Abstract
The purpose of this study was to perform a meta-analysis of current literature to determine whether lowering blood pressure (BP) during the acute phase of an ischemic stroke improves short- and long-term outcomes.
PubMed, Cochrane, and Embase were searched until September 5, 2014 using combinations of the search terms: blood pressure reduction, reduced blood pressure, lowering blood pressure, ischemic stroke, acute stroke, and intra-cerebral hemorrhage. Inclusion criteria were randomized controlled trial and patients with acute stroke (ischemic or hemorrhagic) treated with an antihypertensive agent or placebo. Outcome measures were change in systolic and diastolic BP (SBP, DBP) after treatment, and short- and long-term dependency and mortality rates.
A total of 459 studies were identified, and ultimately 22 studies were included in the meta-analysis. The total number of participants in the treatment groups was 5672 (range, 6–2308), and in the control groups was 5416 (range, 6–2033). In most studies, more than 50% of the participants were males and the mean age was more than 60 years. The mean follow-up time ranged from 5 days to 12 months. As expected, treatment groups had a greater decrease in BP than control groups, and this effect was seen with different classes of antihypertensive drugs. Short-term and long-term dependency rates were similar between treatment and control groups (short-term dependency: pooled odds ratio [OR] = 1.041, 95% confidence interval [CI]: 0.936–1.159, P = 0.457; long-term dependency: pooled OR = 1.013, 95% CI: 0.915–1.120, P = 0.806). Short-term or long-term mortality was similar between the treatment and control groups (short-term mortality: pooled OR = 1.020, 95% CI: 0.749–1.388, P = .902; long-term mortality: pooled OR = 1.039, 95% CI: 0.883–1.222, P = 0.644).
Antihypertensive agents effectively reduce BP during the acute phase of an ischemic stroke, but provide no benefit with respect to short- and long-term dependency and mortality.
INTRODUCTION
Elevated blood pressure (BP) (systolic BP [SBP] >140 mmHg) is seen in over 60% of patients during the acute phase of a stroke (ischemic or hemorrhagic).1,2 The elevated BP may be related to pre-existing hypertension, which is seen in ≥50% of patients, stress, increased intracranial pressure, or autonomic dysfunction as a result of the stroke itself.2,3 Elevated BP during the acute phase of a stroke has been associated with poor short-term and long-term outcomes,1,4,5 and an increased risk of early recurrence.6 Thus, it would seem logical that lowering BP with antihypertensive medications in patients with an elevated BP during a stroke would improve outcomes. However, lowering BP may reduce already compromised cerebral blood flow, and may increase the size of the infarct by reducing flow to the penumbra zone (viable but underperfused tissue surrounding the infarct).2,3 Although the issue has been examined for almost 30 years, it remains unclear whether elevated BP during the acute phase of a stroke should be treated with antihypertensive medications.
Studies examining lowering BP during the acute phase of a stroke have provided conflicting results. Some randomized controlled trials (RCTs) have indicated that lowering BP was safe, and associated with benefits such as improved long-term mortality.7–9 Other studies, however, have shown no benefit of lowering BP during the acute phase of a stroke.10–13 Furthermore, data from some studies have suggested a harmful effect of BP lowering.12,14–16 Two recent Cochrane Database Systematic reviews examined interventions for altering BP in acute stroke and vasoactive drugs for acute stroke and concluded there is insufficient evidence that lowering BP during the acute phase of a stroke produces any improvement in functional outcomes.17,18
Thus, the purpose of this study was to perform a meta-analysis of current literature to determine that lowering BP during the acute phase of an ischemic stroke improves short-term and long-term outcomes.
MATERIALS AND METHODS
Ethic Statement
Meta-analyses do not involve human subjects and do not require institutional review board review (J Grad Med Educ. 2011 March; 3(1): 5–6.).
Literature Search Strategy
This systematic review and meta-analysis was conducted in accordance with PRISMA guidelines,19 and the methodology set forth in the Cochrane Handbook for Systematic Reviews of Interventions.20 PubMed, Cochrane, and Embase were searched until September 5, 2014 using combinations of the search terms: blood pressure reduction, reduced blood pressure, lowering blood pressure, ischemic stroke, acute stroke, and intra-cerebral hemorrhage. Two independent reviewers searched the databases using the keywords to identify potentially relevant articles, and article titles and abstracts were screened based on the inclusion and exclusion criteria. The reference lists of potentially relevant articles were also hand-searched. Where there was uncertainty regarding eligibility, a third reviewer was consulted and a decision arrived at by consensus. The full text of potentially relevant articles was then reviewed by the 2 independent reviewers, and when there was uncertainty regarding inclusion or exclusion of a study, a third reviewer was consulted and a decision arrived at by consensus.
Selection Criteria and Data Extraction
Criteria for inclusion in the meta-analysis were: RCT; patients with acute ischemic stroke; treated with an antihypertensive agent versus placebo; blood pressure was recorded. Non-randomized trials, letters, comments, editorials, case reports, and non-English publications were excluded. Studies that only recruited patients with hemorrhagic stroke were excluded. If a study recruited patients with both ischemic and hemorrhagic stroke and did not provide subgroup data with respect to patients with an ischemic stroke, data of patients with both types of strokes were analyzed together.
Data extracted from studies that met the inclusion criteria included the name of the first author, year of publication, trial name (if any), type of patients, intervention, treatment protocol, number of patients in the treatment and control groups, age of patients, percent males, SBP and diastolic BP (DBP) before and after treatment and time point when BP was monitored, short- and long-term dependency and mortality rates, and long-term stroke-related deaths. If clarifications were required with respect to any information or data of the included studies, the corresponding author was contacted.
Quality Assessment
The methodological quality of each study was assessed using the risk-of-bias assessment tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0)20 by 2 reviewers. Briefly, 6 domains are evaluated: random sequence generation, allocation concealment, blinding of patients and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting risk. Risks of bias figures were generated using Cochrane Review Manager software 5.3.
Outcome Measures and Data Analysis
The outcome measures were change in SBP and DBP after treatment, and short- and long-term dependency and mortality rates. For SBP and DBP, pre- and post-treatment measurements were summarized as either mean ± standard deviation (SD), mean with 95% confidence interval (95% CI), or mean difference of mean change between groups with 95% CI. An effect size difference in means of change from pre- to post-treatment between groups was presented with corresponding standard error (SE) and 95% CI. The effect size of difference in means of change from pre- to post-treatment between groups <0 indicated there was a greater change in SBP or DBP in the treatment group than in the control group, whereas a value >0 indicated there was less of a change in the treatment group. A value of 0 indicated the change was similar between the 2 groups. For dependency and mortality rates, data were summarized as n/N (%) for each group and each study, and an odds ratio (OR) with corresponding 95% CI was calculated. An OR >1 indicates the treatment group had a higher rate than the control group, whereas an OR <1 indicates the treatment group had a lower rate than the control group. An OR = 1 implies the rate was similar between treatment and control groups.
Heterogeneity among the studies was assessed by calculating the Cochran Q and the I2 statistic. A Cochran Q with P < 0.121 or an I2 statistic >50%22 was considered to indicate heterogeneity between studies. When obvious heterogeneity between studies was observed, a random-effects model of analysis (DerSimonian-Laird method)23 was used, otherwise a fixed-effects model was used (Mantel-Haenszel method). Sensitivity analysis was carried out based on the leave-one-out approach for SBP and DBP. Publication bias was assessed by constructing funnel plots and by Egger test. The absence of publication bias is indicated by the data points forming a symmetric funnel-shaped distribution, and a 1-tailed significance level P > 0.05 in Egger test. All statistical analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat; Englewood, NJ).
RESULTS
Literature Search
A flow diagram of study selection is shown in Figure 1. A total of 459 studies were identified though the database search, and 415 non-relevant studies were excluded. Subsequently, 44 full-text articles were reviewed, and 22 studies were excluded, the reasons for which are shown in Figure 1. Thus, 22 studies7,8,10–14,24–38 were included in the meta-analysis. The full texts of all the relevant articles were readily available, and all of the articles contained the necessary data to conduct the meta-analysis. We did not have to contact the corresponding author of any articles.
