Abstract
This study investigated the preoperative independent risk factors associated with survival and recurrence for patients with hepatocellular carcinoma (HCC) who underwent hepatic resection.
In total, 384 consecutive patients who underwent curative hepatic resection for single primary HCC were studied. Predictive factors associated with 1-, 3-, and 5-year survival and recurrence-free survival (RFS) were assessed using a univariate log-rank test and multivariate Cox proportional hazards regression model.
Gamma-glutamyl transferase (GGT) > 100 U/L was identified as a preoperative independent risk factor affecting 1-, 3-, and 5-year survival whereas GGT > 50 U/L and indocyanine green retention 15 min (ICG-R15) > 10% were identified as preoperative independent risk factors affecting 1-, 3-, and 5-year RFS. The 384 patients studied had a 1-, 3-, and 5-year RFS rate of 72.8%, 43.3%, and 27%, respectively. Patients with GGT > 50 U/L had a 1-, 3-, and 5-year RFS rate of 64.5%, 36.0%, and 21.7%. These patients had lower survival rates than did patients with GGT ≤ 50 U/L (P < 0.05). Patients with GGT > 50 U/L and ICG-R15 > 10% had a 1-, 3-, and 5-year RFS rate of 62.4%, 29.5%, and 14.1%, respectively. These patients had lower survival rates than did patients in the other 2 groups with different levels of GGT and ICG (P < 0.05, respectively). The same was also true for patients with a tumor < 5 cm in size.
Combined information in the form of high levels of GGT and ICG-R15 is a preoperative predictor that warrants full attention when evaluating tumor recurrence postoperatively.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the second leading cause of cancer deaths worldwide, with an estimated global incidence of 782,000 new cases and nearly 746,000 deaths in 2012.1 In the present era of using hepatic resection for HCC, although improved diagnostic procedures, surgical techniques, and perioperative management have contributed to better outcomes,2–7 the high rate of recurrence after hepatic resection is a problem that impacts patient prognosis, with a cumulative rate of recurrence of 50% and 60% at 3 years and 60% and 80% at 5 years.8–12 Thus, the challenge for surgeons is how to predict recurrence and take interventional measures early on.
To date, certain characteristics of the prognosis of HCC have been investigated, and aspects such as microvascular invasion (MVI), poor differentiation, and tumor size have been identified as significant risk factors affecting prognosis after hepatic resection.13–16 Recently, numerous studies of different subgroups of patients, such as patients with hepatitis B virus (HBV)-related HCC, noncirrhotic HCC, or multinodular tumors, have focused on factors that predict postoperative survival and recurrence in patients with HCC.17–21 However, the characteristics of HCC and/or the patient's condition varied in those studies, so clinically and biologically significant predictors of tumor recurrence postoperatively are still unclear. In addition, evaluating predictive factors for recurrence of HCC is difficult given patients with multinodular tumors. Thus, the present study investigated independent prognostic risk factors associated with survival and recurrence in patients with single primary HCC with the specific aim of identifying preoperative predictors of tumor recurrence postoperatively.
METHODS
Subjects and Follow-Up
Subjects consisted of 384 consecutive patients who underwent curative hepatic resection for single primary HCC from 1997 to 2007 at the University of Tokyo Hospital, Tokyo, Japan, and this study was approved by the Institutional Review Boards of the University of Tokyo. For specimen of all cases, the surgical margins were negative. All patients were regularly screened for recurrence through monitoring of HCC-specific tumor markers of alpha-fetoprotein (AFP) and des-gamma-carboxy-prothrombin (DCP) every 1 to 2 months, ultrasonography every 2 months, and dynamic computed tomography every 4 months, as previously reported.22,23 There are no cases who died within 1 month after surgery.
