Abstract
The impact of antimicrobial treatment on the outcome of carbapenem nonsusceptible Klebsiella pneumoniae (CnsKP) infections needs to be elucidated. This nationwide, multicenter study was conducted to evaluate the impact of appropriate antimicrobial therapy on 14-day mortality among patients with CnsKP infection in Taiwan.
Patients with CnsKP infections from 11 medical centers and 4 regional hospitals in Taiwan were enrolled in 2013. Carbapenem nonsusceptibility was defined as a minimum inhibitory concentration of ≥2 mg/L for imipenem or meropenem. Predictors of 14-day mortality were determined using the Cox proportional regression model. The influence of infection severity on the impact of appropriate use of antimicrobials on 14-day mortality was determined using the Acute Physiology and Chronic Health Evaluation (APACHE) II score.
Overall 14-day mortality was 31.8% (49/154). Unadjusted mortality for appropriate antimicrobial therapy was 23.1% (18/78 patients). Appropriate therapy was independently associated with reduced mortality (hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.24–0.80; P = 0.007). A subgroup analysis revealed that the benefit of appropriate therapy was limited to patients with higher APACHE II scores (HR for patients with scores >15 and ≤35, 0.46; 95% CI 0.23–0.92; and for those with scores >35, 0.14; 95% CI, 0.02–0.99).
In conclusion, appropriate antimicrobial therapy significantly reduces 14-day mortality for CnsKP infections. Survival benefit is more notable among more severely ill patients.
INTRODUCTION
Klebsiella pneumoniae is an important pathogen of community- and nosocomial-acquired infection worldwide.1,2 Carbapenems have been used widely against severe bacterial infections because of the increasing prevalence of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, and thereby contribute to carbapenem nonsusceptibility among these organisms, especially for K pneumoniae.3 The emergence of carbapenem-resistant K pneumoniae infection has alerted the medical community of the urgent need for effective treatment owing to its high mortality (reported to be 18%–60%).4
One of the 2 main mechanisms of carbapenem-resistant K pneumoniae is overproduction of AmpC β-lactamases or ESBL with decreased porin penetration. The other resistance mechanism is through carbapenemases.3,5K pneumoniae carbapenemase (KPC)-producing K pneumoniae has become a significant problem in terms of public health and clinical outcome owing to its rapid spread around the world.6 The emergence and rapid spread of KPC-2-producing K pneumoniae has also become an important problem in Taiwan since 2012.7
Because of its extensive resistance to most β-lactams, the treatment options for carbapenem-resistant K pneumoniae infection are usually limited to colistin, tigecycline, or aminoglycosides, according to susceptibility in vitro.8 Some studies have reported no significant survival benefit in patients treated with active antimicrobial agents,9–11 whereas others have shown positive results.12,13 Because of the heterogeneity of disease severity, infection focus, and difficulty in conducting a randomized controlled study to eliminate all possible unmeasured confounding factors, the impact of antimicrobial treatment on the outcome of carbapenem-resistant K pneumoniae infection needs to be elucidated.
We conducted a nationwide, multicenter study to evaluate the impact of appropriate antimicrobial therapy on 14-day mortality among patients with carbapenem nonsusceptible K pneumoniae (CnsKP) infection in Taiwan. Particular attention was focused on the outcome related to different levels of disease severity.
METHODS
Study Site
This retrospective cohort study was conducted from January 2013 to December 2013 at 15 hospitals, including 11 medical centers (National Taiwan University Hospital, Taipei Veterans General Hospital, Tri-Service General Hospital, and Linkou Chang Gung Memorial Hospital in the north; Chung Shan Medical University Hospital, and China Medical University Hospital in the center of the country; Chi Mei Medical Center, National Cheng Kung University Hospital, Kaohsiung Chang Gung Memorial Hospital and Kaohsiung Medical University Hospital in the south; Buddhist Tzu Chi General Hospital in the east). The other 4 hospitals were Keelung Chang Gung Memorial Hospital, Taoyuan Armed Forces General Hospital, Chiayi Chang Gung Memorial Hospital, and Kaohsiung Municipal Hsiaokang Hospital. The study protocol was approved by the review board of each participating hospital.