FIGURE 1.
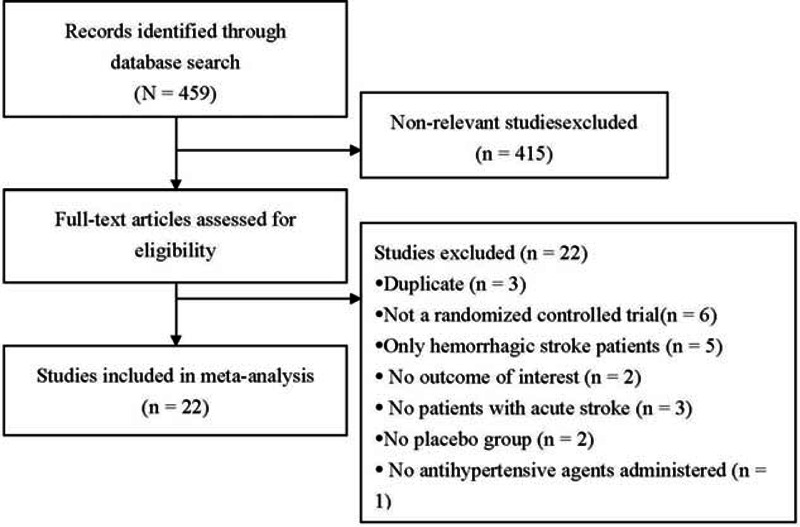
Flow diagram of study selection.
Study Characteristics
The basic characteristics of the studies are shown in Table 1 , and outcomes are summarized in Tables 2 and Tables 3 . The ages of the participants were generally very similar between the treatment groups and control groups, within and between the studies. The total number of participants in the treatment groups was 5672 (range, 6–2308), and in the control groups was 5416 (range, 6–2033). In most studies, >50% of the participants were males and the mean age was >60 years. The mean follow-up time ranged from 5 days to 12 months. In the majority of studies, 80% to 100% of the patients had an ischemic stroke, whereas in a few studies, there were >50% of patients with an ischemic stroke.
TABLE 1.
Characteristics of Studies Included in the Meta-analysis
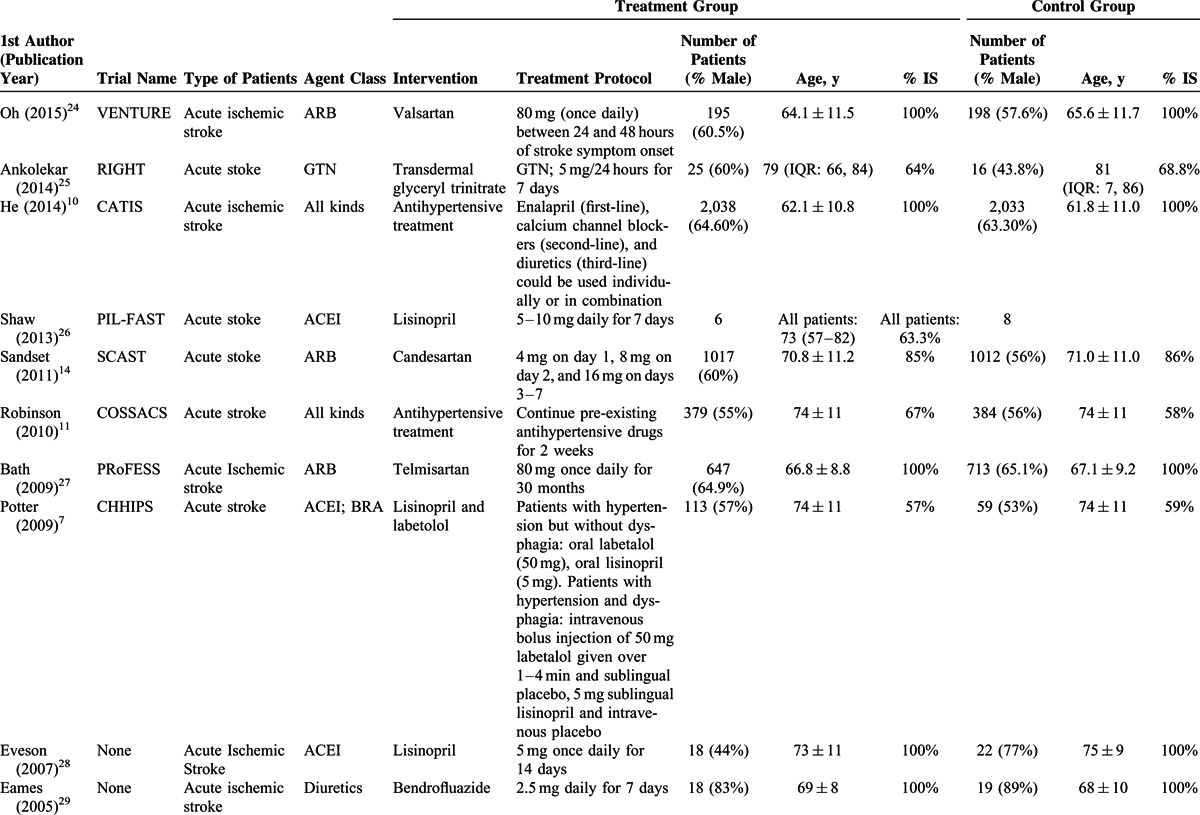
TABLE 1 (Continued).
Characteristics of Studies Included in the Meta-analysis
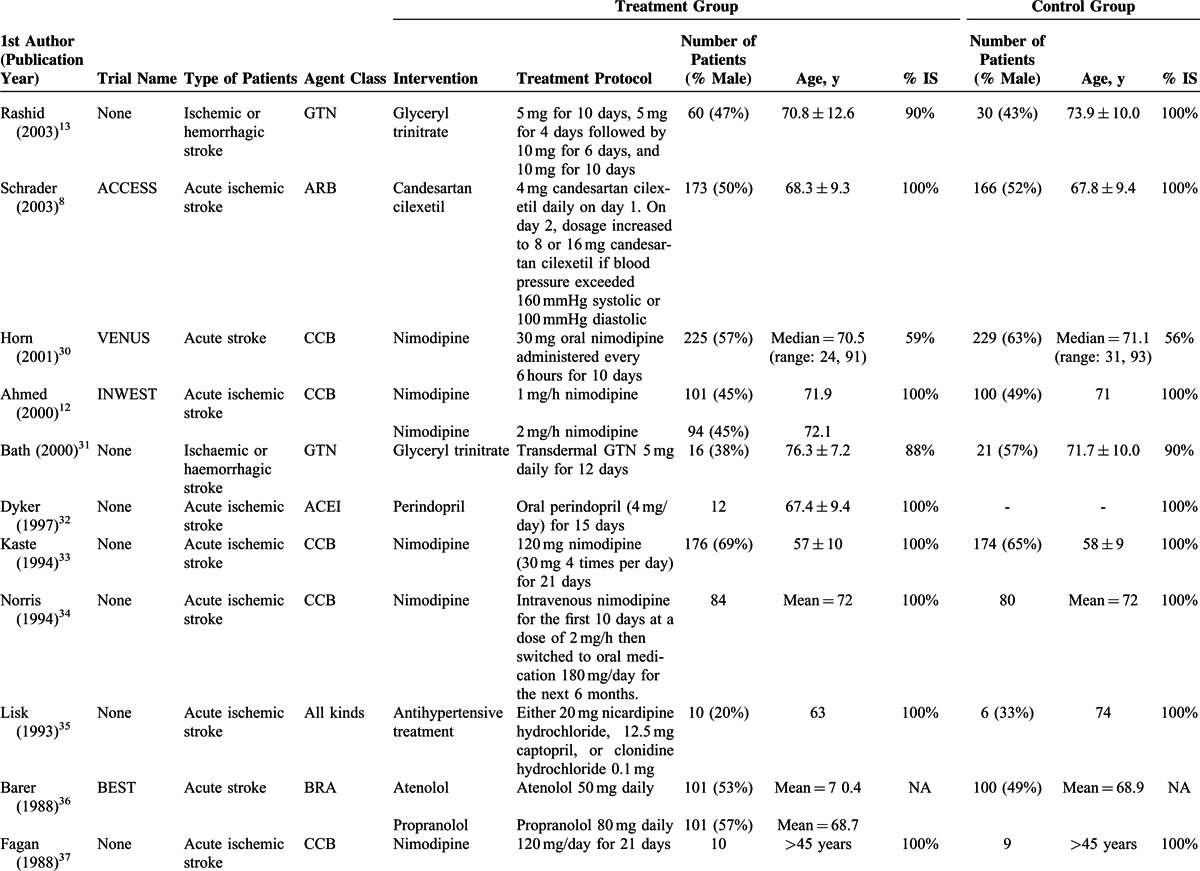
TABLE 1 (Continued).
