Abstract
Granulopoiesis abnormalities have been described in association with thyroid disorders (TD). However, data regarding systematic evaluation of adult neutropenia and concurrent or prior TD are scarce.
To investigate the frequency of TD among patients presenting with neutropenia, and the immunophenotypic and immunologic profile of neutropenic patients with concomitant thyroidopathy.
Two hundred eighteen consecutive neutropenic patients were prospectively evaluated in our outpatient Hematology Clinic, with a detailed laboratory screen, including thyroid function tests, antineutrophil antibodies, blood lymphocytes immunophenotyping, and detection of T-cell clonality by PCR.
Among 218 patients with neutropenia, 95 (43.6%) had TD, 65 chronic immunologic neutropenia, 20 clonal proliferation of T-large granular lymphocytes (T-LGL), 5 autoimmune disorders, and 33 other diagnoses. TD-patients had an increased frequency of recurrent infections compared with other patients (P = 0.045). The following correlations were found: negative correlation between FT3 and absolute neutrophil count (ANC) (r2 = −0.274, P = 0.007), negative correlation between TPO-Abs/TG-Abs and C4 (r2 = −0.16, P = 0.045; r2 = −0.266, P = 0.001), and CD4+ counts were inversely correlated to T4 and positively to TSH (r2 = −0.274, P = 0.024; r2 = 0.16, P = 0.045). In addition, TD-patients had significantly higher percentages of CD4+ lymphocytes (P = 0.003). Among TD-patients, 23.4% had Hashimoto thyroiditis (HT), 4.1%, Graves disease (GD), 8.2% nontoxic multinodular goiter (NTMG), 5% subclinical hypothyroidism, and 2.8% had undergone total thyroidectomy associated with nodules (TTM). Thirteen TD-patients displayed T-LGL. Patients with autoimmune thyroidopathy had an increased frequency of concomitant autoimmune manifestations (P = 0.03). Significant differences between the different thyroidopathies included: HT-patients had higher percentages of B-lymphocytes, while the opposite was evident for the TTM-subgroup (P = 0.009, 0.02); GD-patients showed an increase of the proportion of NK cells and a decrease in the percentage of TCRγδ+ lymphocytes (P = 0.001, 0.045); and NTMG-patients had significantly higher ANC (P = 0.004) compared to other thyroidopathies. Antineutrophil antibodies were found in 37.2% of TD-patients tested. Anti-TPO titers were significantly higher in patients with positive antineutrophil antibodies (P = 0.04).
The frequency of TD among neutropenic patients may be higher than previously reported. The existence of antineutrophil antibodies, as well as the different distribution of lymphocyte subsets among patients with different TD, suggests both humoral and cellular mechanisms in the pathophysiology of thyroid disease-associated neutropenia.
INTRODUCTION
Hematologic disorders, especially single lineage abnormalities, have been described in association with thyroid disorders (TD).1,2 Coexisting folic acid or B12 deficiency may result in a reduction of neutrophils, especially in patients with autoimmune TD. Additionally, hyperthyroidism-associated neutropenia may be caused by either decreased neutrophil circulation time3 or reduced marrow granulocytic reserve.4 Furthermore, immune-mediated acute neutropenia or even agranulocytosis is a well-known serious side effect of antithyroid drugs.5 However, reports on systematic evaluation of adults with neutropenia and concurrent or prior thyroid disease are limited. Moreover, data on the distribution of peripheral lymphocyte subsets in patients with thyroid disease with or without neutropenia are scarce.6,7
The present prospective study, expanding through a 4-year period aims to investigate the frequency of TD among patients presenting with neutropenia in the outpatient setting, describing in parallel their clinical and laboratory characteristics. Furthermore, we wanted to get insight into the pathophysiology of thyroid-associated neutropenia by evaluating possible differences in the distribution of peripheral lymphocyte subsets, the presence of antineutrophil autoantibodies and other immunologic parameters.
PATIENTS AND METHODS
In the present prospective observational and registrational study, we analyzed the clinical and laboratory findings of 218 consecutive patients, who presented with neutropenia as the dominant hematologic abnormality, to the Outpatient Hematology Clinic of our Department between 2010 and 2013. The study has been approved by the Ethics Committee of Athens Laikon General Hospital. Informed consent was not considered necessary, since all the tests performed are included in our routine work-up for the investigation of neutropenia. All patients enrolled in this study had an absolute neutrophil count (ANC) below 2 × 109/L, documented in at least 3 consecutive occasions within the last 3 months before study entry and were referred to our Outpatient Clinic by their family doctor. As the sole selection criteria was their referral from their primary care physician, cases with pancytopenia, systemic symptoms, or other prominent abnormalities would have been referred as inpatients. Patients’ evaluation included detailed medical history, physical examination, complete blood counts, blood smear morphology, folate, vitamin B12 and iron status, serum liver enzymes, serum proteins, serology for HBV, HCV, HIV, EBV and toxoplasmosis, antinuclear (ANA) and anti-DNA antibodies (Abs) by immunofluorescence, rheumatoid factor (RF), complement factors C3 and C4 by nephelometry, and abdominal and thyroid ultrasonography. Peripheral blood lymphocyte subsets were analyzed by 3-color flow cytometry using Abs against CD2, CD3, CD4, CD8, CD19, CD20, CD56, CD57, CD16, T-cell receptor (TCR)αβ, and TCRγδ conjugated with the appropriate fluorochromes. Briefly blood samples were incubated with the Abs for 20 minutes in the dark, washed twice in PBS/FCS. Subsequently, red blood cells were lysed and samples were analyzed immediately in a FACSCalibur (BD Biosciences, Becton, Dickinson and Company, San Jose, CA, USA) cytometer. The appropriate isotypic controls were used.
Antineutrophil antibodies (anti-PMN Abs) were detected by granulocyte immunofluorescence test (GIFT), granulocyte agglutination test (GAT), and lymphocyte immunofluerence test (LIFT) methodology, using polyclonal rabbit F(ab′)2 anti-human IgG-FITC (DakoCytomation).8,9 For both GIFT and GAT, neutrophils from a panel of 3 healthy donors with known phenotype were isolated from fresh whole blood using density gradient separation on the day of testing. In the GAT, patients’ serum was incubated with the control neutrophils for 4 to 6 hours at 30°C on a microtiter plate, and cell agglutination was subsequently evaluated by microscopy. In the GIFT, patients’ serum was incubated with the control neutrophils, which in this case had been previously treated with 1% paraformaldehyde to prevent nonspecific binding of Abs to granulocyte Fc-receptors and to stabilize the cell membranes. Following incubation for 30 minutes at 37°C, the flourochrome-conjugated secondary Ab was added to detect binding of antineutrophil Abs to granulocytes. The evaluation was performed by fluorescence microscopy. In order to distinguish anti-HLA class I from anti-PMN Abs, we used the LIFT methodology. This test was analogous to GIFT, but the evaluation was done by flow cytometry.