Recurrence was defined as the appearance of a new lesion with radiologic features consistent with HCC and was confirmed using at least 2 imaging modalities. Intrahepatic metastasis was identified according to the Liver Cancer Study Group of Japan: General Rules for the Clinical and Pathological Study of Primary Liver Cancer.24 The present study defined 1-, 3-, and 5-year recurrence-free survival (RFS) as the interval from surgery to the date of diagnosis of the first recurrence or a follow-up examination during the period in question, and 1-, 3-, and 5-year survival was calculated based on the time from surgery to death or follow-up during the period in question.
Data Collection and Analysis
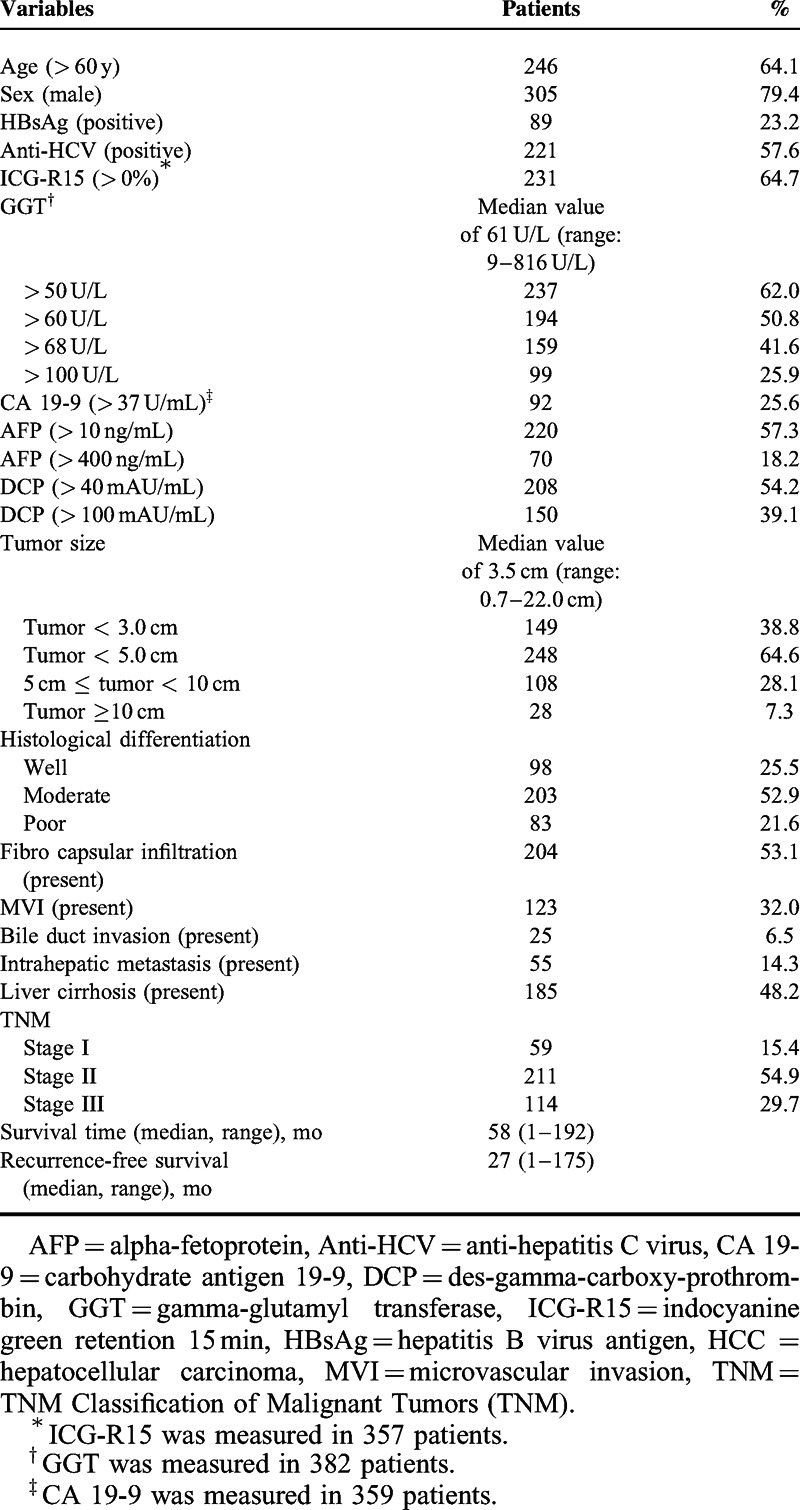
Data on the following preoperative variables were retrospectively analyzed: age, sex, hepatitis B virus antigen, anti-hepatitis C virus, indocyanine green retention 15 minutes after administration (ICG-R15), gamma-glutamyl transferase (GGT), carbohydrate antigen 19-9 (CA 19-9), AFP, and DCP. In addition, tumor size and the postoperative pathological variables of histological differentiation, fibrocapsular infiltration, MVI (including cases of portal vein invasion or hepatic vein invasion), bile duct invasion, intrahepatic metastasis, liver cirrhosis, and TNM Classification of Malignant Tumors (TNM) stage were also examined (Table 1).
TABLE 1.
Baseline Characteristics of 384 Patients With Single Primary HCC After Hepatic Resection
Statistical analysis was performed with the statistical software package SPSS version 22.0 for Windows (SPSS, Chicago, IL). Continuous variables were expressed as the median (range) and compared between groups using the Wilcoxon rank-sum test. Categorical data were compared using the χ2 test. Survival curves were generated by the Kaplan–Meier method and compared using a log-rank test. Multivariate regression analysis was performed with the Cox proportional hazards regression model using a backward elimination procedure in order to identify risk factors affecting postoperative survival and tumor recurrence. To prevent overfitting, only factors that were significantly associated with postoperative survival or tumor recurrence (with P < 0.05 according to univariate comparison) were included in the multivariate analysis. Results of the multivariate analysis are presented as hazard ratio (HR) with a corresponding 95% confidence interval (CI). All statistical tests were 2 sided, and P < 0.05 was considered to indicate a significant difference.
RESULTS
Baseline Characteristics
In total, 384 patients with single primary HCC who underwent curative hepatic resection (305 male patients and 79 female patients) and who had a median age of 65 years (range: 19–85 years) were studied (Table 1). With a median follow-up of 57.5 months (range: 1–174.5 months), 253 patients (65.9%) had recurrence of HCC and 108 patients (28.1%) died. The 1-, 3-, and 5-year survival rates were 96.4%, 83.5%, and 68.9%, and the 1-, 3-, and 5-year RFS rates were 72.8%, 43.3%, and 27.0%, respectively. The overall survival rate and overall RFS rate was 71.9% and 34.1%, respectively.
Risk Factors Affecting 1-, 3-, and 5-Year Survival Postoperatively
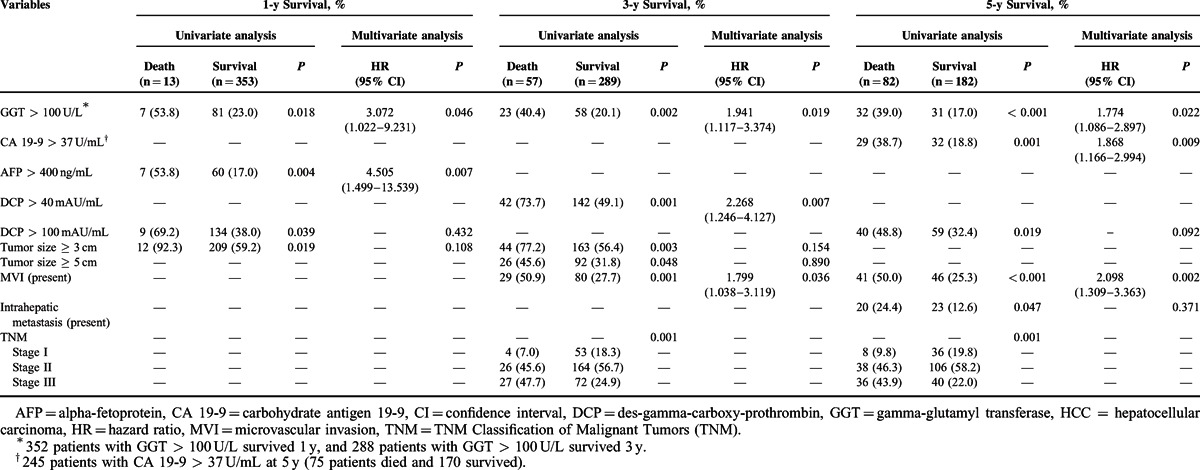
To identify risk factors affecting 1-, 3-, and 5-year survival postoperatively, all selected preoperative variables and postoperative pathological variables listed in Table 1 were included in univariate analysis. Items with P < 0.05 in univariate analysis were selected as variables for inclusion in multivariate regression analysis using the Cox proportional hazard model. As shown in Table 2, GGT > 100 U/L was a statistically significant independent risk factor affecting 1-, 3-, and 5-year survival, whereas high levels of AFP, DCP, and CA 19-9 were statistically significant independent risk factors affecting 1-, 3-, and 5-year survival, respectively. Patients with MVI had a poorer prognosis over time.