Characteristics of Studies Included in the Meta-analysis
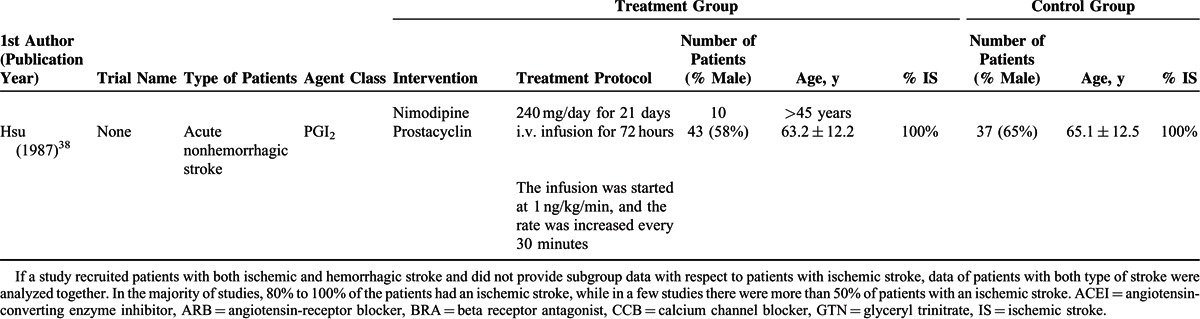
TABLE 2.
Primary Outcome (Blood Pressure) of the Studies Included in the Meta-analysis
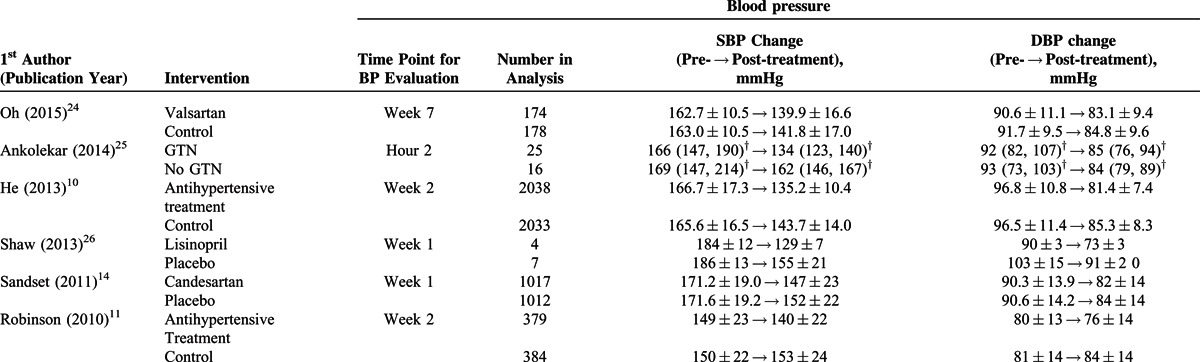
TABLE 2 (Continued).
Primary Outcome (Blood Pressure) of the Studies Included in the Meta-analysis
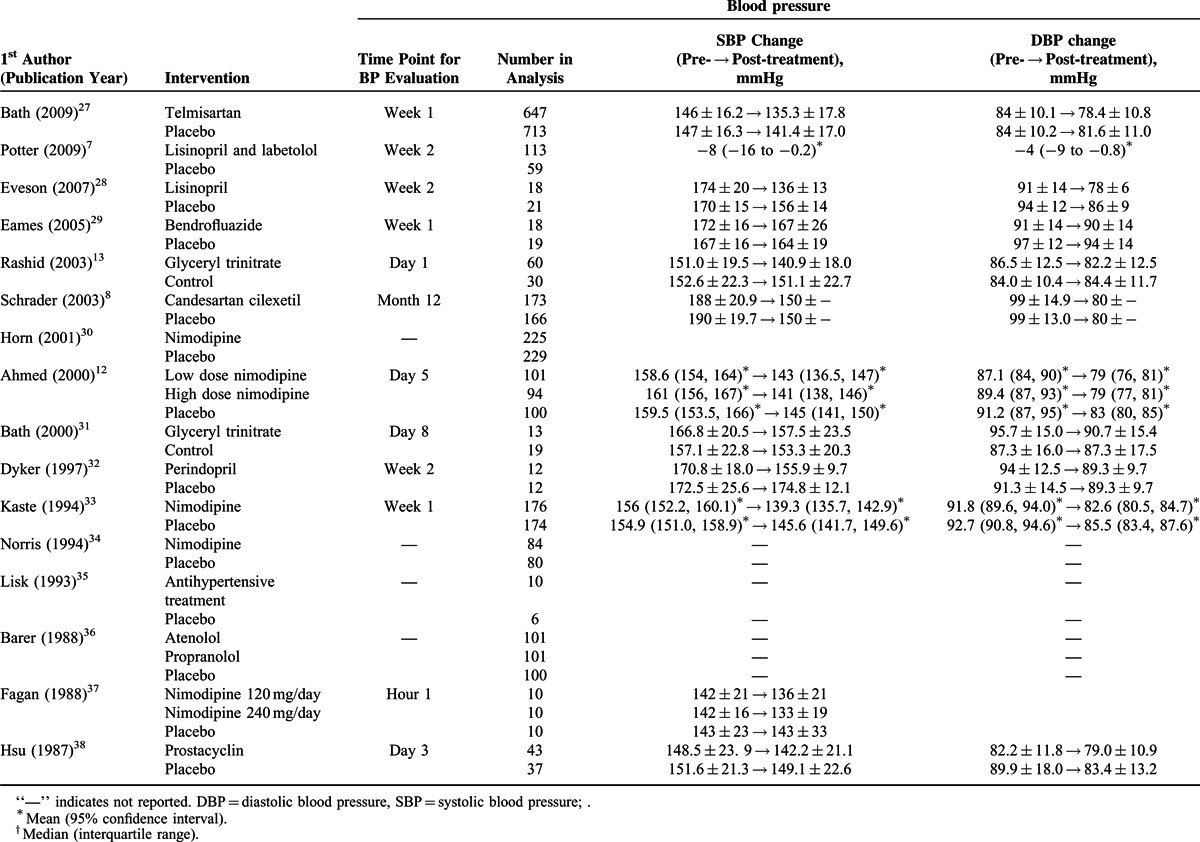
TABLE 3.
