Abstract
Dementia is among various diseases affecting the elderly, who is also at a high risk for fractures. This study aimed to evaluate the association between fracture history and sequential risk of dementia in Taiwan.
A retrospective cohort study was designed using the claims data of the entire insured residents covered by Taiwan's universal health insurance from 1998 to 2010. A total of 66,797 patients with fractures and 133,594 control subjects without fractures were matched in terms of age (±5 years), sex, and index year and then recruited. Fractures and dementia were defined in accordance with the International Classification of Diseases, 9th Revision, Clinical Modification. The influence of fractures on the risk of dementia was analyzed using a Cox proportional hazards model.
After a 12-year follow-up period, 2775 and 3991 incident cases of dementia were reported in exposed and unexposed cohorts, respectively. The overall incidence rate of dementia in individuals with fractures was 41% higher than that in individuals without fractures (6.05 vs 4.30 per 1000 person-years) at an adjusted hazard ratio of 1.38 (95% confidence interval 1.32–1.45) after age, sex, urbanization, and individual disorders or comorbidities were adjusted. Considering fracture location, we found that patients with hip fractures were at a slightly high risk for dementia. The occurrence of multiple fractures at a single visit was also significantly associated with an increased risk of dementia.
Fracture history is regarded as an independent risk factor of dementia in individuals aged ≥65 years, particularly those who suffered from multiple fractures and/or fractures located in the hip. Further studies are needed to support an independent role of fracture in dementia considering the clinical information and other comorbidities.
INTRODUCTION
Dementia is characterized by slow progressive memory loss, impaired cognitive function, and inability to perform personal daily activities. Approximately 35.6 million individuals suffered from dementia worldwide in 2010; the number of affected individuals is expected to increase twice in every 20 years and may reach 65.7 million in 2030 and 115.4 million in 205038; as such, dementia is considered as one of the common reasons that individuals are admitted in long-term care units for. This condition is also reported as one of modern society's greatest public health problems. However, the exact pathogenesis of dementia remains unknown. Previous studies identified possible risk factors, including advanced age, female sex, low educational level, family history, and apolipoprotein E genotype.61 Other risk factors, such as cardiovascular diseases, depression, head trauma, and diabetes mellitus, have also been identified.45
Studies have shown that individuals who suffer from dementia or cognitive impairment are at a substantially higher risk for sustaining a hip fracture than those who are cognitively intact.13,16,21,39,49 In Australia, the prevalence rate of dementia in fall-related incidents leading to hip fracture hospitalizations is approximately 24% to 29%, and patients suffering from hip fracture and dementia exhibit a greater mortality rate than those without dementia.51 Other studies have reported that delirium, which is one of the main predictors of dementia, may occur following hip fracture surgery.14,25,35,36 However, studies have yet to determine whether or not a previous fracture increases the risk of dementia. Therefore, we conducted this nationwide population-based retrospective cohort study by using the database of a universal insurance program to evaluate the association between fracture history and the risk of dementia.
METHODS
Study Design and Participants
The Taiwan National Health Insurance (NHI) program was established in 1995; in this system, 13 insurance programs are consolidated in 1 universal system of health care for all residents. The NHI program exhibited a coverage rate of approximately 99% among 23.74 million Taiwan residents and provided contracts to 97% of hospitals and clinics in 2009 (http://nhird.nhri.org.tw/en/index.htm).
Patient data from the NHI research database (NHIRD) were scrambled to generate relevant information. We performed this retrospective cohort study by using a randomly selected population of 1 million insured subjects. All of the patients aged ≥20 years and diagnosed with fracture from 1998 to 2010 were identified as the fracture cohort. The remaining patients without fracture history during the same period were grouped as the nonfracture cohort. However, patients who revealed a history of dementia at recruitment were excluded from both cohorts. The nonfracture cohort was frequency-matched at a ratio of 1:2 for sex, age (every 5 years), and index-year with the fracture cohort. To calculate all of the incident cases of dementia, we set the follow-up period from 1998 until withdrawal or until December 31, 2010, whichever came first. We then analyzed whether or not fracture is associated with an increased risk of developing dementia. The study was approved by the Institutional Review Board of China Medical University (CMU-REC-101-012).
