Abstract
Malignant struma ovarii (MSO) is a rare malignant ovarian germ cell tumor that has been scarcely reported by thyroid surgeons focusing on treatment. There are no golden standards for its treatment. There has not been any Chinese case included in the English language literatures. This is the first study by collecting all Chinese cases with clinical information. We emphasize on using I131 therapy after operation.
Presented is a case of struma ovarii with malignant histologic features who underwent definitive initial surgery of reproductive system tumors and a total thyroidectomy combined with thyroid-stimulating hormone (TSH)-suppressive therapy following treatment with I131. Furthermore, a Chinese full-text database literature search for cases of MSO was performed, and advisable clinical data were collected following our treatment advice.
Clinical data from 34 additional cases were compiled. As Chinese genetic background and environment are different from those of Western countries, our clinical data closely mirror theirs in some aspects. In addition, we provide a rare gene mutation type of MSO by the case from our department.
Integrating literatures with the experience of thyroid surgeons, we recommend “multidisciplinary joint treatment” for MSO, namely traditional radical initial surgery of ovarian cancer and a total thyroidectomy combined with TSH-suppressive therapy following treatment with I131 for those who do not desire preservation of fertility.
INTRODUCTION
Böttlin first described thyroid tissue in an ovarian teratoma in 1888. In 1976, Fox and Langley accurately reviewed the literature on malignant struma ovarii (MSO). Thirty-three years later (in 2009), an analysis of 88 cases (including 43 cases of proliferative struma ovarii) with MSO was conducted, which is so far the largest series reported. In this study, nearly all cases were collected from separate institutions, with no >2 cases from one individual hospital.1 Due to the scarcity of MSO and the lack of statistical data from non-Western countries, the epidemiological data are not precise.
Currently, there are no typical clinical manifestations and laboratory studies of MSO. Most cases are presented to the department of gynecology with complaints of pelvic mass with or without pain. Imaging studies are normally used for diagnosis of an ovarian mass and surgery follows. Without golden diagnostic standards, final diagnosis criteria are similar to the cervical thyroid based on nuclear and architectural features. Besides, metastasis from the cervical thyroid gland cancer must be excluded.
Presented is a case of struma ovarii with malignant histologic features who underwent definitive initial surgery of reproductive system tumors and a total thyroidectomy combined with thyroid-stimulating hormone (TSH)-suppressive therapy following treatment with I131. Furthermore, a Chinese full-text database literature search for cases of MSO was performed, and advisable clinical data were collected following our treatment advice.
CASE REPORT
A 46-year-old woman G2P2 experienced persistent and dull pain and had a left salpingo-oophorectomy for an adnexal mass in her community hospital. After a diagnosis of MSO was established, she went to gynecology hospital 2 months later to receive further treatment, involving hysterectomy, right salpingo-oophorectomy, pelvic lymphadenectomy, paraaortic lymph node sampling, omentectomy, appendectomy, enterolysis, and repair of intestine. The right ovary was measured 5 cm × 5cm × 4 cm and adherent to the pelvic sidewall. Texture is soft. The section revealed a variegated appearance. Final pathology revealed that there were multiple sites suffering from metastasis of thyroid papillary carcinoma (Figures 1 and 2) with vascular cancer embolus and formation of psammoma bodies, embracing right ovary, uterus, great omentum, intestinal wall, bilateral oviduct, bilateral pelvic sidewall, and pelvic lymph node. The tumor was found to be KRAS mutation-positive.
FIGURE 1.
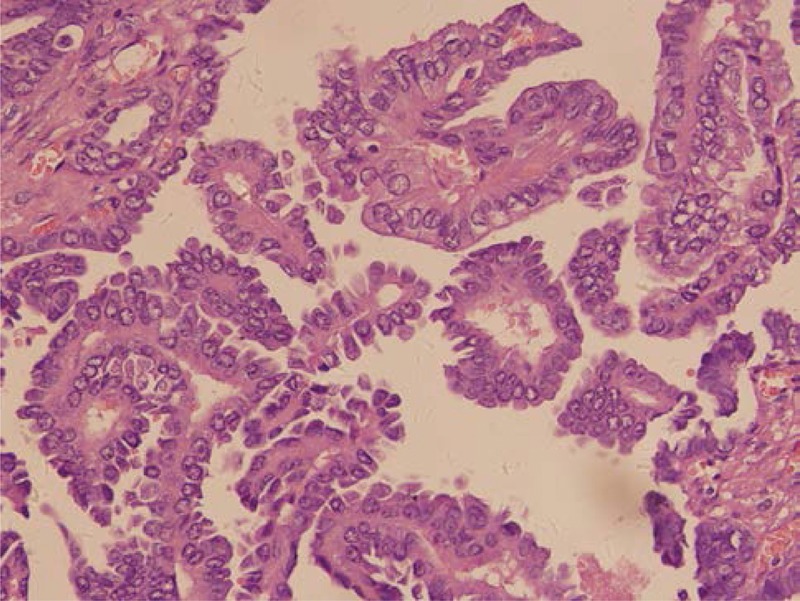
Final pathology revealed that the right ovary suffered from thyroid papillary carcinoma (H&E, ×400).
FIGURE 2.
