Abstract
The aim of this study was to assess the impact of postoperative complications after esophagectomy on long-term outcome.
The treatment of esophageal cancer has recently been improved; however, esophagectomy with thoracotomy and laparotomy carries considerable postoperative morbidity and mortality. The real impact of postoperative complications on overall survival is still under evaluation.
A retrospective analysis was performed on patients with esophageal cancer who underwent esophagectomy with thoracotomy and laparotomy, with R0 or R1 resection between January 1997 and December 2012. Of 402 patients, we analyzed the following parameters 284 patients who could be followed up for over 5 years: stage of disease, neoadjuvant therapies, surgical approaches, surgical complications, postoperative medical complications, and overall and relapse-free survivals using medical records.
Of the 284 patients, 64 (22.5%) had pneumonia, 55 (19.4%) had anastomotic leakage, and 45 (15.8%) had recurrent laryngeal nerve paralysis (RLNP). Pneumonia had a significant negative impact on overall survival (P = 0.035); however, anastomotic leakage and RLNP did not affect overall survival. Multivariate analysis revealed that the presence of pneumonia was predictive of poorer overall survival; the multivariate hazard ratio was 1.456 (95% confidence interval 1.020–2.079, P = 0.039).
Pneumonia has a negative impact on overall survival after esophagectomy. Strategies to prevent pneumonia after esophagectomy should improve outcomes in this operation.
INTRODUCTION
Esophageal cancer is the sixth leading cause of cancer-related mortality worldwide because of its high malignant potential and poor prognosis.1 The postoperative 5-year survival rate in patients with American Joint Committee on Cancer stage I esophageal cancer is approximately 90%, and it decreases to 45% in patients with stage II disease, 20% in those with stage III disease, and 10% in those with stage IV disease.2
Although the efficacy of chemoradiotherapy for esophageal cancer has been reported,3 esophagectomy remains the most important treatment option for esophageal cancer. The recent improvement in long-term survival after esophagectomy can be attributed to advancements in surgical techniques for extended lymph node dissection and perioperative management.4 However, esophagectomy is a highly invasive procedure with several serious postoperative complications, including pneumonia, anastomotic leakage, and recurrent laryngeal nerve paralysis (RLNP), which may result in multiorgan failure.5,6 A significant increase in morbidity and mortality after these invasive procedures has been reported.5–7
Although most authors agree that postoperative complications affect perioperative mortality, the possible long-term impact on overall survival remains unclear.8,9 Pneumonia is a serious medical complication, and anastomotic leakage and RLNP are serious surgical complications after esophagectomy; however, the impact of these complications after esophagectomy on long-term outcome has not been fully discussed.10–13 In this study, we hypothesized that these complications affect the long-term outcome after esophagectomy. The aim of this study was to assess the impact of these complications after esophagectomy on the long-term outcome.
PATIENTS AND METHODS
Patients
Between January 1997 and December 2012, 402 patients underwent esophagectomy with thoracotomy and laparotomy, with R0 or R1 resection at Keio University Hospital, Tokyo Japan. In this study, transhiatal resection and R2 resection were excluded to enable the assessment of the impact of postoperative complications on the long-term outcome. Of the patients who met the inclusion criteria, we retrospectively analyzed the records of 284 patients who could be followed up for over 5 years (Table 1 ). Survival results were obtained from the medical records and calculated from the date of operation. For the survival assessment, patients were followed through November 30, 2014, which constituted the censoring date. This study was conducted with the approval of the Ethics Committee of Keio University School of Medicine, and the clinical trial information was UMIN000016468.
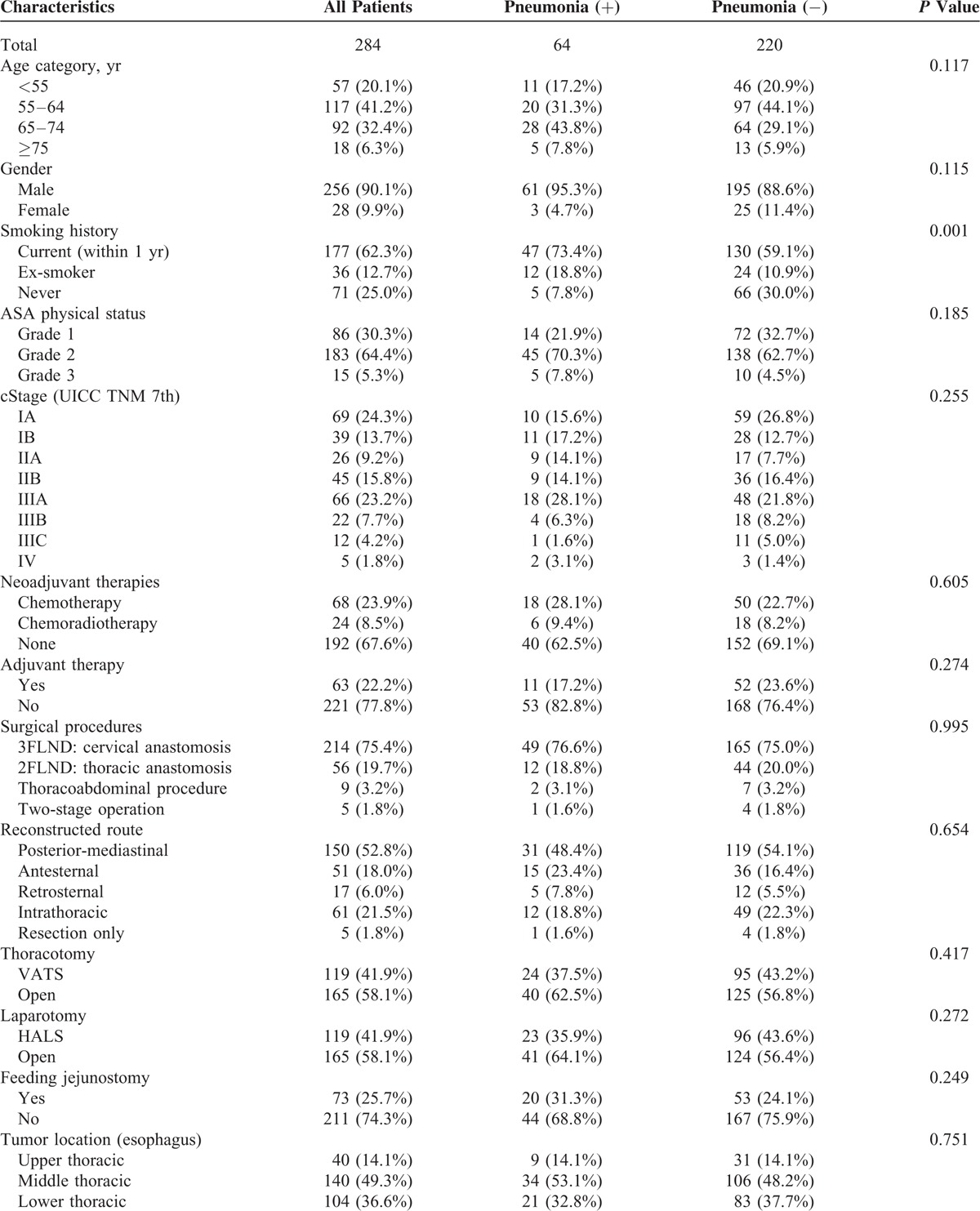
TABLE 1.