Definition of Variables
The explored variables included age, sex, urbanization, and comorbidity. Urbanization was categorized into 4 levels based on the population density of a residential area, in which level 1 was considered as the most urbanized and level 4 was considered as the least urbanized. The International Classification of Diseases, Revision 9, Clinical Modification (ICD-9-CM) codes was used to define the diagnoses. The fracture cohort consisted of patients diagnosed with fracture (ICD-9-CM 800–829) based on primary discharge diagnosis. The nonfracture cohort consisted of other insured individuals without fractures. We identified new dementia events (ICD-9-CM 290, 294.1, 331.0) from outpatient and inpatient medical records and confirmed these events with at least 3 medical visits to increase the validity of the diagnoses. Physicians diagnosed dementia based on medical history, psychological tests, physical and neurological exams, blood tests, and brain imaging to rule out other diseases with dementia-like symptoms. The diagnostic criteria used in Taiwan were in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.59 Patients with at least 1 service claim from 1998 to 2010 for either outpatient or inpatient care at recruitment were evaluated for comorbidities, such as diabetes (ICD-9-CM 250), hypertension (ICD-9-CM 401–405), stroke (ICD-9-CM 430–438), coronary artery disease (ICD-9-CM 410–414), head injury (ICD-9-CM 850–854, 959.01), depression (ICD-9-CM 296.2, 296.3, 300.4, and 311), and cognitive impairment (ICD-9 331.83, 438.0, 310.8, and 294.9). To evaluate the severity of fractures, we also analyzed the numbers and locations of fractures, such as vertebrae (ICD-9-CM 806.20–806.9), upper limbs (ICD-9-CM 810, 812–813), hip (ICD-9-CM 820), and thigh/leg/ankle (ICD-9-CM 821, 823–825). We defined multiple fractures as ≥2 fractures in a single visit. A secondary fracture was defined as the recurrence of fracture after more than a year.
Statistical Analysis
The distribution of categorical variables and the proportions of comorbidities were compared and examined using the χ2 test between fracture and nonfracture cohorts. The incidence rate ratio (IRR) and 95% confidence interval (CI) of the risk of dementia were evaluated using Poisson regression models. Cox proportional hazards models were used to investigate the association between fracture history and the risk of developing dementia over time, after age, sex, urbanization, and comorbidities of diabetes, hypertension, stroke, coronary artery disease, head injury, depression, and cognitive impairment were adjusted. We further analyzed whether or not the risk of dementia varies when these factors were stratified with the duration of the follow-up period after fractures were diagnosed. For individuals aged ≥65 years, the risk of dementia stratified with varied locations and numbers of fractures were investigated. We compared the effects of fractures on the risk of Alzheimer disease (ICD-9-CM 331.0) and dementia. Statistical analyses were performed using the SAS statistical package (version 9.2 for Windows; SAS Institute Inc, Cary, NC). A 2-tailed P < 0.05 was considered significant.
RESULTS
Demographics and Sample Characteristics
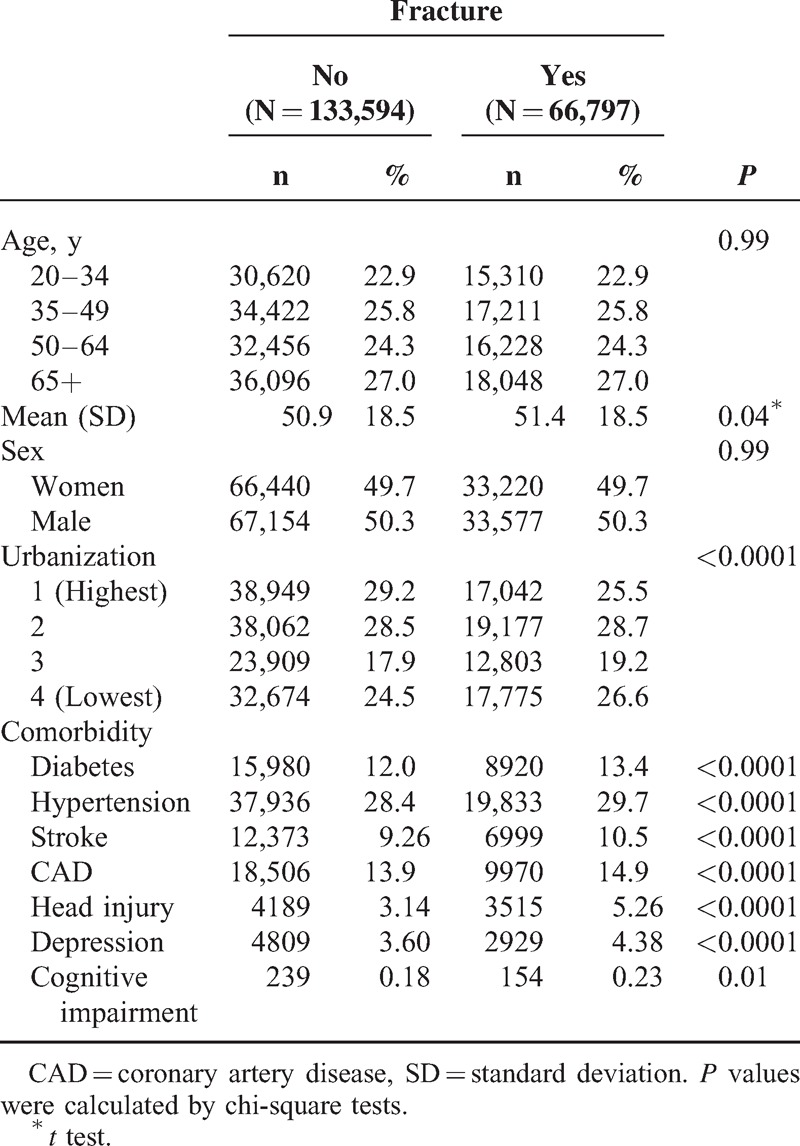
A total of 66,797 and 133,594 individuals were included in the fracture cohort and the nonfracture cohort, respectively (Table 1). Both cohorts were similar in terms of age and sex distribution with mean age values of 51.4 years in the fracture cohort and 50.9 years in the nonfracture cohort. The prevalence of comorbidities including all of the individual disorders was higher in the fracture cohort than in the nonfracture cohort (Table 1).
