, the liver donor pool is small. Thus, hepatic resection (HR) represents a popular alternative treatment for early-stage HCC. The treatment outcomes for HCC patients are affected by multiple variables, including tumor burden, the Child-Pugh score of liver function reserve, and the performance status of the patient.5 In 1996, the Barcelona Clinic Liver Cancer (BCLC) classification staging system was developed to account for these 3 variables by establishing BCLC groups.6,7 The BCLC staging system was recently utilized to stratify HCC patients according to the guidelines established by the American Association for the Study of Liver Disease.8 The stratification capacity of the BCLC system for predicting prognosis has been cross-validated in several cohorts of HCC patients.9,10 In addition to estimating prognosis, the main advantage of the BCLC staging system is that it establishes links between staging and treatment indications.11 This staging system recommends different treatment options for each stage of the disease,7 stating that transarterial chemoembolization (TACE) should be considered for patients with BCLC-B HCC and certain patients with BCLC-C HCC. Curative HR is indicated only for patients with early-stage HCC and satisfactory liver function (BCLC-A).12 Although the BCLC staging system classifies patients with a single nodule as BCLC-A, according to the Milan criteria, a solitary target with a diameter of >5 cm should be excluded from LT, and RFA should not be recommended when the diameter of a solitary target is >5 cm. Notably, not all solitary HCCs can be resected (such as those >10 or 15 cm). Thus, in our study, we defined the BCLC-B stage as patients with Child scores of A or B with large, single-focus HCC (>5 cm) and those with multifocal HCC, which is defined as >3 tumors of any size or 2 to 3 tumors with a maximal diameter of >3 cm in the absence of vascular invasion or extrahepatic spread.13
The indication of liver resection for BCLC-B HCC remains controversial, although it has been assessed in many studies.14 The guidelines from the European Association for the Study of Liver Disease and the American Association for the Study of Liver Disease do not recommend HR for treating BCLC stage B/C HCC.2,7 However, many studies conducted in North America, Europe, and Asia have proposed that liver resection can be safely performed in patients with large or multinodular HCC and good liver function,15–17 and some reports have advocated that BCLC stage C HCCs are also candidates for HR.12 Therefore, using a large cohort of patients from West China, we compared the efficacy and safety of TACE with that of HR for treating BCLC stage B HCC.
METHODS
Between July 2002 and November 2008, 6568 patients were diagnosed with HCC and underwent treatment at the West China Hospital of Sichuan University. In the present study, we only included intermediate HCC patients with Child-Pugh A or B scores who underwent liver resection or TACE. Patients with other initial treatments for HCC, extrahepatic metastasis, or diminished liver function (Child C) were excluded from our study. Patients who underwent LT, RFA, or other therapies were also excluded. Based on the inclusion and exclusion criteria, 923 patients (14.1%) were enrolled in this retrospective study (Figure 1). We divided the 923 patients into 2 groups based on the therapy administered as follows: the liver resection group (n = 433) and the transarterial chemoembolization group (n = 490). Next, we retrospectively analyzed the 2 groups to compare long-term outcomes in terms of 1-, 3-, and 5-year overall survival rates. The ethical conduct of this study was approved by our departmental review board (West China Hospital of Sichuan University) in agreement with the 1990 Declaration of Helsinki and subsequent amendments, and all patients signed informed consent before TACE or resection.
FIGURE 1.
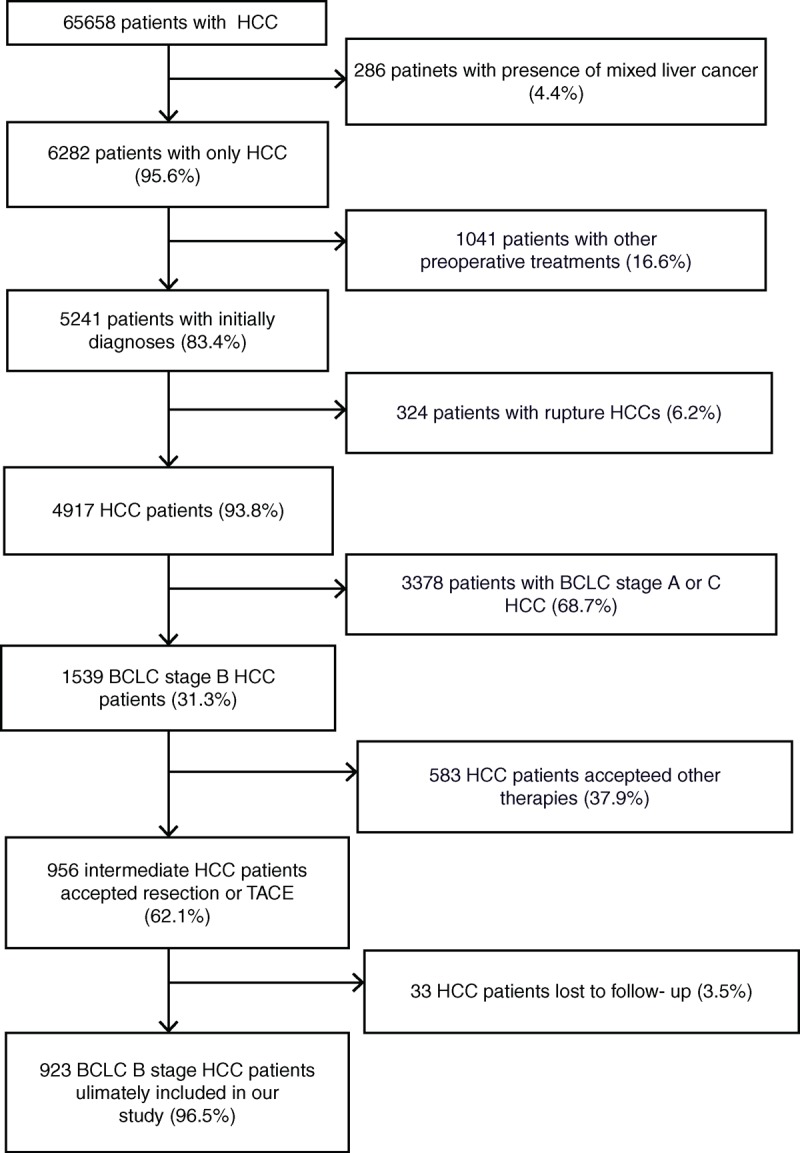
The databases included 6568 HCC patients. Based on the inclusion and exclusion criteria, 923 HCC patients were selected for baseline-adjusted analyses. HCC = hepatocellular carcinoma.