Clinicopathological Characteristics
Surgical Procedures
As per the routine clinical protocol, an epidural cannula was inserted into each patient for the administration of intraoperative and postoperative analgesia. All surgeries were performed under general anesthesia with selective intubation to block the right lung.
In our hospital, most operations included 3-field lymph node dissection (3FLND) with an anastomosis in the neck (75.4%). This operation includes a right transthoracic subtotal esophagectomy and dissection of cervical (bilateral supraclavicular region), mediastinal (periesophagus and around the trachea, including the bilateral recurrent laryngeal nerve), and abdominal (perigastric and around the celiac axis) lymph nodes. The other procedures that were used in this study were 2FLND with an anastomosis in the right thorax (19.7%) and a thoracoabdominal procedure (3.2%). The 2FLND procedure consisted of a laparotomy and a right thoracotomy with an anastomosis in the right thorax. The thoracoabdominal procedure consisted of a single left thoracoabdominal incision with an anastomosis in the left thorax.
The thoracic procedures were performed through a right thoracic incision (58.1%) or by video-assisted thoracic surgery (VATS) (41.9%) in the left lateral decubitus position during 1997–2008 or novel hybrid position during 2009–2012.14 The upper mediastinal procedure was performed by initially placing the patients in the left lateral decubitus position, and the middle and the lower mediastinal procedures were performed by rotating the operating table in the prone position.14 VATS procedures were performed through a minithoracotomy (4–5 cm) with 4 or 5 trocars.14
The abdominal procedures were performed through an upper midline abdominal incision (58.1%) or by hand-assisted laparoscopic surgery (HALS) (41.9%).14 HALS procedures were performed through a transverse minilaparotomy (7 cm) in the right upper quadrant, with 1 port below the navel and 2 ports in the left abdomen.14
The anastomosis was performed in the neck or thorax. In patients with cervical anastomoses, the anastomoses were completed using a circular stapler or were hand-sewn. In patients with intrathoracic anastomoses, the anastomoses were completed using a circular stapler.15
After surgery, each patient was admitted to the intensive care unit (ICU), and mechanical ventilation was continued overnight. If the patient's cardiopulmonary condition was stable, the patient was extubated on postoperative day (POD) 1 and was admitted to the general surgical ward on POD 2. Postoperative analgesia was provided through patient-controlled epidural analgesia. After an evaluation of the anastomosis using contrast on POD 7, oral intake of thick liquids was initiated and gradually changed to jellylike food and then to solid food. Patients were discharged when they could successfully ingest solid food.14
Morbidity and Mortality Following Esophagectomy
Postoperative complications were categorized using the Clavien–Dindo (CD) classification as follows16: grade I was any deviation from the normal postoperative course without the need for pharmacologic treatment or surgical, endoscopic, or radiologic intervention; grade II required pharmacologic treatment with drugs; grade III required surgical, endoscopic, or radiologic intervention; grade IV was life-threatening complications that required ICU management; and grade V was death. In this study, pneumonia and anastomotic leakage beyond CD classification grade II and RLNP and other surgical or medical complications beyond CD classification grade I were considered as postoperative complications.
Each patient underwent periodic physical and laboratory examinations at 2- or 3-month intervals. Computed tomography (CT) was performed every 6 months and periodic endoscopy was performed annually. Relapse-free survival was calculated from the date of operation to the date of relapse detected by CT or endoscopy, or death.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics version 22 (IBM, Armonk, NY). Categorical data were analyzed using the Fisher's exact test or χ2 test when appropriate. Quantitative data were analyzed using an unpaired Student t test and the Mann–Whitney U test. A P value of <0.05 was considered statistically significant. Survival outcomes were analyzed using the Kaplan–Meier method and log-rank tests. Univariate comparisons of survival time were based on Cox regression. The variables with P values <0.05 were included in a stepwise Cox regression model.
RESULTS
Patient Characteristics
The clinicopathological characteristics of all patients are shown in Table 1 . The median age was 62 (range 34–82). Most patients were male (90.1%) and had squamous cell carcinoma (89.8%). Almost 1/3 (32.4%) of the patients received neoadjuvant treatment for locally advanced tumors; this involved chemotherapy alone in 23.9% and chemoradiotherapy in 8.5%. The majority of chemotherapy treatments consisted of 5-fluorouracyl and cisplatin regimens, and the majority of chemoradiotherapy treatments consisted of concomitant regimens based on 5-fluorouracyl-cisplatin and 40 Gy of radiation based on the previous studies.4,17 Seven patients (2.5%) required additional surgery because the prior endoscopic submucosal dissections resulted in noncurative resections. Overall, 279 patients (98.2%) had undergone 1-stage operations, and 5 patients (1.8%) had undergone 2-stage operations. For these 5 patients who had undergone 2-stage operations, both operations were considered in this study to assess postoperative mortality and morbidity. The patients were staged using the International Union against Cancer (UICC 7th edition) tumor-node-metastasis (TNM) classification system.18 According to the clinical staging of the esophageal cancers, 108 (38.0%) patients were cStage I, 71 (25.0%) were cStage II, 100 (35.2%) were cStage III, and 5 (1.8%) were cStage IV.
