Abstract
The global epidemiological landscape of childhood acute gastroenteritis (AGE) is changing after the introduction of 2 effective rotavirus vaccines in 2006. A comprehensive evaluation for viral etiology of childhood AGE in Taiwan, where rotavirus vaccination was provided by the private sector since 2006, is lacking.
From 2009 to 2011, children younger than 5 years of age with AGE who were hospitalized at 3 sentinel hospitals were enrolled in this surveillance study. Stool specimens were tested for rotavirus, norovirus, enteric adenovirus, and astrovirus. The epidemiologic and clinical information was collected by questionnaire-based interviews and chart reviews.
Viral agents were detected in 1055 (37.5%) of 2810 subjects, with rotavirus (21.2%) being the leading cause of disease, followed by norovirus (14.9%), enteric adenovirus (3.74%), astrovirus (2.10%), and a mixture of at least 2 of 4 above-mentioned viruses (4.06%). The majority (56%) of the viral AGE occurred in children <2 years of age. Rotavirus and norovirus were detected more frequently in cool seasons (P < 0.0001 for both), whereas no seasonal variation was observed for adenovirus and astrovirus. Adult households with diarrhea and a Vesikari score >10 were independent factors respectively associated with an increased risk of norovirus (adjusted odds ratio [aOR] 9.034, P = 0.0003) and rotavirus (aOR, 3.284, P < 0.0001) infections. Rotavirus immunization and female gender were protective factors against rotavirus (aOR, 0.198, P < 0.0001) and astrovirus (aOR, 0.382, P = 0.0299) infections, respectively.
Rotavirus and norovirus are the 2 most important viral agents of childhood AGE in Taiwan with partial rotavirus immunization. In addition, different enteric viruses are associated with distinct epidemiologic and clinical features.
INTRODUCTION
Acute gastroenteritis (AGE) is a very common pediatric illness and remains a significant cause of childhood morbidity and mortality worldwide.1,2 Children younger than 5 years of age are most vulnerable to severe AGE. In 2010, it is estimated that 2.7 diarrhea episodes per child, yearly, occurred in children aged 0 to 4 years, and acute diarrhea accounted for 700,000 pediatric deaths in 2011 worldwide.2 Since 2006, the disease burden of AGE and rotavirus-associated AGE has been significantly reduced following the implementation of a rotavirus immunization program in the United States.3 Norovirus has now became the leading cause of medically attended AGE in US children.4 The changing epidemiology of pediatric diarrhea has also been impacted by the frequent emergence of new variants of enteric viruses and their associated global AGE epidemics.5,6
Before the introduction of rotavirus vaccines in 2006, rotavirus was the leading cause of severe diarrhea in Taiwanese children.7 Similar to the conditions in the United States and other countries, with the increasing use of immunizations against rotavirus, it appeared that the incidence of severe rotavirus AGE in children was decreasing in Taiwan.8 However, complete information regarding enteric viral agents and epidemiologic features of severe pediatric AGE is lacking9, and comprehensive investigation on a national scale and across multiple seasons has not yet been performed since the introduction of the rotavirus vaccine. To monitor the epidemiologic changes in pediatric AGE in the postvaccine era, we conducted a 3-year sentinel surveillance study to investigate the viral etiologies and their associated epidemiological and clinical features in severe childhood AGE.
METHODS
Study Design
This was a multicenter, prospective surveillance study evaluating the epidemiology of acute diarrhea due to various viral and bacterial etiologies in Taiwanese children. The study was conducted in 3 sentinel hospitals from January 1, 2009 to December 31, 2011. Children were eligible for the surveillance study if they met all of the following criteria: hospitalized in 1 of the 3 hospitals; aged 2 to 60 months; and presented with diarrhea without any known underlying disease. Diarrhea was defined as the occurrence of 3 or greater episodes of unformed stool within a period of 24 hours of acute illness. Active surveillance of acute diarrhea was conducted by monitoring the reception log of stool samples subjected to microbiology tests in the clinical microbiology laboratory in each of the 3 hospitals. Those who met the inclusion criteria, and for whom a written informed consent was obtained from the parents or guardians, were enrolled in the study. The surveillance study was approved by the institutional review boards of the National Health Research Institute of Taiwan and each of the 3 study sites.