Dependency, Mortality, and Long-term Stroke-related Death Rates of the Studies Included in the Meta-analysis
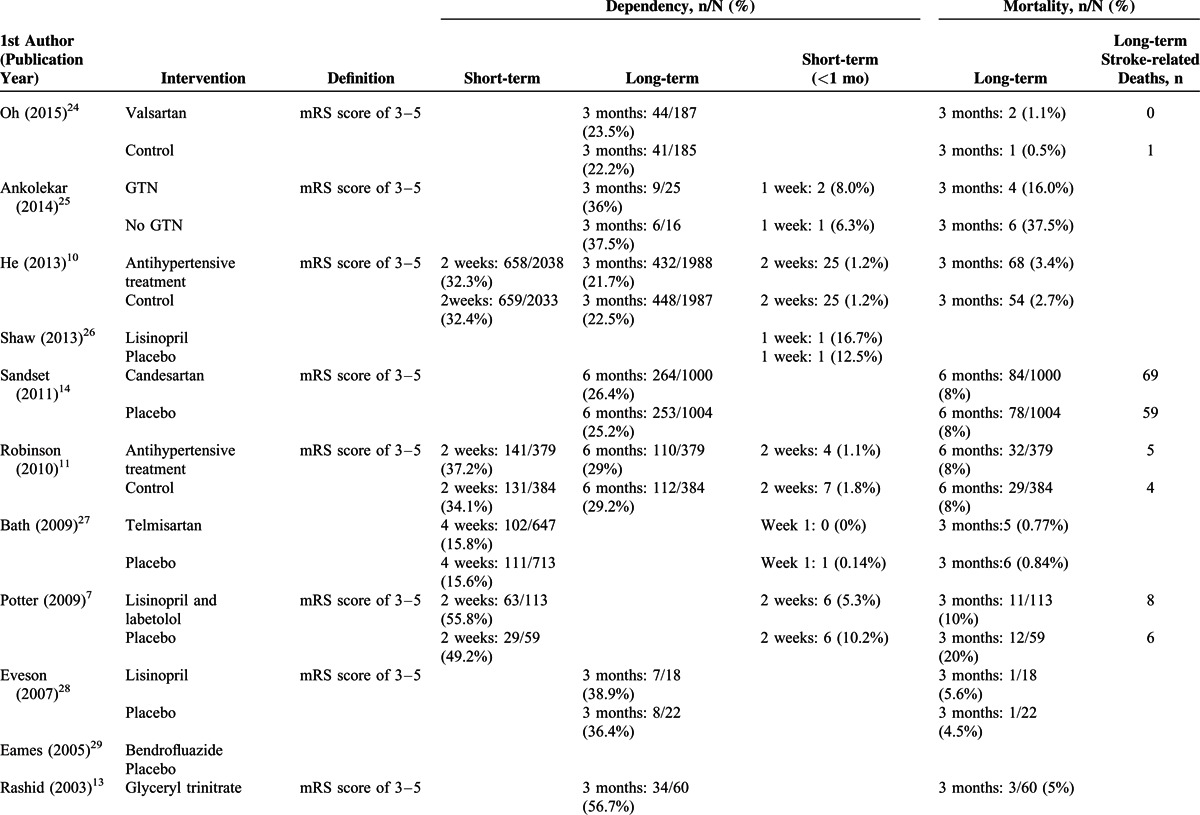
TABLE 3 (Continued).
Dependency, Mortality, and Long-term Stroke-related Death Rates of the Studies Included in the Meta-analysis
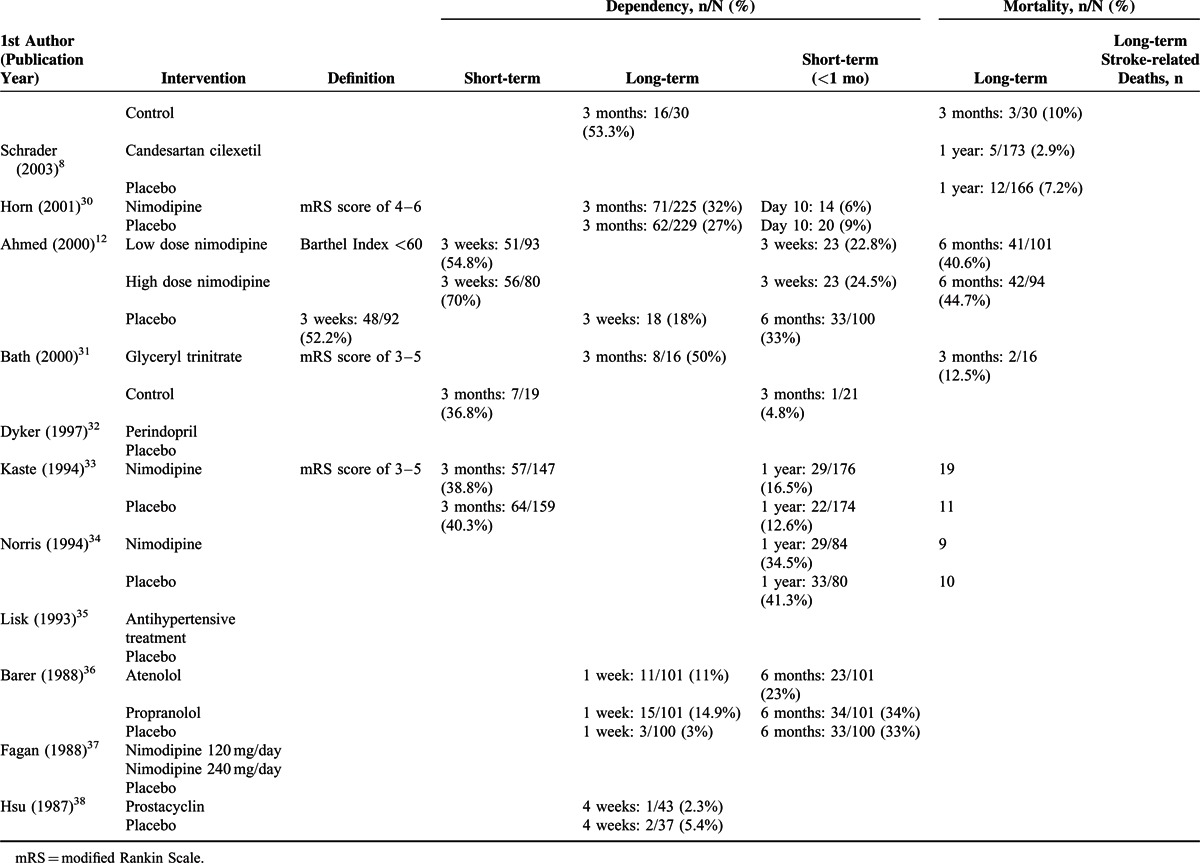
Outcomes
Change of BP
A total of 16 studies7,10–14,24,26–29,32,33,37,38 with complete pre- and post-treatment BP data were included in the analysis (Figure 2). The study by Potter et al7 examined angiotensin-converting enzyme inhibitors (ACEIs) and beta receptor antagonist (BRAs) separately, and thus the 2 classes of drugs were analyzed separately. A random-effects model was used since significant heterogeneity among studies was observed in both SBP and DBP (SBP: Cochran Q = 76.13, I2 = 78.98%, P < 0.001; DBP: Cochran Q = 154.39, I2 = 90.28%, P < 0.001). The pooled difference in means of BP levels was significantly different between the treatment and control groups, and treatment was associated with a greater decrease in SBP and DBP (SBP: difference in means = −7.808, 95% CI: −10.572 to −5.044, P < 0.001, Figure 2A; DBP: difference in means = −4.262, 95% CI: −6.359 to −2.166, P < 0.001, Figure 2B).
FIGURE 2.