TABLE 2.
Risk Factor Analysis of the 1-, 3-, and 5-y Survival for 384 Patients With Single Primary HCC After Hepatic Resection
Patients with GGT > 100 U/L had a 1-, 3-, and 5-year survival rate of 92.0%, 71.6%, and 49.2%, respectively, and these rates significantly differed in comparison to patients with GGT ≤ 100 U/L, who had a 1-, 3-, and 5-year survival rate of 97.8%, 87.1%, and 75.0% (P < 0.05 for the 1-, 3-, and 5-year survival of the 2 groups) (Figure 1A).
FIGURE 1.
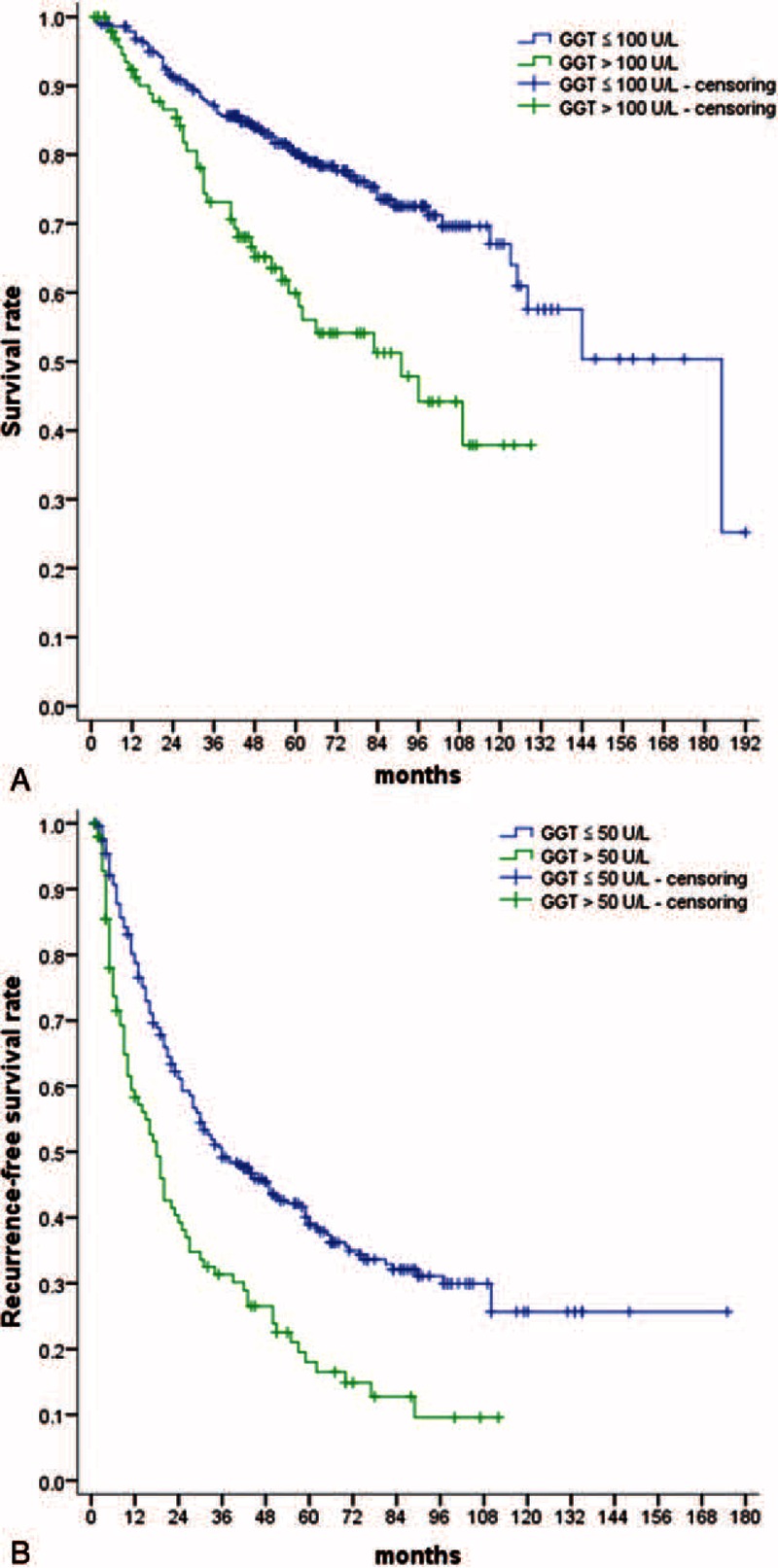
Curves of survival and RFS for 384 patients with single primary HCC after hepatic resection. (A) Survival curves for patients with GGT ≤ 100 U/L or GGT > 100 U/L. (B) RFS curves for patients with GGT ≤ 50 U/L or GGT > 50 U/L. GGT = gamma-glutamyl transferase, HCC = hepatocellular carcinoma, RFS = recurrence-free survival.
In addition, GGT > 100 U/L was also identified as a statistically significant independent risk factor associated with overall survival (HR = 1.971, 95% CI = 1.296–2.997, P = 0.002). Patients with GGT > 100 U/L had an overall survival rate of 60.6%, which was poor than that in patients with GGT ≤ 100 U/L (75.6%) (P = 0.006).