HCC diagnosis in the TACE group was based on 2 imaging scans (contrast-enhanced ultrasound, double-phase helical computed tomography scan, or magnetic resonance imaging) and serum alpha-fetoprotein (AFP) levels. For the resection patients, HCC was diagnosed by retrospective histopathological examination. BCLC stage B was defined as the presence of 1 lesion of >5 cm in diameter or of 2 to 3 lesions (of which at least 1 was >3 cm in diameter) or of >3 lesions of any diameter. Portal hypertension was defined as the presence of esophageal variance or a platelet count of <100 × 109 cells/L in association with splenomegaly.12 HCC resection was performed anatomically by an experienced surgeon, leaving at least 1 cm of nontumor margin. All surgical procedures were performed with the patient under general anesthesia and were guided by ultrasound. Anatomical resection was based on the segmental division of the liver, but nonanatomical resection using a sufficient resection margin was often adopted to ensure that an adequate volume of the liver remained.18 The TACE protocol included the superselective technique, in which tumor-feeding arteries were catheterized with a highly flexible coaxial microcatheter passing through a 4-Fr catheter that had been previously placed within the hepatic artery. A mixture of epirubicin and Lipiodol (average total volume of 50 mL) was then injected under fluoroscopic control, followed by embolization with Spongel particles until vessel stasis was achieved.4,19 The efficacy of liver resection or TACE was evaluated using enhanced imaging scans after 1 and 2 to 3 postoperative months, and AFP levels were measured every 2 to 3 months. The therapy used to treat HCC recurrence, such as re-resection, RFA, re-TACE, or LT was dependent upon the patient's wishes in addition to their liver function, tumor characteristics, and the availability of a liver graft for LT.
Categorical variables were compared using the χ2 and 2-tailed Fisher exact tests. Continuous variables were compared using the Mann–Whitney U test. The effects of resection and TACE were compared by univariate analysis. Multivariate analysis was performed using Cox regression hazard analysis to calculate the hazard ratios and P values of independent variables for overall survival. The continuous data were expressed as the median values with interquartile ranges. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. A 2-tailed P < 0.05 was considered to be statistically significant. All statistical analyses were performed using the SPSS 17.0 statistical package (SPSS Inc, Chicago, IL).
RESULTS
Baseline Characteristics of Patients and Tumors
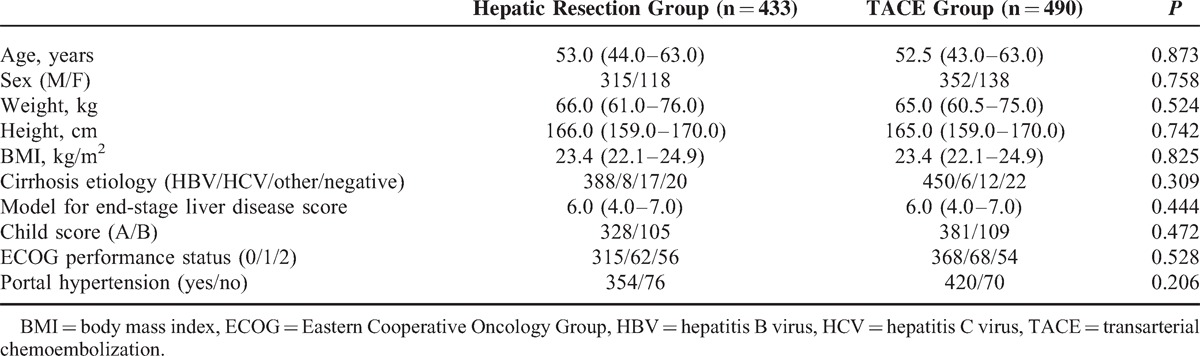
Among the 923 patients with BCLC stage B HCC enrolled in this study, 433 received liver resection and 490 received TACE. Procedures were typically performed by the attending physician based on the tumor characteristics, liver function, and the patients’ condition and religious beliefs. Using the BCLC staging system, we defined BCLC stage B as the presence of one lesion of >5 cm in diameter, 2 to 3 lesions (of which at least 1 was >3 cm in diameter), or >3 lesions of any diameter. We defined portal hypertension as the presence of esophageal variance or a platelet count of <100 × 109 cells/L in association with splenomegaly. Most of the patients in both groups showed portal hypertension because of the high prevalence of hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. For all patients, the shortest follow-up period was 5 years. Table 1 shows the demographics and clinical characteristics of the patients in both groups. Patients who underwent liver resection had an older average age and greater body mass index, but these differences were not statistically significant. No significant between-group differences were observed in patient sex, weight, height, or cirrhosis etiology. Most patients in both groups showed good liver function (Child A), and no difference was observed in the model for end-stage liver disease score or the Child Score. The majority of patients in both groups exhibited a good performance status (grade 0), but 118 patients in the resection group and 122 patients in the TACE group presented with symptoms that were related to tumor burden (Eastern Cooperative Oncology Group [ECOG] PS 1-2).
TABLE 1.