Since this was a prospective study, missing data were negligible in all parameters except of anti-PMN Abs. Anti-PMN Abs were intended to be measured only in patients with thyroidopathy. However, due to the technically challenging procedure of anti-PMN Abs detection in serum, only 43/95 samples were finally considered suitable for reliable analysis. Apart from anti-PMN Abs, the remaining data were available in more than 90% of the patients.
Serum free thyroxine (FT4), total thyroxine (T4), free triiodothyronine (FT3), total triiodothyronine (T3), and thyrotropin (thyroid-stimulating hormone [TSH]) were measured by radioimmunoassay (FT3-CTK RIA, FT4-CTK, SPART, T3-CTK, RIA, T4-CTK, RIA, TSH-CTK-3, IRMA, DiaSorin S.p.A., Italy). The normal range of thyroid hormones was as follows: TSH 0.4 to 4 mU/L, FT3 2.3 to 5.3 pg/mL, FT4 10 to 25 pmol/L, T3 1.23 to 3.08 nmol/L, and T4 58 to 154 nmol/L. The serum titers of antithyrotropin receptor Abs (TRAbs) were determined by a radioreceptor assay (TRAK, RIA, BRAHMS, Diachel S.A., Germany). The normal value of TRAbs was <1.5 IU/L. Antithyroglobulin Abs (anti-TG) and antithyroid peroxidase Abs (anti-TPO) were measured by ELISA. Normal values for anti-TG and anti-TPO were <55 and <30 IU/mL, respectively.
The diagnosis of thyroidopathy was based on clinical examination, laboratory tests, and ultrasonography of the thyroid gland. Patients with thyroid disease were classified in 5 diagnostic categories: Hashimoto thyroiditis (HT), Graves disease (GD), nontoxic multinodular goiter (NTMG), total thyroidectomy associated with nodules (TTM), and antibody-negative subclinical hypothyroidism (AN-SHP). The presence of serum antithyroid Abs, a sonographic heterogeneity of the thyroid gland with a micronodular pattern, with or without hypervascularity and/or goiter were indicative of HT. All patients of this subgroup were euthyroid with or without levothyroxine, for a median of approximately 6 years prior to presentation (median 74 months, range 2 months–18 years). Patients with GD were also euthyroid after cessation of the treatment with antithyroid drugs for at least 5 years. Long-term remission was achieved and further radioactive iodine administration was not needed. They all provided medical records and pathology reports according to which the diagnosis was established by the presence of diffuse goiter, thyrotoxicosis, a uniform pattern of uptake on scan with Tc-99m, positive serum antithyroid Abs, and/or TRAbs. Diagnosis of NTMG was established on the basis of several palpable thyroid nodules, confirmed by ultrasound, and fine needle aspiration in the presence of indicative sonographic features. All NTMG subjects were euthyroid, while serum thyroid Abs were negative. Patients who underwent TTM were receiving replacement therapy with levothyroxine. The thyroid specimens were classified as benign in all TTM patients and showed no histopathological features of chronic lymphocytic thyroiditis. Finally a TSH level above the upper normal limit (>4 mU/L) was considered as subclinical hypothyroidism. Patients in this subgroup had no hypothyroid symptoms, except of tiredness observed in a minority of them. Moreover, they had no laboratory or sonographic signs of autoimmune thyroiditis, that is, they were all antibody negative (AN-SHP). However, even if these criteria were fulfilled, an underlying thyroiditis cannot be definitely excluded. Maximum TSH levels of AN-SHP patients in our study (n = 11) were 6.27 mU/L, and one might argue, that TSH concentrations below 7 mU/L in the elderly could be normal and considered as a physiological adaptation to aging. According to recent studies, the use of age-specific reference ranges for TSH, rather than a common cut-off of 4.0 mU/L has only minor effects on thyroid status, except in the very old (≥85 years old).10 Thus, a TSH level >4 mU/L was considered as subclinical hypothyroidism in the present study. Since only 3 patients with AN-SHP were older than 70 years, we consider the TSH cut-off of 4.0 mU/L as appropriate for the purposes of this study.
The diagnosis of T-cell large granular lymphocyte (T-LGL) expansion was based on the following criteria: relative increase of the percentage of T-cells with a T-LGL phenotype (CD3+/CD8+/CD57+ with or without CD16+), persisting for at least 6 months, and clonal rearrangement of the TCR gamma (γ) or beta (β) gene. Patients who fulfilled the above-named criteria and had in addition an absolute T-LGL count >2 × 109/L were diagnosed as having T-LGL leukemia.11 TCR beta and gamma chain gene rearrangements were studied by polymerase chain reaction (PCR) using the commercially available kits “IdentiClone TCRB Gene Clonality Assay” and “IdentiClone TCRG Gene Clonality Assay” (Invivoscribe Technologies). Fragment analysis was carried out on a Beckman Coulter CEQ8000. Platform and results were interpreted according to the BIOMED-2 study.12
The diagnosis of myelodysplastic syndrome was established based on standard criteria.13 The diagnosis of persistent polyclonal B-cell lymphocytosis was established by blood smear morphology, the identification of polyclonal B cell lymphocytosis by flow cytometry and nonclonal rearrangement of the immunoglobulin heavy chain genes.14
The diagnosis of chronic idiopathic neutropenia was established when other neutropenia states were excluded, in accordance with previous strict diagnostic criteria.9
Drug-related neutropenia was diagnosed in patients under treatment with drugs associated with neutropenia, in whom ANC reached normal values when the drug was withdrawn. Other diagnoses, such as viral infections, connective tissue disorders, and nutritional deficiencies were defined by the relevant clinical and laboratory criteria.
Patients who had T-LGL expansion or a connective tissue disorder, but fulfilled the appropriate criteria for any of the aforementioned categories of thyroidopathy, were classified as suffering from the respective thyroidopathy.
At 6 months intervals patients were followed by clinical examination, complete blood counts and blood smear, and further immunologic tests, if needed.