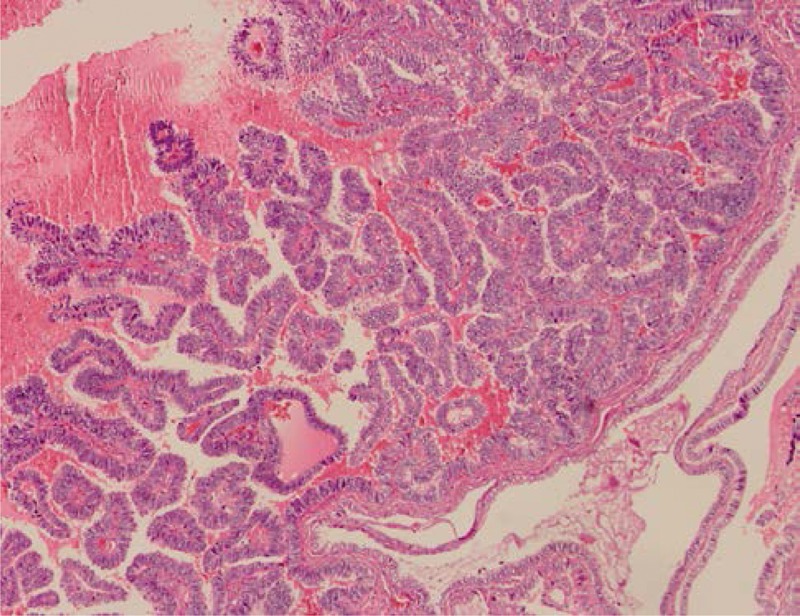
The colloid and cells surrounding the colloid were immunoreactive for thyroglobulin (immunohistochemistry, ×400).
About a month later, the patient was referred to our hospital for further treatment. Neck ultrasonography showed a 5 mm × 3mm × 4 mm cystic and solid nodule in the left lobe of thyroid gland, and enlarged lymph nodes were identified in bilateral submandibular areas, around left cervical great vessel and root of neck without rich flow signals. There was no clear boundary between the cortex and medulla of these lymph nodes. Chest computed tomography showed no abnormalities.
Thus, to benefit I131 therapy and determine whether the thyroid had been involved in carcinoma, total thyroidectomy was performed. Frozen and paraffin sections were read out as nodular goiter and lymph nodes were uneventful (Figure 3). Two months later, the patient underwent I131 treatment. The nuclear medicine whole-body scan before and after I131 ablation showed no evidence of a local and distant metastasis. The patient is now receiving TSH-suppressive therapy followed by imaging studies and serum TSH (S-TSH), T3, T4, FT3, and FT4 levels to evaluate recurrent or metastatic disease. T3, T4, FT3, and FT4 levels were kept in the normal range, and TSH and thyroglobulin level were slightly below normal. She was well and symptom-free 12 months from initial diagnosis.
FIGURE 3.
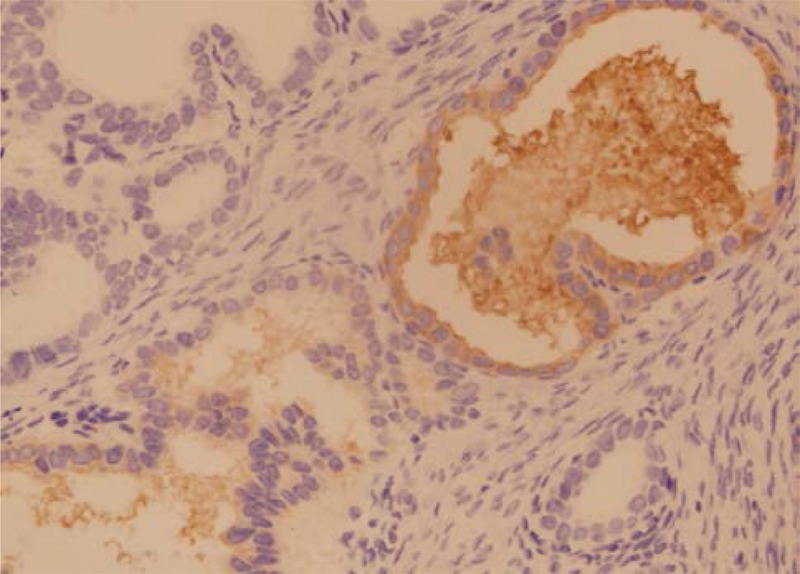
Nodular goiter (H&E, ×100).
METHODS
A literature search of Chinese full-text databases was performed (Database name: Wanfang Data, CNKI, VIP). Key words included malignant struma ovarii, struma ovarii, canceration, thyroid cancer, and thyroid carcinoma. It turned out that articles on MSO published from 1990 to 2010 were available. All articles were then reviewed. All cases of MSO were collected and data focused on clinical features and course. The cases without pathological diagnosis were excluded. Statistical analysis was performed by SPSS Statistics 17.0 (SPSS Inc, Chicago, IL).
Tumor tissue was collected from pathology specimens of the case from our department. It was tested for BRAF and KRAS mutations. Normal thyroid tissue was used as negative control. Genomic DNA was amplified by polymerase chain reaction. Sequences were compared with human genome using BLAST.
RESULTS
The clinical features of 35 cases of MSO, 1 from our institution and 34 collected from the literature, are listed in Table 1.
TABLE 1.