Demographics and Anthropometric Characteristics of the Subjects
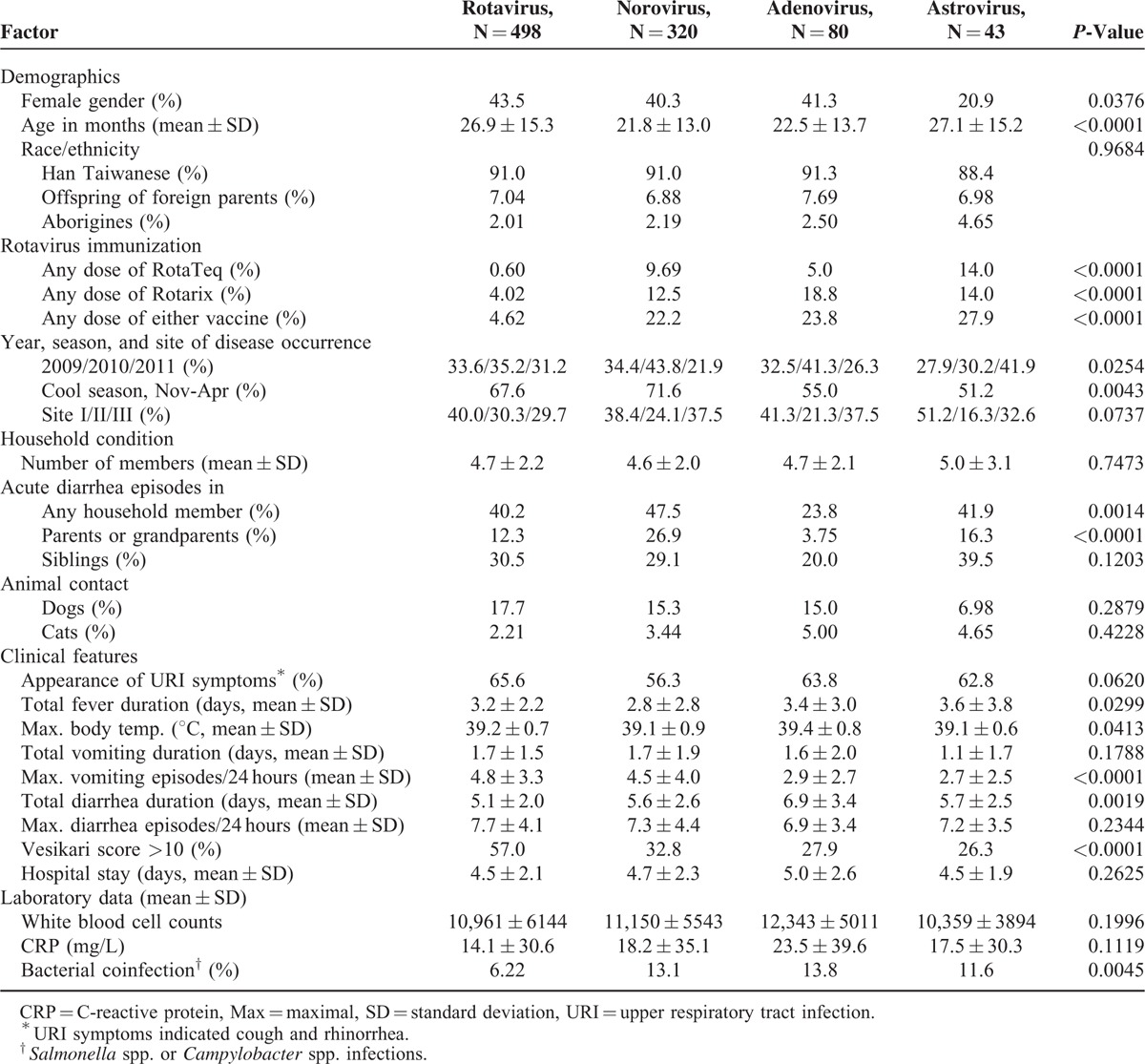
A standardized questionnaire-based interview of the parents or primary caregivers of the enrolled subjects was performed to collect demographic and anthropometric information (Table 1). Information on the clinical features of the diarrhea illness was obtained by chart review. The severity of AGE was defined according to the Vesikari clinical severity scoring system.10 Briefly, the system contains 7 scoring parameters including maximum number and duration of diarrhea, maximum number and duration of vomiting, body temperature, degree of dehydration, and treatment. Each of the 7 parameters is broken into thirds according to an equally divided severity distribution (bottom third = 1, middle third = 2, top third = 3). A sum of the scores of less than 7, 7 to 10, and greater than 10 was considered as mild, moderate, and severe, respectively. The demographic and clinical data were digitized and organized in a computer core laboratory before statistical analysis. The data of air temperature of each month during the period of study were obtained from the Central Weather Bureau in Taiwan.