STATISTICAL ANALYSIS
Comparisons of continuous variables between categories was performed using nonparametric tests (Kruskal-Wallis and/or Mann-Whitney). Correlations between continuous variables were assessed using the Spearman correlation coefficient. Associations of categorical variables were carried out using the chi square (χ2) and Fisher exact test. The results regarding quantitative variables were expressed as the median values and interquartile range (IQR). All statistical analyses were performed with the statistical package SPSS, version 17.00 (SPSS Inc, Chicago, IL). A P-value <0.05 was considered as statistically significant.
RESULTS
Among 218 patients presenting with neutropenia to our Outpatient Clinic, 65 had chronic idiopathic neutropenia, 12 connective tissue disorders, 75 thyroid disease with no other coexisting immunologic or hematologic disorders, 33 T-LGL expansion, and 33 other diagnoses. 13/33 patients with T-LGL expansion and 7/12 patients with connective tissue diseases suffered from thyroidopathy, as well. Hence, overall we detected 95 neutropenic patients with TD (Table 1).
Table 1.
Diagnostic Categories of Neutropenia in 218 Patients Enrolled in the Study
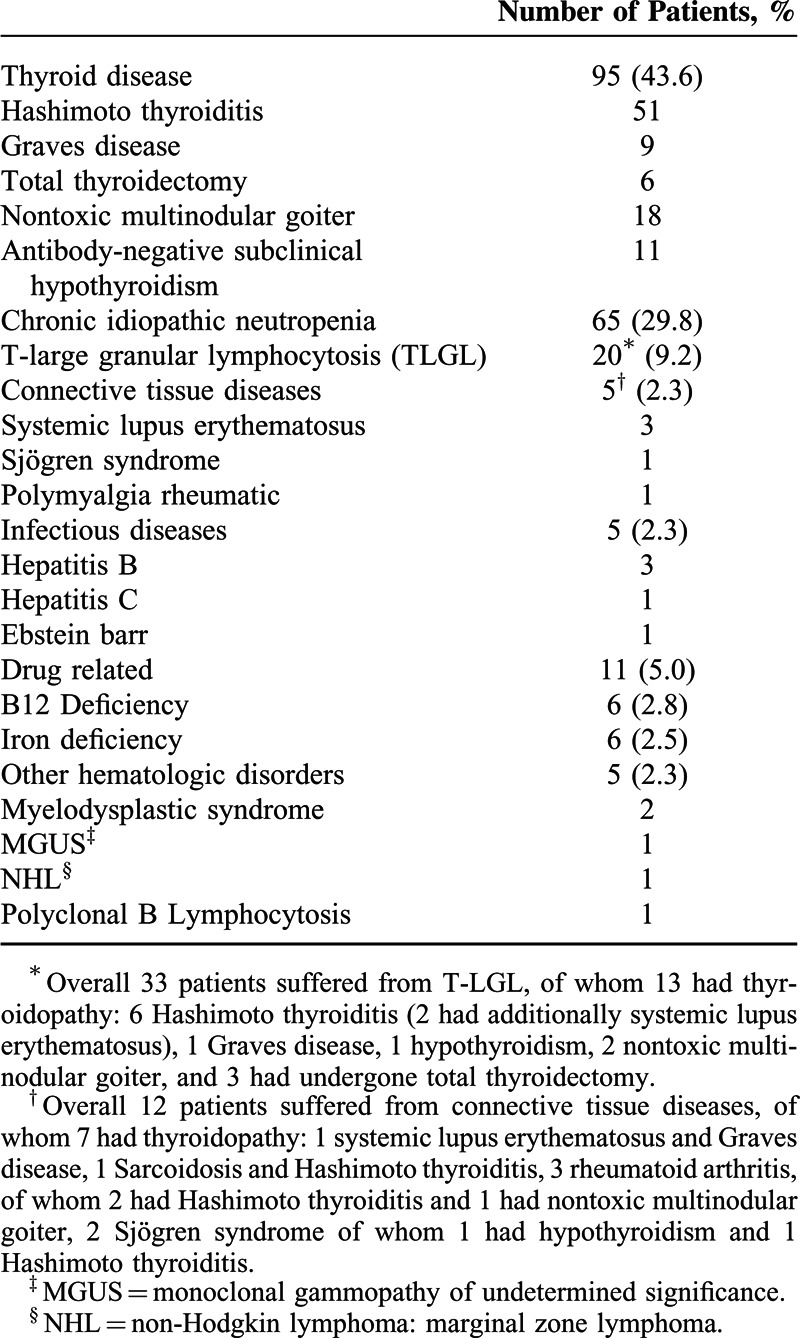
The distribution of the 95 patients with TD in the 5 diagnostic subgroups was as follows: 51 (23.4%) presented HT, 9 (4.1%) GD, 6 (2.8%) TTM, 18 (8.2%) NTMG, and 11 (5%) AN-SHP. The clinical and laboratory characteristics of all neutropenic patients and patients with TD-associated neutropenia are shown in Table 2. The median follow-up time was 28 months (range: 6–80 months).
Table 2.
Clinical and Laboratory Characteristics of All 218 Neutropenic Patients and 95 Neutropenic Patients With Thyroidopathy
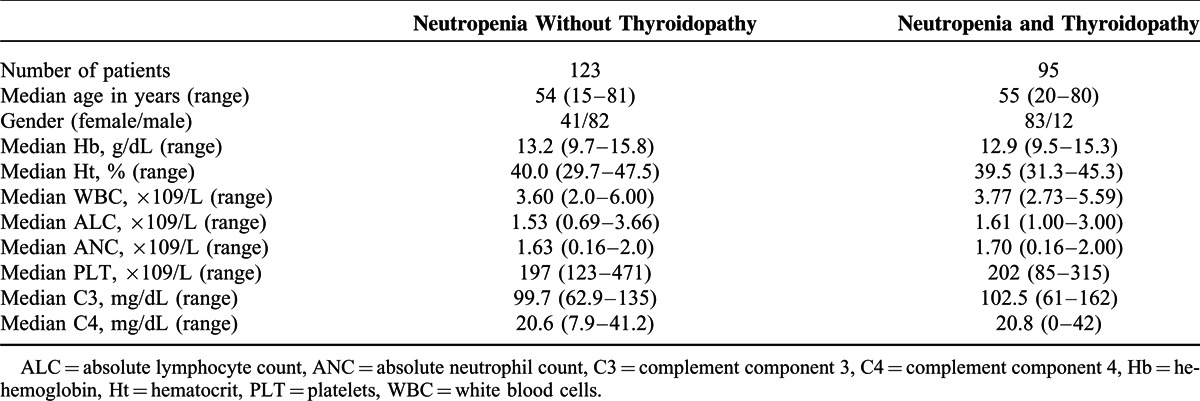
No major differences were detected in the ANC between patients with or without thyroidopathy. However, patients with TD had an increased frequency of recurrent infections (ie, ≥3 episodes/year) (genital, fungal, oral, and respiratory) compared to patients with no thyroidopathy (P = 0.045). Furthermore patients with autoimmune thyroid disease (ATD) had an increased frequency of concomitant autoimmune cutaneous or systemic manifestations (rheumatoid arthritis, systemic lupus erythematosus, Sjögren's syndrome, autoimmune gastritis, and vitiligo) (P = 0.03). The prevalence of specific autoantibodies in patients with ATD did not differ compared with other neutropenic subjects, except for antithyroid Abs.