Risk Factors Affecting 1-, 3-, and 5-Year RFS Postoperatively
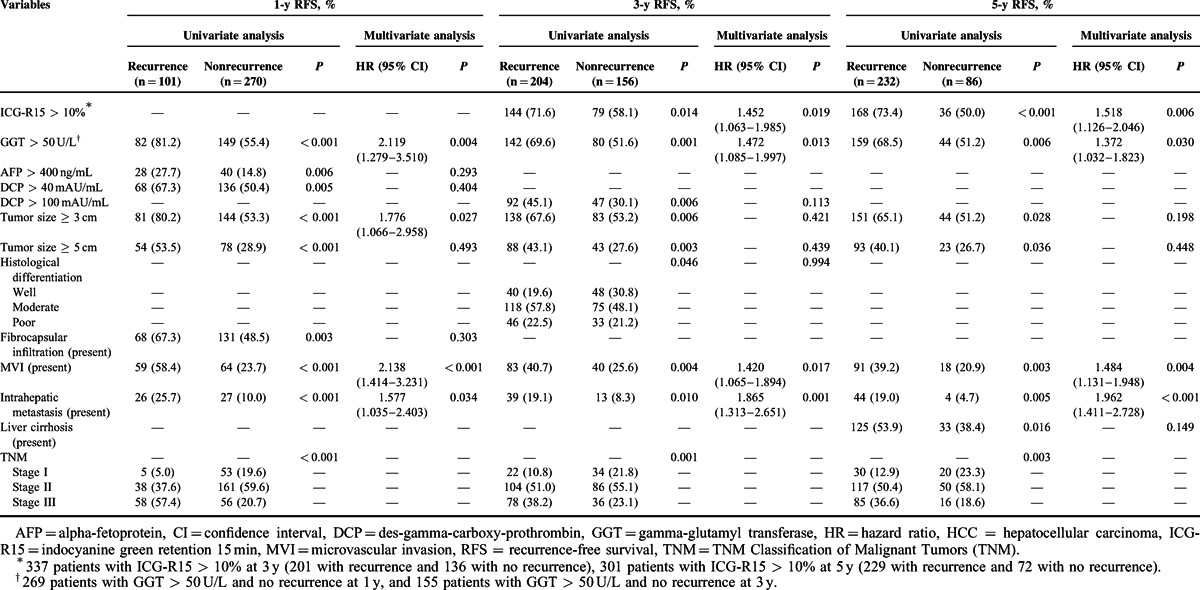
The risk factors affecting 1-, 3-, and 5-year RFS postoperatively were also investigated using univariate analysis and multivariate analysis. As shown in Table 3, GGT > 50 U/L was a statistically significant independent risk factor affecting 1-, 3-, and 5-year RFS, whereas ICG-R15 > 10% was a statistically significant independent risk factor affecting 3- and 5-year RFS. In addition, GGT > 50 U/L (HR = 1.433, 95% CI = 1.092–1.881, P = 0.010) and ICG-R15 > 10% (HR = 1.627, 95% CI = 1.213–2.182, P = 0.001) were also identified as a statistically significant independent risk factor associated with overall RFS. In addition, the presence of MVI or intrahepatic metastasis was also identified as a risk factor associated with tumor recurrence.
TABLE 3.
Risk Factor Analysis of 1-, 3-, and 5-y RFS for 384 Patients With Single Primary HCC After Hepatic Resection
Patients with GGT > 50 U/L had a 1-, 3-, and 5-year RFS rate of 64.5%, 36.0%, and 21.7%, respectively, and these rates significantly differed in comparison to patients with GGT ≤ 50 U/L, who had a 1-, 3-, and 5-year RFS rate of 86.3%, 54.7%, and 36.5%, respectively (P < 0.05 for the 1-, 3-, and 5-year RFS of the 2 groups) (Figure 1B). In addition, patients with GGT > 50 U/L had an overall RFS rate of 27.4%, which was lower than that in patients with GGT ≤ 50 U/L (44.1%) (P = 0.001).
Relationship Between GGT, ICG-R15, and RFS
As shown in Table 3, high levels of GGT and ICG-R15 preoperatively were identified as independent risk factors for postoperative recurrence. Of all 384 patients, 152 patients (42.6%) had GGT > 50 U/L and ICG-R15 >10%, 160 (44.8%) had GGT > 50 U/L and ICG-R15 ≤ 10% or GGT ≤ 50 U/L and ICG-R15 >10%, 45 (12.6%) had GGT ≤ 50 U/L and ICG-R15 ≤ 10%, and 27 were lost to follow-up.
As shown in Figure 2, patients with GGT > 50 U/L and ICG-R15 > 10% had a 1-, 3-, and 5-year RFS of 62.4%, 29.5%, and 14.1%, respectively. These patients had lower RFS rates than did patients in the other 2 groups with different levels of GGT and ICG (P < 0.05, respectively). In addition, of all 384 patients, patients with GGT > 50 U/L and ICG-R15 > 10% had an overall RFS rate of 19.7%, which was lower than that in patients with GGT > 50 U/L and ICG-R15 ≤ 10% or GGT ≤ 50 U/L and ICG-R15 >10% (overall RFS rate of 33.8%), and patients with GGT ≤ 50 U/L and ICG-R15 ≤ 10% (overall RFS rate of 53.3%) (P < 0.05, respectively).
FIGURE 2.

1-, 3-, and 5-year RFS rate for 384 patients, patients with GGT > 50 U/L or GGT ≤ 50 U/L, and patients with the combination of GGT (cutoff of 50 U/L) and ICG-R15 (cutoff of 10%). GGT = gamma-glutamyl transferase, ICG-R15 = indocyanine green retention 15 min, RFS = recurrence-free survival.
Risk Factors Affecting 1-, 3-, and 5-Year RFS Postoperatively for Patients With a Tumor < 5 cm in Size
Numerous studies have reported that a large tumor is a factor influencing the recurrence of HCC. In the 384 patients in the present study, the median tumor size was 3.5 cm (range: 0.7–22.0 cm). One hundred thirty-six patients (35.4%) had a tumor ≥ 5.0 cm in size. In order to exclude the influence of a larger tumor size and further investigate the relationship between clinicopathological variables and recurrence, 248 patients with tumor < 5 cm in size were further analyzed using univariate and multivariate analysis.