Microbiologic Methods
During the study period, CnsKP isolates were collected from clinical specimens and sent for culture to the microbiological laboratories of participating hospitals. Only the first episode of CnsKP infection was collected for each patient. Nonsusceptibility to carbapenem was defined as a minimum inhibitory concentration (MIC) of ≥2 μg/mL for imipenem or meropenem based on the interpretative criteria from the Clinical and Laboratory Standards Institute (CLSI) published in 2012.14 Isolates collected from each hospital were sent to the National Health Research Institutes (Miaoli, Taiwan) and stored at −70 °C in 10% glycerol Luria–Bertani medium before analyses. The Vitek 2 automated system (bioMérieux, Marcy l’Etoile, France) was used for bacterial identification.
MICs were determined by broth microdilution (Sensititre, Trek Diagnostic Systems, Cleveland, OH) for all antibiotics except tigecycline and colistin, as described previously.7 CLSI M100-S22 interpretive breakpoints were used to evaluate the MIC results for all antimicrobial agents studied, except tigecycline and colistin.14 Susceptibility to colistin was based on the European Committee on Antimicrobial Susceptibility Testing (susceptible, MIC ≤2 mg/L; resistant, MIC >2 mg/L), as described previously.15 MICs for tigecycline were determined using the E-test (AB Biodisk, Solna, Sweden) on Mueller–Hinton media. Susceptibility to tigecycline was defined based on criteria set by the US Food and Drug Administration (susceptible, MIC ≤2 mg/L; resistant, MIC ≥8 mg/L), as described previously.16
Isolates were subjected to polymerase chain reaction detection of carbapenemase genes, plasmid-borne AmpC-like genes, and ESBL genes, as described previously.7 Identification of outer-membrane porins (OMPK35 and OMPK36) was carried out as described previously.7
Collection of Clinical Data and Definitions
The clinical data of all consecutive patients infected with CnsKP from different specimens were collected retrospectively. Definitions of CnsKP infection were as described previously.17 Patients younger than 20 years and those with incomplete medical records were excluded. Surveillance cultures were not taken during the study period.
Infections were defined to be “nosocomial-acquired” if the index culture had been collected >48 h after hospital admission. The isolates were defined to be “healthcare-associated” if patients met any of the following criteria: received intravenous therapy at home or in an outpatient clinic during the previous 30 days; received renal dialysis in a hospital or clinic during the previous 30 days; underwent hospitalization for ≥2 days during the previous 90 days; or resided in a nursing home or long-term care facility.18 Severity of illness at the time of onset of infection was assessed by the acute physiology and chronic health evaluation (APACHE) II score, as described previously.19 “Appropriate antimicrobial therapy” was defined as treatment with at least 1 agent for ≥48 h after isolation of a clinical culture specimen to which the isolate was susceptible in vitro.13 “Combination therapy” was defined as administration of ≥2 in vitro active antimicrobial agents for >48 h after isolation of a clinical culture specimen, and the duration of each antimicrobial agent has overlapped for ≥48 h. Tigecycline for an infection of the bloodstream or urinary-tract infection was defined as “appropriate therapy” if the tigecycline MIC was ≤0.5 mg/L at standard dosing.20 The primary outcome measure was 14-day mortality after the onset of infection. Polymicrobial isolation was defined as at least 1 pathogen other than CnsKP isolated from the same specimen. The co-pathogens in polymicrobial infections that were not treated with appropriate antibiotic therapy were excluded in this analysis.
Statistical Analyses
Analyses were processed with Statistical Package for the Social Sciences (SPSS) v19.0 (SPSS, Chicago, IL). Bivariate associations between the binary outcome of 14-day mortality and patient characteristics were analyzed using the χ2 test or Fisher exact test for discrete variables, and the Student t test or Mann–Whitney rank test for continuous variables. The Cox proportional regression model was used to explore factors associated with 14-day mortality. Univariate analyses were done separately for each of the variables to ascertain the hazard ratio (HR) and 95% confidence interval (CI). All biologically plausible variables with P ≤ 0.10 in the univariate analysis were considered for inclusion in the Cox regression model for the multivariate analysis.