TABLE 1.
Univariate Analysis of Epidemiological Factors and Clinical Features in Pediatric Diarrhea of Distinct Viral Origin
Stool Collection and Microbiological Tests
Stool samples were collected from subject patient during the period of hospitalization. All of the stool specimens were screened for rotavirus VP6 antigen using an enzyme immunoassay kit (RIDASCREEN Rotavirus, R-Biopharm AG, Darmstadt, Germany) at each study site. All specimens were then sent to the reference laboratory at the Centers for Disease Control, Taiwan (Taiwan CDC) for confirmatory testing of rotavirus and further detection of norovirus, enteric adenovirus, astrovirus by well-established polymerase chain reaction (PCR) methods as described elsewhere.11–15 For identification of enteric bacteria, the culture methods were used in both the clinical microbiology laboratory at each sentinel sites and the reference laboratory of Taiwan CDC. All stool samples were frozen and stored at −80°C until the time of assay.
Statistical Analysis
The descriptive statistics were performed using an SAS 9.3 for windows (SAS Institute, Inc., Cary, NC). Comparison of categorical variables between 4 viral agents was performed with a χ2 test or with the Fisher exact test where appropriate, whereas differences among the numerical variables were analyzed by an 1-way ANOVA test. Multinomial logistic regression analysis was applied to explore factors associated with infection of rotavirus, norovirus, and astrovirus using adenovirus as referent. The trends in the proportions of specific viral agents in 5 defined age groups were tested by the Mantel–Haenszel χ2 method. Statistical significance was defined as a P-value of <0.05.
RESULTS
Epidemiology of Viral Gastroenteritis in Taiwanese Children, 2009 to 2011
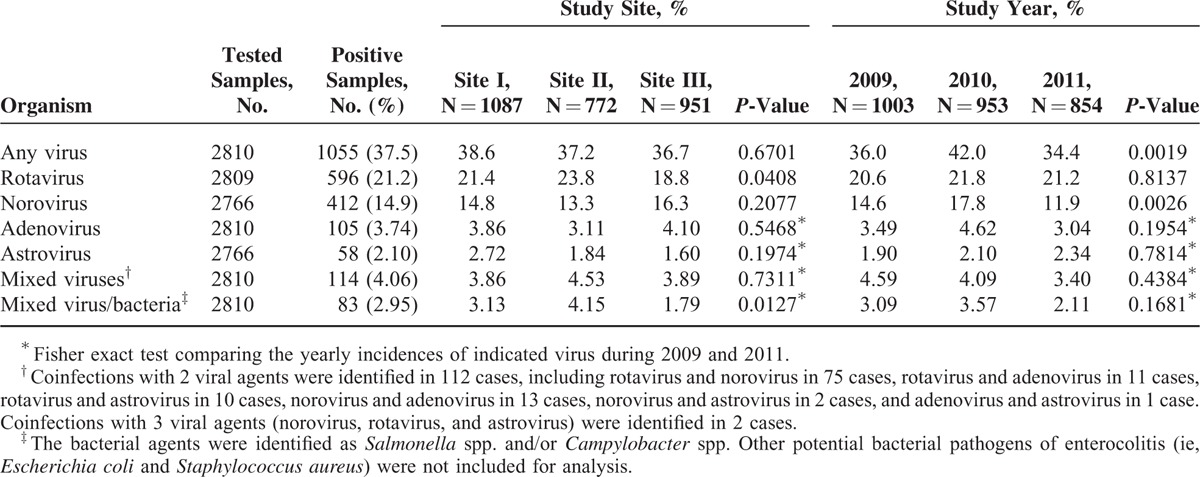
From January 2009 to December 2011, a total of 2810 children aged 2 to 60 months (mean age, 22.1 ± 13.9 months) with acute diarrhea were enrolled in this surveillance cohort. Of these included children, 42.9% were female. The age and gender distributions were not significantly different for the subjects across the different study sites (P = 0.7521 and 0.2501, respectively) and different years of enrollment (P = 0.3561 and 0.1950, respectively). Viral agents were detected in 1055 (37.5%) subjects, with rotavirus (21.2%) representing the leading cause of disease, followed by norovirus (14.9%), adenovirus (3.74%), astrovirus (2.10%), and mixed viruses (4.06%). Infections with bacterial pathogens including Salmonella spp. and Campylobacter spp. were identified in 27.4% and 2.25% of subjects, respectively. The detailed distribution of viral etiologies among subjects from distinct study sites from different years is displayed in Table 2. The prevalence of rotavirus infection differed across the 3 study sites (range, 18.8–23.8%, P = 0.0408) but did not change significantly according to year of enrollment (P = 0.8137). The prevalence of norovirus was relatively consistent among the 3 study sites (P = 0.2077) but varied significantly across study years (range, 11.9–17.8%, P = 0.0026). Adenovirus and astrovirus were identified at low frequencies, and each infection accounted for less than 5% of diarrhea cases within any single site or any year of study. The case distributions across sites and years of study were not significantly different for either virus (Table 2).