TABLE 1.
Comparison of Demographics and Comorbidity Between Fracture Patients and Controls
Association Between Fractures and Incidence of Dementia
After the 12-year follow-up period, a total of 2775 and 3991 incident cases of dementia were reported in exposed and unexposed cohorts, respectively. Mean durations during the index year of fracture and dementia incidence were 6.86 (SD = 3.38) and 6.95 (SD = 3.36) in exposed and unexposed cohorts, respectively. The incidence rate and adjusted hazard ratio (AHR) between the exposed cohort and the unexposed cohort are shown in Table 2. The overall incidence rate of dementia was 41% higher in the exposed cohort than in the unexposed cohort (crude rate of 6.05 vs 4.30 per 1000 person-years, respectively) with a crude HR of 1.41 (95% CI 1.37–1.45). After adjusting for age, sex, urbanization, and individual disorders or comorbidities, we found that AHR was 1.39 (95% CI 1.32–1.45). Approximately 1.3- to 1.5-fold risk of dementia was shown after the factors were stratified with the follow-up duration. We also observed a similar positive association after stratification with sex, age, and urbanization was performed. The incidence rates of dementia were the highest in the elderly (age ≥65 years) in both cohorts (16.3 vs 23.1 per 1000 person-years). The IRR of dementia for the youngest age group (20–34 years) was also high (IRR = 4.95, 95% CI 4.56–5.39) with an AHR of 4.48 (95% CI 1.96–10.2). A significantly increased risk of dementia was similarly observed regardless of the presence of comorbidities. The results of the log-rank test and cumulative incidence curve of dementia showed that the patients with fractures had a significantly higher incidence of dementia than the subjects in the comparison cohort (log-rank P < 0.001) (Figure 1).
TABLE 2.
Incidence and Adjusted HR of Dementia Stratified By Sex, Age, and Comorbidity Compared Between Patients With Fracture and Without Fracture

FIGURE 1.
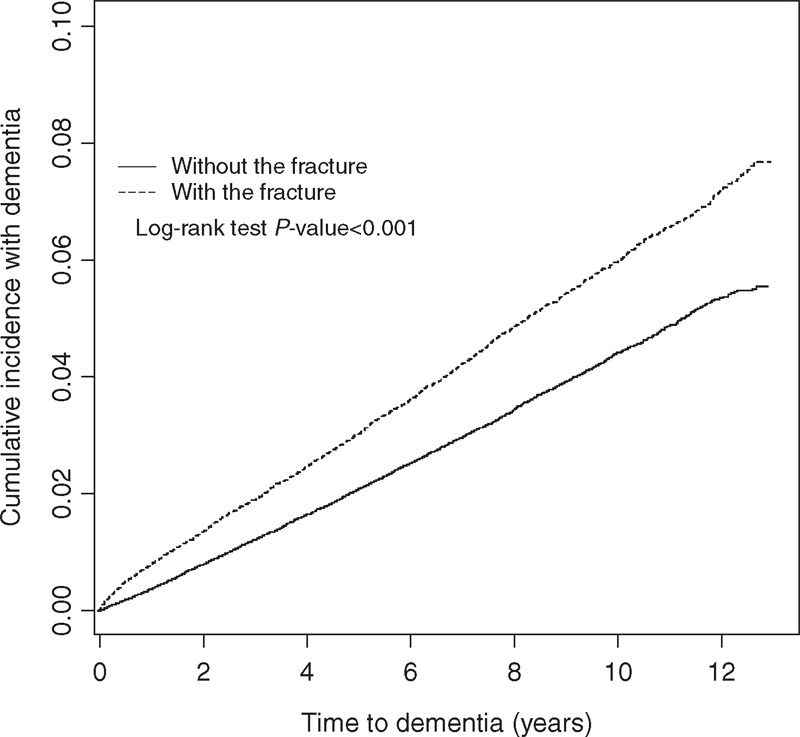
Cumulative incidence of dementia compared between with (dashed line) and without (solid line) fracture.