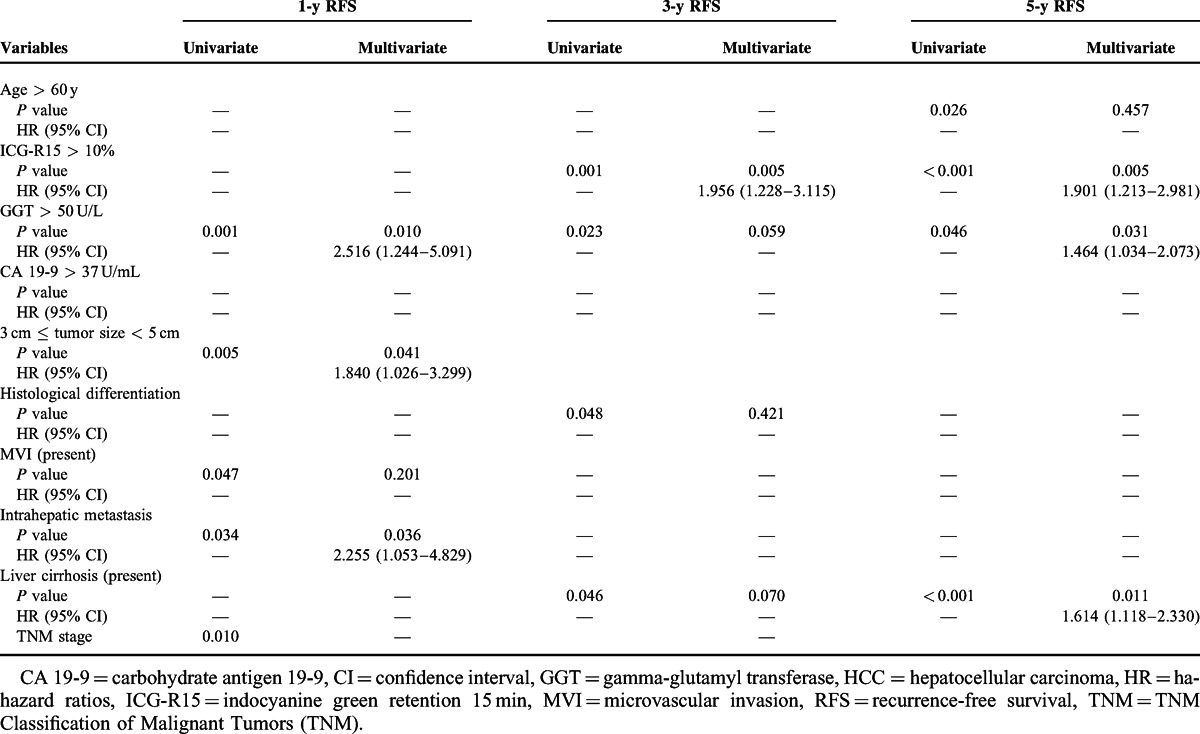
As shown in Table 4, multivariate regression analysis using the Cox proportional hazard model with backward elimination identified the following variables as independent risk factors affecting RFS in 248 patients with a tumor < 5 cm in size: GGT > 50 UL for 1- and 5-year RFS, ICG-R15 > 10% for 3- and 5-year RFS; a tumor ≥ 3.0 cm in size and the presence of intrahepatic metastasis for 1-year RFS, and the presence of liver cirrhosis for 5-year RFS.
TABLE 4.
Univariate and Multivariate Analysis of Risk Factors Affecting the 1-, 3-, and 5-y RFS for 248 Patients With HCC < 5 cm in Size
Relationship Between GGT, ICG-R15, and RFS for Patients With a Tumor < 5 cm in Size
Of 248 patients with a tumor < 5 cm in size, 94 (41.8%) had GGT > 50 U/L and ICG-R15 >10%, 104 (46.2%) had GGT > 50 U/L and ICG-R15 ≤ 10% or GGT ≤ 50 U/L and ICG-R15 >10%, 27 (12.0%) had GGT ≤ 50 U/L and ICG-R15 ≤ 10%, and 23 were lost to follow-up.
As shown in Figure 3, 248 patients had a 1-, 3-, and 5-year RFS rate of 80.3%, 49.3%, and 31.2%, respectively. Patients with GGT > 50 U/L had a 1-, 3-, and 5-year RFS worse than those with GGT ≤ 50 U/L (P < 0.05). Patients with GGT > 50 U/L and ICG-R15 > 10% had a 1-, 3-, and 5-year RFS of 68.8%, 30.0%, and 13.3%, respectively. These patients had lower survival rates than did patients in the other 2 groups with different levels of GGT and ICG (P < 0.05, respectively).
FIGURE 3.
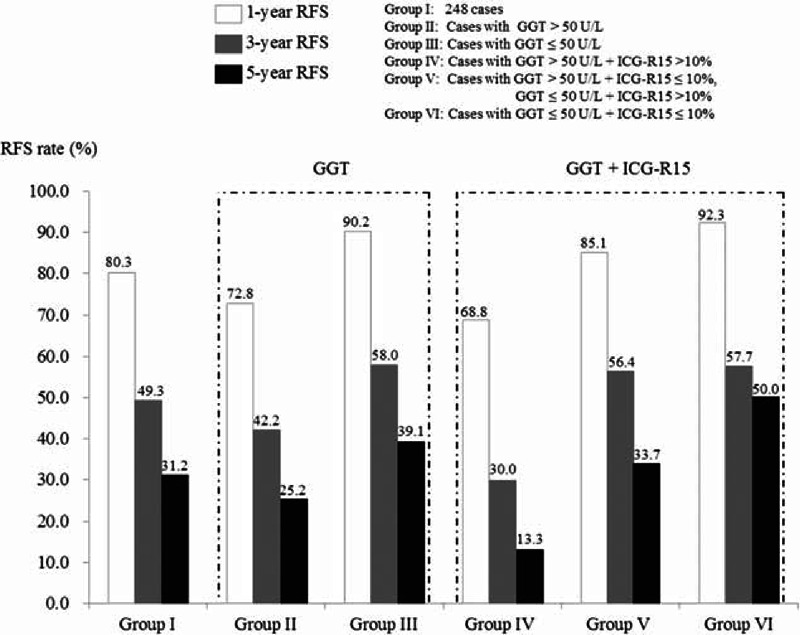
1-, 3-, and 5-year RFS rate for 248 patients with a tumor <5 cm in size, patients with GGT > 50 U/L or GGT ≤ 50 U/L, and patients with the combination of GGT (cutoff of 50 U/L) and ICG-R15 (cutoff of 10%). GGT = gamma-glutamyl transferase, ICG-R15 = indocyanine green retention 15 min, RFS = recurrence-free survival.
DISCUSSION
Owing to the high rate of recurrence after hepatic resection, the risk factors for tumor recurrence must be ascertained. That information could help to take interventional measures earlier and facilitate better surveillance to reduce the rate of recurrence and improve the quality of care for patients with HCC.25–28 The present study examined the postoperative pathological variables of MVI and intrahepatic metastasis. Several studies have confirmed that MVI and intrahepatic metastasis are risk factors associated with an increased risk of recurrence and decreased survival.29–32 The present study yielded similar findings.
The present study focused on preoperative predictors of postoperative survival and recurrence in patients with single primary HCC who underwent hepatic resection. GGT > 100 U/L was identified as a preoperative independent risk factor associated with survival, and GGT > 50 U/L and ICG-R15 > 10% were identified as preoperative independent risk factors associated with tumor recurrence. Patients with GGT > 50 U/L and ICG-R15 > 10% had a worse 1-, 3-, and 5-year RFS, and this was also true for patients with a tumor < 5 cm in size. These results suggest that combined information in the form of high levels of GGT and ICG-R15 warrants full attention as a preoperative predictor associated with tumor recurrence for patients with single primary HCC undergoing hepatic resection.