Baseline Characteristics of the Patients Submitted to Hepatic Resection or TACE
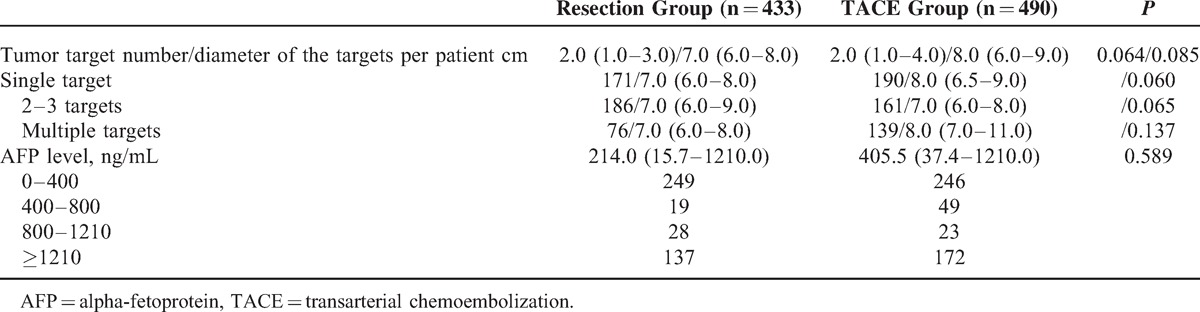
As illustrated in Table 2, the average tumor target number per patient in the TACE group was greater than that found in the resection group, and the diameter per target was larger in the TACE group; however, these differences were not statistically significant (P > 0.05). According to the BCLC staging system, we divided the 2 groups into 3 subgroups as follows: single target, 2 to 3 targets, and multiple targets. Single-target patients displayed a slightly smaller target diameter in the resection group compared with the TACE group (P = 0.060), and the differences between the TACE and 2- to 3-target (P = 0.065), and multiple target (P = 0.137) groups were also not statistically significant. Regarding the preoperative AFP level, which is a biomarker for HCC, we divided all patients into 4 groups according to AFP level and found no significant difference between the TACE and resection groups (P = 0.072).
TABLE 2.
Comparison of Tumor Characteristics
Mortality, Morbidity, and Postoperative Outcomes
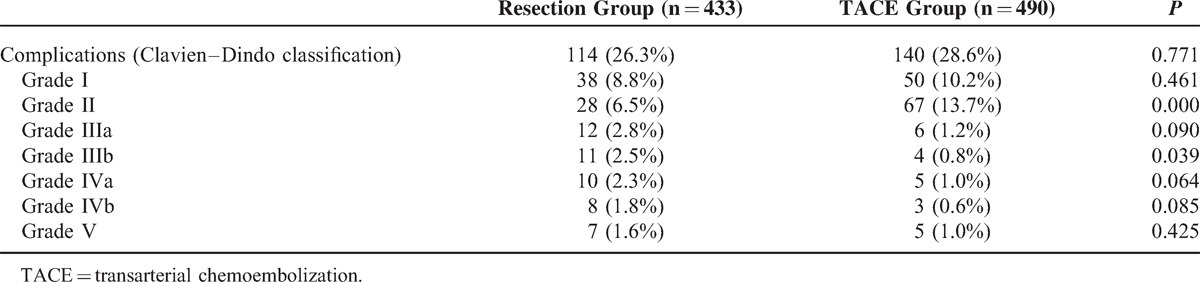
The in-hospital mortality rate was 1.6% in the resection group and 1.0% in the TACE group (P = 0.425). No significant differences were observed between the 2 groups for 30-day mortality (2.3% and 2.0% in the resection and TACE groups, respectively) (P = 0.780). The Clavien–Dindo classification of surgical complications was used to assess postoperative complications. No significant difference was observed in the postoperative complication rate between the 2 groups (P = 0.771, Table 3). However, when we compared minor (grade I–II) and serious (grade III-V) complications, the resection group displayed a significantly greater number of serious complications (4.7% vs 11.1%, respectively, P < 0.01) and a lower number of minor complications (15.2% vs 23.9%, respectively, P = 0.001) compared with the TACE group. In a subgroup analysis, the complication rates of grade I, IIIa, IVa, IVb, and V were not significantly different, but the TACE group had a higher prevalence of grade II (13.7% vs 6.5%, respectively, P < 0.01) and a lower prevalence of grade IIIb (0.8% vs 2.5%, respectively, P = 0.039) complications compared with the resection group. The most common complication reported by the grade II patients was embolism syndrome (49 cases), which included fever, vomiting, and pain (Table 3).
TABLE 3.
Postoperative Recovery of the 2 Patient Groups
The median hospital stay in the resection group was 9.0 (5.0–12.0) days, which was much longer than that of the TACE group (4.0, 3.0–5.0 days) (P < 0.01). The median number of days required for the patients in the 2 groups to return to a normal work schedule was not significantly different (23.0, 16.0–32.0 vs 18.0, 12.0–26.0, respectively, P = 0.143).
Comparison of the Between-group Overall Survival Rates
As shown by at least 5 years of follow-up data, the patients in the resection group displayed significantly better overall survival rates than those in the TACE group (Figure 2, P < 0.01). However, the TACE and resection groups showed similar survival rates at 1 year posttreatment (84.1% vs 85.2%), whereas the benefit of resection on overall survival was more obvious at 3 years (71.1% vs 62.2%, respectively) and even more so after 5 years (61.2% vs 45.1%, respectively). As shown in the Figure 2, the differences between the 2 groups became more dramatic as time progressed. During the 5-year follow-up period, the patients in the resection group who died had a median overall survival time of 24.8 months, whereas those in the TACE group had a median overall survival time of 26.9 months (P = 0.231). The most common cause of death was tumor recurrence (85.1% in the resection group and 81.8% in the TACE group), followed by liver failure. The 1-, 3-, and 5-year tumor recurrence rates were 11.3%, 20.1%, and 32.3%, respectively, in the resection group.
FIGURE 2.