Associations Between Thyroid Function Parameters and Markers of Immunity
In the entire group of subjects (n = 218) the following significant associations were found: serum levels of FT3 inversely correlated to ANC (r2 = −0.274, P = 0.007) (Figure 1A), absolute CD4+ lymphocyte counts correlated positively to TSH levels (r2 = 0.16, P = 0.045) (Figure 1B) and negatively to T4 levels (r2 = −0.274, P = 0.024) (Figure 1C), and serum TPO-Abs/TG-Abs titers inversely correlated to C4 levels (r2 = −0.16, P = 0.045 and r2 = −0.266, P = 0.001, respectively).
FIGURE 1.
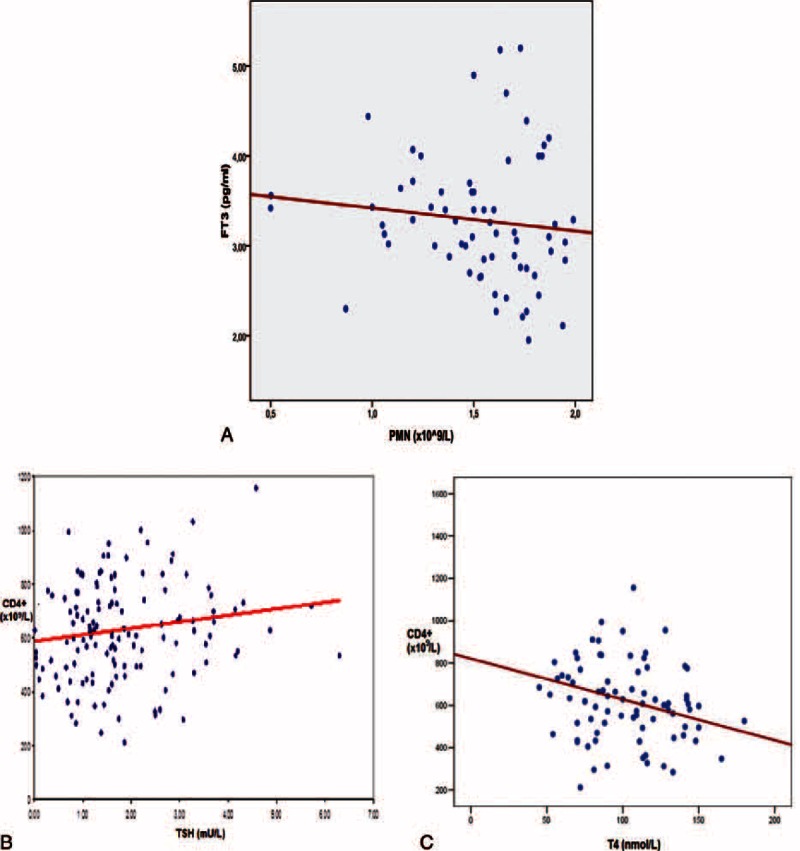
(A) Negative correlation between FT3 levels and absolute neutrophil counts (r2 = −0.274, P = 0.007). (B) Positive correlation between TSH levels and absolute CD4+ counts (×109/L), (r2 = −0.16, P = 0.045). (C) Negative correlation between T4 levels and absolute CD4+ counts (×109/L), (r2 = −0.274, P = 0.024). FT3 = free triiodothyronine, T4 = thyroxine (total), TSH = thyroid-stimulating hormone, PMN = polymorphonuclear/neutrophil cells (absolute counts), CD4+ = subsets of T lymphocytes (absolute counts).
Immunophenotypic Findings in Patients With Neutropenia and Thyroidopathy
Next we investigated possible differences in the distributions of lymphocyte subsets in patients with thyroidopathy (Table 3). The percentage of CD4+ T-lymphocytes was significantly higher in patients with thyroid disease compared with the remaining diagnostic categories (P = 0.003), although absolute CD4+ counts did not differ.
Table 3.
Clinical Characteristics, Endocrine, Hematologic, and Immunophenotypic Findings in Neutropenic Patients With Thyroid Disorders
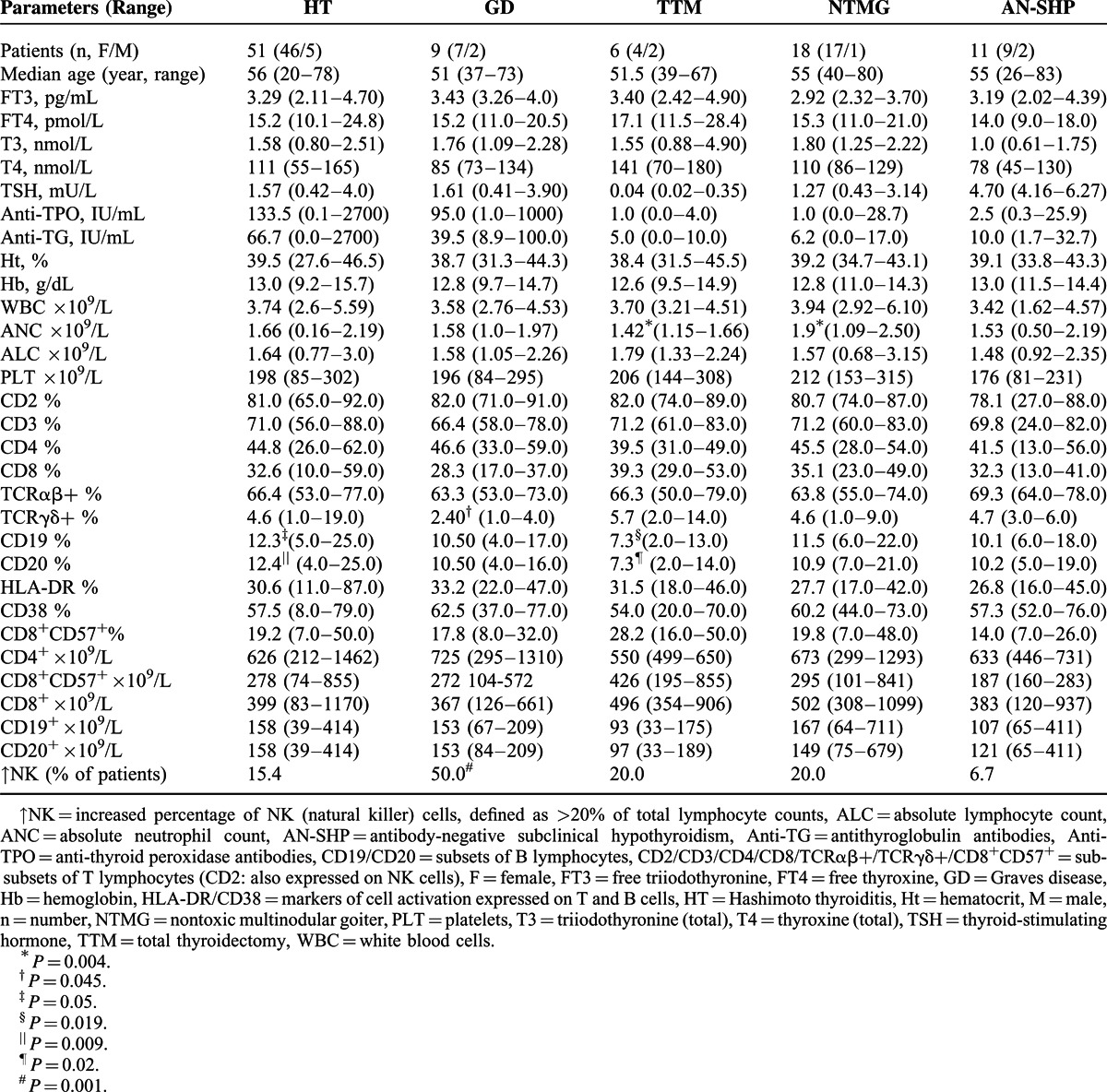
Among patients with TD there was a statistically significant difference in the distribution of B-lymphocytes: in patients with HT (who were euthyroid), the percentage of B-lymphocytes was significantly higher (P = 0.009), compared to other subgroups of thyroidopathy. On the contrary, the TTM-subgroup had significantly lower percentages of B-lymphocytes (P = 0.020). Of note, all patients of the TTM-subgroup were receiving high dose-replacement therapy with levothyroxine and presented with