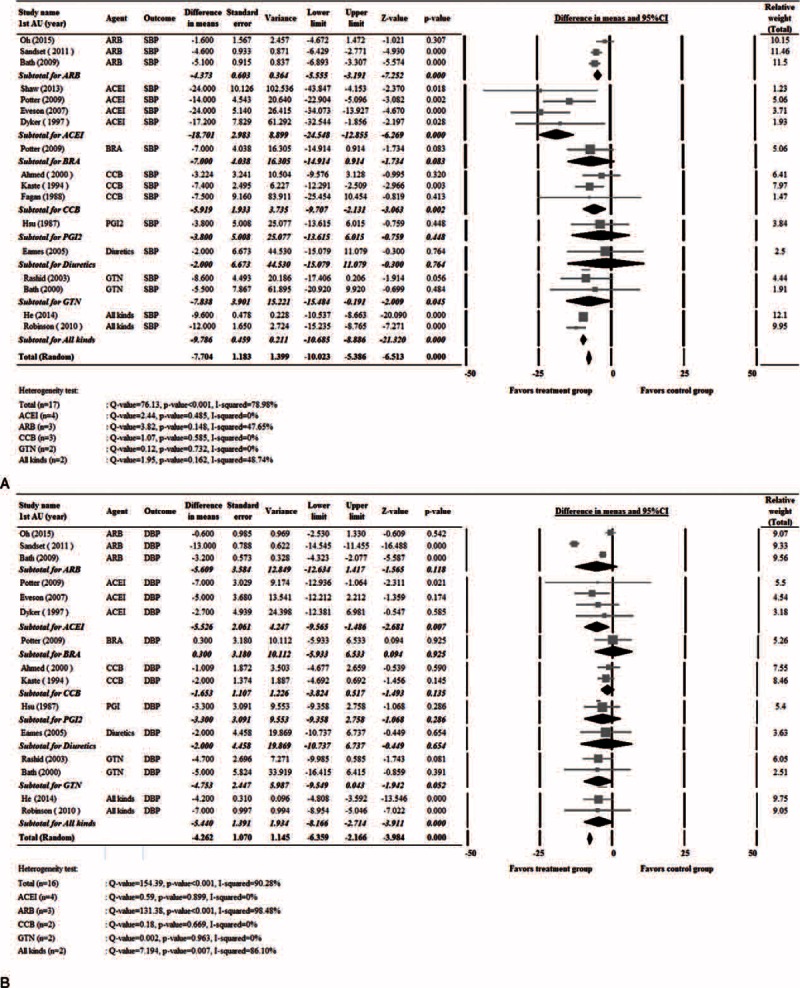
Forest plots of blood pressure levels between patients that received treatment and controls. (A) Systolic blood pressure. (B) Diastolic blood pressure. 1st AU = first author, Std = standardized, diff = difference, CI = confidence interval.
Change of BP by Treatment Type
Subgroup analysis of BP changes was performed based on the types of antihypertensive administered. Of the studies, 3 used angiotensin-receptor blockers (ARBs),14,24,27 4 ACEIs,7,26,28,32 1 a BRA,7 3 calcium channel blockers (CCBs),12,33,37 1 prostacyclin (PGI2, epoprostanol),38 1 a diuretic,29 2 glyceryl trinitrate (GTN),13,31 and 2 multiple drugs10,11 (Table 1 ).
Figure 2A shows that the pooled difference in means of SBP levels was significantly different between the treatment and control groups for ARBs, ACEIs, CCBs, GTN, and multiple drugs. Treatment was associated with a greater decrease in SBP (ARB: difference in means = −4.37, 95% CI: −5.56 to −3.19, P < 0.001; ACEI: difference in means = −18.70, 95% CI: −24.55 to −12.86, P < 0.001; CCB: difference in means = −5.919, 95% CI = −9.71 to −2.13, P = 0.002; GTN: difference in means = −7.84, 95% CI: −15.48 to −0.19, P = 0.045; multiple drugs: difference in means = −7.70, 95% CI: −10.02 to −5.39, P < 0.001).
Figure 2B shows the pooled difference in means of DBP levels was significantly different between the treatment and control groups for ACEI and multiple drugs. Treatment was associated with a greater decrease in DBP (ACEI: difference in means = −5.53, 95% CI: −9.57 to −1.49, P = 0.007; multiple drugs: difference in means = −5.44, 95% CI: −6.36 to −2.17, P < 0.001).
Short-Term and Long-Term Dependency
Five studies7,10–12,27 with complete short-term dependency (2–3 weeks) data (Figure 3A), and 10 studies10,11,13,14,24,25,30,31,33 with complete long-term dependency (>3 months) data (Figure 3B) were included in the analysis. No significant heterogeneity among the studies was noted; hence, a fixed-effects model of analysis was used (short-term dependency: Cochran Q = 3.241, I2 = 0%, P = ).518; long-term dependency: Cochran Q = 3.241, I2 = 0%, P = 0.518). Similar short-term and long-term post-treatment rates of dependency between the treatment and control groups were noted (short-term dependency: pooled OR = 1.041, 95% CI: 0.936–1.159, P = 0.457; long-term dependency: pooled OR = 1.013, 95% CI: 0.915–1.120, P = 0.806) (Figure 3).
FIGURE 3.
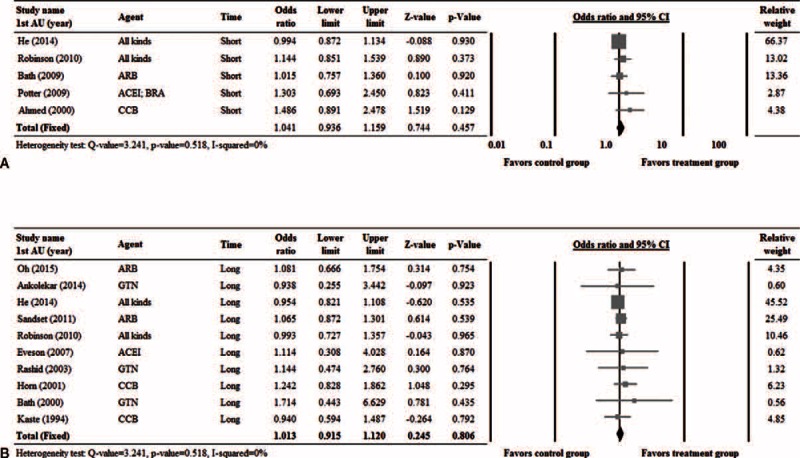
Forest plots of the rates of short-term (A) and long-term (B) dependency compared between patients that received treatment and controls. 1st AU = first author, CI = confidence interval.
Short-Term and Long-Term Mortality
Ten studies7,10–12,25–27,30,36,38 with complete short-term mortality data (Figure 4A), and 13 studies7,8,10–14,25,27,31,33,34,36 with complete long-term mortality data (Figure 4B) were included in the analysis. Significant heterogeneity was noted among the studies with short-term mortality data; thus, a random-effects model of analysis was used. No obvious heterogeneity was noted among the studies with long-term mortality data; thus, a fixed-effects model of analysis was used (short-term: Cochran Q = 11.62, I2 = 22.57%, P = 0.235; long-term: Cochran Q = 17.81, I2 = 27.01%, P = 0.165) There was no significant difference in short-term or long-term mortality between the treatment and control groups (short-term mortality: pooled OR = 1.020, 95% CI: 0.749–1.388, P = .902; long-term mortality: pooled OR = 1.039, 95% CI: 0.883–1.222, P = .644) (Figure 4).
FIGURE 4.
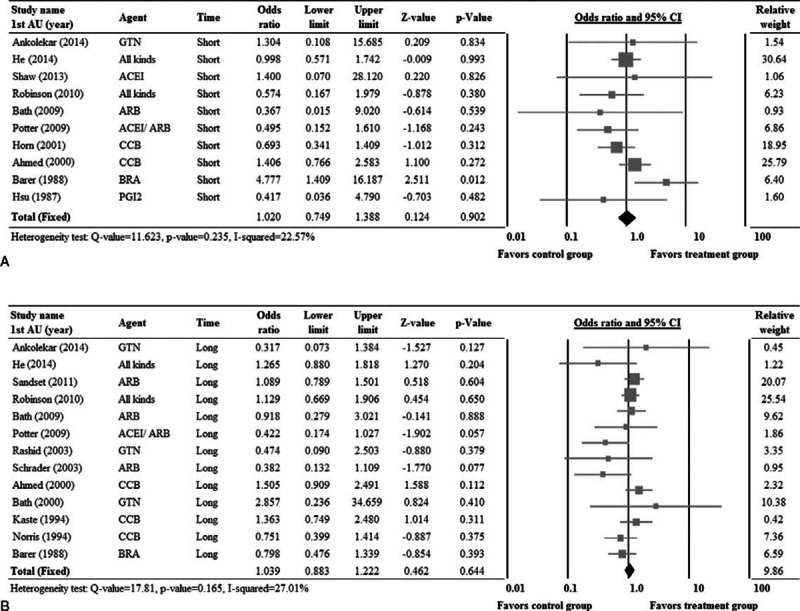
Forest plots of the rates of short-term (A) and long-term (B) mortality compared between patients that received treatment and controls. 1st AU = first author; CI = confidence interval.