RESULTS
Microbiologic Characteristics of CnsKP Isolates
A total of 154 patients infected with CnsKP were identified during the study period. The samples were as follows: sputum (n = 73), bronchoalveolar lavage (n = 2), pleural effusion (n = 2), urine (n = 38), ascites (n = 5), synovial fluid (n = 1), tip of a central venous catheter (n = 2), tip of abdominal drain (n = 2), blood (n = 20), wound (n = 7), and bile (n = 2). Fifty-two isolates (33.8%) had genes that encoded carbapenemases: KPC-2 (n = 43), KPC-3 (n = 1), VIM-1 (n = 5), IMP-8 (n = 2), KPC-2, and IMP-8 (n = 1). The most common mechanism of carbapenem non-susceptibility was production of AmpC-mediated β-lactamases or ESBL plus porin defects, which was identified in 102 cases.
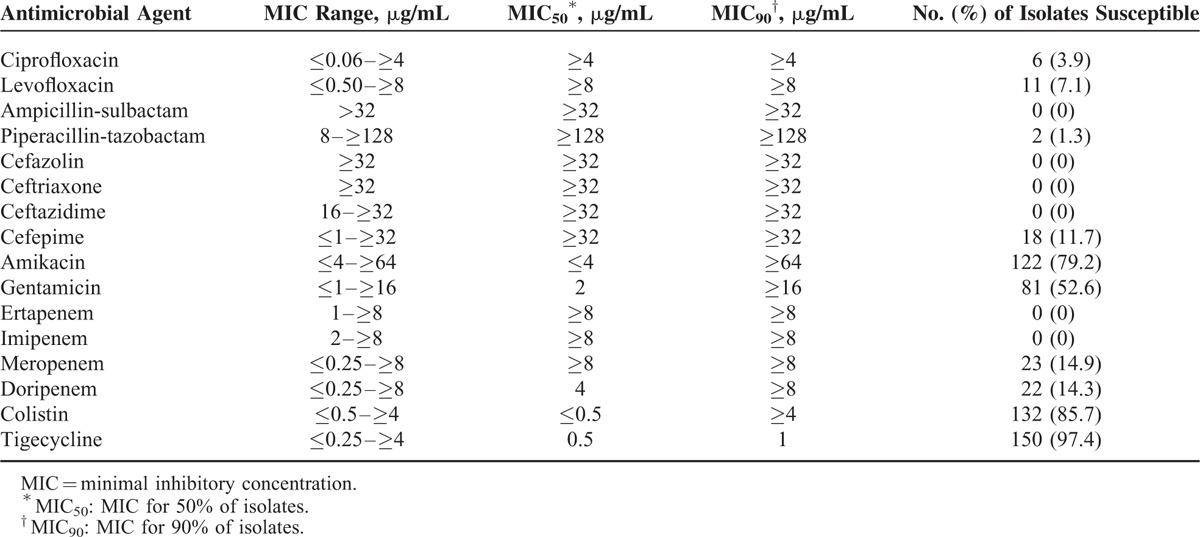
The MIC ranges, MIC50 values, and MIC90 values of various antimicrobial agents against CnSKP isolates are listed in Table 1. The MIC of imipenem was ≥8 μg/mL for 120 (78.0%) isolates, 4 μg/mL for 11 (7.1%) isolates, and 2 μg/mL for 23 (14.9%) isolates. Most isolates were susceptible to tigecycline (n = 150, 97.4%), colistin (n = 132, 85.7%) and amikacin (n = 123, 79.9%), but showed moderate susceptibility to gentamicin (n = 81, 52.6%).
TABLE 1.
In Vitro Activities of Tested Antimicrobial Agents Against 154 CnsKP Isolates
Factors Associated with 14-day Mortality in Patients with CnsKP
The 14-day mortality associated with CnsKP infection was 31.8% (49/154). The overall in-hospital mortality associated with CnsKP infection was 53.2% (82/154). The median duration of hospital stay after isolation of these pathogens was 19.5 days (interquartile range: 7–43).