iatrogenic hyperthyroidism. GD patients displayed a significant increase of the percentage of natural killer (NK) cells (P = 0.001) and a decrease in the percentage of TCRγδ+ lymphocytes (P = 0.045). GD patients had been on antithyroid drug treatment for a period of 6 to 18 months. At the time of the study, none was thyrotoxic and TRAbs were found negative in all but one patient. Patients with NTMG, who were euthyroid, had significantly higher ANC (P = 0.004). Finally, patients with AN-SHP presented no significant differences regarding ANC or lymphocyte subsets.
T-LGL Expansion
Among the 218 patients, 33 displayed clonal proliferation of T-LGLs by PCR for the β and γ TCR rearrangement. Of note, none of the 33 patients fulfilled the strict criteria of T-LGL leukemia. Concomitant thyroid abnormalities were noted in 13/33 patients (39.4%): HT, the most common abnormality, occurred in 6 patients, GD in 1, NTMG in 2, and AN-SHP in 1 patient, while 3 patients had undergone TTM associated with nodules.
Anti-PMN Abs
Investigation for Anti-PMN Abs was carried out in 43 out of 95 patients with TD: 26 with HT, 5 with GD, 6 with TTM, 2 with NTMG, and 4 with AN-SHP. Overall anti-PMN Abs were detected in 16 patients (37.2%): 14 patients had autoimmune thyroidopathy (13 HT, 1 GD) and 2 NTMG. Anti-PMN Abs were positive by GIFT methodology in all patients, while in 4, anti-PMN Abs could be detected by GAT as well. LIFT was negative in all cases.
Patients with ATD had an increased incidence of anti-PMN Abs. This difference was of borderline significance, possibly due to small patient number (P = 0.07).
Furthermore anti-TPO titers were significantly higher in patients with positive anti-PMN Abs (median anti-TPO levels: 100 IU/mL, IQR: 425), compared to those without (median anti-TPO levels: 1.2 IU/mL, IQR: 54.75, P = 0.04), (Figure 2).
FIGURE 2.
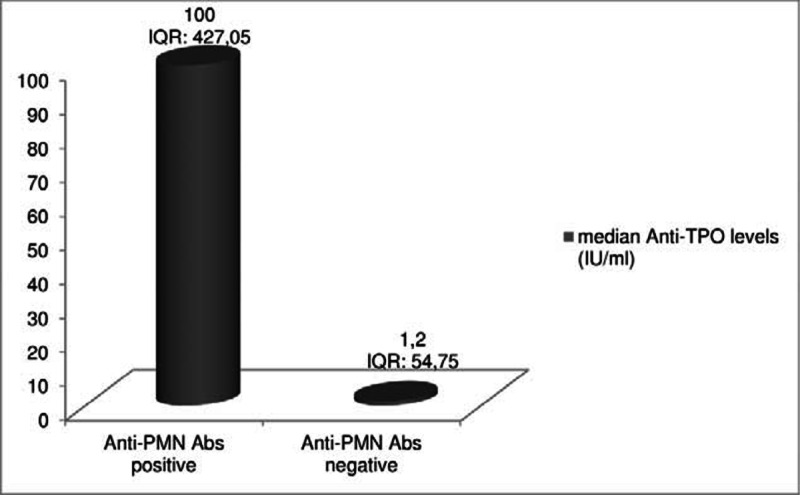
Anti-TPO Abs titers were significantly higher in patients, who had positive Anti-PMN Abs, compared with patients with negative Anti-PMN Abs tests (P = 0.04). Anti-PMN Abs = antineutrophil (antipolymorphonuclear) antibodies, Anti-TPO = antithyroid peroxidase antibodies, IQR = interquartile range.
