Abstract
Research information on the risk of stroke in patients with dialysis-dependent end-stage renal disease (ESRD) who have undergone parathyroidectomy (PTX) is scant.
We used a nationwide health insurance claims database to select all patients with dialysis-dependent ESRD age 18 years and older for the study population. Of the patients with ESRD, we selected 1083 patients who had undergone PTX between 1998 and 2006 as the PTX group and frequency-matched 1083 patients with ESRD by sex, age, years since the disease diagnosis, and the year of undergoing PTX as the non-PTX group.
We used a multivariate Cox proportional hazards regression analysis to measure the risk of stroke for the PTX group compared with the non-PTX group after adjusting for sex, age, premium-based income, urbanization, and comorbidity.
The mean follow-up periods were 6.08 and 5.38 years for the PTX and non-PTX groups, respectively. After adjusting for previously mentioned variables, significant risk reductions of stroke (adjusted hazard ratio = 0.57, 95% confidence interval = 0.41–0.79), particularly those of hemorrhagic stroke (adjusted hazard ratio = 0.34, 95% confidence interval = 0.20–0.57), with PTX were observed. Chronologically, the risk of stroke in the PTX group decreased in the second year after PTX and persisted for >3 years.
PTX reduces the risk of stroke, particularly that of hemorrhagic stroke, in patients with dialysis-dependent ESRD. Other factors for risk reduction include sex (females), an age <65 years, and the presence of comorbidity.
INTRODUCTION
The incidence of stroke in dialysis-dependent patients with end-stage renal disease (ESRD) is up to 10 times greater than that in the general population and is associated with a poor prognosis.1,2 Patients on dialysis have a 3-fold higher risk of death following acute stroke, independent of traditional risk factors.1,3,4 The risk of hemorrhagic stroke is significantly higher in patients on dialysis who have had a stroke.5,6 Cerebral hemorrhage occurs 10 years earlier in patients on chronic dialysis.7 More than two-thirds of patients with cerebral hemorrhage die within 3 months of the onset of a cerebrovascular event.8 The risks of stroke are similar in patients regardless of dialysis modality.9 Other risk factors for stroke in dialysis-dependent patients include the initiation of dialysis,2,7 an older age, hypertension (HTN), diabetes mellitus (DM), a previous stroke, and atrial fibrillation (AF).8,9
Secondary hyperparathyroidism (SHPT) is one of the major problems for patients on long-term dialysis and is associated with increased vascular calcification, cardiovascular (CV) risk, and mortality.10–12 Levels of serum parathyroid hormone (PTH) >600 pg/mL are associated with a 21% increase in all-cause mortality risk.11 An elevated PTH level predicts a greater likelihood of prevalent and incident CV events, including myocardial infarction (MI), stroke, and CV death.12,13
Parathyroidectomy (PTX) reduces the risk of CV death and all-cause mortality.10,14–16 Sharma et al14 reported a 33% reduced risk of CV death after PTX. Iwamoto et al17 reported that the CV death-free survival rate is significantly improved in the PTX group than in the non-PTX group (94.6% vs 76.3%). Kestenbaum et al18 demonstrated that the long-term relative risk of death among patients undergoing PTX was 10% to 15% lower than that among patients not undergoing PTX. However, in a report by Conzo et al,19 PTX did not alter CV morbidity and mortality rates among patients on hemodialysis with SHPT; delayed treatment was considered a possible factor.
The relationship between PTX and incident stroke in patients with ESRD receiving regular dialysis warrants further attention; however, no previous study has addressed this topic. This study aimed to investigate the impact of PTX on the risk of incident stroke in patients with dialysis-dependent ESRD in a retrospective cohort study by using a nationwide health insurance database. We hypothesized that undergoing PTX reduces the risk of incident stroke in patients with ESRD.
METHODS
Data Sources
The National Health Insurance (NHI) program of Taiwan is a single-payer and compulsory health care program introduced in 1995. By 2008, the NHI program provided coverage for >99% of the population of Taiwan, and >92% of health care organizations in Taiwan had signed health service contracts.20
The data analyzed in this study were retrieved from the Taiwan NHI Research Database (NHIRD), a database maintained by the Taiwan National Health Research Institutes, and containing comprehensive outpatient and inpatient information, such as demographic data, dates of visits, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes, and complete prescription details. Dialysis-dependent ESRD is included in the list of catastrophic illnesses of the NHI program. Patients with dialysis-dependent ESRD can apply for a catastrophic illness certificate, with which they can have all ESRD-related health care copayments waived; therefore, >99% of patients with dialysis-dependent ESRD are included in the data sets of the Registry for Catastrophic Illness Patient Database (RCIPD), which was used in this study to select all patients with dialysis-dependent ESRD as the study population. The data sets of the study consisted of the registry of beneficiaries, ambulatory and inpatient care claims, and the RCIPD from 1996 to 2011 from the NHIRD. To protect patient privacy, all personal identification numbers were encrypted before electronic files were released. This study was approved by the institutional review board of China Medical University (CMU-REC-101-012).
Study Patients
Figure 1 depicts the study framework. We included all patients with incident ESRD age 18 years and older, based on the records of ESRD in the RCIPD (ICD-9-CM code 585) and who began regular renal replacement therapy (ie, dialysis or renal transplant) for >90 days from January 1, 1998, to December 31, 2004. All the patients with ESRD who newly underwent PTX were selected and constituted the PTX group. The exclusion criteria included a previous history of stroke (ICD-9-CM code 430–438), renal transplantation (ICD-9-CM code V42.0), parathyroid tumor (ICD-9-CM code 194.1 and 227.1), or other parathyroid disorders (ICD-9-CM code 252.8) during 1998–2006. The date of first PTX was considered the index date. We selected patients with ESRD who did not undergo PTX to form the non-PTX group, based on identical exclusion criteria, by conducting 1:1 random frequency matching by sex, age (in 5-year bands), years since ESRD diagnosis, and the year of undergoing PTX.