GGT is an important enzyme catalyzing the hydrolysis of glutathione and the transfer of gamma-glutamyl residues, and GGT has been widely used as a marker enzyme for some neoplasms, such as lung tumors and ovarian tumors.33–36 GGT was investigated and selected as a liver function test or liver enzyme in the 1960s and 1970s. Numerous clinical studies have noted a high level of abnormal GGT in patients with primary or secondary liver cancer.37 According to a study by Tsutsumi et al,38 analysis of GGT mRNA expression may provide a useful tool for diagnosis of HCC in its early stage as GGT mRNA may shift from type A to type B during the development of HCC.38 However, GGT is found to be abnormal in most patients with liver disease regardless of the cause, and a wide range of diseases and conditions (such as pancreatitis, obesity, and excessive alcohol intake) can also cause high levels of serum GGT.39–41 Thus, GGT was not considered to be a useful tumor marker for the detection of malignant liver disease for a long time.
Although GGT levels have a low level of specificity as a diagnostic marker of malignant liver disease, GGT has critical clinical significance as a prognostic maker with which to evaluate treatment and promptly facilitate selection of further treatment. This finding was revealed by studies based on different subgroups of patients published over the past 5 years. According to a study by Sheen et al,42 patients who had HCC with type B GGT mRNA had worse outcomes, earlier recurrence, and more postrecurrence deaths. Several studies of patients with HCC undergoing hepatic resection have revealed a correlation between elevated levels of GGT and worse survival for patients with HBV-related HCC, Child-Pugh A liver function, or multinodular tumors.18,43,44 In addition, several studies have revealed the predictive value of GGT in patients with unresectable HCC who were treated with transcatheter arterial chemoembolization or chemotherapy.17,20,21,45 In the present study of patients with single primary HCC who underwent hepatic resection, receiver operating characteristic curves were plotted to identify the optimal cutoff value of GGT was 50 U/L for RFS and 100 U/L for survival. After analysis, GGT > 50 U/L was identified as a preoperative independent risk factor affecting 1-, 3-, and 5-year RFS; GGT > 100 U/L was identified as a preoperative independent risk factor affecting 1-, 3-, and 5-year survival. These findings further confirm the role of GGT as a preoperative independent risk factor associated with survival and tumor recurrence in patients with HCC.
Findings from numerous studies have suggested 2 possible molecular mechanisms for the association between GGT and the recurrence of HCC and poor survival. One is that GGT may be associated with worse liver function via induction of DNA instability and subsequent oncogenesis, whereas the other is that GGT may be associated with the degree of malignancy of HCC, such as vascular invasion, tumor metastasis, or a worse grade of tumor differentiation.46 GGT can facilitate DNA damage, genomic instability, and genetic mutation by increasing the uptake of iron,47 and iron has been identified as playing a role in carcinogenesis.48 This mechanism is thought to lead to the death of normal liver cells or the loss of normal liver function. GGT is reported to play a prooxidant role and the subsequent production of reactive oxygen species (ROS) may promote certain intracellular and extracellular molecular signals.49 Recently, ROS were reported to promote an epithelial-to-mesenchymal transition via the Snail-E-cadherin pathway50 and induce inflammation and invasion via the nuclear factor kappa B pathway.51,52 A study of U937 lymphoma cells found that GGT may play a role in antiapoptotic signaling.53 Another study confirmed that cysteinylglycine, which is catalyzed by GGT, is able to form complexes with cisplatin and that such adducts are not readily transported through the cell membrane.54 These mechanisms are thought to account for the progression of HCC, and the molecular mechanisms of the association between GGT and the recurrence of HCC and poor survival should be investigated further.
ICG-R15 is a common parameter for preoperative assessment of preserved hepatic function.55 ICG-R15 is reported to be an early indicator of hepatic dysfunction and it has been used preoperatively to plan the extent of partial hepatectomy by predicting the risk of dysfunction after surgery.56–58 ICG-R15 with a cutoff value of 10% as an upper limit of normal has been widely used in clinical practice in Japan.59–61 In the present study, ICG-R15 > 10% was identified as a preoperative independent risk factor affecting 3- and 5-year RFS. Furthermore, patients with GGT > 50 U/L and ICG-R15 > 10% had a worse 1-, 3-, and 5-year RFS, and the same was also true for patients with a tumor < 5 cm in size. These results suggest that combined information in the form of high levels of GGT and ICG-R15 could be a more convenient and accurate preoperative predictor with which to evaluate tumor recurrence postoperatively.
In conclusion, the present study identified the preoperative variables of GGT > 50 U/L and ICG-R15 > 10% as independent risk factors for tumor recurrence in patients with single primary HCC who underwent hepatic resection. Patients with high levels of GGT and ICG-R15 had a worse 1-, 3-, and 5-year RFS, and the same was also true for patients with a tumor < 5 cm in size. Therefore, combined information in the form of high levels of GGT and ICG-R15 warrants full attention when evaluating tumor recurrence postoperatively. This information should help surgeons to take more effective interventional measures earlier in order to reduce recurrence and improve the quality of care for patients with HCC.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, CA 19-9 = carbohydrate antigen 19-9, CI = confidence interval, DCP = des-gamma-carboxy-prothrombin, GGT = gamma-glutamyl transferase, HCC = hepatocellular carcinoma, HR = hazard ratio, ICG-R15 = indocyanine green retention 15 min, MVI = microvascular invasion, RFS = recurrence-free survival, ROS = reactive oxygen species.
WT and NK designed the research; PS, YI, and ZW conducted the data analyses; PS produced the initial draft of the article. KH, YS, and JA were involved in the interpretation of the analyses and writing of the article. All authors approved the final version of the article and take responsibility for its content.