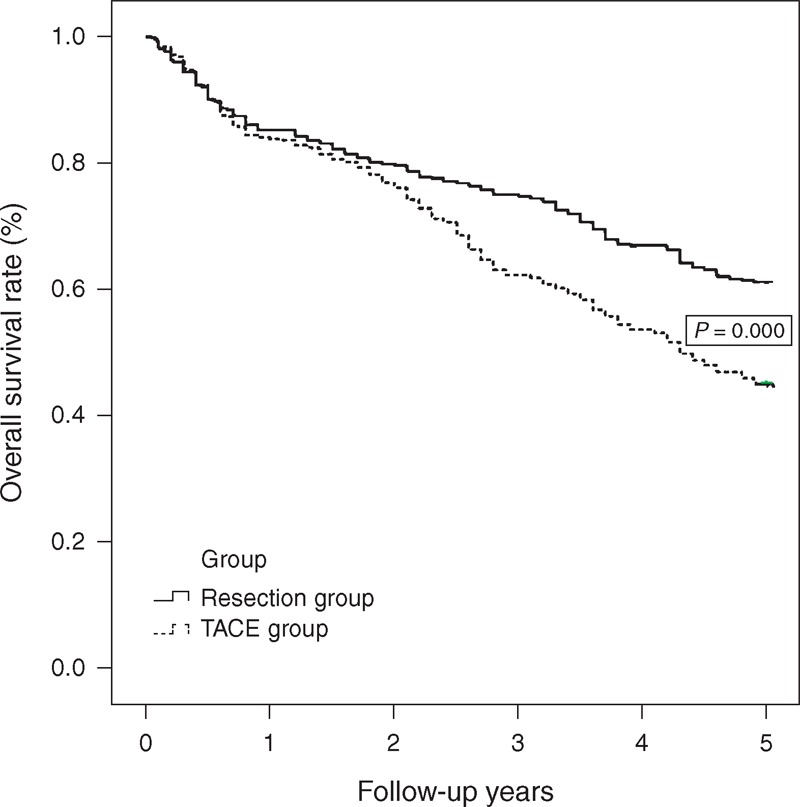
Overall survival curves for BCLC stage B HCC patients who received TACE or resection. Patients in the TACE group showed 1-year survival rates similar to those of the patients in the resection group (>0.05). However, a more marked benefit of resection on overall survival was observed at 3 and 5 years following treatment (P0.000). These between-group differences increased dramatically over time. BCLC = Barcelona Clinic Liver Cancer, HCC = hepatocellular carcinoma, TACE = transarterial chemoembolization.
The 490 patients in the TACE group were divided into 2 groups according to their compact Lipiodol pattern, including the Lipiodol retention group (173 patients) and non-Lipiodol retention group (317 patients). Lipiodol labeling was considered to be compact when the oily contrast medium was clearly visible and well dispersed throughout the tumor, and it was considered to be noncompact in all other cases. When we compared the long-term outcomes of these 2 groups, the overall survival rate of the patients with Lipiodol retention was much greater at 1, 3, and 5 years after treatment compared with the noncompact Lipiodol retention group (Figure 3, P = 0.001).
FIGURE 3.
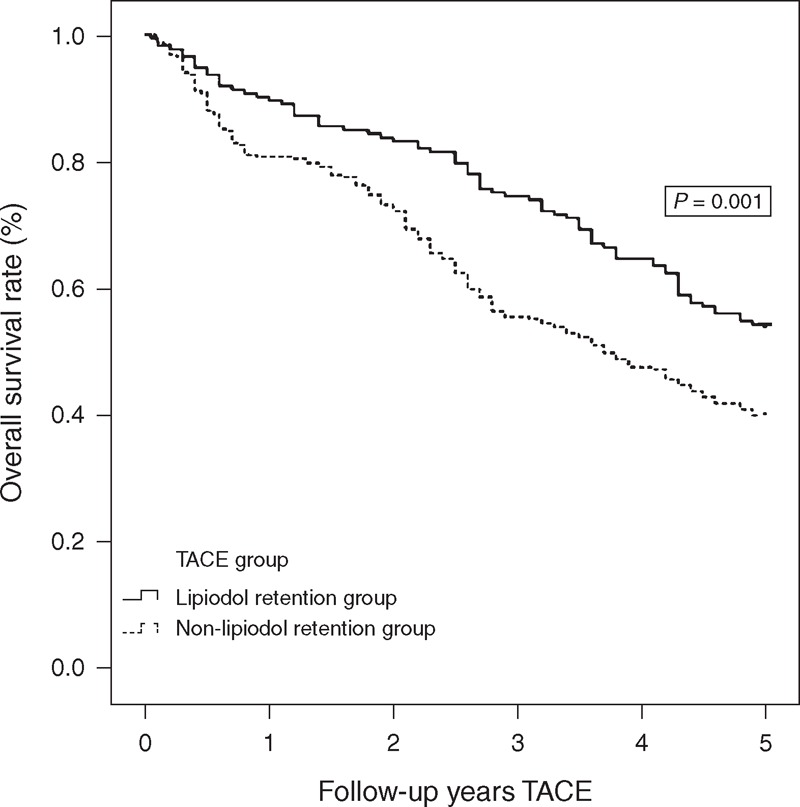
Overall survival curves of subgroups of BCLC stage B HCC patients who received TACE or resection. The overall survival rate of patients with Lipiodol retention was much greater than that of the noncompact Lipiodol retention group (P = 0.001). BCLC = Barcelona Clinic Liver Cancer, HCC = hepatocellular carcinoma, TACE = transarterial chemoembolization.
Subgroup Analysis by Tumor Number
Patients in the 2 groups were divided into 3 subgroups according to their BCLC stage B classifications as follows: 1 lesion of >5 cm in diameter, 2 to 3 lesions (of which at least 1 was >3 cm in diameter), or >3 lesions of any diameter. First, we compared the survival rates of the patients with only 1 tumor target (171 patients in the resection group and 190 patients in the TACE group). The 1-, 3-, and 5-year overall survival rates for the 171 patients in the resection group were 91.8%, 84.2%, and 70.8%, respectively, which were significantly greater than those of the 190 patients in the TACE group (87.9%, 76.3%, and 57.9%, respectively) (Figure 4A, P = 0.010). For the BCLC stage B HCC patients with 2 to 3 tumor targets, the 1-, 3-, and 5-year overall survival rates were 86.5%, 78.5%, and 65.1%, respectively, in the resection group and 86.3%, 67.7%, and 50.3%, respectively, in the TACE group (Fig. 4B, P = 0.002). However, the overall survival rates of the HCC patients with multiple tumors (>3 targets) did not significantly differ between the groups (1-, 3-, and 5-year overall survival rates of 68.4%, 46.0%, and 40.8%, respectively, in the resection group and 76.3%, 36.7%, and 21.6%, respectively, in the TACE group) (Fig. 4C, P = 0.064).