FIGURE 1.
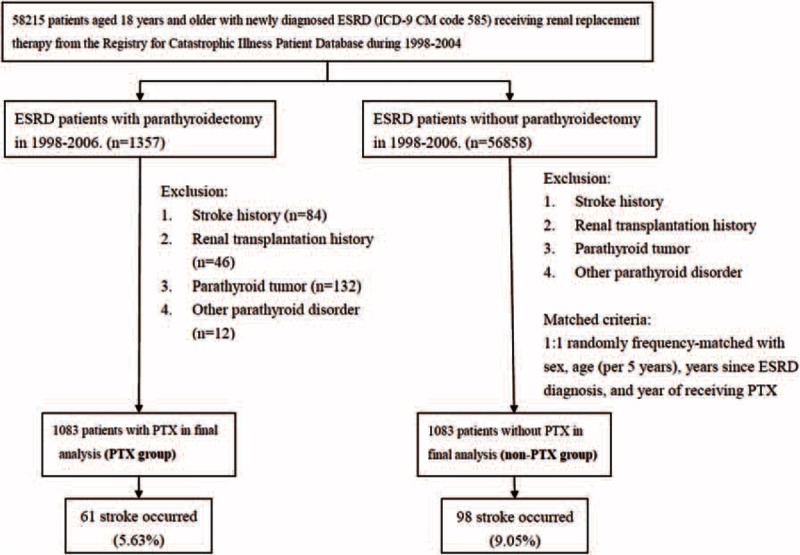
Flow chart showing selection of study subjects. ESRD = end-stage renal disease; PTX = parathyroidectomy.
The demographic factors studied included sex, age, monthly income surrogated as premium-based income, and urbanization. The amount of premium-based income was categorized into 3 levels: <NT$15,000, NT$15 000–29 999, and ≥NT$30,000. The urbanization level was defined according to a Taiwan National Health Research Institutes report (levels 1 and 4 were the highest and lowest levels of urbanization, respectively). The information and characteristics (ICD-9-CM code) of comorbidity, including DM (ICD-9-CM 250), hyperlipidemia (HL; ICD-9-CM 272.0–272.4), HTN (ICD-9-CM 401–405), ischemic heart disease (IHD; ICD-9-CM 410–414), AF (ICD-9-CM 427.31), congestive heart failure (CHF; ICD-9-CM 398.91, 425, and 428), chronic obstructive pulmonary disease (COPD; ICD-9-CM 491–494 and 496), obesity (ICD-9-CM 278), and alcohol-related diseases (ICD-9-CM 291, 303, 305, 571.0, 571.1, 571.2, 571.3, 790.3, and V11.3), were collected from the NHIRD.
The main outcome was the occurrence of stroke during the follow-ups, which was defined by at least once admission record of stroke by ICD-9 disease code in the discharge diagnosis, including hemorrhagic stroke (ICD-9-CM 430–431), ischemic stroke (ICD-9-CM 433–434), transient ischemic attack (ICD-9-CM 435), and unspecified acute stroke (ICD-9-CM 436). Participants were followed from the index date to the date of stroke diagnosis, withdrawal from the insurance program, death, or the end date of the database (December 31, 2011).
Statistical Analyses
First, we compared the distributions of demographic variables and comorbidities between the PTX and non-PTX groups. The chi-square test was used to determine the differences between the 2 groups regarding the distribution of categorical variables. Differences in mean age between the 2 groups were examined using a Student t test. The incidence density rate (per 1000 person-years) of stroke was calculated by dividing the number of incident strokes by person-years at risk in both the groups. The follow-up person-years were calculated for each patient since the index date until the stroke diagnosis, at the end of 2011, or until withdrawal from the insurance system. The cumulative incidence of stroke was computed using the Kaplan–Meier method, and differences in cumulative incidences between the 2 groups were tested using a log-rank test. We used a multivariable Cox proportional hazards regression analysis to measure the adjusted hazard ratio of stroke for the PTX group compared with the non-PTX group after adjusting for sex, age, premium-based income, urbanization, and comorbidity (DM, HL, HTN, IHD, AF, CHF, COPD, obesity, and alcohol-related diseases). All statistical analyses were performed using the SAS 9.3 statistical package (SAS Institute, Cary, NC). The 2-sided level of significance was set at 0.05.