Complications
The postoperative results are shown in Table 2. There were 146 surgical complications in 114 patients (40.1%), including anastomotic leakage, RLNP, wound infection, pyothorax, chylothorax, and necrosis of the conduit. In addition, there were 121 medical complications in 97 patients (34.2%), including pneumonia, arrhythmia, systemic inflammatory response syndrome, cerebral infarction, and thromboembolic events.
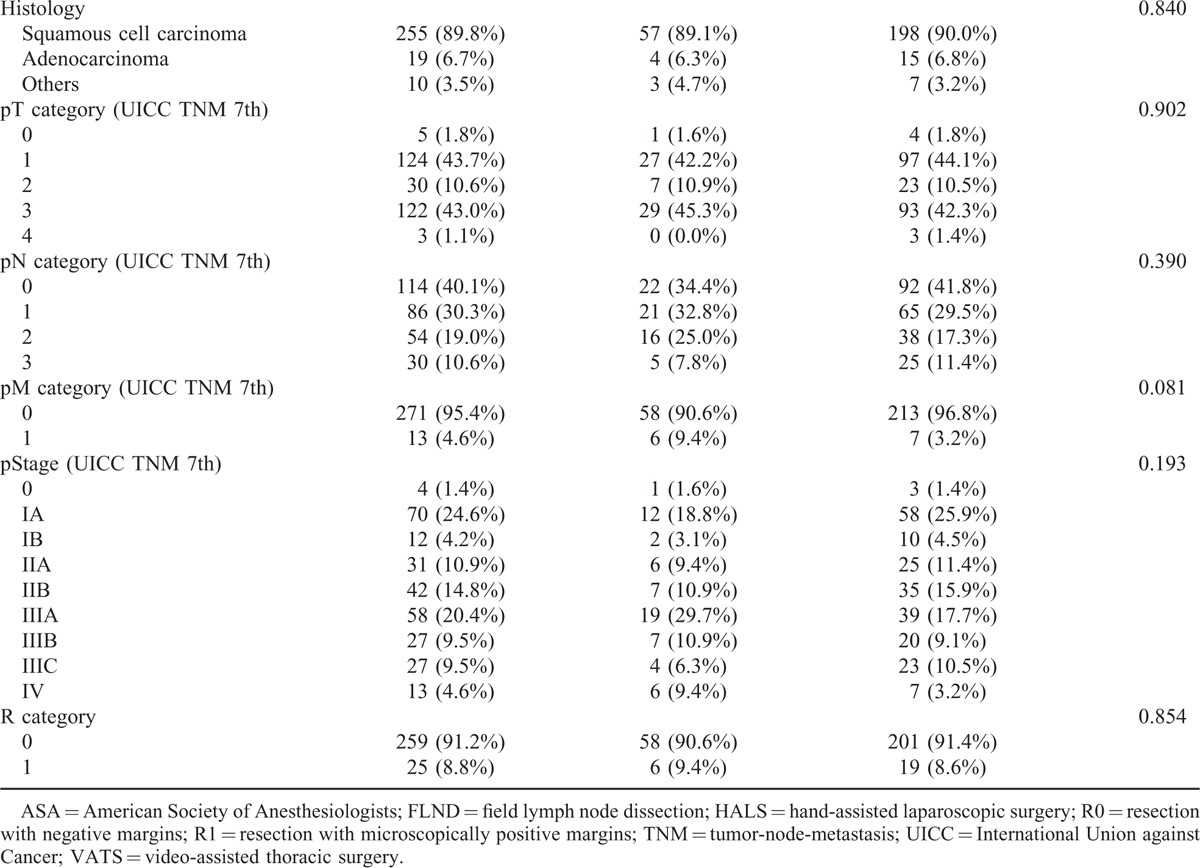
TABLE 1 (Continued).
Clinicopathological Characteristics
Relation of Complications to Outcomes
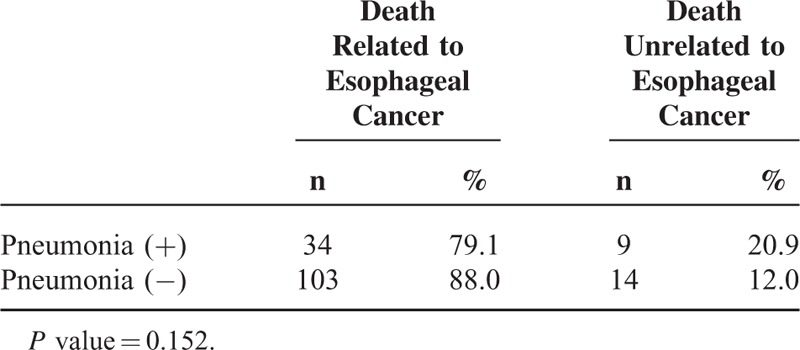
Compared with patients who did not have postoperative complications, pneumonia, necrosis of the conduit, or cerebral infarction had a significantly negative impact on overall survival of the patients. Because there were few patients with necrosis of the conduit or cerebral infarction, the patients with pneumonia were analyzed in detail. Patients with pneumonia had a significantly worse overall survival rate (P = 0.035) (Figure 1A); however, pneumonia did not significantly impact the relapse-free survival (P = 0.108) (Figure 1B). At 12 months, there was a 8.9% difference in overall survival between patients with pneumonia (76.6%) and those without it (85.5%). The magnitude of the overall survival decrement associated with pneumonia expanded at 5 years after the operation, when the difference in overall survival between the 2 groups was 11.7% (40.6% vs 52.3%). Based to the relationship between pneumonia and the cause of death related to esophageal cancer, there was no significant difference in the cause of death related to esophageal cancer between the patients with or without pneumonia (Table 3). The subgroup analyses of overall survival and relapse-free survival regarding age and clinical stage between the patients with or without pneumonia are shown in Figure 2. In the subgroup analyses, pneumonia had a significantly negative impact on overall survival of the patients aged over 65 (Figure 2B) or on those in cStage I (Figure 2D); however, pneumonia did not significantly impact the relapse-free survival of the patients aged over 65 (Figure 2C) or on those in cStage I (Figure 2E). On the other hand, pneumonia did not significantly impact the overall survival of the patients aged under 64 (Figure 2A) or on those in cStage II/III/IV (Figure 2F). With regard to serious surgical complications, anastomotic leakage (Figure 1C) and RLNP (Figure 1D) did not affect overall survival. There was no relationship between pneumonia and anastomotic leakage (P = 0.828) or RLNP (P = 0.266). The characteristics for patients with and without pneumonia are also shown in Table 1 . Smoking history was strongly correlated with the development of pneumonia (P = 0.001). All current smokers stopped smoking at least 1 month before the operation. Table 4 shows the relationship between major surgical complications and patient characteristics. Anastomotic leakage was significantly less with the 2FLND procedure and more with upper thoracic esophagus. RLNP was significantly less with chemoradiotherapy and more with VATS procedure.