TABLE 2.
Distribution of Viral Etiologies of Acute Pediatric Diarrhea in Three Medical Centers in Taiwan From 2009 to 2011
Age-Specific Distribution of Viral Etiologies in Pediatric Diarrhea
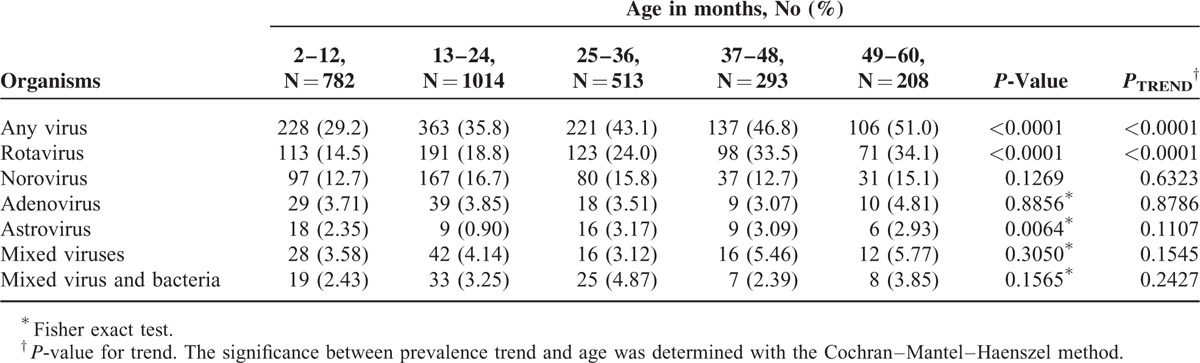
The majority of pediatric diarrhea cases requiring hospitalization occurred in children younger than 2 years of age in this cohort and accounted for 56.0% (591/1055) of subjects with viral gastroenteritis, 51.0% (304/596) of subjects with rotavirus gastroenteritis, and 64.1% (264/412) of subjects with norovirus gastroenteritis. The increased proportion of viral to all-cause gastroenteritis when age advanced was highly significant for rotavirus (P for trend < 0.0001). These proportions did not change significantly in distinct age groups for norovirus (P = 0.1269) and adenovirus (P = 0.8856). Additionally, there was no obvious trend of proportion changes for astrovirus with increasing age (P for trend = 0.1107) (Table 3).
TABLE 3.
Age-Specific Etiologies of Pediatric Diarrhea Requiring Hospitalization in Taiwan, 2009–2011
Temporal Distribution of Enteric Viruses and Correlation With Air Temperature
Viral gastroenteritis occurred more frequently in cool seasons (November to April) when the average air temperature was below 25°C compared to the warm seasons (May to October) when the average temperature was above 25°C (52.4% vs. 23.6%, P < 0.0001). This increased incidence of viral gastroenteritis occurring more frequently in cool seasons was significant for rotavirus (29.8% vs. 13.2%, P < 0.0001) and norovirus (22.7% vs. 7.8%, P < 0.0001) but not for adenovirus (4.41% vs. 3.31%, P = 0.0735, Fisher exact test) or astrovirus (2.42% vs. 1.80, P = 0.2883, Fisher exact test) (Figure 1).
FIGURE 1.