DISCUSSION
The association between thyroidopathy and neutropenia was first described more than 100 years ago and subsequently confirmed by other investigators.15 However, there has been little mention of this association in the recent literature, except when due to thionamide therapy.5 The present study revealed that thyroidopathy represents the most common disorder among apparently healthy patients with mild-to-moderate neutropenia, comprising 43.6% of this cohort of individuals. This frequency is significantly higher than the 1 reported in the recent study by Lima et al,16 who identified TDs in 8.2% among 97 neutropenic individuals, of whom 7 patients had hypothyroidism and 1 GD. The main strength of our study is the fact that all patients were consecutive ones, presenting with neutropenia in the outpatient setting, and that they were prospectively studied with a detailed and homogenous laboratory screen. It is of interest, that the incidence of autoimmune TDs in neutropenic patients is considerably higher compared with the one reported in healthy individuals. Specifically, we identified HT in 23.4% of all neutropenic patients enrolled in this study, whereas the frequency of HT in several large series of healthy subjects ranges between 10.7% and 13.4%.17,18 At this point, it has to be mentioned that the referral pattern may differ between studies, such as the one by Lima et al and ours. Certainly, this is not a population-based study and referral bias is likely to be present, since patients were referred to a Tertiary Center. However, in our country, family physicians, biopathologists, internists, and other specialists can directly refer patients to tertiary Hematology Clinics without prior detailed work-up. Thus, this series of consecutive patients with incidental, persisting neutropenia can be considered as representative of asymptomatic neutropenia in the outpatient setting. Patients with systemic symptoms, the ones with pancytopenia or other prominent abnormalities are most likely investigated as inpatients and were not referred to the Outpatient Clinic. This might explain the low incidence of myelodysplastic syndromes or systemic diseases, such as systemic lupus erythematosus.
No major differences were detected in the ANC between the different diagnostic categories of neutropenia, both in our analysis and the study of Lima et al.16 However, we found that patients with TD had an increased frequency of recurrent infections, although not life-threatening. To the best of our knowledge, this is the first study demonstrating that thyroidopathy may be involved in the development of infections in patients with neutropenia. In this regard, a potential basis for this observation could be that the production of proinflammatory cytokines, circulating immune complexes and cell-bound anti-PMN Abs may disrupt the normal function, phagocytosis, chemotaxis, adherence, and metabolic activity of neutrophils.19,20
Three possible major mechanisms of thyroid-associated neutropenia should be discussed in relation to our findings: direct thyroid hormone-induced toxicity, humoral, and cellular mechanisms.
Excess of thyroid hormones could indeed have a direct toxic effect on neutrophils, since serum levels of FT3 inversely correlated to the ANC in the entire group of patients. In addition, patients of the TTM subgroup, who had iatrogenic hyperthyroidism, presented the lowest ANC, whereas patients with NTMG, who were euthyroid and had no detectable antithyroid Abs, displayed the highest ANC. In this regard, Shaw and Mehta21 suggest that thyroid hormones may have a direct effect in early stages of hematopoiesis disturbing the maturation and differentiation of pluripotent stem cells. Furthermore, experimental data have demonstrated that exposure to both higher and lower than normal concentrations of thyroid hormone induced apoptosis in human CD34+ enriched progenitor cells.22 Pancytopenia associated with a reversible myelodysplastic syndrome has also been reported in patients with hyperthyroidism due to a direct toxic mechanism.23–25
We suggest that humoral mechanisms are more likely to be involved in the pathophysiology of thyroid disease-associated neutropenia at least in patients with ATD based on 2 major findings of our study: the statistically significant inverse correlation between TPO-Abs/TG-Abs titers and levels of C4 complement factor, and the finding of significantly higher anti-TPO titers in patients with anti-PMN Abs compared with the ones without.
The first finding may indirectly reflect the possible role of immune complexes,26 leading to complement consumption. Elevated levels of circulating immune complexes have been reported in 63% of patients with HT.27 Further data indicate that anti-TG Abs in patients with ATD, facilitate the formation of complement-activating thyroglobulin/anti-TG complexes.28
Our second finding, that is, the significantly higher anti-TPO titers in patients with anti-PMN Abs compared with the ones without, is another interesting finding pointing to the same direction. The association between neutropenia of autoimmune origin and autoimmune thyroidopathy has been mentioned in sparse case reports, but never confirmed in a systematic study. This association may be an indirect proof of the autoimmune nature of neutropenia at least in a proportion of patients with ATD. It has been suggested that, since these Abs behave in a manner similar to TRAbs, it is possible that these 2 types of Abs are directed against the same or similar antigens expressed on more than one tissue. The presence of anti-PMN Abs in serum of patients with GD has been recognized by Weitzman in 1985.29 However, in this study anti-PMN Abs were found in 50 to 55% of patients with GD, while only 2 subjects were neutropenic. The investigators assumed that some of the Abs may not alter the survival of neutrophils against which they are directed, or increased bone marrow production might compensate a shortened cell lifespan. In our study, anti-PMN Abs were detected in 37.2% of patients with TD, the great majority of whom presented with ATD. It is noteworthy that the non-expression of the target antigen by the neutrophils used in the assays, the very low titer of the circulating autoantibodies, and/or a very high affinity, causing the Abs to be essentially bound to their targets may not allow the detection of anti-PMN Abs in some patients.30 In concordance with previous observations, we also found an increased frequency of concomitant autoimmune cutaneous or systemic manifestations in patients with ATD.31 These results are suggestive of disturbances of immune regulation, that may predispose to several immune-mediated hematologic and systemic disorders.