The incidence rate of dementia was higher in patients with comorbidities than in patients without comorbidities. The positive associations of fracture and dementia ranged from 1.3- to 1.5-fold risk after other potential risk factors were adjusted (data not shown). Table 3 shows the incidence rates and AHR of dementia according to the number and location of fractures in individuals aged ≥65 years. The patients with hip fractures exhibited a 60% higher risk of dementia (AHR 1.60, 95% CI 1.43–1.79), those with fracture in the vertebrae revealed a 47% higher risk (AHR 1.47, 95% CI 1.11–1.94), those with thigh/leg/ankle fracture presented a 35% higher risk (AHR 1.35, 95% CI 1.18–1.55), and those with an upper limb fracture displayed a 29% higher risk of dementia (AHR 1.29, 95% CI 1.17–1.43) compared with the unexposed cohort. It suggested that those with hip fractures had slightly high risk of dementia. Patients with multiple fractures also exhibited a significantly increased risk of dementia. We further distinguished the incidence of Alzheimer disease from dementia and analyzed the data in Table 4. Similar risks of positive associations were observed in Alzheimer disease and dementia (AHR 1.29, 95% CI 1.04–1.61 and AHR 1.39, 95% CI 1.32–1.46, respectively).
TABLE 3.
Incidence and Adjusted HR of Dementia Stratified by Different Parts and Numbers of Fracture in Patients aged ≥65 years

TABLE 4.
Comparisons of HR Between Patients With and Without Fracture for Different Outcomes (Dementia and Alzheimer's Disease)

DISCUSSION
The current study demonstrated that fracture history could lead to an increased risk of dementia, particularly in individuals aged ≥65 years and suffered from multiple fractures and/or fracture/s in the hip.
Dementia and fractures are 2 adverse conditions commonly affecting the elderly; both conditions are associated with the same frailty-related risk factors, such as old age, vitamin D deficiency, presence of apolipoprotein E4, and lifestyle.13,32 Patients with low bone mineral density are at a high risk for fractures, and this condition is associated with low hypothalamic volume in the early onset of Alzheimer disease; this result suggests that the central mechanisms of bone remodeling may be disrupted by neurodegeneration.31
The cause of dementia remains uncertain and may include multiple factors. To our knowledge, no histological or animal study has shown that fracture can directly cause sequential dementia. The possible biological mechanism may be associated with the formation of excess superoxide radicals during fracture healing. In the first few weeks after fracture, oxidative stress likely occurs, as excess superoxide radicals are produced to remove debris.50 The overproduction of reactive oxygen species induces endothelial dysfunction, thereby leading to vascular dementia and causing increased amounts of amyloid-beta peptides in patients with Alzheimer disease.5 After fracture, consequences, such as decline in physical function, chronic pain, and prolonged inflammatory cytokine secretion during fracture repair, may contribute to dementia.
The quality of life after an individual suffers from fracture has an important influence on the development of dementia. For instance, a decline in physical function and changes in appearance may contribute to a loss of social engagement and physical exercise; as a result, the quality of life of patients is impaired. Fracture has also been considered as an independent influencing factor of the development of functional decline in the elderly regardless of prefracture health conditions.47 Chronic pain after an individual suffers from fracture also contributes to cognitive decline in later life. After suffering from fracture, 7% of patients likely develop complex regional pain syndrome, and none of these patients are relieved from this symptom after 1 year.3 Chronic pain also affects approximately 48.4% of patients after these individuals suffer from pelvic fracture.40 The interaction of pain and psychiatric depression is a vicious cycle, that is, pain causes depression and depression causes pain.7,11,18,28,34 Furthermore, chronic pain can be considered as a brain disease, in which alterations in neural networks affect multiple aspects of brain function, structure, and chemistry; such changes also induce behavioral and psychiatric symptoms.6,55 Pain also affects cognitive function and elicits changes in the central nervous system, specifically on emotional processing.