Clinical Features (n = 35)
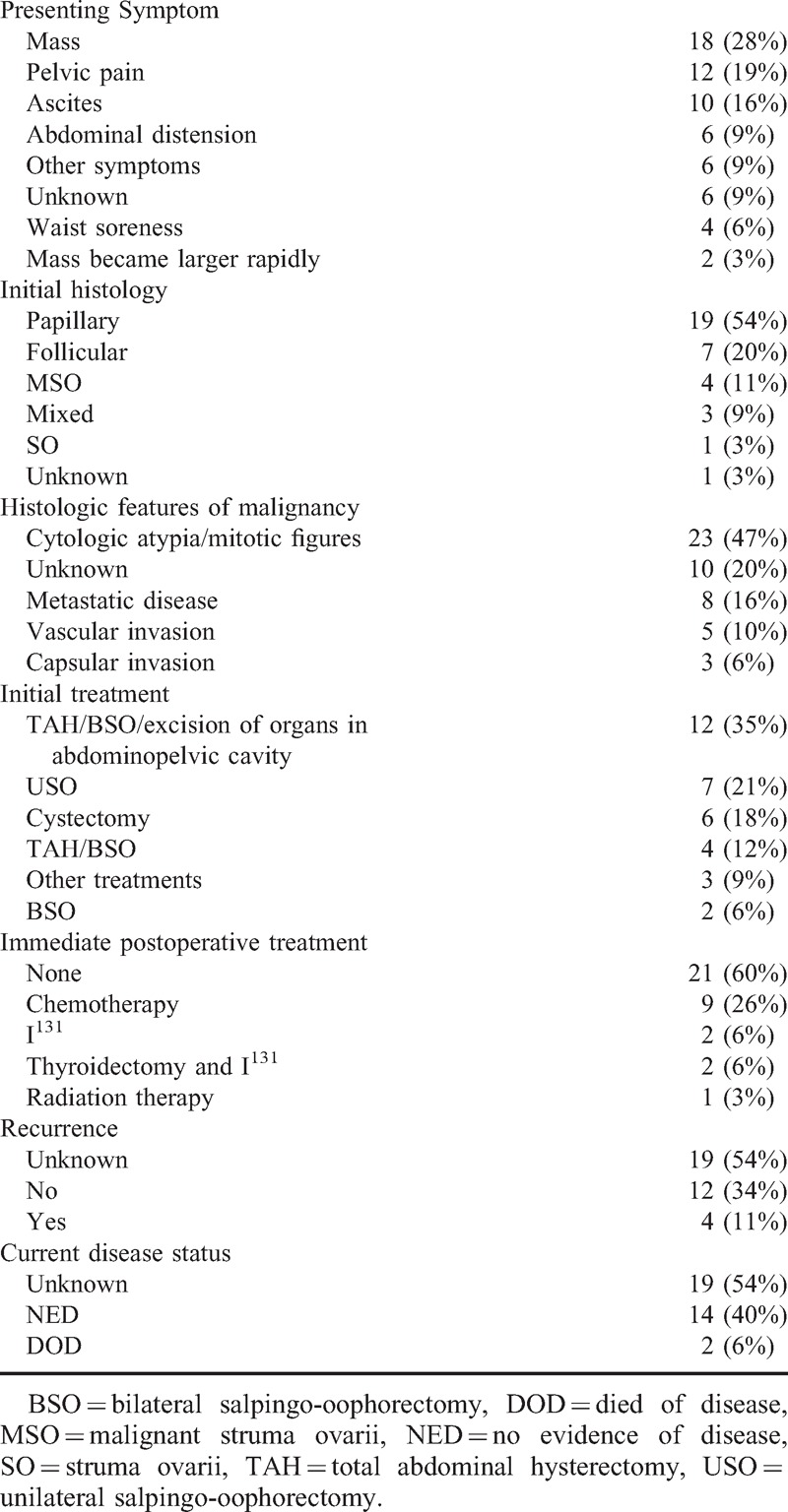
The mean age of patients (n = 27) was 42.7 years (range 26–65 years). The most prevalent finding on presentation was a pelvic mass (28% of cases). The mean size (n = 29) was 8.2 ± 4.23 cm (range 2.5–20 cm). It frequently involved the left ovary (50%, n = 17) or the right ovary (32%, n = 11). In Chinese and English language articles, no exact incidence of bilaterality of MSO was described. Seventeen percent (6/35) of MSO cases troubled with bilateral strumas in our Chinese literature review. Pelvic pain was the most common presenting symptom (19%) followed by abdominal distension (9%) and various other symptoms (9%) such as anepithymia, dyschesia, nausea, fever, intestinal obstruction, and frequent micturition. Ascites was found in 16% of patients. Mass became larger rapidly in 3% of the cases.
The most common initial treatment was a total abdominal hysterectomy, bilateral salpingo-oophorectomy, and excision of organs in abdominopelvic cavity (35%) followed by unilateral salpingo-oophorectomy (21%). The papillary thyroid carcinoma was the most common malignant cell type (54%) followed by follicular thyroid carcinoma (20%) and mixed follicular/papillary carcinoma (9%). The subclassification of 4 cases (11%) was not reported. Histologic features of malignancy of all cases are presented in Table 1. Eight patients (16%) were suffering from metastatic disease all in abdominopelvic cavity.
Table 2 summarizes the clinical course and treatment of 34 cases that have been previously reported in the literature. The majority had no postoperative treatment after initial surgery. Five patients were monitored of recurrent disease with serum thyroglobulin levels. Among the patients (13/34) who had postoperative therapy, 9 had chemotherapy, 2 had I131 therapy, 1 received adjuvant thyroidectomy and I131 therapy, and 1 had radiation therapy.
TABLE 2.