Sensitivity Analysis and Publication Bias
Sensitivity analysis for change in SBP and DBP was performed using the leave-one-out approach (Figure 5). No obvious influences of individual studies on the pooled estimates for change in SBP and DBP were noted, indicating that the pooled estimates for the outcomes were robust.
FIGURE 5.
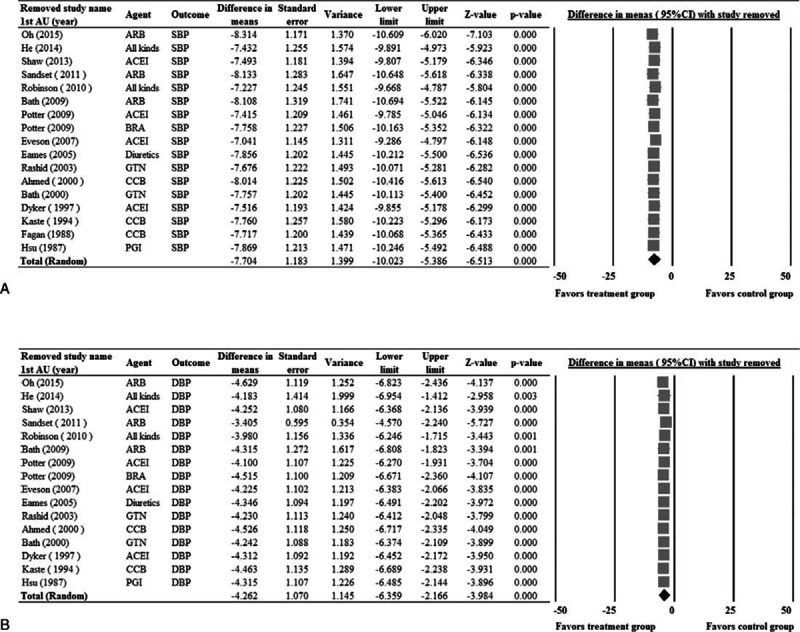
Sensitivity analysis for systolic blood pressure (A), and diastolic blood pressure (B) using the leave-one-out approach. 1st AU = first author, diff = difference, CI = confidence interval.
Funnel plots and the results of Egger test for SBP and DBP are shown in Figure 6. Egger test indicated there was no publication bias with respect to SBP and DBP among the studies (1-tailed P = 0.461 and 0.471, respectively). In addition, no publication bias with respect to long-term mortality was found (data not shown).
FIGURE 6.
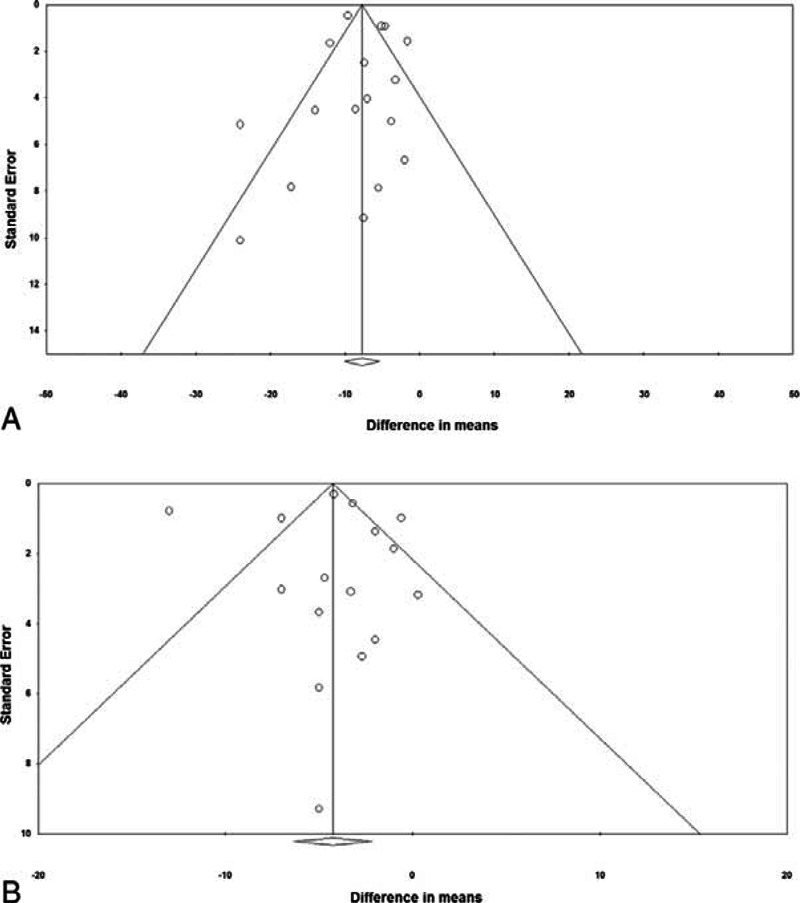
Funnel plots for systolic blood pressure (A), and diastolic blood pressure (B). One-tailed P values from Egger test were 0.461 and 0.471 for systolic blood pressure and diastolic blood pressure, respectively.
Quality Assessment
Results of the quality assessment of the included studies indicated there was generally low risk of bias (Figure 7). However, only 5 studies described the process of allocation concealment and only 5 included an intention-to-treat analysis.
FIGURE 7.
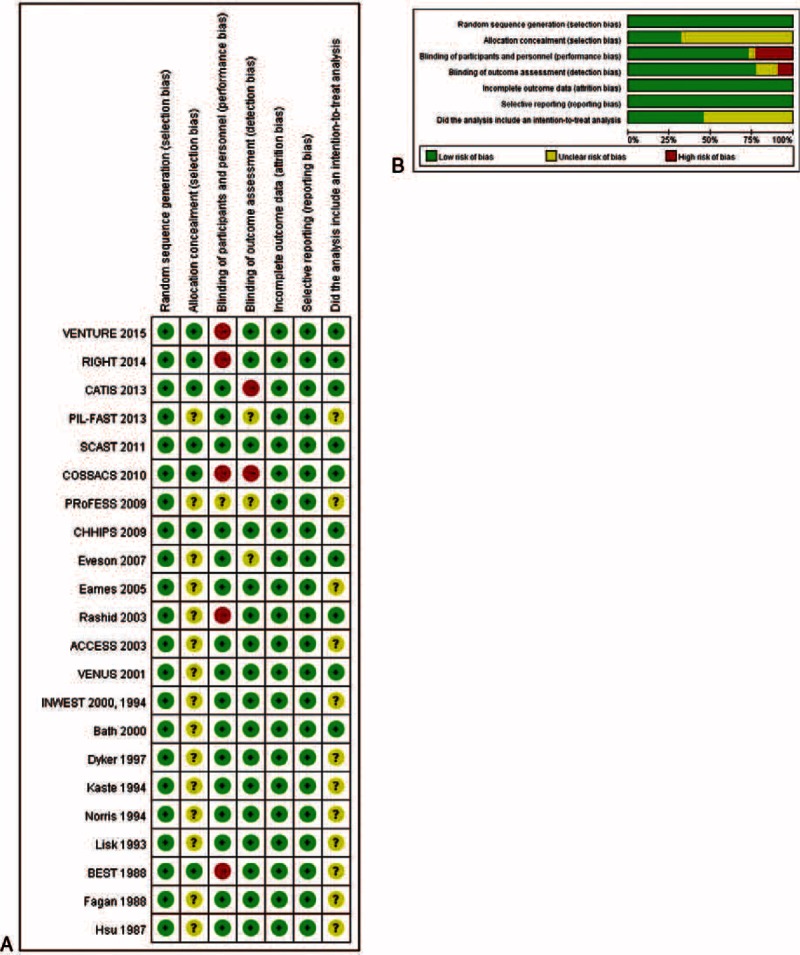
Quality assessment of the included studies. (A) Risk of bias summary. (B) Risk of bias graph.