Regarding possible cellular mechanisms involved in thyroid disease-associated neutropenia, several investigators have reported changes in peripheral lymphocyte subsets in ATD, but results are conflicting.32,33 Furthermore, there are no data regarding immunophenotypic findings in neutropenic patients with concomitant thyroidopathy, apart from rare case reports.6,7 In the present analysis, a monoclonal proliferation of T-LGLs could be detected in 13 patients with thyroidopathy (13.7%), equally distributed between patients with or without ATD. T cell clonality was evidenced by molecular methods and a relative increase in T-LGLs (CD3+/CD8+/CD57+), but the criteria for T-LGL leukemia were not fullfilled.11 The pathogenetic role of T-LGL clones in the induction of neutropenia is well established in many disease states, such as T-LGL leukemia, aplastic anemia, and myelodysplastic syndromes, mainly through increased apoptosis. On the other hand, clonal proliferation of T-LGL has been noted in patients with altered B-cell function and autoimmunity and may be associated with autoimmune endocrine abnormalities.34,35
Another interesting finding from the present immunophenotypic analysis was the relative increase of CD4+ T-lymphocytes in patients with thyroid disease compared to the remaining neutropenic patients. A similar finding has been reported in GD.36
The evaluation of lymphocyte subsets revealed different patterns in the different thyroid disease subgroups. In patients with HT an increase in B-cells was evident, compared to the other thyroidopathies. This finding, taking together the higher levels of antithyroid Abs among anti-PMN Abs-positive patients may indicate the important role of humoral mechanisms in the pathophysiology of thyroid disease-associated neutropenia. A different immunophenotypic profile was encountered in neutropenic GD patients: they displayed an increase of the proportion of NK cells, as well as a decrease in the percentage of TCRγδ+ lymphocytes compared to the remaining thyroidopathies. Unfortunately, there are no reported data regarding number or activity of NK cells in neutropenic subjects with GD, whereas numerous studies have mentioned a significant decrease of TCRγδ+ cells in patients with GD. In addition, some authors underline that this finding does not relate to thyroid hormone concentrations and does not normalize after methimazole or radical therapy of GD.37 It is interesting that other investigators have found a higher proportion of TCRγδ+ cells among the thyroid gland-infiltrating lymphocytes in GD,38 as well as in affected organs in other autoimmune diseases.39 The TTM-subgroup showed decreased B-lymphocytes: of note, all TTM patients were receiving high dose-replacement therapy with levothyroxine and presented with subclinical iatrogenic hyperthyroidism. In concordance to our observation, a negative correlation between levels of thyroxine and both CD4+ and CD19+ lymphocytes has been reported suggesting that hyperthyroidism per se would exert a suppressor effect on both.33 Overall, we assume, that the hematologic disturbances in the TTM subgroup may reflect the effect of thyroid hormone excess on neutrophils. On the contrary, patients with HT and GD were euthyroid subjects, with underlying autoimmune thyroidopathy (though of different pathophysiologic origin) and their immunophenotypic and immunologic characteristics point to humoral and cellular mechanisms, that are likely to be involved in the pathophysiology of thyroid disease-associated neutropenia.
Several limitations of this study should be acknowledged. Firstly, the possible referral bias has been already addressed at the beginning of the discussion. Secondly, the number of patients with GD (n = 9) and TTM (n = 6) are small, so that firm conclusions cannot be drawn. However, this reflects the rarity of these two conditions.
In conclusion, the frequency of thyroid disease among patients with mild to moderate neutropenia is higher than previously reported. A comprehensive evaluation of thyroid function tests as well as antithyroid Abs in patients with neutropenia is mandatory, even in the absence of related symptoms. This may help to identify this indolent etiology of neutropenia in a substantial proportion of cases, avoiding patients’ stress and cumbersome and expensive laboratory tests. Furthermore, our data may indirectly reflect the possible role of immune complexes in thyroid disease-associated neutropenia. The increased prevalence of anti-PMN Abs, their correlation with anti-TPO Abs, as well as the different distribution of lymphocyte subsets among patients with thyroid diseases point to humoral and cellular mechanisms in the pathophysiology of thyroid disease-associated neutropenia. Further investigation is required to elucidate the underlying pathophysiological mechanisms that associate neutropenia with TD.
Acknowledgements
The authors have nothing to disclose.
Footnotes
Abbreviations: Abs = antibodies, ANC = absolute neutrophil count, AN-SHP = antibody-negative subclinical hypothyroidism, Anti-TG = antithyroglobulin antibodies, Anti-PMN Abs = antineutrophil antibodies, Anti-TPO = anti-thyroid peroxidase antibodies, ATD = autoimmune thyroid disease, CBC = complete blood counts, FT3 = free triiodothyronine, FT4 = free thyroxine, GAT = granulocyte agglutination test, GD = Graves disease, GIFT = granulocyte immunofluorescence test, HT = Hashimoto thyroiditis, IQR = interquartile range = LIFT, lymphocyte immunofluerence test = NK, natural killer = NTMG, nontoxic multinodular goiter = T3, total triiodothyronine = T4, total thyroxine = TCR, T-cell receptor = TD, thyroid disorders = T-LGL(s), T-cell large granular lymphocyte(s) = TRAbs, anti-thyrotropin receptor antibodies = TSH, thyroid-stimulating hormone = TTM, total thyroidectomy associated with nodules.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Ansell JE. The Blood in Hypothyroidism. In: Braverman LE, Utiger RD, eds. Werner and Ingbar's The Thyroid: A Fundamental and Clinical Text. 7th ed Philadelphia: Lippincott-Raven;1996(a):821–825. [Google Scholar]
- 2.Ansell JE. The Blood in Thyrotoxicosis. In: Braverman LE, Utiger RD, eds. Werner and Ingbar's The Thyroid: A Fundamental and Clinical Text. 7th ed Philadelphia: Lippincott-Raven;1996(b):637–644. [Google Scholar]
- 3.Eakin DL, Peake RL, Weiss GB. Effect of therapy on the neutropenia of hyperthyroidism. South Med J 1983; 76:335–337.340. [DOI] [PubMed] [Google Scholar]
- 4.Ponassi A, Morra L, Caristo G, et al. Disorders of granulopoiesis in patients with untreated Graves’ disease. Acta Haematol 1983; 70:19–23. [DOI] [PubMed] [Google Scholar]
- 5.Pearce SH. Spontaneous reporting of adverse reactions to carbimazole and propylthiouracil in the UK. Clin Endocrinol (Oxf) 2004; 61:589–594. [DOI] [PubMed] [Google Scholar]