FIGURE 1.
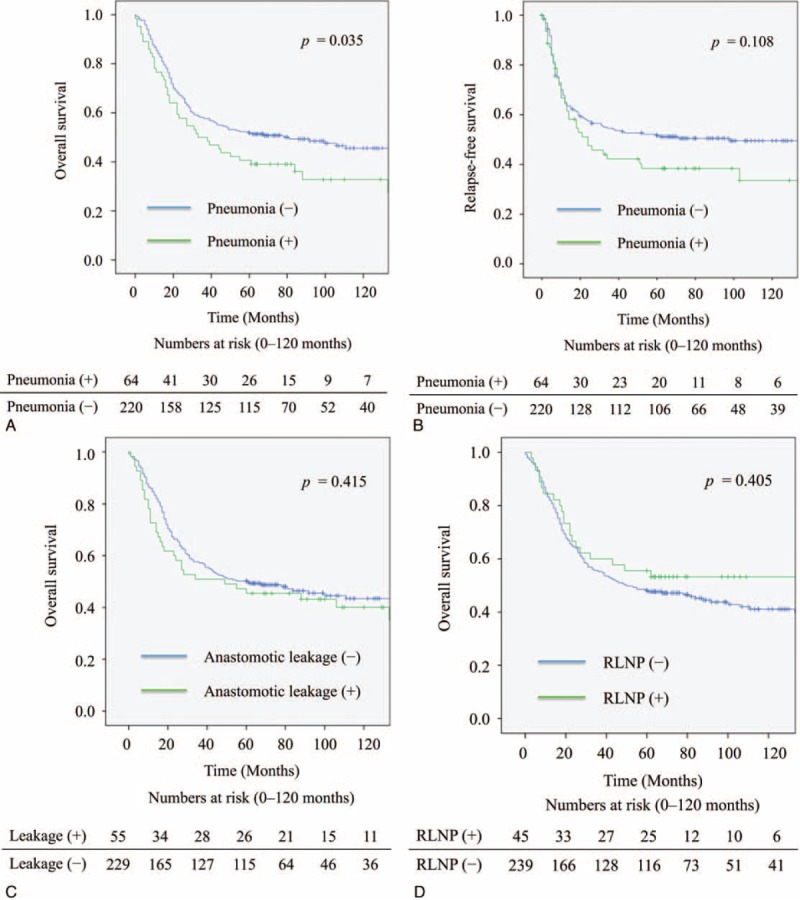
Kaplan–Meier survival curves for patients undergoing esophagectomy. A: Overall survival curves comparing those with and without pneumonia. B: Relapse-free survival curves comparing those with and without pneumonia. C: Overall survival curves comparing those with and without anastomotic leakage. D: Overall survival curves comparing those with and without recurrent laryngeal nerve paralysis. RLNP = recurrent laryngeal nerve paralysis.
TABLE 2.
Postoperative Mortality and Morbidity
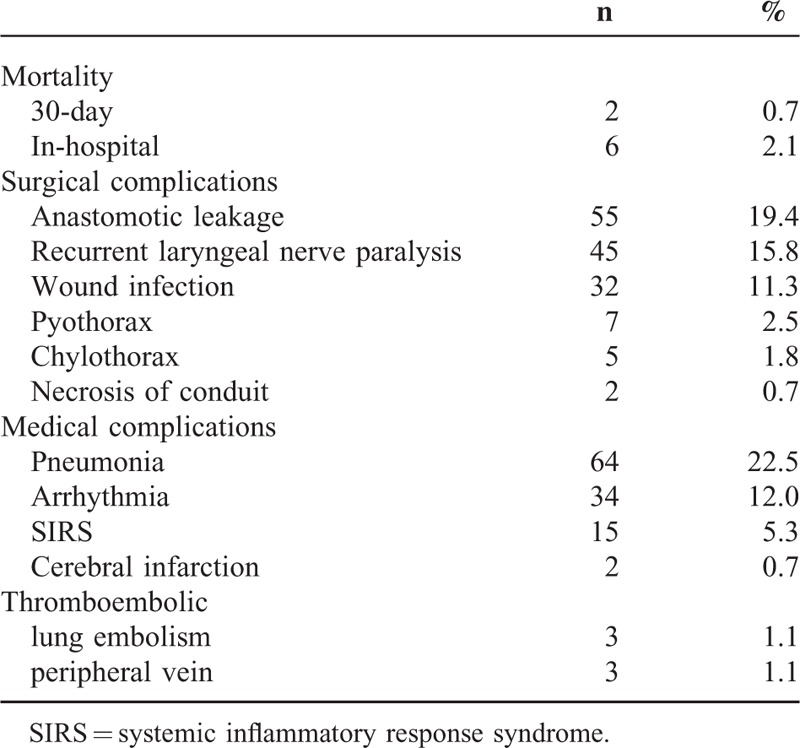
FIGURE 2.