RESULTS
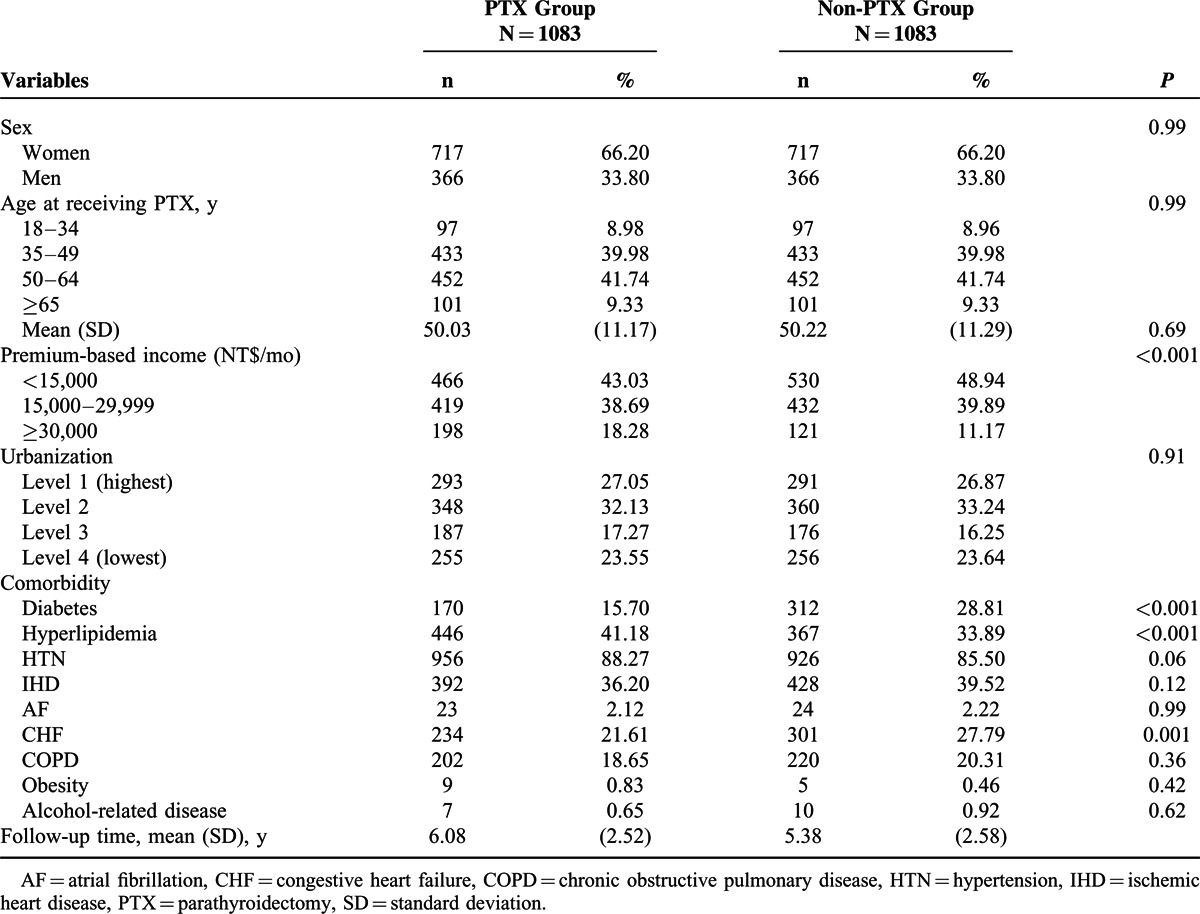
There were 58,215 patients with incident ESRD during 1998–2004. After exclusion and matches, as detailed in Figure 1, the PTX and matched non-PTX groups consisted of 1083 patients each between 1998 and 2006. Women accounted for 66.20% of the patients in both the groups. The mean age in both the groups was 50.03 versus 50.22 years (P = 0.69) (Table 1). Compared with the non-PTX group, the PTX group was less likely to have a low income, diabetes, and CHF, but more likely to have HL.
TABLE 1.
Demographic Factors and Comorbidity of Patients With End-Stage Renal Disease According to PTX Status
The average time from incident ESRD to undergoing PTX for the PTX group was 4.65 ± 1.97 years; the mean follow-up periods from undergoing PTX to the stroke event were 6.08 ± 2.52 and 5.38 ± 2.58 years for the PTX and matched non-PTX groups, respectively.
The risk of stroke (hazard ratio [HR] = 0.55), particularly that of hemorrhagic stroke (HR = 0.35), was lower in the PTX group compared with that in the non-PTX group (9.26 vs 16.81 per 1000 person-years, respectively; Table 2). After adjusting for sex, age, premium-based income, urbanization, and comorbidity, the risks of stroke in the PTX group were 43% lower than those in the non-PTX group (adjusted HR = 0.57, 95% confidence interval [CI] = 0.41–0.79); the risk reduction of hemorrhagic stroke was even higher, up to 66%, in the PTX group (adjusted HR = 0.34, 95% CI = 0.20–0.57). The Kaplan–Meier analysis revealed that the non-PTX group had a higher cumulative incidence rate of stroke than the PTX group did (log-rank test, P < 0.001) (Figure 2).
TABLE 2.
Incidence Density Rates and Hazard Ratios of Stroke Between PTX and Non-PTX Groups
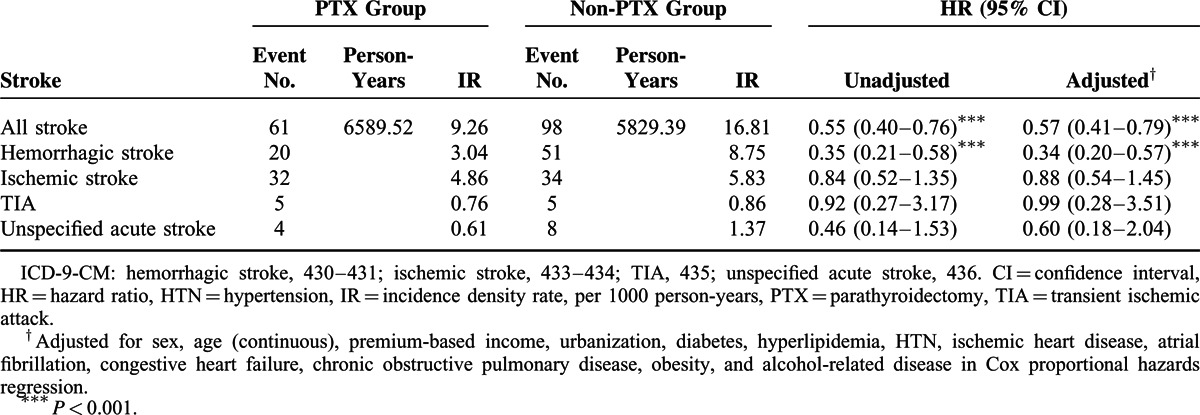
FIGURE 2.