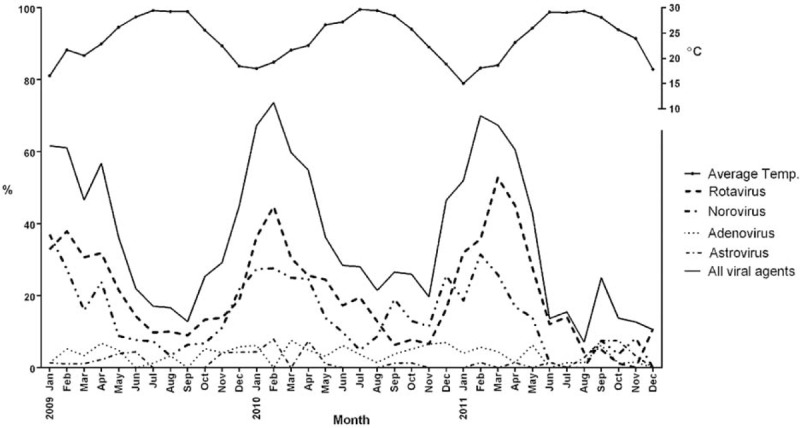
The average air temperatures and monthly distributions of viral etiologies in Taiwanese children with AGE requiring hospitalization from 2009 to 2011.
Etiology-Specific Features for Distinct Viral Gastroenteritis
Among 1055 subjects with confirmed viral infections, 468 (44.4%) had a Vesikari score >10 indicating severe gastroenteritis. The mean hospital stay was 4.65 ± 2.26 days (range, 1–22 days), and the mean duration for fever, vomiting, and diarrhea was 3.12 ± 2.59, 1.68 ± 1.71, and 5.40 ± 2.31 days, respectively. The epidemiological and clinical features of subjects infected with the 4 distinct viral agents are displayed in Table 1. The 4 groups of subjects differed significantly in demographics and immunization history against rotavirus. The subjects also differed according to the rates of household members with acute diarrhea, with the highest incidence (47.5%) for norovirus infection. The appearance of symptoms of upper respiratory tract infection was very common and occurred in 56.3% to 65.6% of the subjects with viral gastroenteritis. Coinfection with bacteria was less frequently identified in rotavirus infections (6.22%, P = 0.0045). Nevertheless, rotavirus was associated with the greatest disease severity, as indicated by a higher incidence of subjects with a Vesikari score >10 in rotavirus infections (57.0%) compared to infections with the other 3 viruses (range, 26.3–32.8%, P < 0.0001).
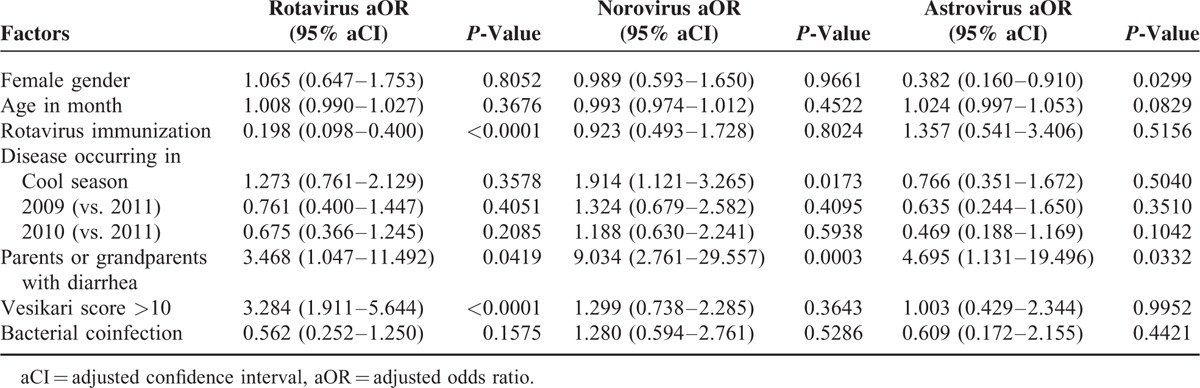
Multinomial logistic regression analysis identified different panels of factors associated with distinct types of viral gastroenteritis (Table 4). In particular, immunization against rotavirus was associated with a decreased incidence of rotavirus infection (adjusted odds ratio [aOR] 0.198, P < 0.0001), whereas a Vesikari score >10 increased the risk of rotavirus infections (aOR, 3.284, P < 0.0001). In addition, parents or grandparents with diarrhea was associated with 9-fold increased risk of norovirus infection (aOR, 9.034, P = 0.0003). The cold season was a significant factor predicting norovirus infection (aOR, 1.914, P = 0.0173), whereas female gender was a protective factor against astrovirus infection (aOR, 0.382, P = 0.0299).