FIGURE 4.
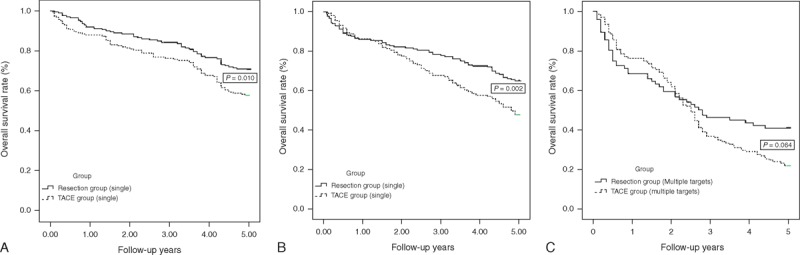
(A–C) Overall survival curves of subgroups of BCLC stage B HCC patients who received TACE or resection according to tumor target number: The benefit of resection on overall survival was much more obvious in the HCC patients with 1 to 3 targets (P < 0.05), but there was no significant difference in patients with >3 HCC targets (P = 0.064). BCLC = Barcelona Clinic Liver Cancer, HCC = hepatocellular carcinoma, TACE = transarterial chemoembolization.
Univariate and Multivariate Analyses
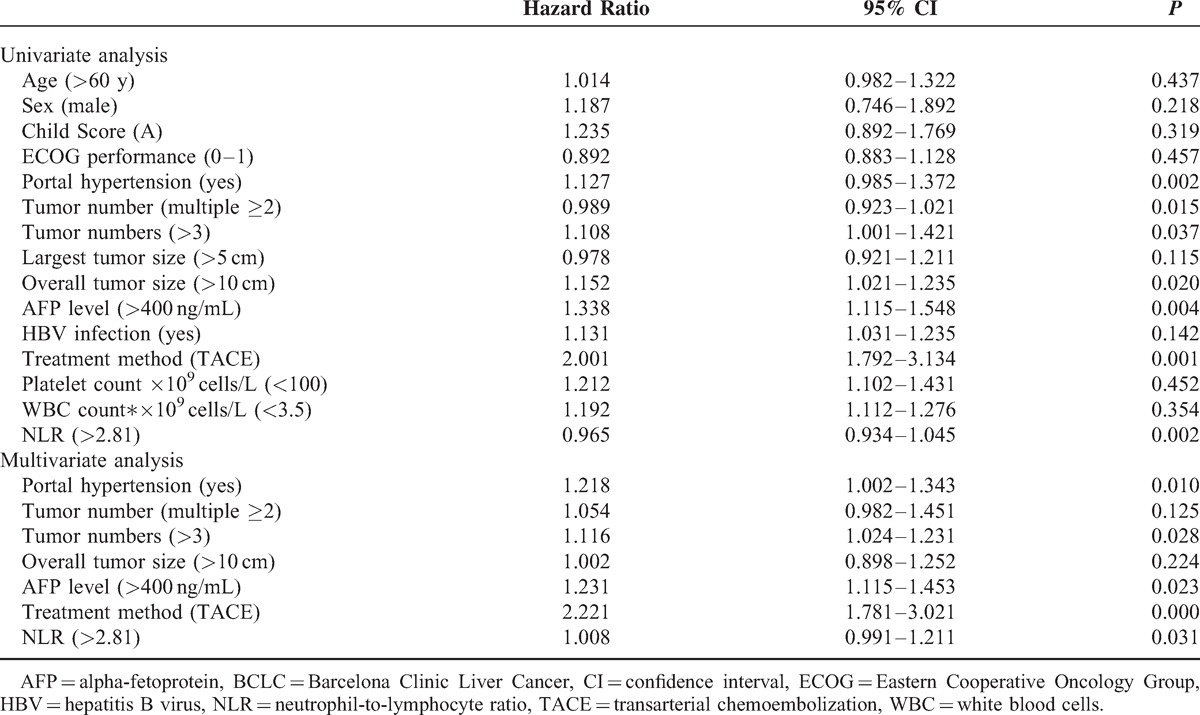
Univariate survival analysis was performed using 15 of the following variables (Table 4): age, sex, Child score, ECOG performance, portal hypertension, tumor number, largest tumor size, overall tumor size, AFP level, HBV infection, treatment method, platelet count ×109 cells/L, WBC count ×109 cells/L, and neutrophil-to-lymphocyte ratio (NLR). Portal hypertension, multiple tumor targets, overall tumor size of >10 cm, AFP level of >400 ng/mL, TACE treatment, and NLR of >2.81 were predictors of survival in the total population. Multivariate analysis demonstrated that portal hypertension (hazard ratio [HR] = 1.218, 95% confidence interval [CI] 1.002–1.343, P = 0.010), >3 tumor targets (HR = 1.116, 95% CI 1.024–1.231, P = 0.028), AFP level of >400 ng/mL (HR = 1.231, 95% CI 1.115–1.453, P = 0.023), TACE treatment (HR = 2.221, 95% CI 1.781–3.021, P < 0.01), and NLR of >2.81 (HR = 1.008, 95% CI 0.991–1.211, P = 0.031) were predictors of overall survival for the BCLC-B HCC patients.
TABLE 4.