A fracture may increase the rate of degeneration via inflammation that contributes to cognitive decline. Neuroendocrine responses mediate macrophage function after trauma.33 For example, interleukin-6 (IL-6) regulates the differentiation of osteoblasts and osteoclasts; IL-6 also promotes angiogenesis by stimulating the release of vascular endothelial growth factors.62 IL-6 concentrations remain high for several months after fracture41,44; such high concentrations may cause bone remodeling and bone loss.43 IL-6 has also been considered as a key factor in inflammation in the pathophysiology of depressive symptoms after individuals suffer from hip fracture.37 High levels of IL-6 have also been reported in elderly patients suffering from hip fracture and impaired mental status.4 Furthermore, increased IL-6 levels are associated with an increased risk of Alzheimer disease.30 In addition to these factors, inflammation and increased peripheral cytokine levels are associated with depression-like symptoms and neuropsychological disturbances in humans.9,63
The present study found a higher risk of dementia in younger individuals or males with fractures than in other patients. Patients with low or major limb fractures also exhibited an increased risk of dementia compared with individuals suffering from upper limb or spine fractures. Various mechanisms, chronic pain, decreased physical activities, and prolonged inflammatory conditions after patients suffered from fracture may partially explain the differences in the risk of dementia in terms of sex, fracture site, and age. For example, young subjects who experience fracture may suffer from chronic pain and system inflammation for a long period; therefore, these individuals may exhibit an early deterioration of physical status. Alzheimer disease may also exhibit an early onset, in which symptoms are manifested before patients reach 65 years of age. However, only a few population-based studies on the epidemiology of young-onset dementia have been reported. For instance, Harvey et al17 estimated that the prevalence of dementia at the onset age of 30 and 65 years is 54 cases per 100,000 individuals (95% CI 45–64); by comparison, the prevalence rate of dementia at the onset age of 30 to 44 years is 12.1 cases per 100,000 individuals. Using a 2-step postal survey with a population of 2,966,000 individuals, Ikejima et al40 found a prevalence of 42.3 cases per 100,000 individuals (95% CI 39–45) in the Ibaraki prefecture in Japan between the ages of 18 and 65 years. In the previous study, the most frequent cause of early-onset dementia is vascular dementia (42.5%).19 Kelley et al23 also evaluated 235 patients with progressive cognitive decline between the ages of 17 and 45 years; the result showed that patients with an onset age of <35 years mainly manifest metabolic causes with inborn errors. Neurodegenerative etiologies are also common when individuals reach 35 years of age. Furthermore, mutations in the genes encoding amyloid precursor protein, presenilin 1, and presenilin 2 are responsible for early-onset autosomal-dominant Alzheimer disease.15 One of the functions of amyloid precursor protein is related to the suppression of osteoblastogenesis and bone formation,60 implicating amyloid precursor protein as a risk factor of osteoporosis and bone fracture. This finding could partially explain the association between fracture and young onset of dementia. However, the incident cases of dementia affecting 20-year to 34-year age group were small in our present analysis; as such, a wide range of CI was obtained. Hence, further studies should be conducted to investigate the association between fracture and dementia in young populations. In women, the low risk of dementia can be explained by the protective effect of estrogen, which elicits an anti-inflammatory effect that decreases the damage caused by cytokine secretion; this hormone also functions against neurodegeneration.20,29,42,46,48,53,57 In addition, surgery for a fracture induces sequential inflammatory processes.24,54,58 Further studies should also be conducted to determine whether or not other aging processes, physical conditions, and inflammation mediate fractures and cognitive decline.
Multiple factors can cause dementia after an individual suffers from fracture. Each factor may also exhibit a different effect with time. Interventions to prevent dementia in later life should be multidisciplinary and comprise medical, social, physical, and psychological strategies. For example, antioxidants, as nutrient supplements, help prevent dementia after an individual suffers from fracture.56 Vitamin E also produces beneficial effects on new bone formation by reducing lipid peroxidation during early fracture healing26,52; foods rich in vitamin E also reduce the long-term risk of dementia.10 Furthermore, clinical applications, including early successful stabilization, nutrient supplementation, postoperative rehabilitation, and regular exercise,12 help reduce the risk of dementia. Physical activity also reduces the risk of developing dementia, and a higher level of midlife fitness is possibly associated with a low risk of dementia in later life. Moreover, physical activity or exercise is considered as an important protective factor reducing the risk of dementia.1,2,8,27 In a previous study, the association among cognitive impairment and exercise, cognitive activities, and socialization was investigated and the result showed that exercise is associated with a low risk of cognitive impairment in the 10-year follow-up period, but this low risk is not statistically significant in the 5-year follow-up period.22
The strengths of this study include the use of population-based data that are highly representative of a general population. However, several factors limit this study. First, the NHIRD does not contain detailed information regarding BMI, smoking habit, alcohol consumption, educational status, patient's living conditions (nursing home vs community), and preinjury physical and mental status, which may be considered as risk factors of dementia. In addition, all of the data in the NHIRD are confidential. Thus, relevant clinical variables, such as bone mineral density, fracture severity, surgical methods, and serum laboratory data were unavailable for analysis. Second, the evidence derived from a retrospective cohort study is generally lower in statistical quality than that from randomized trials because of potential bias related to adjustments for confounding variables. Third, we cannot exclude the possibility of bidirectional relationship between facture and dementia because dementia usually has a long preclinical phase. In addition, we could not recruit individuals with mild dementia or others who did not seek medical care in our present analysis. Individuals with fracture are more likely to consult a doctor than those without fracture, and this difference may cause detection bias because of frequent examinations. These factors may also weaken the true association. If individuals who suffer from fracture were more likely to undergo diagnosis for dementia, these individuals could pose a high risk of dementia in early follow-up periods. However, similar risks of approximately 1.3- to 1.6-fold were observed in our analysis. Nevertheless, the data regarding fracture and dementia diagnoses were reliable.