- 6.Fumeaux Z, Beris P, Borisch B, et al. Complete remission of pure white cell aplasia associated with thymoma, autoimmune thyroiditis and type 1 diabetes. Eur J Haematol 2003; 70:186–189. [DOI] [PubMed] [Google Scholar]
- 7.Rizzi R, Pastore D, Liso A, et al. Autoimmune myelofibrosis: report of three cases and review of the literature. Leuk Lymphoma 2004; 45:561–566. [DOI] [PubMed] [Google Scholar]
- 8.Bux J. Human neutrophil alloantigens. Vox Sang 2008; 94:277–285. [DOI] [PubMed] [Google Scholar]
- 9.Papadaki HA, Pontikoglou C. Pathophysiologic mechanisms, clinical features and treatment of idiopathic neutropenia. Expert Rev Hematol 2008; 1:217–229. [DOI] [PubMed] [Google Scholar]
- 10.Kahapola-Arachchige KM, Hadlow N, Wardrop R, et al. Age-specific TSH reference ranges have minimal impact on the diagnosis of thyroid dysfunction. Clin Endocrinol (Oxf) 2012; 77:773–779. [DOI] [PubMed] [Google Scholar]
- 11.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008:272–275. [Google Scholar]
- 12.van Dongen JJ, Langerak AW, Brüggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003; 17:2257–2317. [DOI] [PubMed] [Google Scholar]
- 13.Bennett JM, Komrokji RS. The myelodysplastic syndromes: diagnosis, molecular biology and risk assessment. Hematology 2005; 10:258–269. [DOI] [PubMed] [Google Scholar]
- 14.Troussard X, Cornet E, Lesesve JF, et al. Polyclonal B-cell lymphocytosis with binucleated lymphocytes (PPBL). Onco Targets Ther 2008; 1:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocher T. Blutintersuchungen bei morbus Basedow mit Beitrigen zur Frühdiagnose und Theorie der Krankheit. Arch Klin Chirurg 1908; 87:131–157. [Google Scholar]
- 16.Lima CS, Paula EV, Takahashi T, et al. Causes of incidental neutropenia in adulthood. Ann Hematol 2006; 85:705–709. [DOI] [PubMed] [Google Scholar]
- 17.O’Leary PC, Feddema PH, Michelangeli VP, et al. Investigations of thyroid hormones and antibodies based on a community health survey: the Busselton thyroid study. Clin Endocrinol (Oxf) 2006; 64:97–104. [DOI] [PubMed] [Google Scholar]
- 18.Staii A, Mirocha S, Todorova-Koteva K, et al. Hashimoto thyroiditis is more frequent than expected when diagnosed by cytology which uncovers a pre-clinical state. Thyroid Res 2010; 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balázs C, Bokk A, Kiss E. Inhibition of metabolic activity of polymorphonuclear granulocytes by thyroid stimulating antibodies. J Endocrinol Invest 1992; 15:465–469. [DOI] [PubMed] [Google Scholar]
- 20.Engelich G, Wright DG, Hartshorn KL. Acquired disorders of phagocyte function complicating medical and surgical illnesses. Clin Infect Dis 2001; 33:2040–2048. [DOI] [PubMed] [Google Scholar]
- 21.Shaw B, Mehta AB. Pancytopenia responding to treatment of hyperthyroidism: a clinical case and review of the literature. Clin Lab Haematol 2002; 24:385–387. [DOI] [PubMed] [Google Scholar]
- 22.Grymuła K, Paczkowska E, Dziedziejko V, et al. The influence of 3,3′,5-triiodo-L-thyronine on human haematopoiesis. Cell Prolif 2007; 40:302–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lima CS, Zantut Wittmann DE, Castro V, et al. Pancytopenia in untreated patients with Graves’ disease. Thyroid 2006; 16:403–409. [DOI] [PubMed] [Google Scholar]
- 24.Akoum R, Michel S, Wafic T, et al. Myelodysplastic syndrome and pancytopenia responding to treatment of hyperthyroidism: peripheral blood and bone marrow analysis before and after antihormonal treatment. J Cancer Res Ther 2007; 3:43–46. [DOI] [PubMed] [Google Scholar]
- 25.Duquenne M, Lakomsky D, Humbert JC, et al. Pancytopenia resolved by the treatment of hyperthyroidm. Presse Med 1995; 24:807–810. [PubMed] [Google Scholar]
- 26.Hayashi S, Kiyokawa T, Aochi H, et al. Characterization of elevated neutrophil-associated IgG in various autoimmune disorders: not anti-neutrophil autoantibodies, but possibly immune complexes, bind to neutrophils. Autoimmunity 1997; 26:195–203. [DOI] [PubMed] [Google Scholar]
- 27.Kappelgaard E, Nielsen H, Bech K, et al. Circulating immune complexes in Hashimoto's thyroiditis. Correlation to HLA and autoantibodies. Allergy 1983; 38:433–439. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen CH, Leslie RG, Jepsen BS, et al. Natural autoantibodies and complement promote the uptake of a self antigen, human thyroglobulin, by B cells and the proliferation of thyroglobulin-reactive CD4(+) T cells in healthy individuals. Eur J Immunol 2001; 31:2660–2668. [DOI] [PubMed] [Google Scholar]
- 29.Weitzman SA, Stossel TP, Harmon DC, et al. Antineutrophil antibodies in Grave's disease. Implications of thyrotropin binding to neutrophils. J Clin Invest 1985; 75:119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coppo P, Ghez D, Fuentes V, et al. Antineutrophil cytoplasmic antibody-associated neutropenia. Eur J Intern Med 2004; 15:451–459. [DOI] [PubMed] [Google Scholar]
- 31.Weetman AP. Diseases associated with thyroid autoimmunity: explanations for the expanding spectrum. Clin Endocrinol (Oxf) 2011; 74:411–418. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen BK, Feldt-Rasmussen U, Bech K, et al. Characterization of the natural killer cell activity in Hashimoto's and Graves’ diseases. Allergy 1989; 44:477–481. [DOI] [PubMed] [Google Scholar]
- 33.Corrales JJ, Orfao A, Miralles JM, et al. The relationship between hyperthyroidism and the distribution of peripheral blood T, NK and B-lymphocytes in multinodular goiter. Horm Metab Res 1994; 26:104–108. [DOI] [PubMed] [Google Scholar]
- 34.Dhodapkar MV, Lust JA, Phyliky RL. T-cell large granular lymphocytic leukemia and pure red cell aplasia in a patient with type I autoimmune polyendocrinopathy: response to immunosuppressive therapy. Mayo Clin Proc 1994; 69:1085–1088. [DOI] [PubMed] [Google Scholar]
- 35.Bareau B, Rey J, Hamidou M, et al. Analysis of a French cohort of patients with large granular lymphocyte leukemia: a report on 229 cases. Haematologica 2010; 95:1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bossowski A, Urban M, Stasiak-Barmuta A. Analysis of changes in the percentage of B (CD19) and T (CD3) lymphocytes, subsets CD4, CD8 and their memory (CD45RO), and naive (CD45RA) T cells in children with immune and non-immune thyroid diseases. J Pediatr Endocrinol Metab 2003; 16:63–70. [DOI] [PubMed] [Google Scholar]
- 37.Sasián S, Rojano J, Gavilán I, et al. Serial analysis of circulating T gamma/delta lymphocyte subpopulations in Graves’ disease. Endocr Res 1998; 24:285–295. [DOI] [PubMed] [Google Scholar]
- 38.Santamaria P, Lewis C, Barbosa JJ. Molecular heterogeneity of a Graves’ thyroid-infiltrating T cell population rich in CD8+ and gamma delta+ T cells. J Endocrinol Invest 1993; 16:913–920. [DOI] [PubMed] [Google Scholar]
- 39.Olive C, Gatenby PA, Serjeantson SW. Variable gene usage of T cell receptor gamma- and delta chain transcript expressed in synovia and peripheral blood of patients with rheumatoid arthritis. Clin Exp Immunol 1992; 87:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]