Univariate and Multivariate Analyses of Overall Survival in BCLC Stage B Patients
Follow-up Treatment
The follow-up analyses indicated that among treatments administered after initial TACE treatment or resection, including re-TACE, re-resection, high-intensity focused ultrasound, LT, RFA, alcohol injection, transarterial chemoinfusion, gamma knife, and sorafenib, the most common follow-up treatment was TACE. No significant difference was observed between the 2 groups (P = 0.216). In the TACE group, the tumors of 48 (9.8%) patients were successfully downstaged to BCLC stage A, and radical therapies, including LT if liver graft was available, resection, or RFA, were considered to be acceptable. Regarding the overall survival rates between the groups, the 1-, 3-, and 5-year survival rates were significantly higher in the downstaged patients (95.8%, 87.5%, and 66.7%, respectively) compared with the remaining HCC patients (82.8%, 59.5%, and 42.8%, respectively) (Figure 5, P = 0.002).
FIGURE 5.
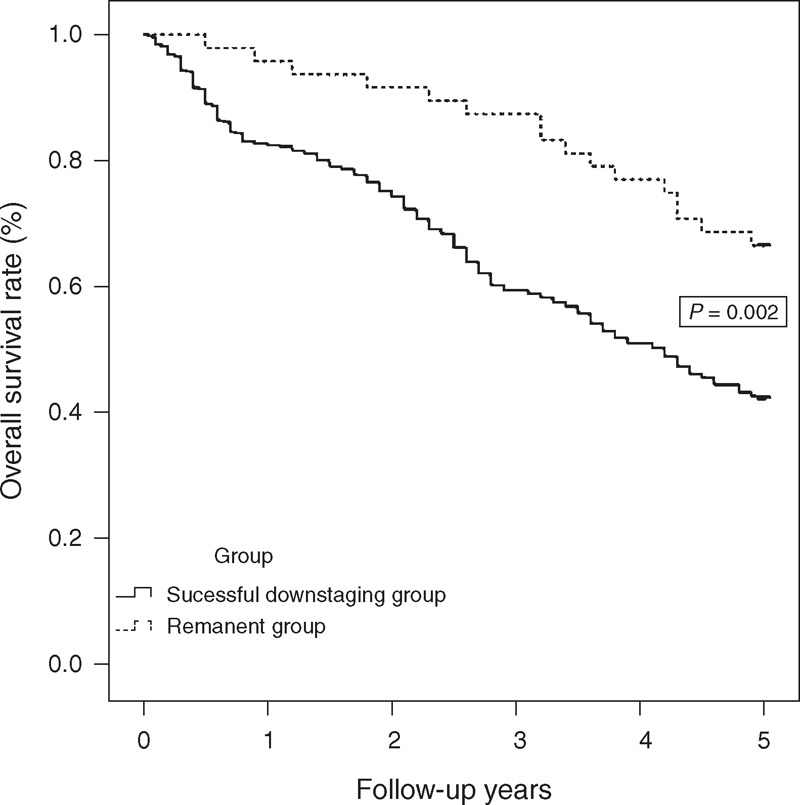
Overall survival curves of patients with successful downstaging who received TACE. The HCC patients who underwent successful TACE showed much better overall survival rates compared with the other patients (P = 0.002). HCC = hepatocellular carcinoma, TACE = transarterial chemoembolization.
DISCUSSION
Numerous staging systems have been proposed for the prognostic classification of HCC, including the Okuda20 system, tumor node metastasis,21 Cancer of the Liver Italian Program,22 Japan Integrated Staging,23 Chinese University Prognostic Index,24 and BCLC staging system.6 Because the BCLC staging system links 5 different HCC stages with the appropriate therapeutic treatment options, it is endorsed by the European Association for the Study of the Liver2 and the American Association for the Study of Liver Diseases,8 and it has been widely adopted as the staging system of choice in many countries. The BCLC-proposed treatment for intermediate-stage HCC suggests TACE as the first-line therapy because it presents with the most improved 2-year survival rate compared with more conservative treatment methods. HR for BCLC stage B HCC is considered to be a poor option associated with an unfavorable prognosis.25 However, some studies have demonstrated good results after resection in BCLC B HCC patients. Moreover, according to AASLD guidelines, TACE is the first-line therapy recommended for intermediate-stage patients; however, in select cases, alternative treatment options and strategies, including surgery, should be considered.
In the present study, most HCC cases (91%) were related to HBV infection, which is similar to other reports on Chinese patients. A majority of patients in both groups (83.9%) showed portal hypertension due to HBV or HCV infection. Several reports have indicated that elderly patients generally prefer the less-invasive TACE procedure.26 The mean age in this study was comparable between the 2 groups, and most of the HCC patients in our study were younger than those in other reports. Few elderly HCC patients were included in our study because of the lack of medical security for the elderly in China compared with other developed countries.27–30
We also evaluated and compared postoperative complications using the Clavien–Dindo classification system. Although there was a comparable postoperative complication rate between the 2 groups, the resection group displayed a much greater rate of serious complications and fewer minor complications. The following reasons could have accounted for this imbalance: first, all resection procedures were performed with the patients under general anesthesia, whereas local anesthesia was used for the TACE patients; second, the resection procedure is much more invasive and risky compared with TACE;31 and third, some serious complications, such as postoperative bleeding and biloma, were specific to resection, whereas embolism syndromes were specific to TACE and were minor (grades I or II).32 These factors may have led to longer hospitalization stays for the patients in the resection group.