Clinical Course
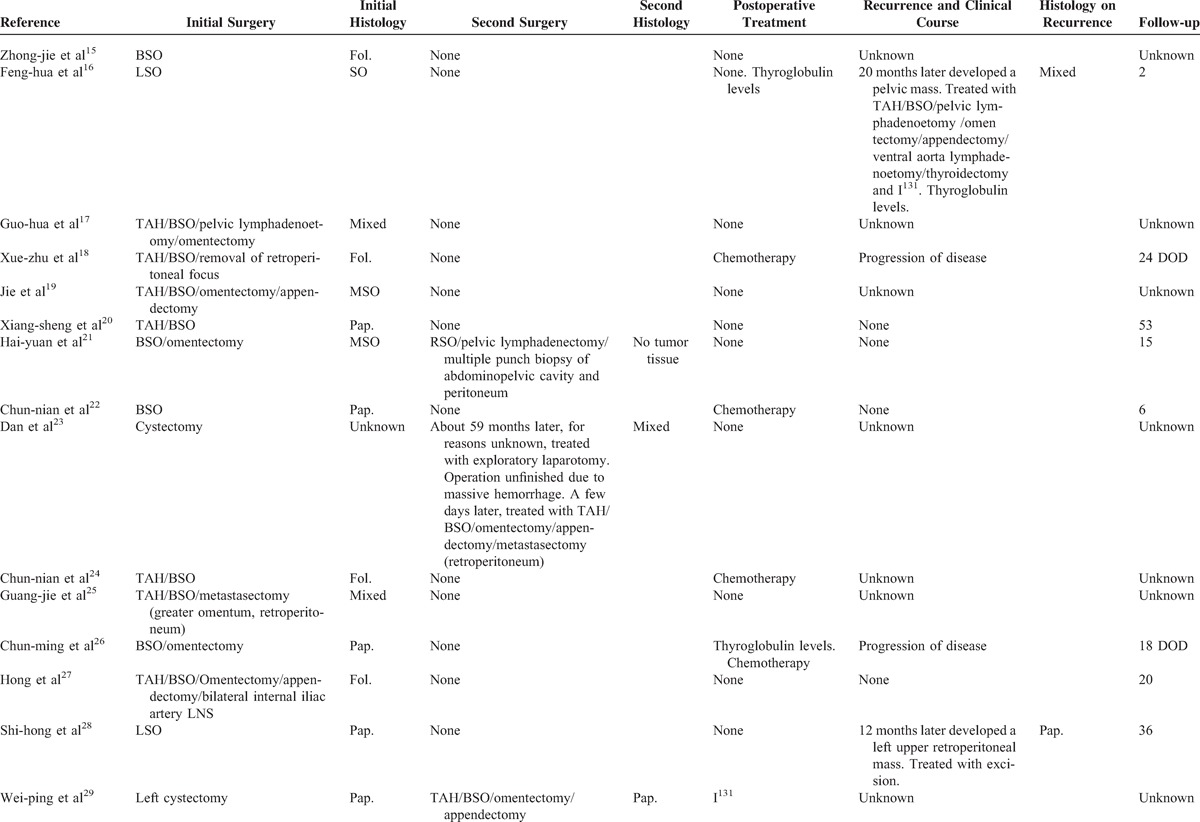
Of the 34 patients, only 15 reported the exact follow-up. Among these 15 patients, 3 had no evidence of disease following initial surgery, and 1 had no evidence of disease after immediate second surgery. During the follow-up period, recurrence occurred in 13% (2/15) of the cases after 20 and 12 months, separately. All two recurrences were with no initial adjunctive therapy. Cures (Table 2 ) after recurrence varied for the 2 patients and they had a complete response to treatment initially. The patients had a median progression-free survival of 31 months (range 12–53, without adjuvant treatment) or 32 months (range 2–96, with adjuvant treatment). To the date of literature reports, 2 women had died of disease, 13 had no evidence of disease, and the status of 19 women was unknown. Of total 34 women, 2 received thyroidectomy and I131 therapy and 2 received I131 therapy alone. Among these 4 women, 2 had no evidence of disease at 2 and 8 months, respectively, and 2 had no exact follow-up period.
Table 3 compared the clinical features of Chinese and English language articles. Chinese language articles included the clinical features of 35 cases of MSO, 1 from our institution and 34 collected from the literature. The information of English language articles was collected from Christopher's review.2 Although Chinese genetic background and environment are different from those of Western countries, our clinical data closely mirror theirs in some aspects: the mean ages are all around 42 years. Size of mass is 8.2 ± 4.23 cm (Chinese) and 10.5 ± 4.69 cm (English). The 2 most common presenting symptoms are presence of pelvic mass (the most common) and pelvic pain. There is no comparability of recurrence, and current disease status of Chinese and English language articles for 54% of the data is unknown.
TABLE 2 (Continued).
Clinical Course
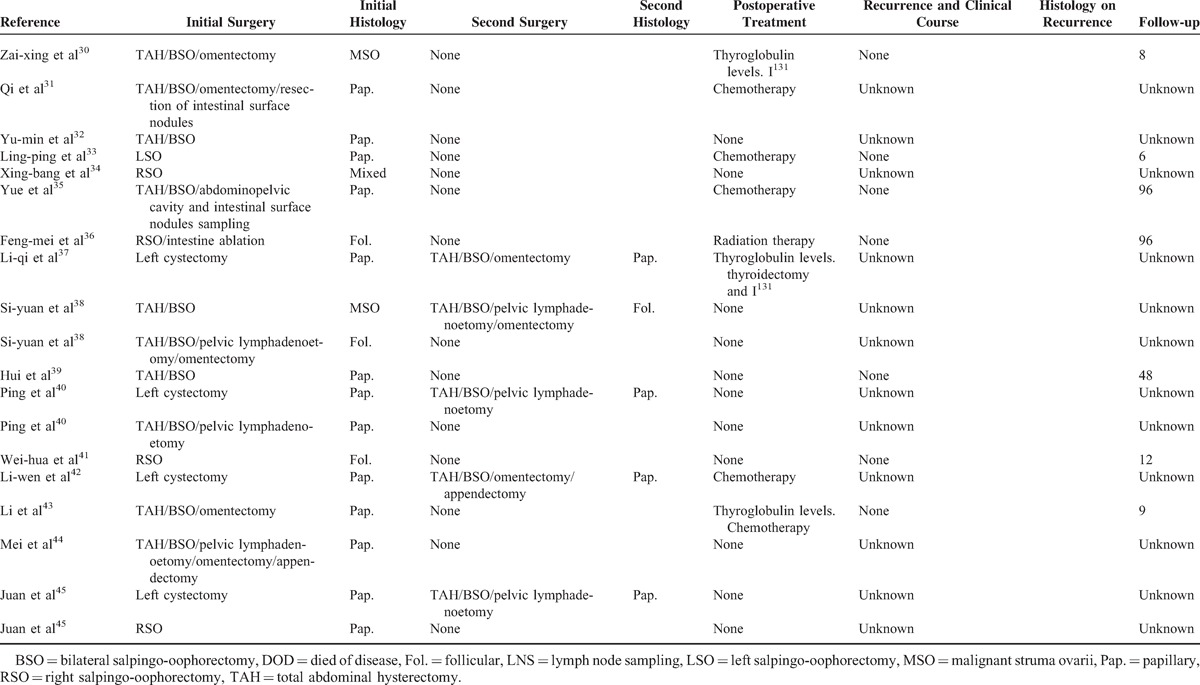
TABLE 3.
