Abstract
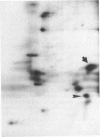
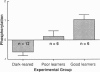
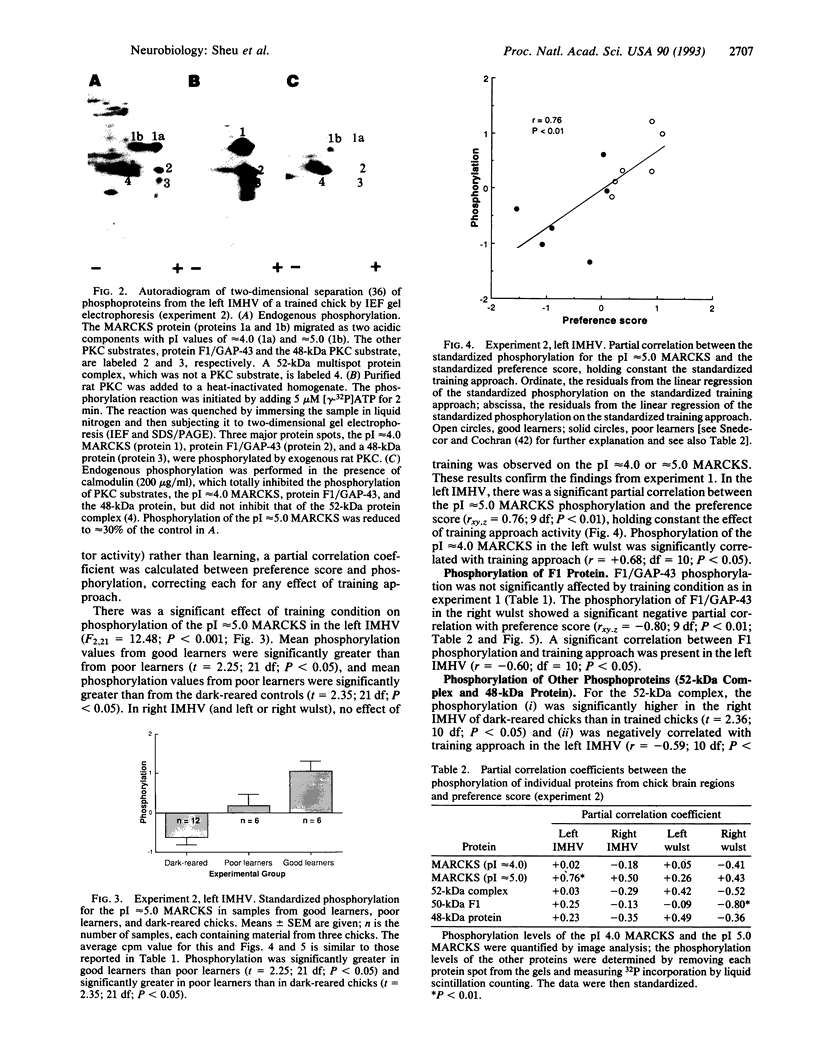
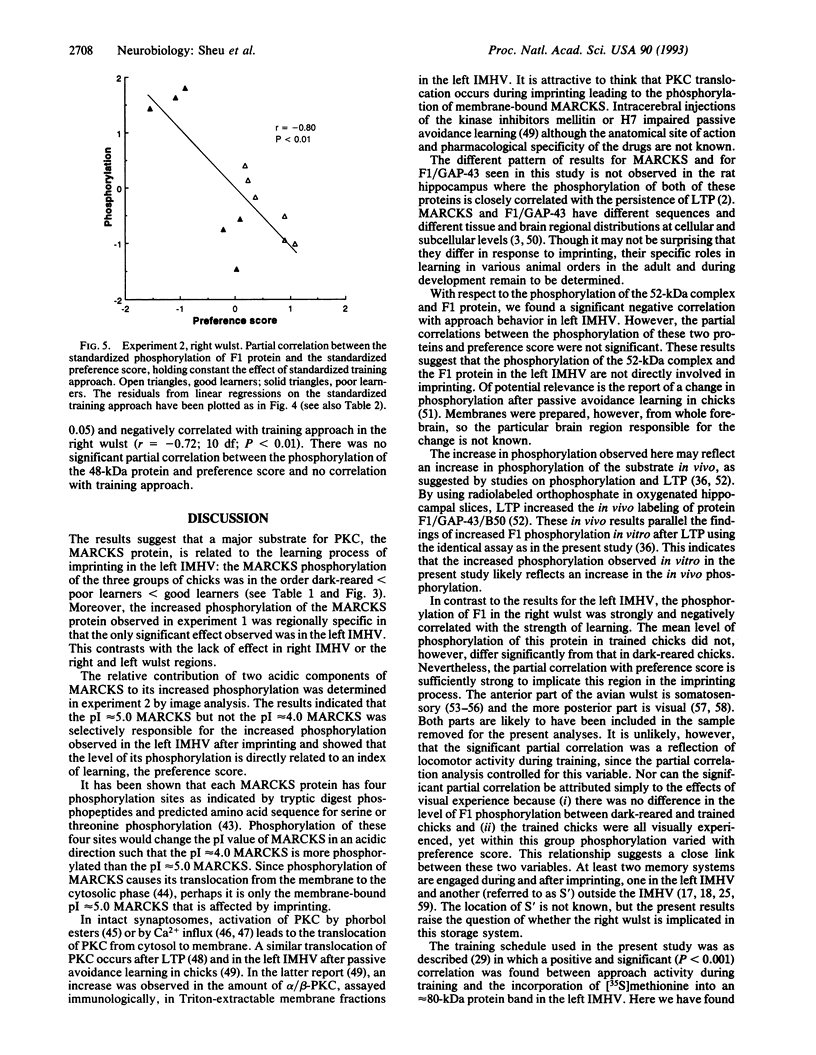
The effect of imprinting, an early form of exposure learning, on the phosphorylation state of the protein kinase C substrates myristoylated alanine-rich C-kinase substrate (MARCKS) and protein F1/43-kDa growth-associated protein (F1/GAP-43) was studied in two regions of the chick forebrain. One region, the intermediate and medial part of the hyperstriatum ventrale (IMHV), is probably a site of long-term memory; the other, the wulst, contains somatic sensory and visual projection areas. After imprinting, a significant increase in MARCKS protein phosphorylation was observed in the left IMHV but not the right IMHV. No significant alteration in F1/GAP-43 was observed in IMHV. MARCKS was resolved into two acidic components of pI approximately 5.0 and approximately 4.0. Phosphorylation of the pI approximately 5.0 MARCKS but not the pI approximately 4.0 MARCKS was significantly altered by imprinting. The partial correlation between preference score (an index of learning) and phosphorylation, holding constant the effect of approach activity during training, was significant only for the pI approximately 5.0 MARCKS in the left IMHV. A significant negative partial correlation between preference score and F1/GAP-43 phosphorylation in the right wulst was observed. Because the imprinting-induced alteration in MARCKS is selective with respect to phosphoprotein moiety, hemispheric location, and brain region, we propose that these alterations may be central to the learning process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Akers R. F., Routtenberg A. Calcium-promoted translocation of protein kinase C to synaptic membranes: relation to the phosphorylation of an endogenous substrate (protein F1) involved in synaptic plasticity. J Neurosci. 1987 Dec;7(12):3976–3983. doi: 10.1523/JNEUROSCI.07-12-03976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K. A., Walaas S. I., Wang J. K., Greengard P. Widespread occurrence of "87 kDa," a major specific substrate for protein kinase C. Proc Natl Acad Sci U S A. 1986 May;83(9):2822–2826. doi: 10.1073/pnas.83.9.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. M., Bullock S., Rose S. P. Phosphorylation of synaptic proteins in chick forebrain: changes with development and passive avoidance training. J Neurochem. 1988 May;50(5):1579–1587. doi: 10.1111/j.1471-4159.1988.tb03047.x. [DOI] [PubMed] [Google Scholar]
- Basi G. S., Jacobson R. D., Virág I., Schilling J., Skene J. H. Primary structure and transcriptional regulation of GAP-43, a protein associated with nerve growth. Cell. 1987 Jun 19;49(6):785–791. doi: 10.1016/0092-8674(87)90616-7. [DOI] [PubMed] [Google Scholar]
- Bateson P. P., Jaeckel J. B. Imprinting: Correlations between activities of chicks during training and testing. Anim Behav. 1974 Nov;22(4):899–906. doi: 10.1016/0003-3472(74)90013-x. [DOI] [PubMed] [Google Scholar]