This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan. The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sharfi AR, Elarabi YE. The ’watering-can’ perineum: presentation and management. Br J Urol 1997; 80:933–936. [DOI] [PubMed] [Google Scholar]
- 2.Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg 2011; 253:453–469. [DOI] [PubMed] [Google Scholar]
- 3.Ardiles V, Sanchez Claria R, Mazza OM, et al. Prognostic factors after resection of hepatocellular carcinoma in the non-cirrhotic liver: presentation of 51 cases. Cir Esp 2010; 87:148–154. [DOI] [PubMed] [Google Scholar]
- 4.Smoot RL, Nagorney DM, Chandan VS, et al. Resection of hepatocellular carcinoma in patients without cirrhosis. Br J Surg 2011; 98:697–703. [DOI] [PubMed] [Google Scholar]
- 5.Verhoef C, de Man RA, Zondervan PE, et al. Good outcomes after resection of large hepatocellular carcinoma in the non-cirrhotic liver. Dig Surg 2004; 21:380–386. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Zhao G, Wei K, et al. Adjuvant sorafenib reduced mortality and prolonged overall survival and post-recurrence survival in hepatocellular carcinoma patients after curative resection: a single-center experience. Biosci Trends 2014; 8:333–338. [DOI] [PubMed] [Google Scholar]
- 7.Gao JJ, Song PP, Tamura S, et al. Standardization of perioperative management on hepato-biliary-pancreatic surgery. Drug Discov Ther 2012; 6:108–111. [PubMed] [Google Scholar]
- 8.Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002; 235:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muscari F, Foppa B, Carrere N, et al. Resection of a transplantable single-nodule hepatocellular carcinoma in Child-Pugh class A cirrhosis: factors affecting survival and recurrence. World J Surg 2011; 35:1055–1062. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Rui JA, Wang SB, et al. Factors predictive for long-term survival of male patients with hepatocellular carcinoma after curative resection. J Surg Oncol 2007; 95:298–303. [DOI] [PubMed] [Google Scholar]
- 11.Naito S, Imamura H, Tukada A, et al. Postoperative recurrence pattern and prognosis of patients with hepatocellular carcinoma, with particular reference to the hepatitis viral infection status. Liver Int 2014; 34:802–813. [DOI] [PubMed] [Google Scholar]
- 12.Guo R, Feng X, Xiao S, et al. Short- and long-term outcomes of hepatectomy with or without radiofrequency-assist for the treatment of hepatocellular carcinomas: a retrospective comparative cohort study. Biosci Trends 2015; 9:65–72. [DOI] [PubMed] [Google Scholar]
- 13.Zhu WJ, Huang CY, Li C, et al. Risk factors for early recurrence of HBV-related hepatocellular carcinoma meeting milan criteria after curative resection. Asian Pac J Cancer Prev 2013; 14:7101–7106. [DOI] [PubMed] [Google Scholar]
- 14.Park SK, Jung YK, Chung DH, et al. Factors influencing hepatocellular carcinoma prognosis after hepatectomy: a single-center experience. Korean J Intern Med 2013; 28:428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011; 254:108–113. [DOI] [PubMed] [Google Scholar]
- 16.Hung HH, Lei HJ, Chau GY, et al. Milan criteria, multi-nodularity, and microvascular invasion predict the recurrence patterns of hepatocellular carcinoma after resection. J Gastrointest Surg 2013; 17:702–711. [DOI] [PubMed] [Google Scholar]
- 17.Carr BI, Pancoska P, Branch RA. Low alpha-fetoprotein hepatocellular carcinoma. J Gastroenterol Hepatol 2010; 25:1543–1549. [DOI] [PubMed] [Google Scholar]
- 18.Zhao WC, Zhang HB, Yang N, et al. Preoperative predictors of short-term survival after hepatectomy for multinodular hepatocellular carcinoma. World J Gastroenterol 2012; 18:3272–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faber W, Sharafi S, Stockmann M, et al. Long-term results of liver resection for hepatocellular carcinoma in noncirrhotic liver. Surgery 2013; 153:510–517. [DOI] [PubMed] [Google Scholar]
- 20.Guiu B, Deschamps F, Boulin M, et al. Serum gamma-glutamyl-transferase independently predicts outcome after transarterial chemoembolization of hepatocellular carcinoma: external validation. Cardiovasc Intervent Radiol 2012; 35:1102–1108. [DOI] [PubMed] [Google Scholar]
- 21.Nishikawa H, Nishijima N, Arimoto A, et al. Prognostic factors in patients with hepatitis B virus-related hepatocellular carcinoma undergoing nucleoside analog antiviral therapy. Oncol Lett 2013; 6:1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shindoh J, Hasegawa K, Matsuyama Y, et al. Low hepatitis C viral load predicts better long-term outcomes in patients undergoing resection of hepatocellular carcinoma irrespective of serologic eradication of hepatitis C virus. J Clin Oncol 2013; 31:766–773. [DOI] [PubMed] [Google Scholar]
- 23.Shindoh J, Hasegawa K, Inoue Y, et al. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB (Oxford) 2013; 15:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer. Tokyo: Kanehara-Syuppan; 2008. [Google Scholar]
- 25.Nagasue N, Ono T, Yamanoi A, et al. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg 2001; 88:515–522. [DOI] [PubMed] [Google Scholar]
- 26.Laurent C, Blanc JF, Nobili S, et al. Prognostic factors and longterm survival after hepatic resection for hepatocellular carcinoma originating from noncirrhotic liver. J Am Coll Surg 2005; 201:656–662. [DOI] [PubMed] [Google Scholar]
- 27.Song P, Feng X, Inagaki Y, et al. Clinical utility of simultaneous measurement of alpha-fetoprotein and des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinoma in China: a multi-center case-controlled study of 1,153 subjects. Biosci Trends 2014; 8:266–273. [DOI] [PubMed] [Google Scholar]
- 28.Song P, Feng X, Zhang K, et al. Perspectives on using des-gamma-carboxyprothrombin (DCP) as a serum biomarker: facilitating early detection of hepatocellular carcinoma in China. Hepatobiliary Surg Nutr 2013; 2:227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esnaola NF, Mirza N, Lauwers GY, et al. Comparison of clinicopathologic characteristics and outcomes after resection in patients with hepatocellular carcinoma treated in the United States, France, and Japan. Ann Surg 2003; 238:711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikai I, Arii S, Kojiro M, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer 2004; 101:796–802. [DOI] [PubMed] [Google Scholar]
- 31.Zeng QA, Qiu J, Hong J, et al. Hepatectomy for hepatocellular carcinoma patients with macronodular cirrhosis. Eur J Gastroenterol Hepatol 2012; 24:575–582. [DOI] [PubMed] [Google Scholar]