TABLE 4.
Etiology-Specific Factors of Acute Viral Gastroenteritis Requiring Hospitalization in Taiwanese Children, Identified by Comparing the Factors Between the Indicated Virus, and Adenovirus Using Multinomial Logistic Regression Analysis
DISCUSSION
Results from the present study indicate that rotavirus remained the leading cause of viral gastroenteritis requiring hospitalization in Taiwanese children from 2009 to 2011. Rotavirus vaccines, including Rotarix (GlaxoSmithKline Biologicals, Rixensart, Belgium) and RotaTeq (Merck and Co., Inc., West Point, PA), were introduced into Taiwan as a self-paid vaccine during late 2006. The uptake rate of full-dose vaccine (2 doses for Rotarix and 3 doses for RotaTeq) was estimated to be 19.4% for each annual birth cohort from 2009 to 2011 (merged data from Merck and Co. and GlaxoSmithKline, Taiwan branch). This estimate was generally consistent with the data in the present study showing that 21.6% (609/2810) of all subjects reportedly received at least 1 dose of rotavirus vaccine. Under partial immunization, we demonstrated that 21.2% of the pediatric diarrhea cases requiring hospitalization remained related to rotavirus infections. Nevertheless, this rate was largely reduced compared to data in the prevaccine era. From 2001 to 2006, the rates were at least 2-fold higher, ranging from 43% and 46%, respectively, in 2 large-scale hospital-based studies with similar design and similar age groups (<5 years old).16,17 Results from the Asian Rotavirus Surveillance Network disclosed an even higher rate of 49% (range, 43–53%) in Taiwan from 2001 to 2002.7 Taken together, these data suggest a marked reduction of rotavirus-associated hospitalizations in Taiwan when approximately 20% of the vulnerable population was immunized.
The present study provides comprehensive information on the epidemiologic and clinical features associated with each of the 4 most common enteric viruses in children. The simultaneous evaluation of the viruses generated different panels of etiology-specific features, which are relevant in studies of viral behavior and useful in clinical practice. For instance, immunization with any dose of rotavirus vaccine substantially diminished the probability of rotavirus infection by approximately 80%. In contrast, a Vesikari score >10 was strongly suggestive of rotavirus infection. Subjects with a greater severity of rotavirus infection, compared to those with other enteric viral infections designated by the measurement of Vesikari scores, has been demonstrated elsewhere.18,19 Parents or grandparents with acute diarrhea was a significant factor predicting norovirus infection. The household diarrhea history was of marginal significance for rotavirus and astrovirus infection. The observation was consistent with the previous findings that norovirus was capable of causing severe disease in subjects, irrespective of age, whereas symptomatic infections of rotavirus and enteric adenovirus generally occurred in children younger than 5 years of age.1,20
Norovirus is the most common agent responsible for food-borne gastroenteritis outbreaks worldwide.21,22 It was intriguing to learn that the annual incidence of norovirus infections fluctuated from year to year across the 3 years of the study. This observation occurred in contrast to rotavirus, for which the year-to-year rate was relatively stable (Table 2). It has been well documented that new variants of norovirus emerge frequently and that global pandemics usually occur every few years.5 The epidemic outbreaks associated with emerging variants can have a substantial impact on the local epidemiology of norovirus diseases. Indeed, the emerging pandemic strains in the past decade, including “Hunter” virus, the “2006a and 2006b” strains, and the “Sydney” strain of GII.4 norovirus, which were, respectively, identified in 2004, 2006, and 2012, also caused outbreaks in Taiwan in the indicated years.8,23 Another new epidemic strain, GII.4 2010, was associated with an outbreak of severe norovirus infections in Taiwanese children in 2010.8 This new variant also likely affected the temporal distributions of norovirus in the study years and may have been responsible for the increased number of cases in 2010 in the present study.
Astrovirus infection was reported in a range of 2% to 16% of children hospitalized for diarrhea worldwide,24–26 and a hospital-based survey in northern Taiwan in 1998 to 1999 disclosed that 2.9% of childhood diarrhea was caused by astrovirus.27 In the present study, astrovirus infection accounted for a similar low rate of AGE cases (2.1%). The data indicated that astrovirus was of relatively low virulence and played a limited role in severe diarrhea in Taiwanese children. Interestingly, approximately 80% of subjects with astrovirus infection were male. The particular vulnerability of male children to severe astrovirus infections was also observed in the above-mentioned survey in northern Taiwan, in which male gender accounted for 75% (9/12) of astrovirus-infected subjects.27 Further investigation may be required to explore the biologic role of gender in astrovirus infection. Alternatively, the gender factor may be merely a confounding factor, given that potential host factors including cleanliness or other personal hygiene behaviors were not collected for analysis.