DISCUSSION
The results of this meta-analysis showed, as expected, that antihypertensive agents effectively reduce BP during the acute phase of an ischemic stroke, though only ACEIs and multiple drugs effectively reduced DBP. Importantly, the analysis showed that administration of antihypertensive provided no benefit with respect to short- and long-term dependency and mortality.
In over 60% of patients, BP increases during the acute phase of a stroke and then subsequently decreases over about a 7- to 14-day period in approximately two-thirds of patients, with about one-third remaining hypertensive.1–3 Guidelines have recommend that acute lowering of BP should be delayed unless BP is >220/120 mmHg, >200/100 mmHg with end organ involvement, or >200/120 mmHg with primary intracerebral hemorrhage.17 Studies have also shown that both very low and very high BPs are associated with early and late death and dependency.5,7,28,39 Treatment of moderately elevated BP during the acute phase of a stroke, however, remains controversial with some studies indicating that lowering BP is safe and associated with benefits such as improved long-term mortality,7–9 other showing no benefit of lowering BP,11–13 and still others suggesting that lowering BP is harmful.12,14–16
The randomized, double-blind, placebo-controlled Very Early Nimodipine Use in Stroke (VENUS) trial showed no beneficial effect of nimodipine administered during the acute phase of a stroke,30 which confirmed the results of a prior trial published in 1994.34 Another early study of nimodipine also showed that nimodipine did not improve functional outcomes of ischemic strokes, and was associated with a higher early mortality rate than placebo.33 A more recent study of nimodipine for the treatment of acute stroke showed that reduction of DBP, but not of SBP, was associated with worse neurological outcomes.12
The Controlling Hypertension and Hypotension Immediately Post-Stroke trial compared labetalol, lisinopril, and placebo in 179 patients with acute ischemic or hemorrhagic strokes and found that treatment reduced 3-month mortality by 50% without an increase in serious adverse events.7 The Continue Or Stop post-Stroke Antihypertensives Collaborative Study trial studied patients with acute stroke who were taking antihypertensive medications at the time of the stroke.11 Patients were randomized to either stop or continue the antihypertensive medications, and the results showed that continuation of antihypertensive drugs did not reduce 2-week death or dependency, the cardiovascular event rate, or mortality at 6 months, and that lower BP levels in patients who continued antihypertensive medications were not associated with an increase in adverse events. The authors, however, pointed out that the trial was underpowered due to early termination. The recently published China Antihypertensive Trial in Acute Ischemic Stroke trial randomized 4071 patients with acute ischemic stroke at 26 hospitals in China to receive antihypertensive treatment or discontinue all antihypertensive medications found that BP reduction with antihypertensive medications did not reduce the likelihood of death and major disability at 14 days or at hospital discharge.10
The Scandinavian Candesartan Acute Stroke Trial (SCAST) randomized 2029 patients with acute stroke to receive candesartan or placebo and found no evidence that treatment had a beneficial effect, and may have increased the risk of poor outcome.14 Further analysis of the SCAST data showed that patients with a large decrease or increase/no change in SBP had a significantly increased risk of early adverse events relative to patients with a small decrease (OR = 2.08, 95% CI: 1.19–3.65 and OR = 1.96, 95% CI: 1.13–3.38, respectively), those with an increase/no change in SBP had a significantly increased risk of poor neurological outcomes as compared with the other groups (P = 0.001), and there were no differences in functional outcomes at 6 months.16
Other meta-analyses have examined the effect of lowering BP during the acute phase of a stroke. The most recent analysis published in 2014 by Wang et al15 included data of 13236 patients from 17 trials and found that early BP lowering was associated with a higher 30-day mortality as compared with placebo (relative risk: 1.34, 95% CI: 1.02–1.74, P = 0.03), but had no effect on early neurological deterioration, death within 7 days, long-term mortality, early and long-term dependency, early and long-term combination of death or dependency, and long-term stroke recurrence. A 2009 meta-analysis by Geeganage and Bath40 included 9008 patients from 37 trials, and found large falls or increases in BP were associated with worse outcomes, and that modest reductions in BP may reduce death and combine death or dependency. However, the authors pointed out that because the CIs were wide, an overall benefit or hazard could not be determined. A 2004 systematic review found that high BP in patients with acute ischemic or hemorrhagic stroke was associated with subsequent death, death or dependency, and death or deterioration and that moderate lowering of BP might improve outcomes.4
In the subgroup analysis of different classes of antihypertensive agents, only ACEIs and multiple drugs effectively reduced DBP. However, only 1 study examining BRA, prostaglandins, and diuretics, respectively, was available, only 2 articles were included in the analysis of CCBs and GTN, and the results of Oh et al24 were different from the other 2 studies in the ARB group. Wang et al41 have reported a differential lowering of SBP and DBP with antihypertensive agents and that the absolute benefit increased with age and with lower ratio of DBP to SBP lowering. In addition, in patients with a larger-than-median reduction in SBP, active treatment consistently reduced the risk of all outcomes irrespective of the decrease in DBP or the achieved DBP. Overall, these results suggest that more studies are needed to clarify the effects of different types of antihypertensives on DBP after a stroke.
There are limitations of this study that should be considered. The types of patients, antihypertensive agents used, treatment protocol, and efficacy and safety criteria differed between the included studies. Although the vast majority of patients had ischemic strokes, a small proportion had hemorrhagic strokes. It was not possible to only include patients with ischemic strokes without markedly limiting the number of included studies. Subgroup analysis for dependency and mortality was not performed because the number of studies in each drug subgroup was small with regard to short-term results, and the P values of each study were not significant. Therefore, we decided not to do this analysis for both long-term and short-term results for consistency. The time from symptoms to presentation varied between the studies, we did not examine adverse events of antihypertensive treatment, and patient-related factors were not considered.3 The analysis primarily included patients with ischemic strokes, and thus may not be applicable to patients with hemorrhagic stroke. The time range of the included studies was quite large, with the earliest study from 1987 and the most recent from 2015.
CONCLUSIONS
The results of this study indicate that although antihypertensive agents effectively reduced BP during the acute phase of an ischemic stroke, they do not result in a decrease in short- or long-term dependency or mortality. Further investigation to determine whether BP reduction may be of value in certain subgroups of patients may be warranted.
Acknowledgments
None.
Footnotes
Abbreviations: ACEI = angiotensin converting enzyme inhibitor, ARB = angiotensin-receptor blocker, BP = blood pressure, BRA = beta receptor antagonist, CCB = calcium channel blocker, cI = confidence interval, DBP = diastolic blood pressure, GTN = glyceryl trinitrate, MRS = modified Rankin Scale, OR = odds ration, PGI2 = prostacyclin, SBP = systolic blood pressure, SE = standard error.
RZ, F-DL, and SW contributed equally to this study.