CONCLUSIONS
In conclusion, a fracture could be considered as an independent risk factor of dementia. A comprehensive understanding of the pathways and their relative effects on the outcomes of fractures could provide the basis of the development of effective interventions and potentially improve preventative efforts. Further studies are needed to support an independent role of fracture in dementia considering the clinical information and other comorbidities.
Footnotes
Abbreviations: ICD-9-CM = International Classification of Diseases, Revision 9, Clinical Modification, NHI = National Health Insurance, NHIRD = National Health Insurance research database.
This study was supported by the National Sciences Council, Executive Yuan (Grant Nos. NSC 99–2621-M-039–001 and NSC 100–2621-M-039–001), the Department of Health (Grant Nos. DOH101-TD-B-111–004 and DOH101-TD-C-111–005), the Taiwan Department of Health Clinical Trial and Research Center for Excellence (Grant No. DOH101-TD-B-111–004), the Department of Health Cancer Research Center for Excellence (Grant No. DOH101-TD-C-111–005), and the China Medical University Hospital (Grant No. DMR-102–056). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors disclosed all financial and interpersonal relationships that could be viewed as a potential conflict of interest.
REFERENCES
- 1.Aarsland D, Sardahaee FS, Anderssen S, et al. Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment Health 2010; 14:386–395. [DOI] [PubMed] [Google Scholar]
- 2.Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc 2011; 86:876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beerthuizen A, Stronks DL, Van’t Spijker A, et al. Demographic and medical parameters in the development of complex regional pain syndrome type 1 (CRPS1): prospective study on 596 patients with a fracture. Pain 2012; 153:1187–1192. [DOI] [PubMed] [Google Scholar]
- 4.Beloosesky Y, Hendel D, Weiss A, et al. Cytokines and C-reactive protein production in hip-fracture-operated elderly patients. J Gerontol A Biol Sci Med Sci 2007; 62:420–426. [DOI] [PubMed] [Google Scholar]
- 5.Bennett S, Grant MM, Aldred S. Oxidative stress in vascular dementia and Alzheimer's disease: a common pathology. J Alzheimers Dis 2009; 17:245–257. [DOI] [PubMed] [Google Scholar]
- 6.Borsook D. Neurological diseases and pain. Brain 2012; 135 (Pt 2):320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borsook D, Becerra L, Carlezon WA, Jr, et al. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain 2007; 11:7–20. [DOI] [PubMed] [Google Scholar]
- 8.Defina LF, Willis BL, Radford NB, et al. The association between midlife cardiorespiratory fitness levels and later-life dementia: a cohort study. Ann Intern Med 2013; 158:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DellaGioia N, Hannestad J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci Biobehav Rev 2010; 34:130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devore EE, Grodstein F, van Rooij FJ, et al. Dietary antioxidants and long-term risk of dementia. Arch Neurol 2010; 67:819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elman I, Zubieta JK, Borsook D. The missing p in psychiatric training: why it is important to teach pain to psychiatrists. Arch Gen Psychiatry 2011; 68:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson Sorman D, Sundstrom A, Ronnlund M, et al. Leisure activity in old age and risk of dementia: a 15-year prospective study. J Gerontol B Psychol Sci Soc Sci 2014; 69:493–501. [DOI] [PubMed] [Google Scholar]
- 13.Friedman SM, Menzies IB, Bukata SV, et al. Dementia and hip fractures: development of a pathogenic framework for understanding and studying risk. Geriatr Orthop Surg Rehabil 2010; 1:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Givens JL, Sanft TB, Marcantonio ER. Functional recovery after hip fracture: the combined effects of depressive symptoms, cognitive impairment, and delirium. J Am Geriatr Soc 2008; 56:1075–1079. [DOI] [PubMed] [Google Scholar]
- 15.Guerreiro RJ, Gustafson DR, Hardy J. The genetic architecture of Alzheimer's disease: beyond APP, PSENs and APOE. Neurobiol Aging 2012; 33:437–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harboun M, Dorenlot P, Cohen N, et al. Impact of hip fracture, heart failure and weight loss on the risk of institutionalization of community-dwelling patients with dementia. Int J Geriatr Psychiatry 2008; 23:1245–1252. [DOI] [PubMed] [Google Scholar]