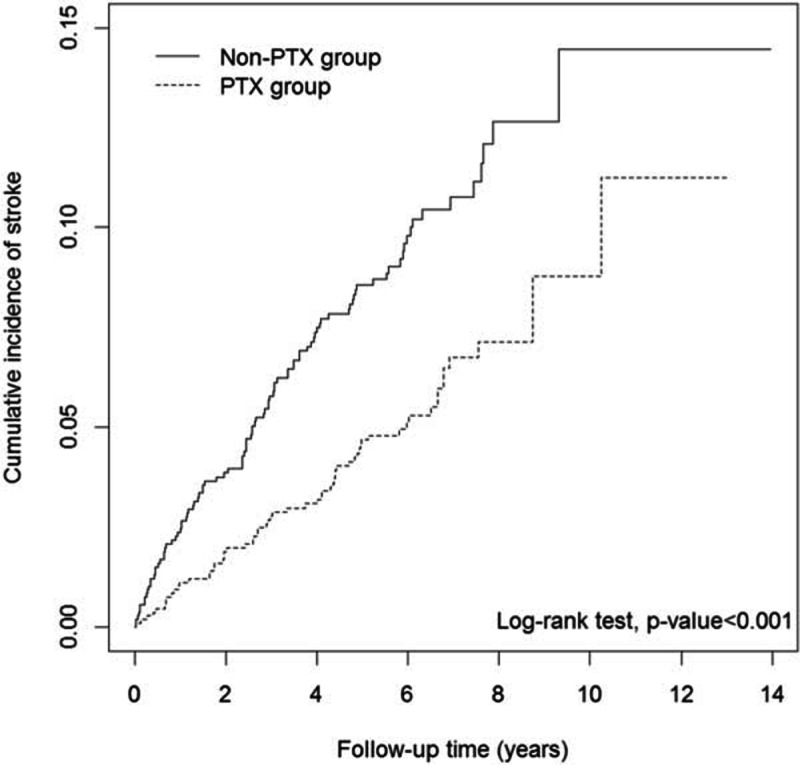
Cumulative incidence curves of stroke for PTX and no PTX groups. PTX = parathyroidectomy.
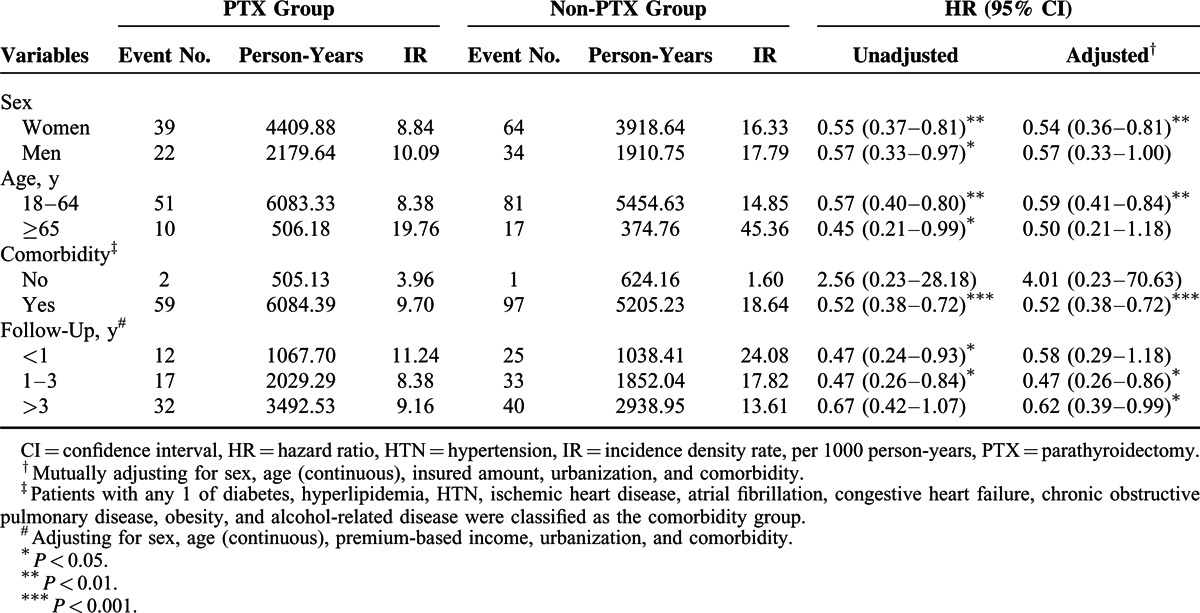
Women in the PTX group had a lower risk of stroke than those in the non-PTX group did (adjusted HR = 0.54, 95% CI = 0.36–0.81) (Table 3). Younger patients in the PTX group, age 18 to 64 years, exhibited a lower risk of stroke than those in the non-PTX group with the same age range did (adjusted HR = 0.59, 95% CI = 0.41–0.84). Regarding comorbidity, patients with a comorbidity in the PTX group had a significantly reduced risk of stroke than those with a comorbidity in the non-PTX group did (adjusted HR = 0.52, 95% CI = 0.38–0.72). When stratified by follow-up periods, the risk of stroke began to decrease significantly in the PTX group in the second year after PTX, and the risk reduction persisted for >3 years (Table 3).