This work was financially supported by the National Natural Science Foundation of China (No. 81271302 and 81070914 to J.R. Liu), the “New One Hundred Talent Project” from Shanghai Jiao Tong University School of Medicine, China (J.R. Liu), and the “Science and Technology Project” of the Shanghai Pudong New Area Health Bureau (Pudong New Area Population and Family Planning Commission) (No. PW 2013D-4, to J.R. Liu), research innovation project from Shanghai municipal science and technology commission (No. 14JC1404300, to J.R. Liu), and Shanghai Jiaotong University School of Medicine students’ innovative experiment project (2014, to J.R. Liu and J.L. Peng).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med 2007; 25:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qureshi AI. Acute hypertensive response in patients with stroke: pathophysiology and management. Circulation 2008; 118:176–187. [DOI] [PubMed] [Google Scholar]
- 3.Alqadri SL, Sreenivasan V, Qureshi AI. Acute hypertensive response management in patients with acute stroke. Curr Cardiol Rep 2013; 15:426.doi: 10.1007/s11886-013-0426-7. [DOI] [PubMed] [Google Scholar]
- 4.Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004; 43:18–24. [DOI] [PubMed] [Google Scholar]
- 5.Leonardi-Bee J, Bath PM, Phillips SJ, et al. IST Collaborative Group. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002; 33:1315–1320. [DOI] [PubMed] [Google Scholar]
- 6.Bogousslavsky J, Victor SJ, Salinas EO, et al. for the European-Australian Fiblast (Trafermin) in Acute Stroke Grouup. Fiblast (trafermin) in acute stroke: results of the European-Australian phase ii/iii safety and efficacy trial. Cerebrovasc Dis 2002; 14:239–251. [DOI] [PubMed] [Google Scholar]
- 7.Potter JF, Robinson TG, Ford GA, et al. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): a randomised, placebo-controlled, double-blind pilot trial. Lancet Neurol 2009; 8:48–56. [DOI] [PubMed] [Google Scholar]
- 8.Schrader J, Luders S, Kulschewski A, et al. The ACCESS Study: evaluation of Acute Candesartan Cilexetil Therapy in Stroke Survivors. Stroke 2003; 34:1699–1703. [DOI] [PubMed] [Google Scholar]
- 9.Anderson CS, Huang Y, Wang JG, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol 2008; 7:391–399. [DOI] [PubMed] [Google Scholar]
- 10.He J, Zhang Y, Xu T, et al. CATIS Investigators. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomised clinical trial. JAMA 2014; 311:479–489. [DOI] [PubMed] [Google Scholar]
- 11.Robinson TG, Potter JF, Ford GA, et al. COSSACS Investigators. Effects of antihypertensive treatment after acute stroke in the Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS): a prospective, randomised, open, blinded-endpoint trial. Lancet Neurol 2010; 9:767–775. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed N, Näsman P, Wahlgren NG. Effect of intravenous nimodipine on blood pressure and outcome after acute stroke. Stroke 2000; 31:1250–1255. [DOI] [PubMed] [Google Scholar]
- 13.Rashid P, Weaver C, Leonardi-Bee J, et al. The effects of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure, cerebral and cardiac hemodynamics, and plasma nitric oxide levels in acute stroke. J Stroke Cerebrovasc Dis 2003; 12:143–151. [DOI] [PubMed] [Google Scholar]
- 14.Sandset EC, Bath PM, Boysen G, et al. SCAST Study Group. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet 2011; 377:741–750. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Tang Y, Rong X, et al. Effects of early blood pressure lowering on early and long-term outcomes after acute stroke: an updated meta-analysis. PLoS One 2014; 9:e97917.doi: 10.1371/journal.pone.0097917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandset EC, Murray GD, Bath PM, et al. Scandinavian Candesartan Acute Stroke Trial (SCAST) Study Group. Relation between change in blood pressure in acute stroke and risk of early adverse events and poor outcome. Stroke 2012; 43:2108–2114. [DOI] [PubMed] [Google Scholar]
- 17.Bath PM, Krishnan K. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database Syst Rev 2014; 10:CD000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geeganage C, Bath PM. Vasoactive drugs for acute stroke. Cochrane Database Syst Rev 2010; 7:CD002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org. Updated March, 2011. [Google Scholar]
- 21.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997; 127:820–826. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 24.Oh MS, Yu KH, Hong KS, et al. Valsartan Efficacy oN modesT blood pressUre REduction in acute ischemic stroke (VENTURE) study group. Modest blood pressure reduction with valsartan in acute ischemic stroke: a prospective, randomized, open-label, blinded-end-point trial. Int J Stroke 2015; doi: 10.1111/ijs.12446. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Ankolekar S, Fuller M, Cross I, et al. Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: the rapid intervention with glyceryl trinitrate in Hypertensive Stroke Trial (RIGHT, ISRCTN66434824). Stroke 2013; 44:3120–3128. [DOI] [PubMed] [Google Scholar]
- 26.Shaw L, Price C, McLure S, et al. Paramedic Initiated Lisinopril For Acute Stroke Treatment (PIL-FAST): results from the pilot randomised controlled trial. Emerg Med J 2013; 31:994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bath PM, Martin RH, Palesch Y, et al. Effects of telmisartan on functional outcome, recurrence and blood pressure in patients with acute mild ischemic stroke: a PRoFESS subgroup analysis. Stroke 2009; 40:3541–3546. [DOI] [PubMed] [Google Scholar]
- 28.Robinson TG, Potter JF. Added to Meta Eveson DJ. Lisinopril for the treatment of hypertension within the first 24 hours of acute ischemic stroke and follow-up. Am J Hypertens 2007; 20:270–277. [DOI] [PubMed] [Google Scholar]
- 29.Eames PJ, Robinson TG, Panerai RB, et al. Bendrofluazide fails to reduce elevated blood pressure levels in the immediate post-stroke period. Cerebrovasc Dis 2005; 19:253–259. [DOI] [PubMed] [Google Scholar]
- 30.Horn J, de Haan RJ, Vermeulen M, et al. Very Early Nimodipine Use in Stroke (VENUS): a randomized, double-blind, placebo-controlled trial. Stroke 2001; 32:461–465. [DOI] [PubMed] [Google Scholar]
- 31.Bath PM, Pathansali R, Iddenden R, et al. The effect of transdermal glyceryl trinitrate, a nitric oxide donor on blood pressure and platelet function in acute stroke. Cerebrovasc Dis 2000; 11:265–272. [DOI] [PubMed] [Google Scholar]
- 32.Dyker AG, Grosset DG, Lees K. Perindopril reduces blood pressure but not cerebral blood flow in patients with recent cerebral ischemic stroke. Stroke 1997; 28:580–583. [DOI] [PubMed] [Google Scholar]
- 33.Kaste M, Fogelholm R, Erilä T, et al. A randomized, double-blind, placebo-controlled trial of nimodipine in acute ischemic hemispheric stroke. Stroke 1994; 25:1348–1353. [DOI] [PubMed] [Google Scholar]
- 34.Norris JW, Le Brun LH, Anderson BA. Intravenous nimodipine in acute ischaemic stroke. Cerebrovasc Dis 1994; 4:194–196. [Google Scholar]
- 35.Lisk DR, Grotta JC, Lamki LM, et al. Should hypertension be treated after acute stroke? A randomized controlled trial using single photon emission computed tomography. Arch Neurol 1993; 50:855–862. [DOI] [PubMed] [Google Scholar]
- 36.Barer DH, Cruickshank JM, Ebrahim SB, et al. Low dose beta blockade in acute stroke (BEST trial): an evaluation. BMJ 1988; 296:737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fagan SC, Gengo FM, Bates V, et al. Effect of nimodipine on blood pressure in acute ischemic stroke in humans. Stroke 1988; 19:401–402. [DOI] [PubMed] [Google Scholar]
- 38.Hsu CY, Faught RJ, Furlan AJ, et al. Intravenous prostacyclin in acute nonhemorrhagic stroke: a placebo-controlled double-blind trial. Stroke 1987; 18:352–358. [DOI] [PubMed] [Google Scholar]
- 39.Bogousslavsky J, Regli F, Zumstein V, et al. Double-blind study of nimodipine in non-severe stroke. Eur Neurol 1990; 30:23–26. [DOI] [PubMed] [Google Scholar]
- 40.Geeganage CM, Bath PM. Relationship between therapeutic changes in blood pressure and outcomes in acute stroke: a metaregression. Hypertension 2009; 54:775–781. [DOI] [PubMed] [Google Scholar]
- 41.Wang JG, Staessen JA, Franklin SS, et al. Systolic and diastolic blood pressure lowering as determinants of cardiovascular outcome. Hypertension 2005; 45:907–913. [DOI] [PubMed] [Google Scholar]