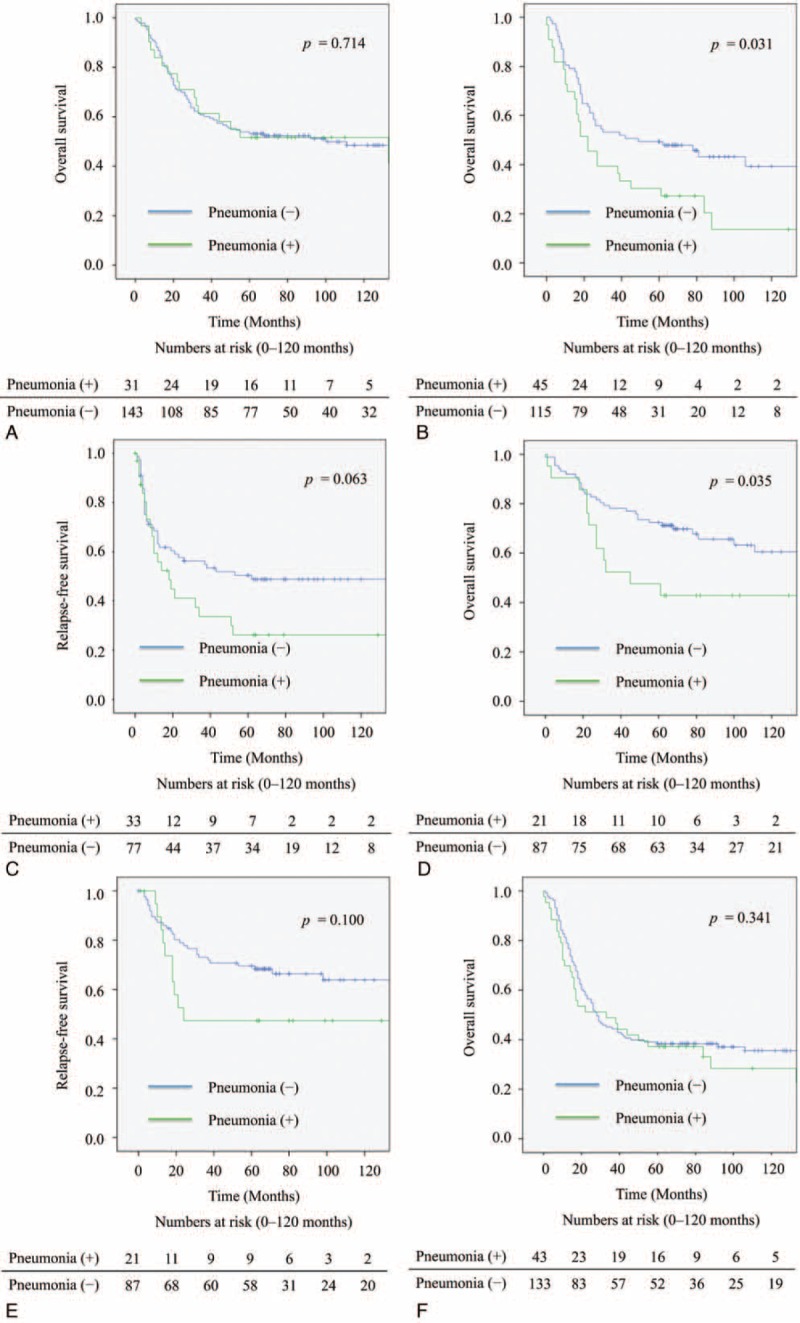
Subgroup analyses of Kaplan–Meier survival curves for patients with or without pneumonia undergoing esophagectomy. A: Overall survival curves for the patients aged under 64. B: Overall survival curves for the patients aged over 65. C: Relapse-free survival curves for the patients aged over 65. D: Overall survival curves for the patients in cStage I. E: Relapse-free survival curves for the patients in cStage I. F: Overall survival curves for the patients in cStage II/III/IV.
TABLE 3.
Cause of Death After Esophagectomy With or Without Pneumonia
In this study, 99 patients (34.9%) with advanced esophageal cancer pathologically and did not receive neoadjuvant therapy were candidates for adjuvant therapy. Among them, 6 with pneumonia (27.3%) and 33 without pneumonia (42.9%) received adjuvant therapy. Patients without pneumonia were more likely to receive adjuvant therapy than those with pneumonia, but the difference was not significant (P = 0.187); and adjuvant therapy did not have an impact on overall survival (P = 0.231).
Univariate and Multivariate Analysis
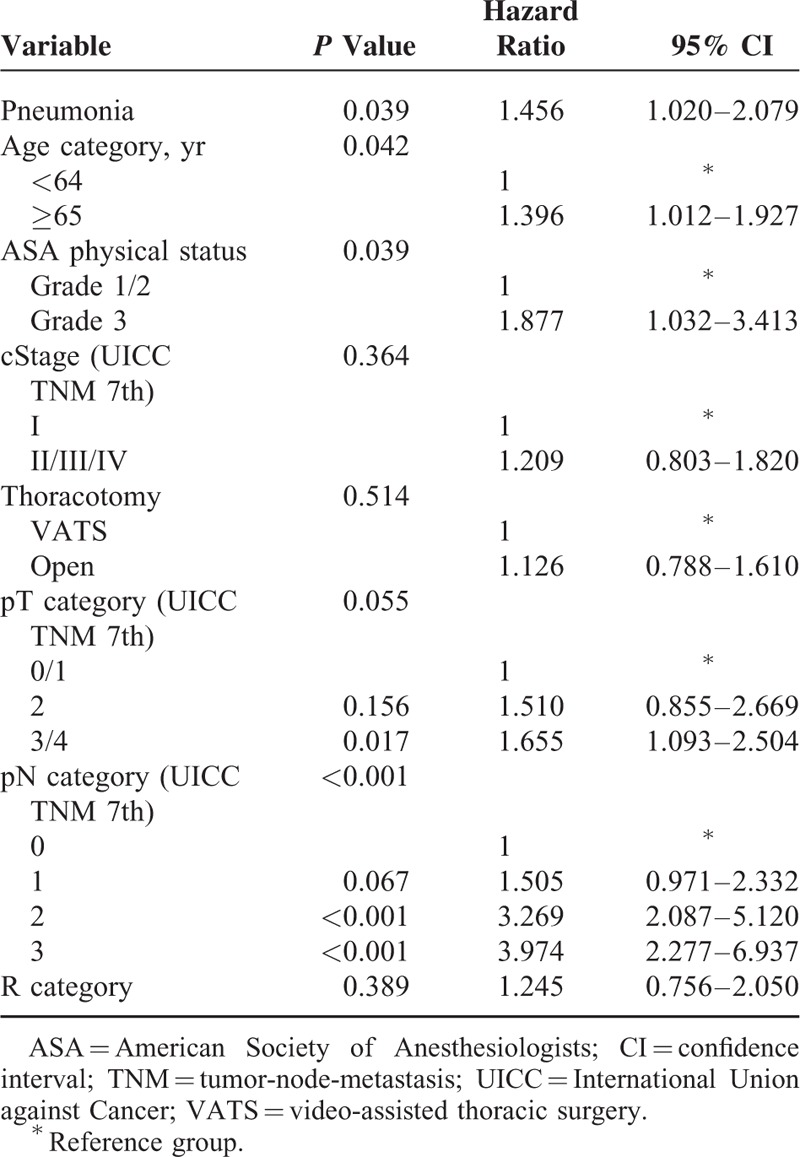
Univariate analysis of the factors that affected the overall survival, except complications, is shown in Table 5. This analysis revealed that age category, American Society of Anesthesiologists (ASA) physical status, clinical stage, surgical procedures, thoracotomy, pathological T-stage, pathological N-stage, and R category were the relevant prognostic factors. Because there was no significant difference in overall survival between 1-stage operations (3FLND, 2FLND, and thoracoabdominal procedure) (P = 0.868) and few patients underwent 2-stage operation, surgical procedures were not included in multivariate analysis. Multivariate analysis including relevant prognostic factors established by univariate analysis and pneumonia revealed that pneumonia (P = 0.039, hazard ratio: 1.456, 95% confidence interval 1.020–2.079), age category (P = 0.042), ASA physical status (P = 0.039), and pathological N-stage (P < 0.001) were the independent prognostic factors (Table 6).