TABLE 3.
Incidence Density Rates and Hazard Ratios of Stroke According to PTX Status Stratified by Sex, Age, Comorbidities, and Follow-Up Periods
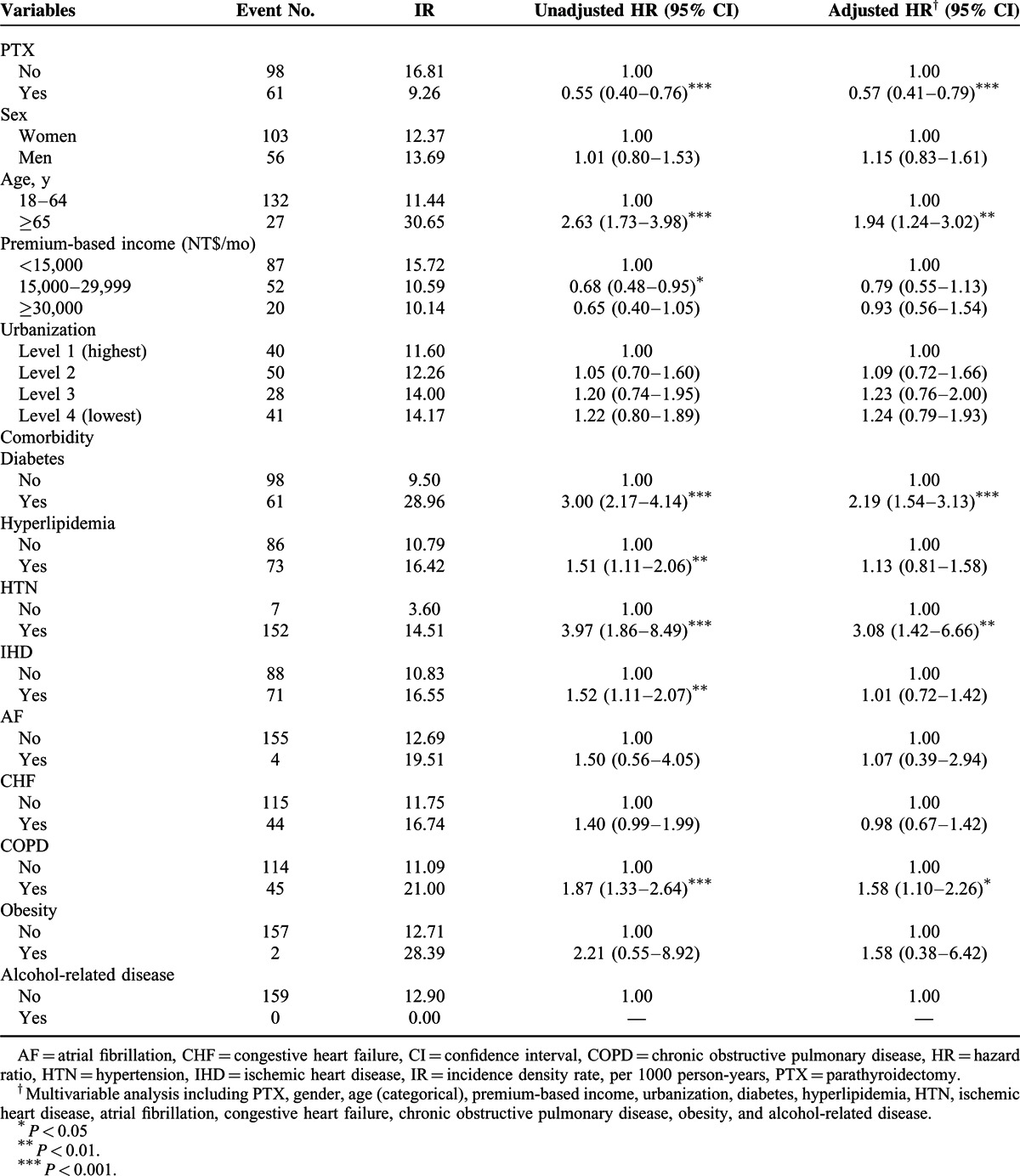
Compared with the patients age 18 to 64 years, those age 65 years and older had a 1.94-fold higher risk of stroke development (95% CI = 1.24–3.02) (Table 4). Other risk factors for stroke, demonstrated by the multivariate Cox proportional hazard model, included diabetes (2.19, 95% CI = 1.54–3.13), HTN (3.08, 95% CI = 1.42–6.66), and COPD (1.58, 95% CI = 1.10–2.26).
TABLE 4.
Cox Model Measured Hazard Ratios and 95% Confidence Interval of Stroke Associated With PTX and Covariates
DISCUSSION
Principal Findings
This nationwide population-based retrospective cohort study was conducted in an area with the highest prevalence of ESRD. After matching for sex, age, years since ESRD diagnosis, and the year of undergoing PTX and adjusting the regression models to control the effects of age, sex, premium-based income, urbanization, and comorbidity (DM, HL, HTN, IHD, AF, CHF, COPD, obesity, and alcohol-related disease), we observed that patients with ESRD who had undergone PTX had a 43% reduced risk of developing stroke compared with those who did not undergo PTX. The risk reduction in developing hemorrhagic stroke was even higher, up to 66%. Female sex, an age <65 years, and the presence of comorbidity were factors associated with reduced risk of stroke in patients with ESRD who had undergone PTX. With the mean follow-up period of 6.08 years after PTX and 5.38 years in the matched non-PTX group, the risk of stroke decreased in the second year after PTX and continued to decrease for >3 years. The other crucial risk factors associated with developing stroke included an age >65 years, diabetes, HTN, and COPD.