- 32.Akamatsu N, Sugawara Y, Kokudo N. Acute liver failure and liver transplantation. Intractable Rare Dis Res 2013; 2:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wetmore LA, Gerard C, Drazen JM. Human lung expresses unique gamma-glutamyl transpeptidase transcripts. Proc Natl Acad Sci USA 1993; 90:7461–7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanigan MH, Frierson HF, Jr, Brown JE, et al. Human ovarian tumors express gamma-glutamyl transpeptidase. Cancer Res 1994; 54:286–290. [PubMed] [Google Scholar]
- 35.Hudson EA, Munks RJ, Manson MM. Characterization of transcriptional regulation of gamma-glutamyl transpeptidase in rat liver involving both positive and negative regulatory elements. Mol Carcinog 1997; 20:376–388. [PubMed] [Google Scholar]
- 36.Brouillet A, Holic N, Chobert MN, et al. The gamma-glutamyl transpeptidase gene is transcribed from a different promoter in rat hepatocytes and biliary cells. Am J Pathol 1998; 152:1039–1048. [PMC free article] [PubMed] [Google Scholar]
- 37.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci 2001; 38:263–355. [DOI] [PubMed] [Google Scholar]
- 38.Tsutsumi M, Sakamuro D, Takada A, et al. Detection of a unique gamma-glutamyl transpeptidase messenger RNA species closely related to the development of hepatocellular carcinoma in humans: a new candidate for early diagnosis of hepatocellular carcinoma. Hepatology 1996; 23:1093–1097. [DOI] [PubMed] [Google Scholar]
- 39.Goldberg DM, Martin JV. Role of gamma-glutamyl transpeptidase activity in the diagnosis of hepatobiliary disease. Digestion 1975; 12:232–246. [DOI] [PubMed] [Google Scholar]
- 40.Ellis G, Worthy E, Goldberg DM. Lack of value of serum gamma-glutamyl transferase in the diagnosis of hepatobiliary disease. Clin Biochem 1979; 12:142–145. [DOI] [PubMed] [Google Scholar]
- 41.Ruppin DC, Frydman MI, Lunzer MR. Value of serum gamma-glutamyltransferase activity in the diagnosis of hepatobiliary disease. Med J Aust 1982; 1:421–424. [DOI] [PubMed] [Google Scholar]
- 42.Sheen IS, Jeng KS, Tsai YC. Is the expression of gamma-glutamyl transpeptidase messenger RNA an indicator of biological behavior in recurrent hepatocellular carcinoma? World J Gastroenterol 2003; 9:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ju MJ, Qiu SJ, Fan J, et al. Preoperative serum gamma-glutamyl transferase to alanine aminotransferase ratio is a convenient prognostic marker for Child-Pugh A hepatocellular carcinoma after operation. J Gastroenterol 2009; 44:635–642. [DOI] [PubMed] [Google Scholar]
- 44.Zhao WC, Fan LF, Yang N, et al. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur J Surg Oncol 2013; 39:858–864. [DOI] [PubMed] [Google Scholar]
- 45.Zhang JB, Chen Y, Zhang B, et al. Prognostic significance of serum gamma-glutamyl transferase in patients with intermediate hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol 2011; 23:787–793. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Song P, Xia J, et al. Can gamma-glutamyl transferase levels contribute to a better prognosis for patients with hepatocellular carcinoma? Drug Discov Ther 2014; 8:134–138. [DOI] [PubMed] [Google Scholar]
- 47.Corti A, Duarte TL, Giommarelli C, et al. Membrane gamma-glutamyl transferase activity promotes iron-dependent oxidative DNA damage in melanoma cells. Mutat Res 2009; 669:112–121. [DOI] [PubMed] [Google Scholar]
- 48.Weinberg ED. The role of iron in cancer. Eur J Cancer Prev 1996; 5:19–36. [PubMed] [Google Scholar]
- 49.Stark AA, Russell JJ, Langenbach R, et al. Localization of oxidative damage by a glutathione-gamma-glutamyl transpeptidase system in preneoplastic lesions in sections of livers from carcinogen-treated rats. Carcinogenesis 1994; 15:343–348. [DOI] [PubMed] [Google Scholar]
- 50.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene 2005; 24:7443–7454. [DOI] [PubMed] [Google Scholar]
- 51.Javed S, Mejias-Luque R, Kalali B, et al. Helicobacter bilis gamma-glutamyltranspeptidase enhances inflammatory stress response via oxidative stress in colon epithelial cells. PLoS One 2013; 8:e73160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer 2010; 102:639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moon DO, Kim BY, Jang JH, et al. K-RAS transformation in prostate epithelial cell overcomes H2O2-induced apoptosis via upregulation of gamma-glutamyltransferase-2. Toxicol In Vitro 2012; 26:429–434. [DOI] [PubMed] [Google Scholar]
- 54.Paolicchi A, Sotiropuolou M, Perego P, et al. gamma-Glutamyl transpeptidase catalyses the extracellular detoxification of cisplatin in a human cell line derived from the proximal convoluted tubule of the kidney. Eur J Cancer 2003; 39:996–1003. [DOI] [PubMed] [Google Scholar]
- 55.Kim HJ, Kim CY, Park EK, et al. Volumetric analysis and indocyanine green retention rate at 15 min as predictors of post-hepatectomy liver failure. HPB (Oxford) 2015; 17:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lisotti A, Azzaroli F, Buonfiglioli F, et al. Indocyanine green retention test as a noninvasive marker of portal hypertension and esophageal varices in compensated liver cirrhosis. Hepatology 2014; 59:643–650. [DOI] [PubMed] [Google Scholar]
- 57.Fung J, Poon RT, Yu WC, et al. Use of liver stiffness measurement for liver resection surgery: correlation with indocyanine green clearance testing and post-operative outcome. PloS One 2013; 8:e72306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zipprich A, Kuss O, Rogowski S, et al. Incorporating indocyanin green clearance into the Model for End Stage Liver Disease (MELD-ICG) improves prognostic accuracy in intermediate to advanced cirrhosis. Gut 2010; 59:963–968. [DOI] [PubMed] [Google Scholar]
- 59.Okabe H, Beppu T, Hayashi H, et al. Rank classification based on the combination of indocyanine green retention rate at 15 min and (99m)Tc-DTPA-galactosyl human serum albumin scintigraphy predicts the safety of hepatic resection. Nucl Med Commun 2014; 35:478–483. [DOI] [PubMed] [Google Scholar]
- 60.Okabe H, Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res 2009; 39:107–116. [DOI] [PubMed] [Google Scholar]
- 61.Akita H, Sasaki Y, Yamada T, et al. Real-time intraoperative assessment of residual liver functional reserve using pulse dye densitometry. World J Surg 2008; 32:2668–2674. [DOI] [PubMed] [Google Scholar]