TABLE 4.
Clinicopathological Characteristics of Patients With Major Surgical Complications
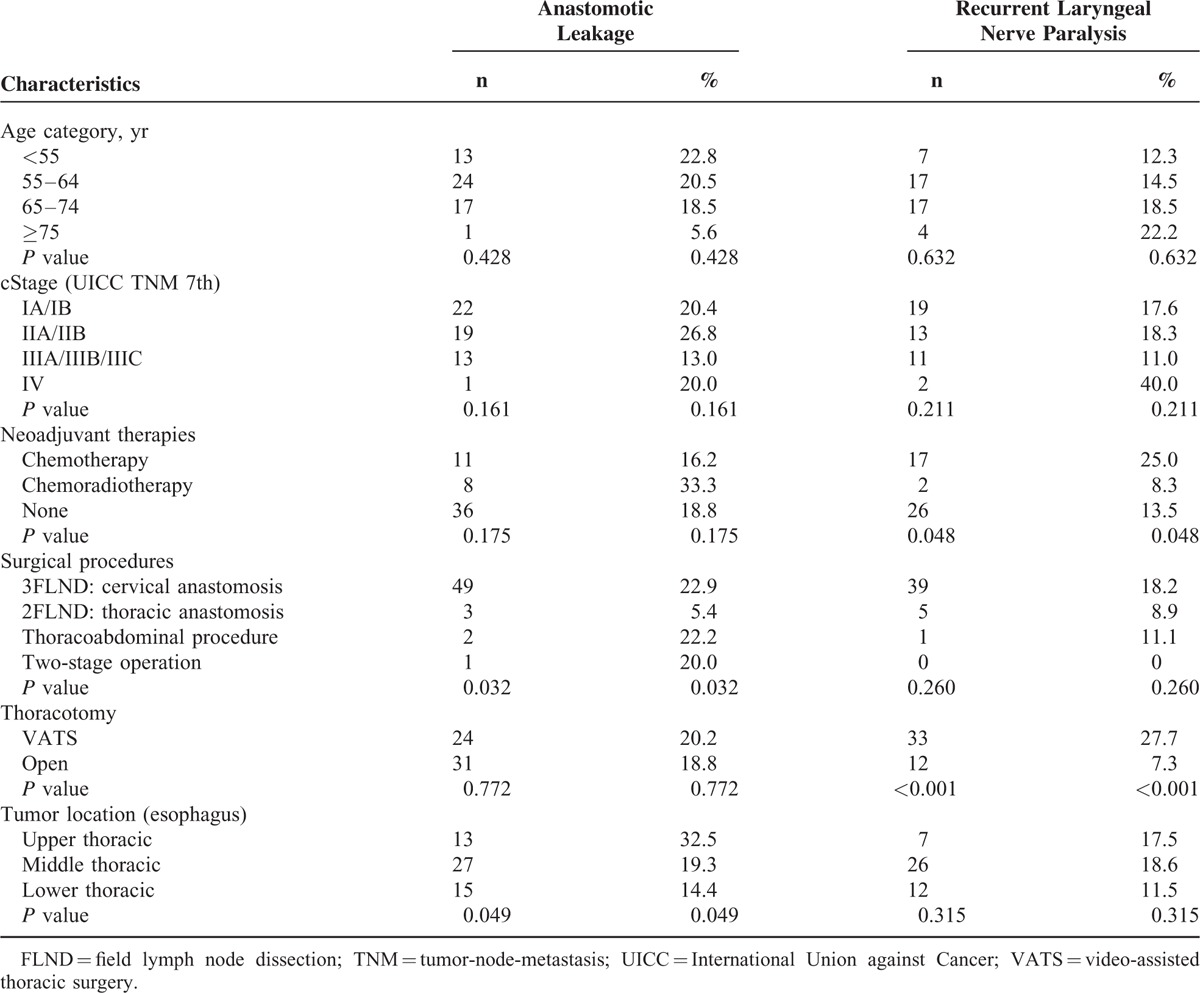
TABLE 5.