Comparison With the Literature
No previous research has investigated stroke in patients with ESRD who had undergone PTX; our findings are complementary to those of previous studies, whereby decreased CV events and a more favorable prognosis were observed in patients on dialysis who had undergone PTX.21–24 Bleyer et al21 reported improvements in vascular calcification after PTX in a small series of patients on dialysis. Shih et al22 reported a series of 21 patients on hemodialysis with severe SHPT who achieved a normotensive and euvolemic status after PTX. Nanasato et al23 reported a restored left ventricular function and an improved cardiac fatty acid metabolism after PTX in a patient on hemodialysis. In a cohort study conducted by Lin et al24 with 53 nondiabetic patients with hemodialysis-dependent ESRD who had intact PTH levels of >800 pg/mL, PTX was associated with improved outcomes in major CV events, including death, cerebrovascular accidents, and MI. Several other investigations have confirmed that PTX improves survival in patients with ESRD. In the retrospective matched study between 88 patients who had undergone PTX and 88 control patients on dialysis with no indication of PTX, conducted by Iwamoto et al,17 the CV death-free survival rate was significantly improved in the PTX group. A retrospective study conducted by Goldenstein et al10 with a cohort of 251 patients with chronic kidney disease with severe SHPT confirms the benefit of PTX on mortality. Sharma et al14 reported that near-total PTX in patients on dialysis was associated with a significant reduction in the long-term risk of death. However, in a systematic review evaluating the evidence for the association between mineral metabolism disturbances and the risk of all-cause mortality, CV mortality, and CV events in chronic kidney disease, none of the studies independently assessed stroke, transient ischemic attack, or acute coronary syndrome.25 In this study, we provided evidence from a nationwide investigation of 1083 cases with matched controls, and for the first time, reported that stroke was the primary outcome in patients with ESRD with a history of previous PTX treatment versus those without a history of previous PTX treatment.
We found COPD to be a risk factor for stroke in patients with ESRD who are on dialysis, possibly because COPD is a result of chronic systemic inflammation and has a close relationship with CV diseases.26 Our findings are consistent with those of a previous report by Yin et al.27
Potential Mechanisms
Accelerated atherosclerosis is the key factor for the high prevalence of CV diseases in patients with renal dysfunction.28 Several studies indicated that high PTH levels could be causally involved in the process leading to the manifestation of CV diseases in patients both normal or impaired renal function.12,29,30 In an animal study, PTH2 receptor messenger RNA was abundantly expressed in the arterial and cardiac endothelium, which supported the close relationships between PTH and CV risk.31 Patients on hemodialysis had advanced atherosclerosis in the carotid arteries compared with matched nondialysis patients.32 A clinical study showed that high PTH levels are closely associated with coronary calcification.33 The serum PTH level was reported to be independently associated with the intima-media thickness of the femoral artery in patients on hemodialysis.34 Iwamoto et al17 found that in the PTX group, both PTH and calcium levels fell significantly, and the prognosis was improved. Bleyer et al21 reported that after PTX in patients with ESRD, the calcification progression slowed down.
Clinical Implication
The prevention of stroke in patients with dialysis-dependent ESRD is challenging. Although antiplatelet therapy, particularly aspirin, is reported to be a safe and effective treatment for ischemic stroke prevention in patients with ESRD undergoing dialysis,35 other studies have claimed that antiplatelet or warfarin treatment cannot lower the risk of ischemic stroke in patients with ESRD.36 In addition, lipid-lowering regimens, which have proven effective in reducing the risks of MI and major cardiac events, failed to provide positive evidence for stroke in patients with dialysis-dependent ESRD.37 Furthermore, few studies have reported an effective regimen for reducing the risk of hemorrhagic stroke in patients with ESRD. Our data provided the first positive evidence that PTX may reduce the risk of stroke, particularly that of hemorrhagic stroke. We considered that PTH represents a new CV risk factor for patients with ESRD, supporting the suggestions by Anderson et al.12 Additional studies may be required to compare the risk reduction of stroke by PTX versus medical treatments for patients with ESRD with SHPT.
LIMITATIONS
Several limitations must be discussed. First, the study was conducted on a health insurance claims database and lacked information on personal health behaviors and certain crucial CV risk factors, such as smoking, body mass index, alcoholism, exercise, and dietary habits. We were unable to control certain residual confounding factors. However, we included alcohol-related disease to adjust for the influence of alcohol intake. We also included HTN, DM, HL, and obesity to adjust for the influence of body mass index. In addition, we used an alternative method to adjust for smoking-related diseases (including IHD and COPD) to minimize the potential confounding effect of smoking. We understood that neither IHD nor COPD could represent detrimental CV effects of smoking; besides, COPD might result from other causes including alpha 1 antitrypsin deficiency. Nevertheless, alpha 1 antitrypsin deficiency is very rare in Chinese population; in addition, similar methods had been accepted in certain previous study.38 Second, limited by the characteristics of the NHIRD, we had no access to lab data (including PTH levels) and details of medical treatment with vitamin D analogs or calcimimetics for patients on hemodialysis, which made it difficult to include SHP patients indicated for PTX. Nevertheless, NHI claims undergo strict review, which provides a reliable method for including ESRD patients with SHPT who are indicated for the operation. Third, patients with ESRD without an indication for PTX might have been included in the non-PTX group, which may have attenuated the potential effects of PTX; therefore, we might have underestimated the risk reduction of stroke with PTX. However, the significant results may suggest a strengthened relationship between PTX and a reduced risk of stroke. Fourth, Study designs comparing the PTX and non-PTX groups might have incurred a selection bias. Female sex, a younger age, the absence of diabetes, and HTN were factors associated with higher rates of PTX.39,40 However, all these factors were matched (age, sex) or adjusted for (diabetes, HTN) in our study design. Finally, the event number in our study was small; hence, we could not further divide our study patients into different modalities of renal replacement therapy. Further research with larger case series and longer follow-up periods might be required.