- 17.Harvey RJ, Skelton-Robinson M, Rossor MN. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry 2003; 74:1206–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Husain MM, Rush AJ, Trivedi MH, et al. Pain in depression: STAR∗D study findings. J Psychosom Res 2007; 63:113–122. [DOI] [PubMed] [Google Scholar]
- 19.Ikejima C, Yasuno F, Mizukami K, et al. Prevalence and causes of early-onset dementia in Japan: a population-based study. Stroke 2009; 40:2709–2714. [DOI] [PubMed] [Google Scholar]
- 20.Inagaki T, Etgen AM. Neuroprotective action of acute estrogens: animal models of brain ischemia and clinical implications. Steroids 2013; 78:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jalbert JJ, Eaton CB, Miller SC, et al. Antipsychotic use and the risk of hip fracture among older adults afflicted with dementia. J Am Med Dir Assoc 2010; 11:120–127. [DOI] [PubMed] [Google Scholar]
- 22.Jedrziewski MK, Ewbank DC, Wang H, et al. The impact of exercise, cognitive activities, and socialization on cognitive function: results from the National Long-Term Care Survey. Am J Alzheimers Dis Other Demen 2014; Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley BJ, Boeve BF, Josephs KA. Young-onset dementia: demographic and etiologic characteristics of 235 patients. Arch Neurol 2008; 65:1502–1508. [DOI] [PubMed] [Google Scholar]
- 24.Kohl BA, Deutschman CS. The inflammatory response to surgery and trauma. Curr Opin Crit Care 2006; 12:325–332. [DOI] [PubMed] [Google Scholar]
- 25.Krogseth M, Wyller TB, Engedal K, et al. Delirium is an important predictor of incident dementia among elderly hip fracture patients. Dement Geriatr Cogn Disord 2011; 31:63–70. [DOI] [PubMed] [Google Scholar]
- 26.Kurklu M, Yildiz C, Kose O, et al. Effect of alpha-tocopherol on bone formation during distraction osteogenesis: a rabbit model. J Orthop Traumatol 2011; 12:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lautenschlager NT, Almeida OP. Physical activity and cognition in old age. Curr Opin Psychiatry 2006; 19:190–193. [DOI] [PubMed] [Google Scholar]
- 28.Leone M, Proietti Cecchini A, Franzini A, et al. Lessons from 8 years’ experience of hypothalamic stimulation in cluster headache. Cephalalgia 2008; 28:787–797.discussion 98. [DOI] [PubMed] [Google Scholar]
- 29.Liang Z, Valla J, Sefidvash-Hockley S, et al. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer's disease patients. J Neurochem 2002; 80:807–814. [DOI] [PubMed] [Google Scholar]
- 30.Licastro F, Pedrini S, Caputo L, et al. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer's disease: peripheral inflammation or signals from the brain? J Neuroimmunol 2000; 103:97–102. [DOI] [PubMed] [Google Scholar]
- 31.Loskutova N, Honea RA, Brooks WM, et al. Reduced limbic and hypothalamic volumes correlate with bone density in early Alzheimer's disease. J Alzheimers Dis 2010; 20:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lui LY, Stone K, Cauley JA, et al. Bone loss predicts subsequent cognitive decline in older women: the study of osteoporotic fractures. J Am Geriatr Soc 2003; 51:38–43. [DOI] [PubMed] [Google Scholar]
- 33.Maddali S, Stapleton PP, Freeman TA, et al. Neuroendocrine responses mediate macrophage function after trauma. Surgery 2004; 136:1038–1046. [DOI] [PubMed] [Google Scholar]
- 34.Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci 2009; 14:5291–5338. [DOI] [PubMed] [Google Scholar]
- 35.Marcantonio ER, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc 2000; 48:618–624. [DOI] [PubMed] [Google Scholar]
- 36.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001; 49:516–522. [DOI] [PubMed] [Google Scholar]
- 37.Matheny ME, Miller RR, Shardell MD, et al. Inflammatory cytokine levels and depressive symptoms in older women in the year after hip fracture: findings from the Baltimore Hip Studies. J Am Geriatr Soc 2011; 59:2249–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2012; 2:a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melton LJ, 3rd, Leibson CL, Achenbach SJ, et al. Fracture risk after the diagnosis of Parkinson's disease: influence of concomitant dementia. Mov Disord 2006; 21:1361–1367. [DOI] [PubMed] [Google Scholar]
- 40.Meyhoff CS, Thomsen CH, Rasmussen LS, et al. High incidence of chronic pain following surgery for pelvic fracture. Clin J Pain 2006; 22:167–172. [DOI] [PubMed] [Google Scholar]