Univariate Analysis of Factor Affecting Overall Survival After Esophagectomy
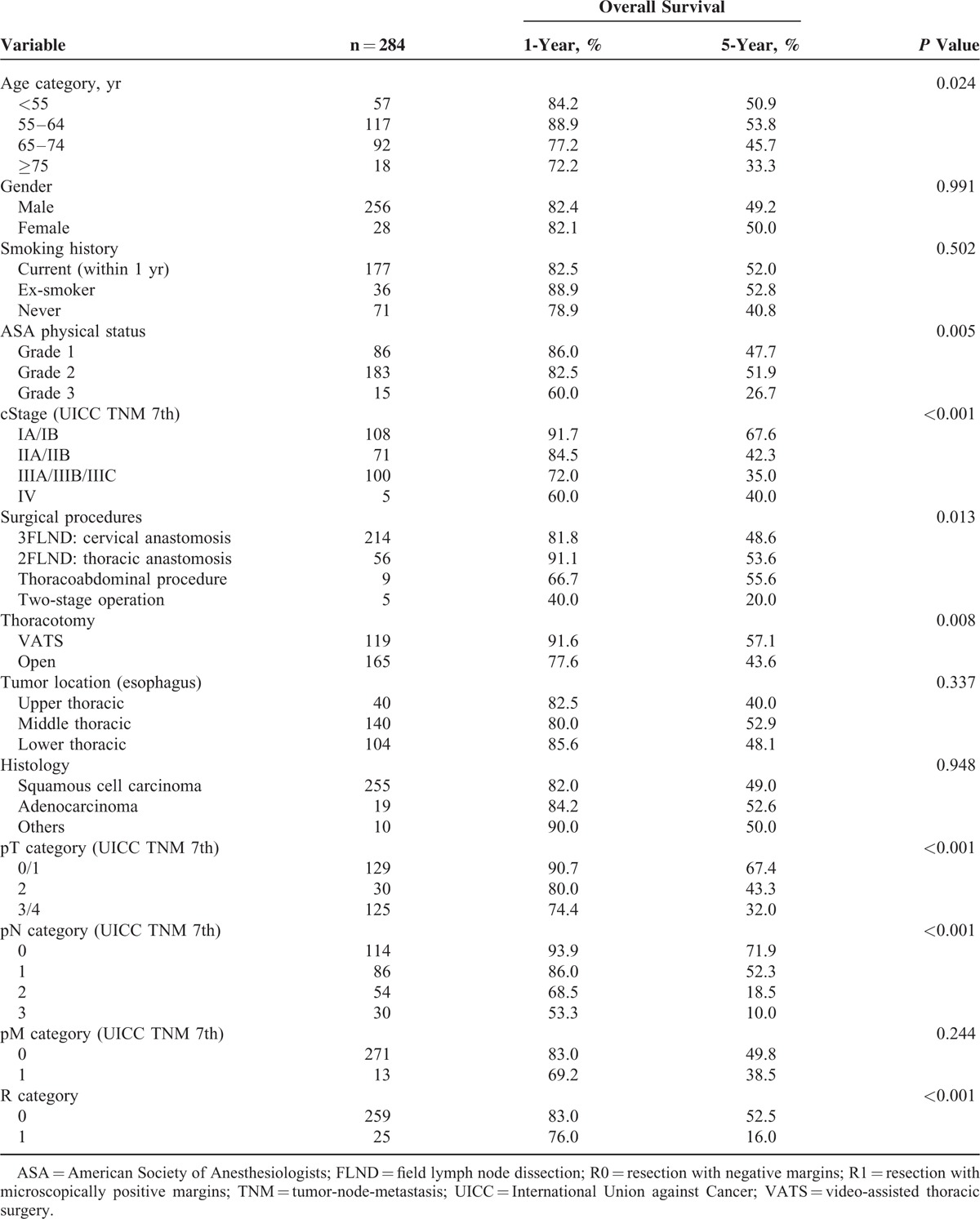
TABLE 6.
Multivariate Analysis of Factor Affecting Overall Survival After Esophagectomy
DISCUSSION
Despite recent improvements in surgical procedures and perioperative management, esophagectomy has reportedly been associated with high rates of morbidity and mortality.2 After Kinugasa et al5 first suggested a potential negative prognostic impact of postoperative respiratory complications, there have been other reports that have indicated the negative impact of postoperative complications on long-term outcomes.11,19 On the other hand, some reports have indicated that postoperative complications did not affect long-term outcomes.8,10,12,13,20 To date, our study is one of the largest studies to analyze the impact of postoperative complications after esophagectomy on long-term outocomes.8,10,11
In this study, we observed that pneumonia after esophagectomy had a negative impact on overall survival but did not affect relapse-free survival. Our results could be explained by 3 factors. First, pneumonia, as a generalized infectious disease, led to a strong impairment of the immunological system that may adversely impact the esophageal cancer recurrence. We had previously reported that infectious complications after esophagectomy were significant factors in increasing the levels of inflammatory cytokines such as interleukin (IL)-6 and IL-8,21 and also reported that increased expression of both IL-8 and its receptor CXCR-2 correlated with tumor progression after esophagectomy.22 It could be explained that pneumonia was related to tumor progression through the development of inflammatory cytokines such as IL-8. Second, pneumonia after esophagectomy worsened the general condition of the patients and led to a cause of death that was unrelated to esophageal cancer, although not significantly in this study. Third, the worsening of the general condition after postoperative pneumonia led to a delay or cessation of additional therapy after esophagectomy and led to esophageal cancer recurrence, although this was not statistically significant. Pneumonia, as a whole, did not affect relapse-free survival or increase the incidence of death unrelated to esophageal cancer, respectively; however, the combination of recurrence and increase in the incidence of death unrelated to esophageal cancer after postoperative pneumonia was thought to have a negative impact on overall survival.
In our subgroup analyses, pneumonia had a significantly negative impact on overall survival of the patients aged over 65 or on those in cStage I. In addition, smoking history was strongly correlated with the development of pneumonia. The results suggested that patients aged over 65 or in cStage I were not suitable for esophagectomy if they were at high risk of esophagectomy such as smoking history, and these patients might be recommended definitive chemoradiotherapy as one of the treatment options instead of esophagectomy.3,23
In reviewing the surgical complications after esophagectomy, anastomotic leakage and RLNP did not affect overall survival. Moreover, there was no relationship between medical complications and surgical complications. This result could be explained by the hypothesis that surgical complications were treated as a local issue and did not cause problems systemically.
In this study, only 1/3 of the patients received neoadjuvant therapy—this seemed to be a low number, particularly in modern regimes as exemplified by the CROSS study.24 This is because, in Japan, adjuvant therapy was adopted before 2000, based on the previous study.25 After that, neoadjuvant therapy was compared with adjuvant therapy in a nation-wide trial,17 therefore, the patients who underwent neoadjuvant therapy were few compared with CROSS study.24 This study has a heavy bias toward squamous cell carcinoma rather than adenocarcinoma, which is more commonly seen in Western countries.24 Although there was no relationship between histological type and pneumonia and no significant difference in overall survival between squamous cell carcinoma and adenocarcinoma, it could not be concluded that there was no relationship in overall survival between histological types because of a small number of patients with adenocarcinoma (6.7%).
In this study, patients undergoing chemoradiotherapy were more likely to have anastomotic leakage than those who underwent chemotherapy or those who did not undergo neoadjuvant therapy; however, anastomotic leakage did not affect overall survival. In recent years, most patients with advanced esophageal cancer have received neoadjuvant chemotherapy or chemoradiotherapy; this increased the R0 resection rate and improved the prognosis.26 Although patients with neoadjuvant chemoradiotherapy required special care for the anastomotic leakage, neoadjuvant chemoradiotherapy was a safe and effective treatment for patients with locally advanced esophageal cancer.