The Comparison of Clinical Features Between Chinese∗ and English Language Articles†
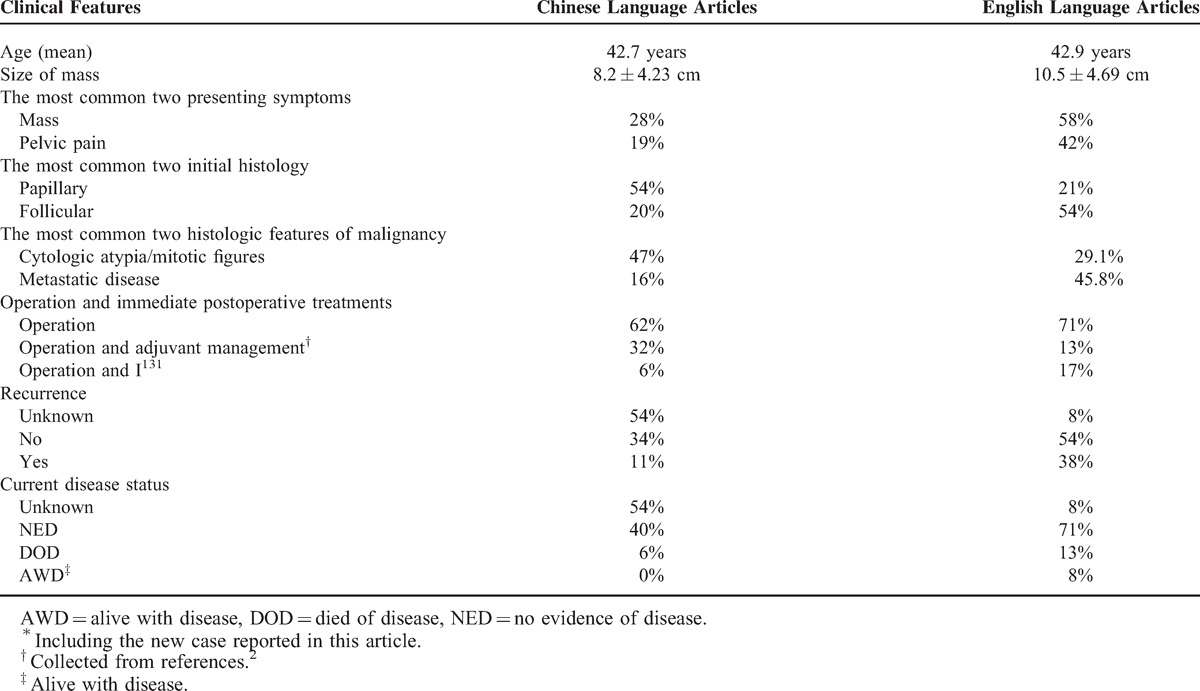
The papillary thyroid carcinoma was the most common malignant cell type (54%) followed by follicular thyroid carcinoma (20%) in Chinese language articles. These results were somewhat against Christopher's report (follicular 13 cases [54%], papillary 5 cases [21%]).2 The two most common histologic features of malignancy are cytologic atypia/mitotic figures and metastatic disease in both Chinese and English language articles. The most common one is cytologic atypia and/or mitotic figures (47%) in Chinese, and metastatic disease (45.8%) in English language articles.
In Table 3, operation and immediate postoperative treatments are divided into 3 groups. Most patients received surgery alone in both Chinese (62%) and English (71%) language articles. Thirty-two percent (Chinese) and 13% (English) patients received adjuvant treatments including chemotherapy and radiation therapy after operation. Seventeen percent (English) patients received I131 therapy after operation, and only 6% patients did that in Chinese language articles.
In Robboy et al's1 88 cases, struma ovarii was not diagnosed preoperatively, although 25 patients with struma ovarii in retrospect had possible symptoms or signs of thyroid hyperfunction. In our 35 cases, only 3 patients suffered from clinical manifestation of hyperthyroidism before seeing a doctor for MSO. One patient was diagnosed with hyperthyroidism 7 years before seeing a doctor for MSO, and was cured with anti-thyroid drug and I131 therapy. So, the patient experienced no clinical manifestation of hyperthyroidism when treating MSO. T3, T4, and TSH levels were all within the normal range before and after surgery. FT3 and FT4 levels were not reported. One patient presented to hospital with palpitations, mild fine tremors, and proptosis. Her heart rate was 110 beats per minute. No diffuse goiters were apparent on inspection, and no abnormal masses were palpable in the thyroid gland. The laboratory data were as follows: T3 = 104.5 nmol/L, T4 = 22.1 nmol/L (before surgery); T3, and T4 levels were within the normal range after surgery. FT3, FT4, and TSH levels were not reported. Symptoms of thyroid hyperfunction fell away. One patient presented to hospital with palpitations. Her heart rate was 110 beats per minute. T4 and FT4 levels were elevated before surgery and within the normal range after surgery. T3, FT3, and TSH levels were not reported. Her symptoms of thyroid hyperfunction disappeared. Except these 3 patients above, the TSH levels in our 31 patients from literatures were within the normal range before and after surgery. The TSH levels were within the normal range before surgery in 1 case from our hospital. She received TSH-suppressive therapy after surgery; TSH level was slightly below normal.