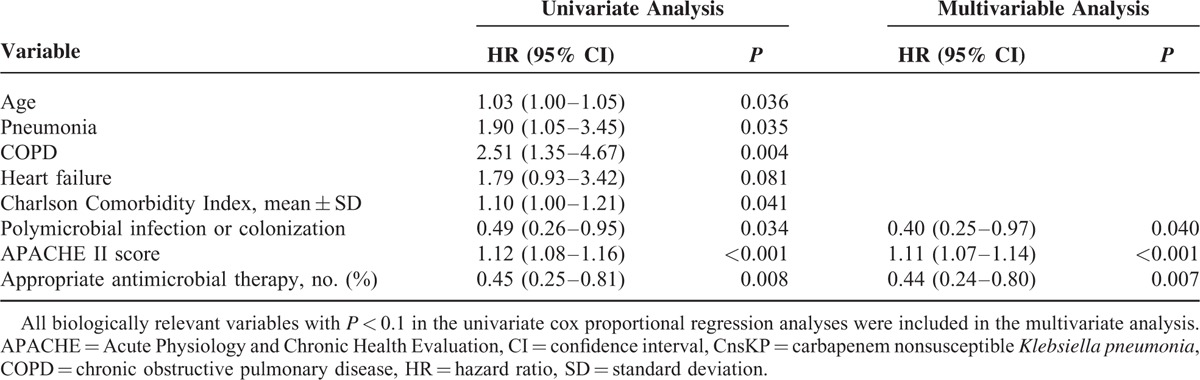
A comparison of the clinical features between survivors and non-survivors 14 days after CnsKP infection is shown on Table 2. In the univariate analysis, advanced age, pneumonia, chronic obstructive pulmonary disease, higher Charlson score, and higher APACHE II score were significantly associated with 14-day mortality. Polymicrobial isolation and appropriate antimicrobial therapy were associated with survival. Multivariate analyses showed the APACHE II score (HR 1.11; 95% CI 1.07–1.14; P < 0.001) to be an independent factor associated with 14-day mortality. Polymicrobial isolation (HR 0.40; 95% CI 0.25–0.97; P = 0.040) and appropriate antimicrobial therapy (HR, 0.44; 95% CI 0.24–0.80; P = 0.007) were independent protective factors for 14-day mortality (Table 3).
TABLE 2.
Characteristics of Patients With CnsKP Infection Stratified by 14-day Mortality
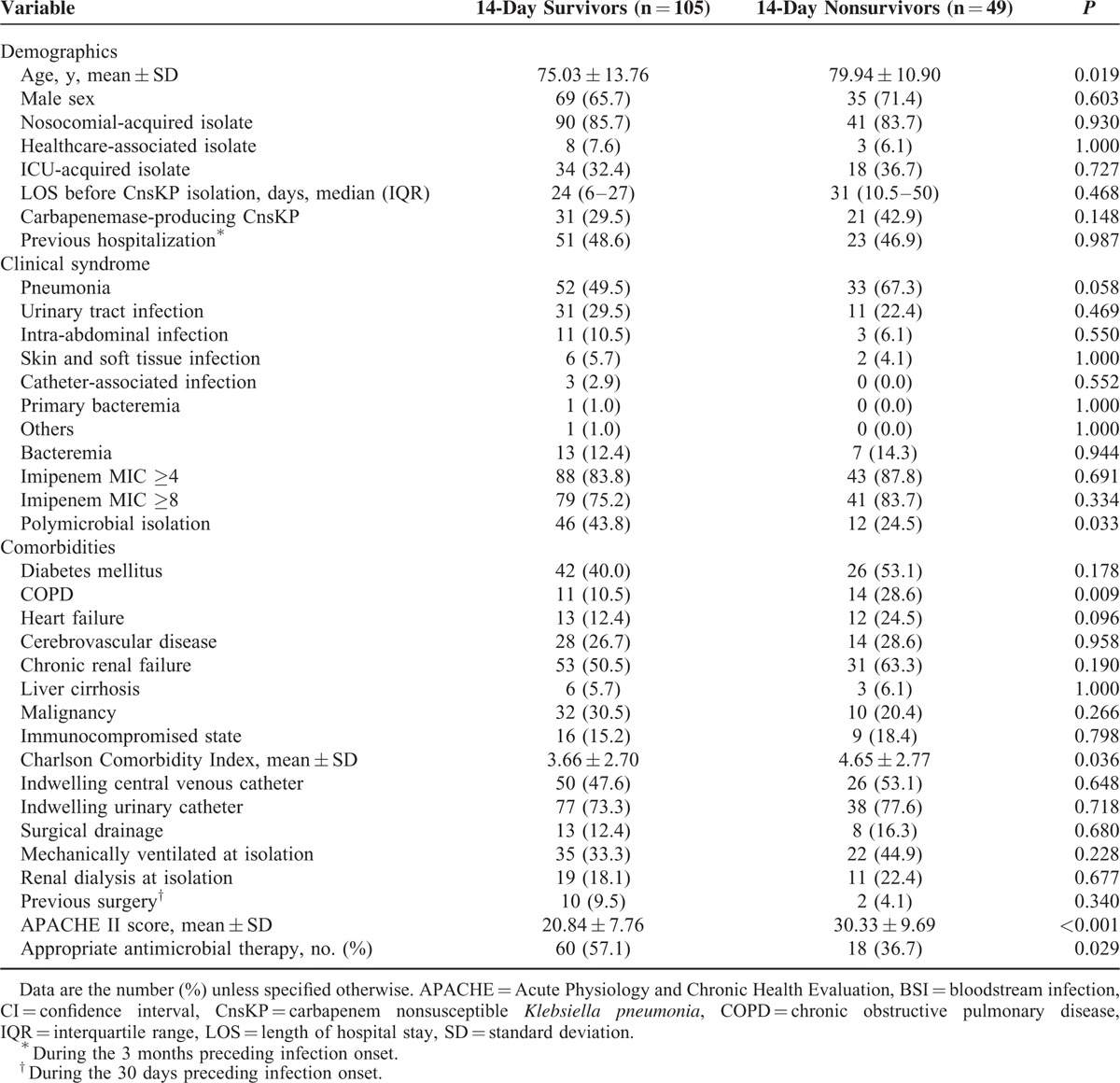
TABLE 3.
Cox Proportional Regression Analysis of Predictors for 14-day Mortality Among 154 Patients With CnsKP Infection
The 14-day mortality of patients receiving appropriate antimicrobial therapy was significantly lower than that for those receiving inappropriate antimicrobial therapy (23.1% vs 40.8%, P = 0.018). We further compared the demographic and clinical characteristics between patients who received appropriate (n = 78) and inappropriate antimicrobial therapy (n = 76). There was no significant difference in age, sex, comorbid conditions, APACHE II score, source of infection, acquisition of infection in the intensive care unit, or days of hospitalization before infection (data not shown). Among patients receiving appropriate antimicrobial therapy, most patients received monotherapy (n = 68, 87.2%). The most common in vitro active antimicrobial used was tigecycline (n = 33, 42.3%), followed by colistin (n = 27, 34.6%). The 14-day mortality of patients receiving appropriate antimicrobial therapy with tigecycline was 15.2% (5/33). The 14-day mortality of patients receiving appropriate antimicrobial therapy with colistin was 33.3% (9/27). The most common combination was colistin plus tigecycline, which was used in 6 cases. The standard dose of tigecycline was administered in patients throughout this study.