- Bateson P. P. The characteristics and context of imprinting. Biol Rev Camb Philos Soc. 1966 May;41(2):177–211. doi: 10.1111/j.1469-185x.1966.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Bateson P. P., Wainwright A. A. The effects of prior exposure to light on the imprinting process in domestic chicks. Behaviour. 1972;42(3):279–290. doi: 10.1163/156853972x00310. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Bolhuis J. J. Mechanisms of avian imprinting: a review. Biol Rev Camb Philos Soc. 1991 Nov;66(4):303–345. doi: 10.1111/j.1469-185x.1991.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Bradley P., Horn G., Bateson P. Imprinting. An electron microscopic study of chick hyperstriatum ventrale. Exp Brain Res. 1981;41(2):115–120. doi: 10.1007/BF00236600. [DOI] [PubMed] [Google Scholar]
- Brown M. W., Horn G. Are specific proteins implicated in the learning process of imprinting? Brain Res Dev Brain Res. 1990 Mar 1;52(1-2):294–297. doi: 10.1016/0165-3806(90)90248-w. [DOI] [PubMed] [Google Scholar]
- Burchuladze R., Potter J., Rose S. P. Memory formation in the chick depends on membrane-bound protein kinase C. Brain Res. 1990 Dec 3;535(1):131–138. doi: 10.1016/0006-8993(90)91831-z. [DOI] [PubMed] [Google Scholar]
- Cimler B. M., Giebelhaus D. H., Wakim B. T., Storm D. R., Moon R. T. Characterization of murine cDNAs encoding P-57, a neural-specific calmodulin-binding protein. J Biol Chem. 1987 Sep 5;262(25):12158–12163. [PubMed] [Google Scholar]
- Cipolla-Neto J., Horn G., McCabe B. J. Hemispheric asymmetry and imprinting: the effect of sequential lesions to the hyperstriatum ventrale. Exp Brain Res. 1982;48(1):22–27. doi: 10.1007/BF00239569. [DOI] [PubMed] [Google Scholar]
- Delius J. D., Bennetto K. Cutaneous sensory projections to the avian forebrain. Brain Res. 1972 Feb 25;37(2):205–221. doi: 10.1016/0006-8993(72)90667-1. [DOI] [PubMed] [Google Scholar]
- Funke K. Somatosensory areas in the telencephalon of the pigeon. I. Response characteristics. Exp Brain Res. 1989;76(3):603–619. doi: 10.1007/BF00248917. [DOI] [PubMed] [Google Scholar]
- Gianotti C., Nunzi M. G., Gispen W. H., Corradetti R. Phosphorylation of the presynaptic protein B-50 (GAP-43) is increased during electrically induced long-term potentiation. Neuron. 1992 May;8(5):843–848. doi: 10.1016/0896-6273(92)90198-m. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Stumpo D. J., Blackshear P. J. Characterization of the phosphorylation sites in the chicken and bovine myristoylated alanine-rich C kinase substrate protein, a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Jul 15;264(20):11912–11919. [PubMed] [Google Scholar]
- Graff J. M., Stumpo D. J., Blackshear P. J. Molecular cloning, sequence, and expression of a cDNA encoding the chicken myristoylated alanine-rich C kinase substrate (MARCKS). Mol Endocrinol. 1989 Nov;3(11):1903–1906. doi: 10.1210/mend-3-11-1903. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Young T. N., Johnson J. D., Blackshear P. J. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21818–21823. [PubMed] [Google Scholar]