- 41.Miller RR, Cappola AR, Shardell MD, et al. Persistent changes in interleukin-6 and lower extremity function following hip fracture. J Gerontol A Biol Sci Med Sci 2006; 61:1053–1058. [DOI] [PubMed] [Google Scholar]
- 42.Numakawa T, Matsumoto T, Numakawa Y, et al. Protective action of neurotrophic factors and estrogen against oxidative stress-mediated neurodegeneration. J Toxicol 2011; 2011:405194.12 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papanicolaou DA, Wilder RL, Manolagas SC, et al. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med 1998; 128:127–137. [DOI] [PubMed] [Google Scholar]
- 44.Pape HC, Schmidt RE, Rice J, et al. Biochemical changes after trauma and skeletal surgery of the lower extremity: quantification of the operative burden. Crit Care Med 2000; 28:3441–3448. [DOI] [PubMed] [Google Scholar]
- 45.Patterson C, Feightner JW, Garcia A, et al. Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. CMAJ 2008; 178:548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereno GL, Balaszczuk V, Beltramino CA. Effect of sex differences and gonadal hormones on kainic acid-induced neurodegeneration in the bed nucleus of the stria terminalis of the rat. Exp Toxicol Pathol 2012; 64:283–289. [DOI] [PubMed] [Google Scholar]
- 47.Piirtola M, Lopponen M, Vahlberg T, et al. Fractures as an independent predictor of functional decline in older people: a population-based study with an 8-year follow-up. Gerontology 2012; 58:296–304. [DOI] [PubMed] [Google Scholar]
- 48.Pozzi S, Benedusi V, Maggi A, et al. Estrogen action in neuroprotection and brain inflammation. Ann N Y Acad Sci 2006; 1089:302–323. [DOI] [PubMed] [Google Scholar]
- 49.Ranstam J, Elffors L, Kanis JA. A mental-functional risk score for prediction of hip fracture. Age Ageing 1996; 25:439–442. [DOI] [PubMed] [Google Scholar]
- 50.Reilly PM, Schiller HJ, Bulkley GB. Pharmacologic approach to tissue injury mediated by free radicals and other reactive oxygen metabolites. Am J Surg 1991; 161:488–503. [DOI] [PubMed] [Google Scholar]
- 51.Scandol JP, Toson B, Close JC. Fall-related hip fracture hospitalisations and the prevalence of dementia within older people in New South Wales, Australia: an analysis of linked data. Injury 2013; 44:776–783. [DOI] [PubMed] [Google Scholar]
- 52.Shuid AN, Mohamad S, Muhammad N, et al. Effects of alpha-tocopherol on the early phase of osteoporotic fracture healing. J Orthop Res 2011; 29:1732–1738. [DOI] [PubMed] [Google Scholar]
- 53.Spampinato SF, Molinaro G, Merlo S, et al. Estrogen receptors and type 1 metabotropic glutamate receptors are interdependent in protecting cortical neurons against beta-amyloid toxicity. Mol Pharmacol 2012; 81:12–20. [DOI] [PubMed] [Google Scholar]
- 54.Tang JX, Mardini F, Janik LS, et al. Modulation of murine Alzheimer pathogenesis and behavior by surgery. Ann Surg 2013; 257:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tosato M, Lukas A, van der Roest HG, et al. Association of pain with behavioral and psychiatric symptoms among nursing home residents with cognitive impairment: results from the SHELTER study. Pain 2012; 153:305–310. [DOI] [PubMed] [Google Scholar]
- 56.Troesch B, Eggersdorfer M, Weber P. The role of vitamins in aging societies. Int J Vitam Nutr Res 2012; 82:355–359. [DOI] [PubMed] [Google Scholar]
- 57.Vegeto E, Belcredito S, Etteri S, et al. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci U S A 2003; 100:9614–9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan Y, Xu J, Ma D, et al. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology 2007; 106:436–443. [DOI] [PubMed] [Google Scholar]
- 59.Wu YT, Lee HY, Norton S, et al. Prevalence studies of dementia in Mainland China, Hong Kong and taiwan: a systematic review and meta-analysis. PLoS One 2013; 8:e66252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia WF, Jung JU, Shun C, et al. Swedish mutant APP suppresses osteoblast differentiation and causes osteoporotic deficit, which are ameliorated by N-acetyl-L-cysteine. J Bone Miner Res 2013; 28:2122–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yaffe K, Tocco M, Petersen RC, et al. The epidemiology of Alzheimer's disease: laying the foundation for drug design, conduct, and analysis of clinical trials. Alzheimers Dement 2012; 8:237–242. [DOI] [PubMed] [Google Scholar]
- 62.Yang X, Ricciardi BF, Hernandez-Soria A, et al. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 2007; 41:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 2011; 25:181–213. [DOI] [PubMed] [Google Scholar]