Minimally invasive esophagectomy is becoming more widespread—41.4% of thoracotomies were performed by VATS procedures in this study.6 There was no significant difference in developing pneumonia and anastomotic leakage between VATS procedure and open procedure; however, VATS procedure significantly increased the risk of RLNP and special care must be taken to avoid this risk. Although VATS procedure improved overall survival by univariate analysis, it was not found to affect overall survival by multivariate analysis. Furthermore, surgical procedures among 1-stage operations did not affect overall survival.
Recent studies have emphasized a notable improvement in morbidity and mortality rates at high-volume referral centers and have recommended that the operation with high rates of morbidity and mortality should be performed only at specialized centers.27 We had previously reported that the 30-day and operative mortality rates after esophagectomy were 1.2% and 3.4%, respectively, using the data from 5354 patients in 713 hospitals included in a Japanese Nationwide web-based database.6 Our hospital is one of the largest high-volume centers that performs esophagectomy in Japan, and in this study, the 30-day and operative mortality rates after esophagectomy were 0.7% and 2.1%, respectively; this indicated that our conclusions were reliable.
The most important limitation of our study was that our study was a retrospective study at a single institution. However, our study had a large sample size and we do believe that the multivariate analysis may overcome some biases.
CONCLUSION
Our study contributes to the discussion regarding whether postoperative complications affect long-term outcome after esophagectomy. We have reported that pneumonia had a negative impact on overall survival after esophagectomy. Esophagectomy remains the most important treatment option for esophageal cancer, and it is mandatory to make an effort to prevent pneumonia for improving overall survival after esophagectomy.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, CD = Clavien–Dindo, CI = confidence interval, CT = computed tomography, FLND = field lymph node dissection, HALS = hand-assisted laparoscopic surgery, ICU = intensive care unit, IL = interleukin, POD = postoperative day, R0 = resection with negative margins, R1 = resection with microscopically positive margins, R2 = resection with macroscopically positive margins, RLNP = recurrent laryngeal nerve paralysis, SIRS = systemic inflammatory response syndrome, TNM = tumor-node-metastasis, UICC = International Union against Cancer, VATS = video-assisted thoracic surgery.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg 2000; 232:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999; 281:1623–1627. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi H, Saikawa Y, Oyama T, et al. Factors influencing the long-term survival in patients with esophageal cancer who underwent esophagectomy after chemoradiotherapy. World J Surg 2010; 34:277–284. [DOI] [PubMed] [Google Scholar]
- 5.Kinugasa S, Tachibana M, Yoshimura H, et al. Postoperative pulmonary complications are associated with worse short- and long-term outcomes after extended esophagectomy. J Surg Oncol 2004; 88:71–77. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014; 260:259–266. [DOI] [PubMed] [Google Scholar]
- 7.Fujita H, Kakegawa T, Yamana H, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg 1995; 222:654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferri LE, Law S, Wong KH, et al. The influence of technical complications on postoperative outcome and survival after esophagectomy. Ann Surg Oncol 2006; 13:557–564. [DOI] [PubMed] [Google Scholar]
- 9.Carrott PW, Markar SR, Kuppusamy MK, et al. Accordion severity grading system: assessment of relationship between costs, length of hospital stay, and survival in patients with complications after esophagectomy for cancer. J Am Coll Surg 2012; 215:331–336. [DOI] [PubMed] [Google Scholar]
- 10.Ancona E, Cagol M, Epifani M, et al. Surgical complications do not affect long-term survival after esophagectomy for carcinoma of the thoracic esophagus and cardia. J Am Coll Surg 2006; 203:661–669. [DOI] [PubMed] [Google Scholar]
- 11.Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg 2004; 198:42–50. [DOI] [PubMed] [Google Scholar]
- 12.Lindner K, Fritz M, Haane C, et al. Postoperative complications do not affect long-term outcome in esophageal cancer patients. World J Surg 2014; 38:2652–2661. [DOI] [PubMed] [Google Scholar]
- 13.D’Annoville T, D’Journo XB, Trousse D, et al. Respiratory complications after oesophagectomy for cancer do not affect disease-free survival. Eur J Cardiothorac Surg 2012; 41:e66–e73. [DOI] [PubMed] [Google Scholar]
- 14.Kaburagi T, Takeuchi H, Kawakubo H, et al. Clinical utility of a novel hybrid position combining the left lateral decubitus and prone positions during thoracoscopic esophagectomy. World J Surg 2014; 38:410–418. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi H, Oyama T, Saikawa Y, et al. Novel thoracoscopic intrathoracic esophagogastric anastomosis technique for patients with esophageal cancer. J Laparoendosc Adv Surg Tech A 2012; 22:88–92. [DOI] [PubMed] [Google Scholar]
- 16.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250:187–196. [DOI] [PubMed] [Google Scholar]
- 17.Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012; 19:68–74. [DOI] [PubMed] [Google Scholar]
- 18.Sobin LGM, Wittekind C. TNM Classification of Malignant Tumors. New York: Wiley-Blackwell; 2009. [Google Scholar]
- 19.Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 2009; 250:798–807. [DOI] [PubMed] [Google Scholar]
- 20.Karl RC, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg 2000; 231:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamura A, Takeuchi H, Matsuda S, et al. Factors affecting cytokine change after esophagectomy for esophageal cancer. Ann Surg Oncol 2015; [E pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Ogura M, Takeuchi H, Kawakubo H, et al. Clinical significance of CXCL-8/CXCR-2 network in esophageal squamous cell carcinoma. Surgery 2013; 154:512–520. [DOI] [PubMed] [Google Scholar]
- 23.Kato H, Sato A, Fukuda H, et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn J Clin Oncol 2009; 39:638–643. [DOI] [PubMed] [Google Scholar]
- 24.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 25.Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. J Clin Oncol 2003; 21:4592–4596. [DOI] [PubMed] [Google Scholar]
- 26.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011; 12:681–692. [DOI] [PubMed] [Google Scholar]
- 27.Brusselaers N, Mattsson F, Lagergren J. Hospital and surgeon volume in relation to long-term survival after oesophagectomy: systematic review and meta-analysis. Gut 2014; 63:1393–1400. [DOI] [PubMed] [Google Scholar]