- Horn G., Bradley P., McCabe B. J. Changes in the structure of synapses associated with learning. J Neurosci. 1985 Dec;5(12):3161–3168. doi: 10.1523/JNEUROSCI.05-12-03161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn G., McCabe B. J., Bateson P. P. An autoradiographic study of the chick brain after imprinting. Brain Res. 1979 May 25;168(2):361–373. doi: 10.1016/0006-8993(79)90176-8. [DOI] [PubMed] [Google Scholar]
- Horn G., McCabe B. J., Cipolla-Neto J. Imprinting in the domestic chick: the role of each side of the hyperstriatum ventrale in acquisition and retention. Exp Brain Res. 1983;53(1):91–98. doi: 10.1007/BF00239401. [DOI] [PubMed] [Google Scholar]
- Horn G. Neural bases of recognition memory investigated through an analysis of imprinting. Philos Trans R Soc Lond B Biol Sci. 1990 Aug 29;329(1253):133–142. doi: 10.1098/rstb.1990.0158. [DOI] [PubMed] [Google Scholar]
- Karns L. R., Ng S. C., Freeman J. A., Fishman M. C. Cloning of complementary DNA for GAP-43, a neuronal growth-related protein. Science. 1987 May 1;236(4801):597–600. doi: 10.1126/science.2437653. [DOI] [PubMed] [Google Scholar]
- Karten H. J., Hodos W., Nauta W. J., Revzin A. M. Neural connections of the "visual wulst" of the avian telencephalon. Experimental studies in the piegon (Columba livia) and owl (Speotyto cunicularia). J Comp Neurol. 1973 Aug;150(3):253–278. doi: 10.1002/cne.901500303. [DOI] [PubMed] [Google Scholar]
- Katz F., Ellis L., Pfenninger K. H. Nerve growth cones isolated from fetal rat brain. III. Calcium-dependent protein phosphorylation. J Neurosci. 1985 Jun;5(6):1402–1411. doi: 10.1523/JNEUROSCI.05-06-01402.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linden D. J., Routtenberg A. The role of protein kinase C in long-term potentiation: a testable model. Brain Res Brain Res Rev. 1989 Jul-Sep;14(3):279–296. doi: 10.1016/0165-0173(89)90004-0. [DOI] [PubMed] [Google Scholar]
- McCabe B. J., Cipolla-Neto J., Horn G., Bateson P. Amnesic effects of bilateral lesions placed in the hyperstriatum ventrale of the chick after imprinting. Exp Brain Res. 1982;48(1):13–21. doi: 10.1007/BF00239568. [DOI] [PubMed] [Google Scholar]
- McCabe B. J., Horn G., Bateson P. P. Effects of restricted lesions of the chick forebrain on the acquisition of filial preferences during imprinting. Brain Res. 1981 Jan 26;205(1):29–37. doi: 10.1016/0006-8993(81)90717-4. [DOI] [PubMed] [Google Scholar]
- McCabe B. J., Horn G., Bateson P. P. Effects of restricted lesions of the chick forebrain on the acquisition of filial preferences during imprinting. Brain Res. 1981 Jan 26;205(1):29–37. doi: 10.1016/0006-8993(81)90717-4. [DOI] [PubMed] [Google Scholar]
- McCabe B. J., Horn G. Learning and memory: regional changes in N-methyl-D-aspartate receptors in the chick brain after imprinting. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2849–2853. doi: 10.1073/pnas.85.8.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan J. G., Horn G. Learning-dependent Changes in the Responses to Visual Stimuli of Neurons in a Recognition Memory System. Eur J Neurosci. 1992 Oct;4(11):1112–1122. doi: 10.1111/j.1460-9568.1992.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Murakami K., Chan S. Y., Routtenberg A. Protein kinase C activation by cis-fatty acid in the absence of Ca2+ and phospholipids. J Biol Chem. 1986 Nov 25;261(33):15424–15429. [PubMed] [Google Scholar]