Impact of Appropriate Antimicrobial Therapy on 14-day Mortality in Patients With Different Severities of Infection
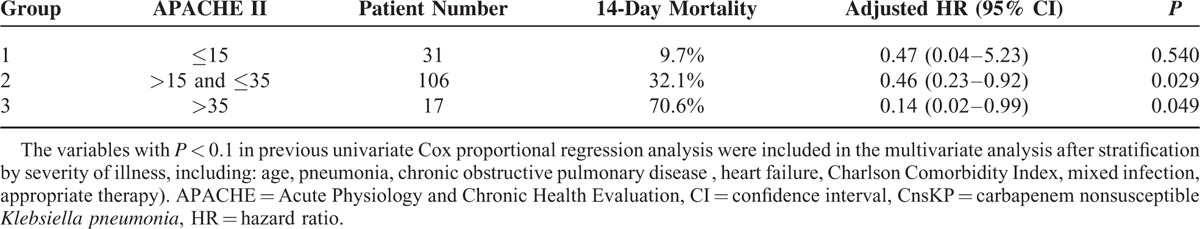
The adjusted HRs by multivariate analysis for 14-day mortality of patients receiving appropriate antimicrobial therapy with APACHE II score >15, >20, >25, >30, and >35 were 0.39 (95% CI 0.21–0.73; P = 0.003), 0.42 (95% CI 0.23–0.80; P = 0.008), 0.32 (95% CI 0.15–0.72; P = 0.006), 0.29 (95% CI 0.09–0.92; P = 0.035), and 0.14 (95% CI, 0.02–0.99, P = 0.049), respectively. Appropriate antimicrobial therapy showed the most notable effect among patients with the most severe illness (APACHE II score >35). Patients were then categorized into 3 groups according to APACHE II score (Table 4). In group 1 (score ≤15), appropriate antimicrobial therapy was not associated with a significantly better outcome. However, among patients in group 2 (score, 16–35) and group 3 (score >35), appropriate antimicrobial therapy significantly reduced 14-day mortality (HR, 0.46 [95% CI, 0.23–0.92] and 0.14 [95% CI 0.02–0.99], respectively).
TABLE 4.
Adjusted Hazard Ratios of Appropriate Antimicrobial Therapy for 14-day Mortality Among Patients With CnsKP Infection, Stratified by Severity of Illness
DISCUSSION
The present study represented one of the largest series focusing on CnsKP infection, and reported 14-day mortality of 31.8%, highlighting the high mortality caused by this pathogen. This retrospective study showed that appropriate antimicrobial therapy reduced 14-day mortality independently after adjustment for various factors. We further demonstrated that appropriate antimicrobial therapy significantly benefited more severely ill patients.
In general, inadequate antibiotic therapy can influence outcome. The detrimental effects of inadequate antibiotic therapy seem to become weaker in patients who are not severely ill as well as the most severely infected patients with short life expectancies.21 It is reasonable to assume that less severely ill patients should have a better prognosis and might recover without specific therapeutic interventions. Nevertheless, one recent study showed that appropriate antimicrobial therapy was the potential life-saving measure for patients with Acinetobacter baumannii bacteremia in the most severe cases.22 The present study was the first to analyze the efficacy of antimicrobial therapy in CnsKP infections with different disease severity stratified by APACHE II score. Our observation that appropriate antimicrobial therapy did not significantly benefit patients with APACHE II scores ≤15 is in accordance with the previous studies. Furthermore, we showed that appropriate antimicrobial therapy benefited patients with more severe illness. Studies on bloodstream infections caused by KPC-producing K pneumoniae CnsKP did not take account of disease severity on the effect of antimicrobial therapy.10,12,13 Recently, Daikos et al23 stratified patients with KPC-producing K pneumoniae bacteremia by the severity of sepsis and underlying diseases, and found the superiority of combination therapy in patients with rapidly fatal underlying disease and septic shock. Tumbarello et al24 also demonstrated treatment with ≥2 drugs displaying activity against KPC-producing K pneumoniae isolates improves survival, mainly in patients with APACHE III scores ≥15. Bass et al25 suggested that appropriate therapy benefited critically ill patients with carbapenem-resistant bacteremia, but the analysis from patients with different severity was not performed. It is evident that objective measurement of disease severity should be included in future clinical studies if evaluating the impact of appropriate antimicrobial therapy on the mortality caused by CnsKP infections.
Treatment of carbapenem-resistant Enterobacteriaceae is derived primarily from experiences in carbapenemase-producing K pneumoniae infection.3,26 Data from the literature suggest that treatment with a single in vitro active agent results in mortality not significantly different from that observed in patients treated with no active therapy, whereas combination therapy with ≥2 in vitro active agents is superior to monotherapy providing a clear survival benefit. The lowest mortality has been observed in patients treated with carbapenem-containing combinations.3,26 Combination therapy has been recommended for CnsKP infection by some experts,4,27 but the inherent limitations of those studies related to their observational design and sample size weaken the evidence to support the use of combination therapies against CnsKP.28 One recent study showed that combination therapy was not superior to monotherapy to treat KPC-producing Enterobacteriaceae infections.29 Because of the regulations for National Health Insurance Reimbursement in Taiwan, only a few cases in the present study received combination therapy. The uncommon practice of combination therapy provided the opportunity to analyze the significance of monotherapy in CnsKP. Our analyses supported the evidence that appropriate monotherapy had an important role in the outcome of CnsKP infection. Our study implied that appropriate therapy is the cornerstone determining the outcome in CnsKP infection.