Although diarrhea can be seen in “respiratory” types of adenovirus infections (ie, types 3 and 7), adenoviral gastroenteritis is mainly caused by the enteric fastidious species types 40 and 41, which were reported in 5% to 15% of cases of acute pediatric diarrhea.28–30 We observed a slightly lower incidence of 3.74% in the present study. Adenovirus shared similar patterns of temporal distributions with astrovirus. In particular, these infections occurred throughout the year during the period of study without the significant seasonal variation seen in cases of rotavirus and norovirus infection.
Among the study subjects, an increasing proportion of rotavirus etiology was noted as their age advanced (Table 3). However, this factor of age for rotavirus etiology was not statistically significant after adjusted by multinomial logistic regression analysis (Table 4). In addition, these data should be interpreted with caution and should not be interpreted as the older children with an increased risk of severe rotavirus infection. Rather, this finding suggests that rotavirus be a more virulent agent than the other 3 viruses, capable of causing severe disease even in the older age group. Young children, particularly those less than 2 years of age, remained the population with the largest disease burden of severe rotavirus gastroenteritis in Taiwan.
In conclusion, this sentinel surveillance study provides a landscape view of the epidemiology of severe viral gastroenteritis in Taiwanese children from 2009 to 2011. Rotavirus remained the leading cause of severe viral gastroenteritis and was associated with the greatest disease severity among subjects with enteric viral infections. However, a marked reduction in rotavirus-associated hospitalization was observed when the population was partially immunized. Norovirus infection was characterized by a substantial fluctuation in yearly incidence, and simultaneous occurrence of diarrhea in adult households. Enteric adenovirus and astrovirus were infrequent enteropathogens in severe childhood AGE. The identified epidemiologic and clinical features associated with each enteric virus are relevant for studies concerning viral behavior and should be useful for healthcare workers dealing with pediatric diarrhea.
Footnotes
Abbreviations: AGE = acute gastroenteritis, Taiwan CDC = Centers for Disease Control, Taiwan, PCR = polymerase chain reaction.
C.-J.C. and F.-T.W. contributed equally to this study.
This study was financially supported in part by research grant of DOH98-DC-1005, MOHW103-CDC-C-315-000201, and MOHW103-CDC-C-315-000801 from Taiwan Centers for Disease Control.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Centers for Disease Control and Prevention. Rotavirus surveillance—worldwide, 2001–2008. Morb Mortal Wkly Rep 2008; 57:1255–1257. [PubMed] [Google Scholar]
- 2.Walker CLF, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013; 381:1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai R, Curns AT, Steiner CA, et al. All-cause gastroenteritis and rotavirus-coded hospitalizations among US children, 2000–2009. Clin Infect Dis 2012; 55:e28–e34. [DOI] [PubMed] [Google Scholar]
- 4.Payne DC, Vinjé J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 2013; 368:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tu ETV, Bull RA, Greening GE, et al. Epidemics of gastroenteritis during 2006 were associated with the spread of norovirus GII.4 variants 2006a and 2006b. Clin Infect Dis 2008; 46:413–420. [DOI] [PubMed] [Google Scholar]
- 6.Lindesmith LC, Donaldson EF, Lobue AD, et al. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med 2008; 5:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson EAS, Bresee JS, Parashar UD, et al. Rotavirus epidemiology: The Asian Rotavirus Surveillance Network. Vaccine 2008; 26:3192–3196. [DOI] [PubMed] [Google Scholar]
- 8.Chen S-Y, Tsai C-N, Chen C-L, et al. Severe viral gastroenteritis in children after suboptimal rotavirus immunization in Taiwan. Pediatr Infect Dis J 2013; 32:1335–1339. [DOI] [PubMed] [Google Scholar]
- 9.Yang S-Y, Hwang K-P, Wu F-T, et al. Epidemiology and clinical peculiarities of norovirus and rotavirus infection in hospitalized young children with acute diarrhea in Taiwan, 2009. J Microbiol Immunol Infect 2010; 43:506–514. [DOI] [PubMed] [Google Scholar]