Serum CA125, alpha fetoprotein, beta Human Chorionic Gonadotropin, and lactate dehydrogenase were measured in 15, 8, 4, and 2 cases before surgery, respectively. The levels were all within the normal range except serum CA125 levels, which were elevated in 4 cases. In these 4 cases, serum CA125 levels were 79.3 U/mL before surgery and 152.1 U/mL after surgery in 1 patient, and 70.71 U/mL before surgery and within the normal range after surgery in another. In the other 2 cases, serum CA125 levels were 95.4 U/mL in 1 patient and exceeded 500 U/mL in another before surgery, and were not measured after surgery. The data above reflect that serum tumor markers that were most often used in diagnosis of ovarian tumors were less significant in diagnosis of MSO.
In addition, a KRAS codon 12 mutation, the GGT→GTT transversion, corresponding to the Gly→Val amino acid change was identified in the absence of other genetic alterations commonly found in papillary thyroid carcinoma.
DISCUSSION
Struma ovarii was first described in 1895. It is defined as a monodermal variant of ovarian teratomas in which thyroid tissue is the predominant (>50%) or exclusive component. Moreover, it also includes cases of mature cystic teratomas with macroscopically identifiable thyroid tissue or containing malignant thyroid tissue, even when the thyroid tissue component is <50% of the lesion. The tissues from our cases were monodermal.
Germ cell tumors of ovary account for 20% to 40% of all ovarian tumors. Mature teratoma of ovary accounts for 10% to 20% of all ovarian tumors. Immature teratoma of ovary is rare, and occurs in approximately 1% of all teratomas. The incidences above reported in Chinese and foreign literatures are similar. MSO is a rare malignant ovarian germ cell tumor that has been scarcely reported by thyroid surgeons focusing on treatment. It was reported in the literature almost exclusively as case reports. In the United States, those biologically malignant are far rarer, with an annual incidence at <1 in 10,000,000 woman years.1 In Chinese language articles, no exact incidence of MSO in China was described. The diagnostic histologic criteria used by most authors resembled the ones used in the eutopic thyroid gland. The present small series of MSO are far from enough to establish uniform treatment modality.
Even so, we can get some clinical information from the results of literature search. In Chinese and English language articles, there are similar regular patterns that appear in age, size of mass, and symptoms. Also, there are some differences: the 2 most common histologic features of malignance are dissimilar; Chinese malignant cell type was somewhat against Christopher's report (follicular, 13 cases [54%]; papillary, 5 cases [21%]),2 but similar to Stanley's report (papillary, 20 cases [43%];, follicular, 4 cases [9%]).1 The possible causes may involve selection bias, small sample size, different genetic background, and environment.
Five percent to 6% and 16% of MSO cases troubled with metastases in English language articles2 and in our Chinese literature review, respectively. The cancer can spread to the contralateral ovary, abdominopelvic lymph nodes, the omentum, the peritoneal cavity (Table 2 ), the lung, scalp, bone, brain, pleura, diaphragm, and liver.1,3,4 Because all of our cases and most other cases1–5 were confirmed postoperatively, the majority of estimate of pelvic lymph nodes and distant metastases is done with the second surgery (Table 2 ). Indeed, the second operation brings hurt and financial burden to patients. Yet, considering the long-term effects, it should be done if feasible. Thyroid surgeons recognize that even for patients who have already received treatment to improve metastasis performance, active treatment should be given for differentiated thyroid cancer.6 Moreover, among our 15 patients with the exact follow-up, 2 had died of disease without radical surgery even though adjuvant treatment was given.
The prognosis after operation is usually good. So whether the adjuvant treatment should be given is the subject of widespread controversy. As seen in Table 3, most patients underwent surgery alone in both Chinese and English language articles. A small number of patients underwent adjuvant managements such as radiation therapy, chemotherapy, and I131 therapy after operation. We do not agree with using external beam radiotherapy as regular adjuvant management of MSO, for it is mainly indicated as initial treatment or for recurrence of unresectable thyroid tumors or local invasion presumed to have macro- or microscopic residual disease, which does not concentrate I131. Cytotoxic chemotherapy also has no role in routine management of papillary and follicular thyroid cancer. Its use is restricted to patients with progressive disease uncontrolled by surgery, I131, or other treatment modalities.6 We and some other researchers recommended I131 as a major adjunctive therapy for MSO.1,2,4 I131 therapy is one of 3 major strategies for the treatment of thyroid cancer and hyperthyroidism, successfully used for >50 years.7 Adults with differentiated thyroid cancer are treated with high doses of radioiodine and have an excellent long-term prognosis. However, there is limited information on the effects of this treatment for MSO. Till date, of our 34 literature cases, 2 women had died of disease. They had received no I131 therapy. Another 3 women had received I131 therapy, 2 of them had no evidence of disease at 2 and 8 months, respectively, and 1 had no exact follow-up period. This reveals that radioactive iodine was not widely used in China for MSO. But it seems that it benefited our patients and others.1,2,4 Regardless of the uncertainty of curative effect of I131 therapy on MSO due to the protracted natural history of the disease, this treatment certainly has a lot of benefits:7 iodine scanning can effectively inspect metastasis and recurrence, and then kill cancer cells; it is easily tolerated by patients; except for occasional hypothyroidism, it almost has no side effect; it is relatively inexpensive. After all, whether I131 should be considered in the first-line treatment for MSO after operation depends on years of more data collection and the choice of individual treating program.
I131 therapy requires another surgery: total thyroidectomy, before using radioactive iodine. The main reasons are as follows: it enhances the effect of I131 therapy and iodine scanning and when all normally functioning thyroid tissues are excised, I131 is then easily absorbed by metastatic or recurring thyroid carcinoma; long-term follow-up is necessary with I131; it excludes a primary thyroid carcinoma.