The major strengths of this research include the following: first, we adopted novel approaches to analyze the relationship between PTX and the risk of stroke, to divide the category of stroke into detailed subgroups, and to confirm higher risks of hemorrhagic stroke in patients with ESRD but with a greater risk reduction after undergoing PTX. Second, we conducted a comprehensive adjustment to control for multiple confounding factors, including traditional CV risk factors and AF, CHF, IHD, and COPD. Third, a large research sample (1083 patients in the PTX group and 1083 matched controls out of 56,858 patients with ESRD) and long follow-up period (mean = 6.08 ± 2.52 and 5.38 ± 2.58 years for the PTX and non-PTX groups, respectively) increased the validity of the data.
CONCLUSION
PTX reduces the risk of stroke, particularly that of hemorrhagic stroke. Other significant factors for risk reduction in patients undergoing PTX include female sex, an age below 65 years, and the presence of comorbidity. Patients aged ≥65 years and with DM, HTN, and COPD are at a higher risk of developing stroke. The risk reduction of stroke in patients with ESRD who have undergone PTX becomes significant after the second year of follow-up and persists thereafter. Thus, PTH may be considered a new CV risk factor for patients with ESRD.
Acknowledgments
We appreciated the professional comments provided by Dr Sung Sheng-Feng.
Footnotes
Abbreviations: CI = confidence interval, ESRD = end-stage renal disease, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NHIRD = National Health Insurance Research Database, PTH = parathyroid hormone, PTX = parathyroidectomy, RCIPD = Registry for Catastrophic Illness Patient Database, SHPT = secondary hyperparathyroidism.
All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript: conception/design—Y-HH, C-HK; provision of study materials—C-HK; collection and/or assembly of data—all authors; data analysis and interpretation—Y-HH, H-JC, C-HK; manuscript writing—all authors; final approval of manuscript—all authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002, Taiwan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have declared that no competing interests exist.
REFERENCES
- 1.Power A, Chan K, Singh SK, et al. Appraising stroke risk in maintenance hemodialysis patients: a large single-center cohort study. Am J Kidney Dis 2012; 59:249–257. [DOI] [PubMed] [Google Scholar]
- 2.Sánchez-Perales C, Vázquez E, García-Cortés MJ, et al. Ischaemic stroke in incident dialysis patients. Nephrol Dial Transplant 2010; 25:3343–3348. [DOI] [PubMed] [Google Scholar]
- 3.Ovbiagele B. Chronic kidney disease and risk of death during hospitalization for stroke. J Neurol Sci 2011; 301:46–50. [DOI] [PubMed] [Google Scholar]
- 4.Ocak G, van Stralen KJ, Rosendaal FR, et al. Mortality due to pulmonary embolism, myocardial infarction, and stroke among incident dialysis patients. J Thromb Haemost 2012; 10:2484–2493. [DOI] [PubMed] [Google Scholar]
- 5.Iseki K, Kinjo K, Kimura Y, et al. Evidence for high risk of cerebral hemorrhage in chronic dialysis patients. Kidney Int 1993; 44:1086–1090. [DOI] [PubMed] [Google Scholar]
- 6.Kawamura M, Fijimoto S, Hisanaga S, et al. Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis 1998; 31:991–996. [DOI] [PubMed] [Google Scholar]
- 7.Murray AM, Seliger S, Lakshminarayan K, et al. Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol 2013; 24:1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wetmore JB, Ellerbeck EF, Mahnken JD, et al. Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol 2013; 23:112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Power A. Stroke in dialysis and chronic kidney disease. Blood Purif 2013; 36:179–183. [DOI] [PubMed] [Google Scholar]
- 10.Goldenstein PT, Elias RM, Pires de Freitas do Carmo L, et al. Parathyroidectomy improves survival in patients with severe hyperparathyroidism: a comparative study. PloS ONE 2013; 8:e68870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52:519–530. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JL, Vanwoerkom RC, Horne BD, et al. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: dependent or independent risk factors? Am Heart J 2011; 162:331.e2–339.e2. [DOI] [PubMed] [Google Scholar]
- 13.Rostand SG, Drueke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 1999; 56:383–392. [DOI] [PubMed] [Google Scholar]
- 14.Sharma J, Raggi P, Kutner N, et al. Improved long-term survival of dialysis patients after near-total parathyroidectomy. J Am Coll Surg 2012; 214:400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacour B, Roullet JB, Liagre AM, et al. Serum lipoprotein disturbances in primary and secondary hyperparathyroidism and effects of parathyroidectomy. Am J Kidney Dis 1986; 8:422–429. [DOI] [PubMed] [Google Scholar]