- 10.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis 1990; 22:259–267. [DOI] [PubMed] [Google Scholar]
- 11.Wu F-T, Liang SY, Tsao KC, et al. Hospital-based surveillance and molecular epidemiology of rotavirus infection in Taiwan, 2005–2007. Vaccine 2009; 27 Suppl. 5:F50–F54. [DOI] [PubMed] [Google Scholar]
- 12.Kojima S, Kageyama T, Fukushi S, et al. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J Virol Methods 2002; 100:107–114. [DOI] [PubMed] [Google Scholar]
- 13.Belliot G, Laveran H, Monroe SS. Detection and genetic differentiation of human astroviruses: phylogenetic grouping varies by coding region. Arch Virol 1997; 142:1323–1334. [DOI] [PubMed] [Google Scholar]
- 14.Noel JS, Lee TW, Kurtz JB, et al. Typing of human astroviruses from clinical isolates by enzyme immunoassay and nucleotide sequencing. J Clin Microbiol 1995; 33:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohayem J, Berger S, Juretzek T, et al. A simple and rapid single-step multiplex RT-PCR to detect Norovirus, Astrovirus and Adenovirus in clinical stool samples. J Virol Methods 2004; 118:49–59. [DOI] [PubMed] [Google Scholar]
- 16.Iturriza-Gómara M, Dallman T, Bányai K, et al. Rotavirus surveillance in Europe, 2005–2008: web-enabled reporting and real-time analysis of genotyping and epidemiological data. J Infect Dis 2009; 200 Suppl. 1:S215–S221. [DOI] [PubMed] [Google Scholar]
- 17.Kawai K, O’Brien MA, Goveia MG, et al. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vaccine 2012; 30:1244–1254. [DOI] [PubMed] [Google Scholar]
- 18.O’Ryan ML, Lucero Y, Prado V, et al. Symptomatic and asymptomatic rotavirus and norovirus infections during infancy in a Chilean birth cohort. Pediatr Infect Dis J 2009; 28:879–884. [DOI] [PubMed] [Google Scholar]
- 19.Wikswo ME, Desai R, Edwards KM, et al. Clinical profile of children with norovirus disease in rotavirus vaccine era. Emerging Infect Dis 2013; 19:1691–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atmar RL, Estes MK. Diagnosis of noncultivatable gastroenteritis viruses, the human caliciviruses. Clin Microbiol Rev 2001; 14:15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamano M, Kuzuya M, Fujii R, et al. Epidemiology of acute gastroenteritis outbreaks caused by noroviruses in Okayama, Japan. J Med Virol 2005; 77:282–289. [DOI] [PubMed] [Google Scholar]
- 22.Belliot G, Lopman BA, Ambert-Balay K, et al. The burden of norovirus gastroenteritis: an important foodborne and healthcare-related infection. Clin Microbiol Infect 2014; 20:724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin F-R, Shen Y-H, Fang C-W, et al. Incidence of and factors associated with false positives in laboratory diagnosis of norovirus infection by amplification of the RNA-dependent RNA polymerase gene. PLoS ONE 2014; 9:e109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter JE, Mitchell DK. Role of astroviruses in childhood diarrhea. Curr Opin Pediatr 2000; 12:275–279. [DOI] [PubMed] [Google Scholar]
- 25.Mustafa H, Palombo EA, Bishop RF. Epidemiology of astrovirus infection in young children hospitalized with acute gastroenteritis in Melbourne, Australia, over a period of four consecutive years, 1995 to 1998. J Clin Microbiol 2000; 38:1058–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao H, Nilsson M, Abreu ER, et al. Viral diarrhea in children in Beijing, China. J Med Virol 1999; 57:390–396. [PubMed] [Google Scholar]
- 27.Lin HC, Kao C-L, Chang L-Y, et al. Astrovirus gastroenteritis in children in Taipei. J Formos Med Assoc 2008; 107:295–303. [DOI] [PubMed] [Google Scholar]
- 28.Christensen ML. Human viral gastroenteritis. Clin Microbiol Rev 1989; 2:51–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mistchenko AS, Huberman KH, Gomez JA, et al. Epidemiology of enteric adenovirus infection in prospectively monitored Argentine families. Epidemiol Infect 1992; 109:539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhnoo I, Wadell G, Svensson L, et al. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol 1984; 20:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]