In the present study, 48 patients who underwent successful TACE as downstaging therapy followed by radical therapy showed a better overall survival rate. Chemoembolization involves the mixing of iodized oil with ≥1 anticancer drugs, such as doxorubicin hydrochloride, epirubicin hydrochloride, mitomycin C, cisplatin, neocarzinostatin, or floxuridine. The mixture is then injected into tumor-feeding vessels, and the vessels are embolized with gelatin sponges. In our series, the main anticancer drug used for chemoembolization was epirubicin. TACE remains as the most commonly used palliative therapy for unresectable HCC, and it has been evaluated as a potentially effective downstaging modality in Europe33 and the United States.34 Thus, it is the recommended treatment strategy for patients with advanced HCC, according to the American Association for the Study of Liver Diseases guidelines.8 Regarding the use of LT for HCC, patient response to TACE as a dynamic criterion is readily identifiable in clinical practice, and it appears to reflect tumor biological properties and aggressiveness.35 Response criteria, such as descriptions of the size and number of nodules, have been shown to be more reliable in predicting HCC recurrence compared with the Milan or University of California,San Francisco (UCSF) criteria.36–38 mRECIST (complete response, partial response, progressive disease, and stable disease) as assessed by imaging scans after TACE is a reproducible and reliable method for differentiating responders from nonresponders.38 Moreover, as described in the present report, tumors with Lipiodol retention are more susceptible to extensive tumor necrosis after TACE. Numerous additional studies have demonstrated similar results. Patients exhibiting compact uptake of Lipiodol into their tumor had a greater probability of survival than those with less compact uptake.26 Therefore, the Lipiodol uptake pattern after TACE can be considered to be a posttreatment prognostic marker that is comparable with mRECIST. Although several groups have argued that preoperative TACE complicates surgery because of chemical hepatitis of the hepatic parenchyma, edema, hemorrhage, and adhesions, a recent review has indicated that there are no increases in morbidity and mortality after TACE in patients who have undergone resection.41 Pre-TACE can also reduce surgical risk by shrinking macroscopic tumor size, enlarging the remaining liver, clarifying the tumor margin and tumor number, and controlling or eliminating micrometastases.39 Thus, tumor response to TACE may be a useful criterion for selecting optimal candidates for HR and for avoiding unnecessary invasive surgery.
Previous randomized controlled trials have demonstrated that TACE is superior to symptomatic treatment in terms of overall survival rate.40 TACE is used as a palliative therapy for unresectable HCC, and previous studies have shown that patients with unresectable HCC, poor liver function, and multiple liver tumors or relatively large tumor sizes are eligible for this procedure.41 Our results revealed that patients in the resection group demonstrated superior survival rates compared with those in the TACE group with 1 to 3 BCLC stage B tumor targets. This finding may have been due to the superiority of HR relative to TACE in tumor ablation, although HR is far more invasive.42 However, the survival rate did not significantly differ between the TACE group and the HR group for BCLC B stage HCC with multiple tumor targets (>3 targets). Most cases of HCC with multiple tumor targets are caused by the spread of intrahepatic metastasis and micrometastases from the primary tumor, and it is unsurprising that initial treatment with hepatectomy fails to cure the majority of these patients.43 The better outcome indicated by this study compared with other reports on the resection of BCLC-B HCC26,43 is mainly due to our strict inclusion criteria, and, in particular, the increased number of patients with a solitary tumor target with a diameter of >5 cm. Downstaging with TACE and additional treatment options can allow for subsequent resection in some patients with intermediate-stage HCC. However, the prospective benefit of curative procedures applied after downstaging has not been tested to date. Furthermore, patients with intermediate-stage HCC represented a heterogeneous population because this classification included patients with wide ranges of tumor burden, liver function (Child-Pugh A or B), and disease etiology.44
The principal limitation of this study was its nonrandomized nature. However, the practical reasons that impaired our ability to perform randomized controlled trials included the differing levels of invasiveness of surgery versus TACE. In addition, most of the HCC patients possessed chronic HBV infection, which is not representative of all HCC patients worldwide. Therefore, larger, randomized, multicenter studies are needed to confirm out results.
In conclusion, surgical resection yielded better survival rates than TACE in intermediate (BCLC stage B) HCC patients with 1 to 3 tumor targets, but this approach produced similar results to TACE when >3 targets were present. Thus, because of the longer hospital stay and increased risk of more serious complications, surgical resection is only recommended for intermediate (BCLC stage B) HCC patients with 1 to 3 targets.
Acknowledgments
The authors thank American Journal Experts for their language editing service.
Footnotes
Abbreviations: AASLD = American Association for the Study of Liver Diseases, AFP = alpha-fetoprotein, BCLC = Barcelona Clinic Liver Cancer, CLIP = Cancer of the Liver Italian Program, CUPI = Chinese University Prognostic Index, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, JIS = Japan Integrated Staging, LT = liver transplantation, MeSH = medical subject headings, NLR = neutrophil–lymphocyte ratio, RFA = radiofrequency ablation, TACE = transarterial chemoembolization.
Furthermore, this article is not based on a previous presentation or meeting.
The authors have reported no conflicts of interest.