Although it is needful to do total thyroidectomy, its surgical risk and financial burden have to be considered. Based on volume–outcome analyses, the most experienced surgeons may have complication rates considerably <1% for total thyroidectomy in thyroid cancer.8 Concerning cosmetic results, an incision between 4 and 6 cm in length has become standard and leaves a scar that is well hidden in the skin crease. Moreover, almost all of the patients with MSO have a normal thyroid, which is much more easily removed than thyroid with pathological changes. This results in shorter length of stay, fewer complications, better cosmetic results, and lower costs. Lifelong treatment with thyroxine (T4) is then required after total thyroidectomy. Clinical and experimental researches have proved that thyroid-cell proliferation relied on TSH; therefore, TSH suppression became a treatment for differentiated thyroid cancer. In treatment, T3, T4, FT3, and FT4 levels were kept in the normal range and TSH and thyroglobulin levels were slightly below normal. A number of reports have clarified that TSH-suppressive treatment with the l-enantiomer of tetraiodothyronine (L-T4) profits high-risk thyroid cancer patients by decreasing progression and recurrence rates, and cancer-related tumor.6–11 It is safe, effective, cheap, and easy to use and hailed as a major success by patients and clinicians. Unfortunately, data on total thyroidectomy in MSO are scarce. Among our total 34 cases, only 2 received it. Only one of them had a follow-up for 2 months. Total thyroidectomy was not widely used according to English language literature as well. But we believe that attributes to longer life expectancy, improved methods, and more experiences of disease detection and treatment in MSO, and there might be an increase in demand for total thyroidectomy in patients.
In conclusion, MSO lacks distinctive clinical or physical findings, and the final diagnosis depends on pathological examination after operation. Even though long-term survival is documented in most patients,1,2,4 it is worthwhile to note that some patients died soon after diagnosis of their diseases.4 Also, among our reviewed 34 cases, 2 had died of disease 24 and 18 months after initial surgery, respectively. This mortality of MSO is not lower than that of thyroid cancer in the neck.12,13 We suggest that women desiring to preserve their fertility choose conservative treatment and take serum thyroglobulin levels as a recurrence marker. If fertility is not taken into consideration, traditional radical initial surgery of ovarian cancer followed by total thyroidectomy combined with TSH-suppressive therapy and I131 therapy should be done by an expert multidisciplinary team. Frozen sections should be used to raise the rate of definite diagnosis of MSO during an operation, obtain an adequate surgical margin, and avoid a second surgery. I131 scanning combined with thyroglobulin levels can inspect metastasis and recurrence with high efficiency. In addition, it is the second time the unique KRAS mutation is described in a papillary thyroid carcinoma arising in MSO to the best of our knowledge.14 We believe that the accumulation of these data will surely help raise the level of clinical diagnosis in MSO, further provide objective basis for the prognosis of MSO, and explore a new therapy target aimed to the BRAF gene by molecular biology.
The defects of this study are small sample size, no follow-up (some cases), lack of details (some cases), and low literature quality (some articles). But considering the rarity of MSO, it is hoped that this research can make a contribution to further work in the field.
Footnotes
Abbreviations: l-T4 = l-enantiomer of tetraiodothyronine, MSO = malignant struma ovarii, TSH = thyroid-stimulating hormone, USO = unilateral salpingo-oophorectomy.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Robboy SJ, Shaco-Levy R, Peng RY, et al. Malignant struma ovarii: an analysis of 88 cases, including 27 with extraovarian spread. Int J Gynecol Pathol 2009; 28:405–422. [DOI] [PubMed] [Google Scholar]
- 2.DeSimone CP, Lele SM, Modesitt SC. Malignant struma ovarii: a case report and analysis of cases reported in the literature with focus on survival and I131 therapy. Gynecol Oncol 2003; 89:543–548. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita M, Ishii T, Ohtori S, et al. Metastasis of malignant struma ovarii to the lumbar spine. J Clinical Neurosci 2010; 17:269–272. [DOI] [PubMed] [Google Scholar]
- 4.Shaco-Levy R, Bean SM, Bentley RC, et al. Natural history of biologically malignant struma ovarii: analysis of 27 cases with extraovarian spread. Int J Gynecol Pathol 2010; 29:212–227. [DOI] [PubMed] [Google Scholar]
- 5.Devaney K, Snyder R, Norris H, et al. Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 cases. Int J Gynecol Pathol 1993; 12:333–343. [DOI] [PubMed] [Google Scholar]
- 6.Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 2006; 154:787–803. [DOI] [PubMed] [Google Scholar]
- 7.Al-Shakhrah IA. Radioprotection using iodine-131 for thyroid cancer and hyperthyroidism: a review. Clin J Oncol Nurs 2008; 12:905–912. [DOI] [PubMed] [Google Scholar]
- 8.Elaraj DM, Sturgeon C. Adequate surgery for papillary thyroid cancer. Surgeon 2009; 7:286–289. [DOI] [PubMed] [Google Scholar]
- 9.Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998; 8:737–744. [DOI] [PubMed] [Google Scholar]
- 10.Singer PA, Cooper DS, Daniels GH, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch Intern Med 1996; 2165–2172. [PubMed] [Google Scholar]