In the present study, the major resistance mechanism of CnsKP was production of AmpC-mediated β-lactamases or ESBL plus porin defects, which is different from the mechanism of carbapenemase production described in the literature.3 The different genetic background behind the MIC may also play a part in prediction of the outcome. Whether the different resistance mechanisms accounted for the efficacy of antimicrobial therapy needs further investigation. Not all laboratories were able to determine the mechanism of resistance in clinical practice. Therefore, the findings of the present study provide evidence that administration of appropriate therapy according to MIC testing is essential to manage these infections.
Our study was limited by its retrospective design. However, our data have important clinical implications because of the limited number of therapeutic options for CnsKP and the weak evidence for the clinical use of these regimens. Our study was also strengthened by the inclusion of a large number of patients with various sites and severities of infection, and a well-defined endpoint of 14-day mortality. Another limitation is that we cannot analyze the role of combination antibiotic therapy due to the rare utilization. Finally, co-isolation with other pathogens was quite common (37.7%, 58/154 patients). The relationship between polymicrobial isolation and the outcome needs more studies to clarify. Some reports have excluded cases of polymicrobial infection or limited the cases in bacteremia, but this method would limit generalizability. Therefore, the present study represents real-life clinical experiences and provides important clinical information.
In conclusion, appropriate antimicrobial therapy (even with a single active agent) was found to reduce 14-day mortality in subjects with CnsKP infection. The survival benefit was more notable among more severely ill patients.
ACKNOWLEDGMENTS
The authors thank Dr. Sai-Cheong Lee of Keelung Chang Gung Memorial Hospital, Dr. Jann-Tay Wang of National Taiwan University Hospital, Dr. Jung-Chung Lin of Tri-Service General Hospital, Dr. Shih-Ta Shang of Taoyuan Armed Forces General Hospital, Dr. Min-Chi Lu of Chung Shan Medical University Hospital, Dr. Jen-Hsien Wang of China Medical University Hospital, Dr. Cong-Yu Huang of Chiayi Chang Gung Memorial Hospital, Dr. Wen-Chien Ko of National Cheng Kung University Hospital, Dr. Chen-Hsiang Lee of Kaohsiung Chang Gung Memorial Hospital, Dr. Ke Chang of Kaohsiung Municipal Hsiaokang Hospital, and Dr. Po-Liang Lu of Kaohsiung Medical University Hospital, for their participation in this study.
The authors thank Ms. Chiu-Mei Yeh for her endorsement and assistance in the statistical analyses.
We also thank for the Medical Science & Technology Building of Taipei Veterans General Hospital for providing experimental space and facilities.
Footnotes
Abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation, CI = confidence interval, CLSI = Clinical and Laboratory Standards Institute, CnsKP = carbapenem non-susceptible Klebsiella pneumoniae, COPD = chronic obstructive pulmonary disease, ESBL = extended-spectrum β-lactamase, HR = hazard ratio, KPC = Klebsiella pneumoniae carbapenemase, MIC = minimum inhibitory concentration.
The results of this study have been presented in the 7th international congress of the Asia Pacific Society of Infection Control (APSIC 2015).
Funding: This work was supported by grants from the Ministry of Science and Technology in Taiwan, Taipei Veterans General Hospital (V103B-016 and V104B-001), Centers for Disease Control, R.O.C. (Taiwan) (DOH101-DC-1204, DOH102-DC-1508, and MOHW103-CDC-C-114–134504), and Szu-Yuan Research Foundation of Internal Medicine.
All authors report no conflicts of interest relevant to this article.