REFERENCES
- 1.Wong KM, Yeh ML, Chuang SC, et al. Survival comparison between surgical resection and percutaneous radiofrequency ablation for patients in Barcelona Clinic Liver Cancer early stage hepatocellular carcinoma. Indian J Gastroenterol 2013; 32:253–257. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001; 35:421–430. [DOI] [PubMed] [Google Scholar]
- 3.Kim JM, Kwon CH, Joh JW, et al. Patients with unresectable hepatocellular carcinoma beyond Milan criteria: should we perform transarterial chemoembolization or liver transplantation? Transplant Proc 2010; 42:821–824. [DOI] [PubMed] [Google Scholar]
- 4.Lei J, Wang W, Yan L. Surgical resection versus open-approach radiofrequency ablation for small hepatocellular carcinomas within Milan criteria after successful transcatheter arterial chemoembolization. J Gastrointest Surg 2013; 17:1752–1759. [DOI] [PubMed] [Google Scholar]
- 5.Lencioni R, Chen XP, Dagher L, et al. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist 2010; 15 Suppl 4:42–52. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999; 19:329–338. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208–1236. [DOI] [PubMed] [Google Scholar]
- 9.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology 2005; 41:707–716. [DOI] [PubMed] [Google Scholar]
- 10.Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol 2006; 44:723–731. [DOI] [PubMed] [Google Scholar]
- 11.Wang JH, Changchien CS, Hu TH, et al. The efficacy of treatment schedules according to Barcelona Clinic Liver Cancer staging for hepatocellular carcinoma - Survival analysis of 3892 patients. Eur J Cancer 2008; 44:1000–1006. [DOI] [PubMed] [Google Scholar]
- 12.Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014; 260:329–340. [DOI] [PubMed] [Google Scholar]
- 13.Zhong JH, Xiang BD, Gong WF, et al. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PloS One 2013; 8:e68193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011; 37:212–220. [DOI] [PubMed] [Google Scholar]
- 15.Ruzzenente A, Capra F, Pachera S, et al. Is liver resection justified in advanced hepatocellular carcinoma? Results of an observational study in 464 patients. J Gastrointest Surg 2009; 13:1313–1320. [DOI] [PubMed] [Google Scholar]
- 16.Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008; 134:1908–1916. [DOI] [PubMed] [Google Scholar]
- 17.Ho MC, Huang GT, Tsang YM, et al. Liver resection improves the survival of patients with multiple hepatocellular carcinomas. Ann Surg Oncol 2009; 16:848–855. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Z, Lei J, Li B, et al. Liver resection and radiofrequency ablation of very early hepatocellular carcinoma cases (single nodule <2 cm): a single-center study. Eur J Gastroenterol Hepatol 2014; 26:339–344. [DOI] [PubMed] [Google Scholar]
- 19.Lei J, Wang W, Yan L. Downstaging advanced hepatocellular carcinoma to the Milan criteria may provide a comparable outcome to conventional Milan criteria. J Gastrointest Surg 2013; 17:1440–1446. [DOI] [PubMed] [Google Scholar]
- 20.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985; 56:918–928. [DOI] [PubMed] [Google Scholar]
- 21.Hermanek P, Hutter RV, Sobin LH, et al. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer 1999; 86:2668–2673. [PubMed] [Google Scholar]
- 22.Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. The Cancer of the Liver Italian Program (CLIP) Investigators. Hepatology 2000; 31:840–845. [DOI] [PubMed] [Google Scholar]
- 23.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 2003; 38:207–215. [DOI] [PubMed] [Google Scholar]
- 24.Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer 2002; 94:1760–1769. [DOI] [PubMed] [Google Scholar]
- 25.El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008; 134:1752–1763. [DOI] [PubMed] [Google Scholar]
- 26.Lee HS, Kim KM, Yoon JH, et al. Therapeutic efficacy of transcatheter arterial chemoembolization as compared with hepatic resection in hepatocellular carcinoma patients with compensated liver function in a hepatitis B virus-endemic area: a prospective cohort study. J Clin Oncol 2002; 20:4459–4465. [DOI] [PubMed] [Google Scholar]
- 27.Hong J, Song Y, Liu J, et al. Perception and fulfillment of cancer patients’ nursing professional social support needs: from the health care personnel point of view. Support Care Cancer 2014; 22:1049–1058. [DOI] [PubMed] [Google Scholar]
- 28.Fang K, Shia BC, Ma S. Health insurance coverage, medical expenditure and coping strategy: evidence from Taiwan. BMC Health Services Res 2012; 12:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei KR, Chen WQ, Zhang SW, et al. Cancer registration in the Peoples Republic of China. Asian Pac J Cancer Prev 2012; 13:4209–4214. [DOI] [PubMed] [Google Scholar]
- 30.Zhang NJ, Guo M, Zheng X. China: awakening giant developing solutions to population aging. Gerontologist 2012; 52:589–596. [DOI] [PubMed] [Google Scholar]
- 31.Choi JY. Treatment algorithm for intermediate and advanced stage hepatocellular carcinoma: Korea. Oncology 2011; 81 Suppl 1:141–147. [DOI] [PubMed] [Google Scholar]
- 32.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology 2010; 52:762–773. [DOI] [PubMed] [Google Scholar]
- 33.Herrero JI, Sangro B, Pardo F, et al. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transplant 2008; 14:272–278. [DOI] [PubMed] [Google Scholar]
- 34.Yao FY, Kinkhabwala M, LaBerge JM, et al. The impact of pre-operative loco-regional therapy on outcome after liver transplantation for hepatocellular carcinoma. Am J Transplant 2005; 5 (4 Pt 1):795–804. [DOI] [PubMed] [Google Scholar]
- 35.Otto G, Schuchmann M, Hoppe-Lotichius M, et al. How to decide about liver transplantation in patients with hepatocellular carcinoma: size and number of lesions or response to TACE? J Hepatol 2013; 59:279–284. [DOI] [PubMed] [Google Scholar]
- 36.Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transplant 2006; 12:1260–1267. [DOI] [PubMed] [Google Scholar]
- 37.Vitale A, D’Amico F, Frigo AC, et al. Response to therapy as a criterion for awarding priority to patients with hepatocellular carcinoma awaiting liver transplantation. Ann Surg Oncol 2010; 17:2290–2302. [DOI] [PubMed] [Google Scholar]
- 38.Bargellini I, Bozzi E, Campani D, et al. Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur J Radiol 2013; 82:e212–e218. [DOI] [PubMed] [Google Scholar]
- 39.Chua TC, Liauw W, Saxena A, et al. Systematic review of neoadjuvant transarterial chemoembolization for resectable hepatocellular carcinoma. Liver Int 2010; 30:166–174. [DOI] [PubMed] [Google Scholar]
- 40.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002; 359:1734–1739. [DOI] [PubMed] [Google Scholar]
- 41.O'Suilleabhain CB, Poon RT, Yong JL, et al. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Brit J Surg 2003; 90:325–331. [DOI] [PubMed] [Google Scholar]
- 42.Bronowicki JP, Boudjema K, Chone L, et al. Comparison of resection, liver transplantation and transcatheter oily chemoembolization in the treatment of hepatocellular carcinoma. J Hepatol 1996; 24:293–300. [DOI] [PubMed] [Google Scholar]
- 43.Luo J, Peng ZW, Guo RP, et al. Hepatic resection versus transarterial lipiodol chemoembolization as the initial treatment for large, multiple, and resectable hepatocellular carcinomas: a prospective nonrandomized analysis. Radiology 2011; 259:286–295. [DOI] [PubMed] [Google Scholar]
- 44.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008; 100:698–711. [DOI] [PubMed] [Google Scholar]