- 11.McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med 2002; 34:554–564. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi M, Yagi H, Earashi M, et al. Recurrence and mortality in patients with differentiated thyroid carcinoma. Int Surg 1995; 80:162–166. [PubMed] [Google Scholar]
- 13.QIAN Bi-yun, HEMin, DONG Shu-fen, et al. Incidence and mortality of thyroid cancers in Tianjin from 1981 to 2001. J Endocrinol Metab 2005; 21:432–434. [Google Scholar]
- 14.Stanojevic B, Dzodic R, Saenko V, et al. Unilateral follicular variant of papillary thyroid carcinoma with unique KRAS mutation in struma ovarii in bilateral ovarian teratoma: a rare case report. BMC Cancer 2012; 12:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.HAN Zhong-jie, JIANG Ying, LIN Chang-wan A case report of malignant struma ovarii. Chinese Journal of Oncology 1998; 20:478. [Google Scholar]
- 16.WEI Feng-hua, DENG Wen-hui, ZHANG Yi Clinical analysis of malignant struma ovarii peritoneal disseminate. Chinese Journal of Medicine 2007; 42:52–54. [Google Scholar]
- 17.DENG Guo-hua, ZHANG Hong-tu, ZHANG Xun A case report of malignant struma ovarii. J Diag Pathol 2009; 16:314. [Google Scholar]
- 18.ZHANG Xue-zhu A case report of unilateral malignant transformation in bilateral struma ovarii. Chinese Journal of Oocology 1998; 20:173. [Google Scholar]
- 19.ZHANG Jie, UANG Li A case report of malignant struma ovarii. Clinical Medicine of China 2006; 22:786. [Google Scholar]
- 20.ZHANG Xiang-sheng, ZHANG Lei-lei, MU Qing Malignant struma ovarii: a clinicopathologic observation. BMC J 2006; 29:457–459. [Google Scholar]
- 21.LIU Hai-yuan, LANG Jing-he, LENG Jin-hua Diagnosis and treatment of struma ovarii with laparoscopic procedure (report of 7 cases). China Journal of Endoscopy 2004; 10:40–42. [Google Scholar]
- 22.HE Chun-nian, XU Cui-qing, CHEN Chen A case report of ovarian mature cystic teratoma complicated with ovarian primary papillary thyroid carcinoma. Chinese Journal of Diagnostic Pathology 2004; 11:307–308. [Google Scholar]
- 23.WANG Dan, CHANG Qing A case report of malignant struma ovarii. Acta Academiae Medicinae Militaris Tertiae 2003; 25:2251–2252. [Google Scholar]
- 24.HE Chun-nian, XU Cui-qing, CHEN Che Malignant struma ovarian: cases report and review of the literature. J Clin Exp Pathol 2005; 20:314–317. [Google Scholar]
- 25.DUAN Guang-jie, YU Dong-mei, LIU Feng-xuan A case report of malignant struma ovarii (follicular variant of papillary carcinoma). Cancer Research on Prevention and Treatment 2004; 31:722. [Google Scholar]
- 26.LI Chun-ming, HE Jun-jie, HE Nai-fen Malignant struma ovarii: a clinicopathologic analysis and literature review. China Healthcare Innovation 2007; 2:20–21. [Google Scholar]
- 27.WU Hong, ZHAO Tan-zhen A case report of ovarian thyroid follicular carcinoma. The Journal of Medical Theory and Practice 1990; 3:35–36. [Google Scholar]
- 28.LIAO Shi-hong A case report of ovarian papillary thyroid carcinoma. Chinese Journal of Ultrasound in Medicine 1991; 7:147. [Google Scholar]
- 29.MIN Wei-ping Struma ovarian: a report of 7 cases and review of the literature. Zhejiang Clinical Medical Journal 2004; 6:204–205. [Google Scholar]
- 30.DENG Zai-xing, YU Wen-ju, XIE Bei Struma ovarii: a clinicopathologic observation of 9 cases. Zhejiang Clinical Medical Journal 2004; 6:370–371. [Google Scholar]
- 31.FU Qi Unilateral malignant transformation in bilateral struma ovarii. Chinese Journal of Obstetrics and Gynecology 1994; 29:693–694. [Google Scholar]
- 32.WANG Yu-min, QU Chuan-gui A case report of bilateral ovarian primary thyroid carcinoma. The Practical Journal of Cancer 1991; 6:334–345. [Google Scholar]
- 33.MENG Ling-ping, LU Tong, WAN Kai-ming A case report of ovarian mature cystic teratoma complicated with ovarian primary papillary thyroid carcinoma. Radiologic Practice 2009; 24:461. [Google Scholar]
- 34.WU Xing-bang, WU Ya, XU Qing-ying A case report of struma ovarii with partial canceration. Journal of Bengbu Medical College 1996; 21:112. [Google Scholar]
- 35.WANG Yue, LI Xiao-ping, ZHANG Chao Ovarian immature teratoma and peritoneal gliomatosis. Chin J Obstet Gynecol 2001; 2:239–242. [Google Scholar]
- 36.LI Feng-mei, BIAN Fu-ping Clinical analysis of 7 cases of struma ovarii. Research of Traditional Chinese Medicine 2002; 18:30–31. [Google Scholar]
- 37.SUN Li-qi, ZHANG Su, WAN Ze-qiu Clinical analysis of 9 cases of struma ovarii. Chinese Journal of Obstetrics and Gynecology 2004; 39:50–51. [Google Scholar]
- 38.ZENG Si-yuan, LI Long-yu, LI Cheng-xin Struma ovarii: A report of 8 cases. China Journal of Cancer Prevention and Treatment 2004; 11:1340–1341. [Google Scholar]
- 39.LIU Hui, PENG Zhi-lan, HU Han Struma ovarii: A report of 11 cases. Journal of West China University of Medical Sciences 2001; 32:323–324. [Google Scholar]
- 40.LU P, YU J-y, WANG J-l Ovarian primary papillary thyroid carcinoma: a clinicopathologic analysis. J Diag Pathol 2006; 13:421–422. [Google Scholar]
- 41.WU Wei-hua, SUN Dong-li, DUAN Chong-ying A case report of malignant struma ovarii. Henan J Oncol 1999; 12:76. [Google Scholar]
- 42.CAI Li-wen A report of 2 cases of benign and malignant struma ovarii with literature review. Zhejiang Clinical Medical Journal 2004; 6:494. [Google Scholar]
- 43.Wang Li. Clinicopathologic analysis of strumal carcinoid of ovary in 10 patients. MMJC 2010; 12:48–50. [Google Scholar]
- 44.PAN Mei, ZHAO Bo-wen, FANG Shu-hua A case report of ovarian nodular goiter with partial papillary canceration and ultrasound representation. Journal of Ultrasound in Clinical Medicine 2006; 8:503. [Google Scholar]
- 45.ZHAO Juan Clinicopathologic observation of 2 cases of malignant struma ovarian and review of the literature. Practical New Medicine 2007; 8:1114–1115. [Google Scholar]