REFERENCES
- 1.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 1998; 11:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meatherall BL, Gregson D, Ross T, et al. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med 2009; 122:866–873. [DOI] [PubMed] [Google Scholar]
- 3.Tzouvelekis LS, Markogiannakis A, Psichogiou M, et al. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrosillo N, Giannella M, Lewis R, et al. Treatment of carbapenem-resistant Klebsiella pneumoniae: the state of the art. Expert Rev Anti Infect Ther 2013; 11:159–177. [DOI] [PubMed] [Google Scholar]
- 5.Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm!. Trends Mol Med 2012; 18:263–272. [DOI] [PubMed] [Google Scholar]
- 6.Patel G, Bonomo RA. Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol 2013; 4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu SK, Wu TL, Chuang YC, et al. National surveillance study on carbapenem non-susceptible Klebsiella pneumoniae in Taiwan: the emergence and rapid dissemination of KPC-2 carbapenemase. PloS one 2013; 8:e69428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Duin D, Kaye KS, Neuner EA, et al. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis 2013; 75:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardakas KZ, Matthaiou DK, Falagas ME, et al. Characteristics, risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in the intensive care unit. J Infect 2015; 70:592–599.(in press). [DOI] [PubMed] [Google Scholar]
- 10.Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daikos GL, Petrikkos P, Psichogiou M, et al. Prospective observational study of the impact of VIM-1 metallo-beta-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 2009; 53:1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950. [DOI] [PubMed] [Google Scholar]
- 13.Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803. [DOI] [PubMed] [Google Scholar]
- 14.Clinical, Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: Twenty-Second Informational Supplement. M100-S22. Wayne PA: CLSI; 2012. [Google Scholar]
- 15.European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 3.0 ed. Available: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_Breakpoint_table_v_3.0.pdf 2013. [Google Scholar]
- 16.Department of Health and Human Services, Food and Drug Administration. Class II special controls guidance document: antimicrobial susceptibility test systems (AST): guidance for industry. Washington, DC. USA. Available: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM169359.pdf 2009. [Google Scholar]
- 17.van Duin D, Perez F, Rudin SD, et al. Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother 2014; 58:4035–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu HS, Wang FD, Tseng CP, et al. Characteristics of healthcare-associated and community-acquired Klebsiella pneumoniae bacteremia in Taiwan. J Infect 2012; 64:162–168. [DOI] [PubMed] [Google Scholar]
- 19.Lin YT, Wang FD, Chan YJ, et al. Clinical and microbiological characteristics of tigecycline non-susceptible Klebsiella pneumoniae bacteremia in Taiwan. BMC Infect Dis 2014; 14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicasio AM, Crandon JL, Nicolau DP. In vivo pharmacodynamic profile of tigecycline against phenotypically diverse Escherichia coli and Klebsiella pneumoniae isolates. Antimicrob Agents Chemother 2009; 53:2756–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harbarth S, Nobre V, Pittet D. Does antibiotic selection impact patient outcome? Clin Infect Dis 2007; 44:87–93. [DOI] [PubMed] [Google Scholar]
- 22.Lee YT, Kuo SC, Yang SP, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis 2012; 55:209–215. [DOI] [PubMed] [Google Scholar]
- 23.Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-Producing Klebsiella pneumoniae bloodstream infections: Lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 2014; 58:2322–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumbarello M, Trecarichi EM, De Rosa FG, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 2015; 70:2133–2143. [DOI] [PubMed] [Google Scholar]
- 25.Bass SN, Bauer SR, Neuner EA, et al. Impact of combination antimicrobial therapy on mortality risk for critically ill patients with carbapenem-resistant bacteremia. Antimicrob Agents Chemother 2015; 59:3748–3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzouvelekis LS, Markogiannakis A, Piperaki E, et al. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 2014; 20:862–872. [DOI] [PubMed] [Google Scholar]
- 27.Daikos GL, Markogiannakis A, Souli M, et al. Bloodstream infections caused by carbapenemase-producing Klebsiella pneumoniae: a clinical perspective. Expert Rev Anti Infect Ther 2012; 10:1393–1404. [DOI] [PubMed] [Google Scholar]
- 28.Paul M, Carmeli Y, Durante-Mangoni E, et al. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother 2014; 69:2305–2309. [DOI] [PubMed] [Google Scholar]
- 29.de Oliveira MS, de Assis DB, Freire MP, et al. Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin Microbiol Infect 2015; 21:179e1–1797e. [DOI] [PubMed] [Google Scholar]