- Nelson R. B., Friedman D. P., O'Neill J. B., Mishkin M., Routtenberg A. Gradients of protein kinase C substrate phosphorylation in primate visual system peak in visual memory storage areas. Brain Res. 1987 Jul 28;416(2):387–392. doi: 10.1016/0006-8993(87)90924-3. [DOI] [PubMed] [Google Scholar]
- Nelson R. B., Linden D. J., Hyman C., Pfenninger K. H., Routtenberg A. The two major phosphoproteins in growth cones are probably identical to two protein kinase C substrates correlated with persistence of long-term potentiation. J Neurosci. 1989 Feb;9(2):381–389. doi: 10.1523/JNEUROSCI.09-02-00381.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. B., Routtenberg A. Characterization of protein F1 (47 kDa, 4.5 pI): a kinase C substrate directly related to neural plasticity. Exp Neurol. 1985 Jul;89(1):213–224. doi: 10.1016/0014-4886(85)90277-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Ouimet C. C., Wang J. K., Walaas S. I., Albert K. A., Greengard P. Localization of the MARCKS (87 kDa) protein, a major specific substrate for protein kinase C, in rat brain. J Neurosci. 1990 May;10(5):1683–1698. doi: 10.1523/JNEUROSCI.10-05-01683.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. P. How chicks make memories: the cellular cascade from c-fos to dendritic remodelling. Trends Neurosci. 1991 Sep;14(9):390–397. doi: 10.1016/0166-2236(91)90027-r. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Chan S. Y., Henzel W., Haskell C., Kuang W. J., Chen E., Wilcox J. N., Ullrich A., Goeddel D. V., Routtenberg A. Primary structure and mRNA localization of protein F1, a growth-related protein kinase C substrate associated with synaptic plasticity. EMBO J. 1987 Dec 1;6(12):3641–3646. doi: 10.1002/j.1460-2075.1987.tb02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Karten H. J. Immunohistochemical analysis of the visual wulst of the pigeon (Columba livia). J Comp Neurol. 1990 Oct 15;300(3):346–369. doi: 10.1002/cne.903000307. [DOI] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M., Rosen A., Nairn A. C., Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991 May 23;351(6324):320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Wang J. K., Walaas S. I., Sihra T. S., Aderem A., Greengard P. Phosphorylation and associated translocation of the 87-kDa protein, a major protein kinase C substrate, in isolated nerve terminals. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2253–2256. doi: 10.1073/pnas.86.7.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild J. M. The avian somatosensory system: connections of regions of body representation in the forebrain of the pigeon. Brain Res. 1987 Jun 2;412(2):205–223. doi: 10.1016/0006-8993(87)91127-9. [DOI] [PubMed] [Google Scholar]
- Wu W. C., Walaas S. I., Nairn A. C., Greengard P. Calcium/phospholipid regulates phosphorylation of a Mr "87k" substrate protein in brain synaptosomes. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5249–5253. doi: 10.1073/pnas.79.17.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]