- 16.Evenepoel P, Claes K, Kuypers D, et al. Impact of parathyroidectomy on renal graft function, blood pressure and serum lipids in kidney transplant recipients: a single centre study. Nephrol Dial Transplant 2005; 20:1714–1720. [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto N, Sato N, Nishida M, et al. Total parathyroidectomy improves survival of hemodialysis patients with secondary hyperparathyroidism. J Nephrol 2012; 25:755–763. [DOI] [PubMed] [Google Scholar]
- 18.Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int 2004; 66:2010–2016. [DOI] [PubMed] [Google Scholar]
- 19.Conzo G, Perna AF, Savica V, et al. Impact of parathyroidectomy on cardiovascular outcomes and survival in chronic hemodialysis patients with secondary hyperparathyroidism. A retrospective study of 50 cases prior to the calcimimetics era. BMC Surg 2013; 13 suppl 2: S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bureau of National Health Insurance DoH, Executive Yuan. National Health Insurance, Taiwan, 2012-2013 Annual Report 2012 2013/6/22 [cited January 20, 2015]: [76 p.]. http://www.nhi.gov.tw/Resource/webdata/13767_1_NHI_2012-2013%20ANNUAL%20REPORT.pdf. [Google Scholar]
- 21.Bleyer AJ, Burkart J, Piazza M, et al. Changes in cardiovascular calcification after parathyroidectomy in patients with ESRD. Am J Kidney Dis 2005; 46:464–469. [DOI] [PubMed] [Google Scholar]
- 22.Shih CJ, Tarng DC, Yang WC, et al. Parathyroidectomy reduces intradialytic hypotension in hemodialysis patients with secondary hyperparathyroidism. Kidney Blood Press Res 2013; 37:323–331. [DOI] [PubMed] [Google Scholar]
- 23.Nanasato M, Goto N, Isobe S, et al. Restored cardiac conditions and left ventricular function after parathyroidectomy in a hemodialysis patient. Parathyroidectomy improves cardiac fatty acid metabolism assessed by 123I-BMIPP. Circ J 2009; 73:1956–1960. [DOI] [PubMed] [Google Scholar]
- 24.Lin HC, Chen CL, Lin HS, et al. Parathyroidectomy improves cardiovascular outcome in nondiabetic dialysis patients with secondary hyperparathyroidism. Clin Endocrinol (Oxf) 2014; 80:508–515. [DOI] [PubMed] [Google Scholar]
- 25.Covic A, Kothawala P, Bernal M, et al. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24:1506–1523. [DOI] [PubMed] [Google Scholar]
- 26.Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of ’overspill’ of inflammatory mediators from the lungs? Review of the evidence. Thorax 2010; 6:930–936. [DOI] [PubMed] [Google Scholar]
- 27.Yin L, Lensmar C, Ingelsson E, et al. Differential association of chronic obstructive pulmonary disease with myocardial infarction and ischemic stroke in a nation-wide cohort. Int J Cardiol 2014; 173:601–603. [DOI] [PubMed] [Google Scholar]
- 28.Hojs Fabjan T, Hojs R. Stroke and renal dysfunction. Eur J Intern Med 2014; 25:18–24. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson IL, Aberg J, Rastad J, et al. Endothelial vasodilatory dysfunction in primary hyperparathyroidism is reversed after parathyroidectomy. Surgery 1999; 126:1049–1055. [DOI] [PubMed] [Google Scholar]
- 30.Rashid G, Bernheim J, Green J, et al. Parathyroid hormone stimulates endothelial expression of atherosclerotic parameters through protein kinase pathways. Am J Physiol Renal Physiol 2007; 292:F1215–F1218. [DOI] [PubMed] [Google Scholar]
- 31.Usdin TB, Bonner TI, Harta G, et al. Distribution of parathyroid hormone-2 receptor messenger ribonucleic acid in rat. Endocrinology 1996; 137:4285–4297. [DOI] [PubMed] [Google Scholar]
- 32.Hojs R. Carotid intima-media thickness and plaques in hemodialysis patients. Artif Organs 2000; 24:691–695. [DOI] [PubMed] [Google Scholar]
- 33.Coen G, Manni M, Mantella D, et al. Are PTH serum levels predictive of coronary calcifications in haemodialysis patients? Nephrol Dial Transplant 2007; 22:3262–3267. [DOI] [PubMed] [Google Scholar]
- 34.Kawagishi T, Nishizawa Y, Konishi T, et al. High-resolution B-mode ultrasonography in evaluation of atherosclerosis in uremia. Kidney Int 1995; 48:820–826. [DOI] [PubMed] [Google Scholar]
- 35.Chen CY, Lee KT, Lee CT, et al. Effectiveness and safety of antiplatelet in stroke patients with end-stage renal disease undergoing dialysis. Int J Stroke 2014; 9:580–590. [DOI] [PubMed] [Google Scholar]
- 36.Chen JJ, Lin LY, Yang YH, et al. Anti-platelet or anti-coagulant agent for the prevention of ischemic stroke in patients with end-stage renal disease and atrial fibrillation-A nation-wide database analyses. Int J Cardiol 2014; 177:1008–1011. [DOI] [PubMed] [Google Scholar]
- 37.Kasiske BL, Wheeler DC. The management of dyslipidemia in CKD: new analyses of an expanding dataset. Am J Kidney Dis 2013; 61:371–374. [DOI] [PubMed] [Google Scholar]
- 38.Yeh CC, Wang HH, Chou YC, et al. High risk of gastrointestinal hemorrhage in patients with epilepsy: a nationwide cohort study. Mayo Clin Proc 2013; 88:1091–1098. [DOI] [PubMed] [Google Scholar]
- 39.Kestenbaum B, Seliger SL, Gillen DL, et al. Parathyroidectomy rates among United States dialysis patients: 1990-1999. Kidney Int 2004; 65:282–288. [DOI] [PubMed] [Google Scholar]
- 40.Chuang CH, Wang JJ, Weng SF, et al. Epidemiology and mortality among dialysis patients with parathyroidectomy: Taiwan National Cohort Study. J Nephrol 2013; 26:1143–1150. [DOI] [PubMed] [Google Scholar]


